Trichomonas vaginalis Virus: Current Insights and Emerging Perspectives
Abstract
1. Introduction
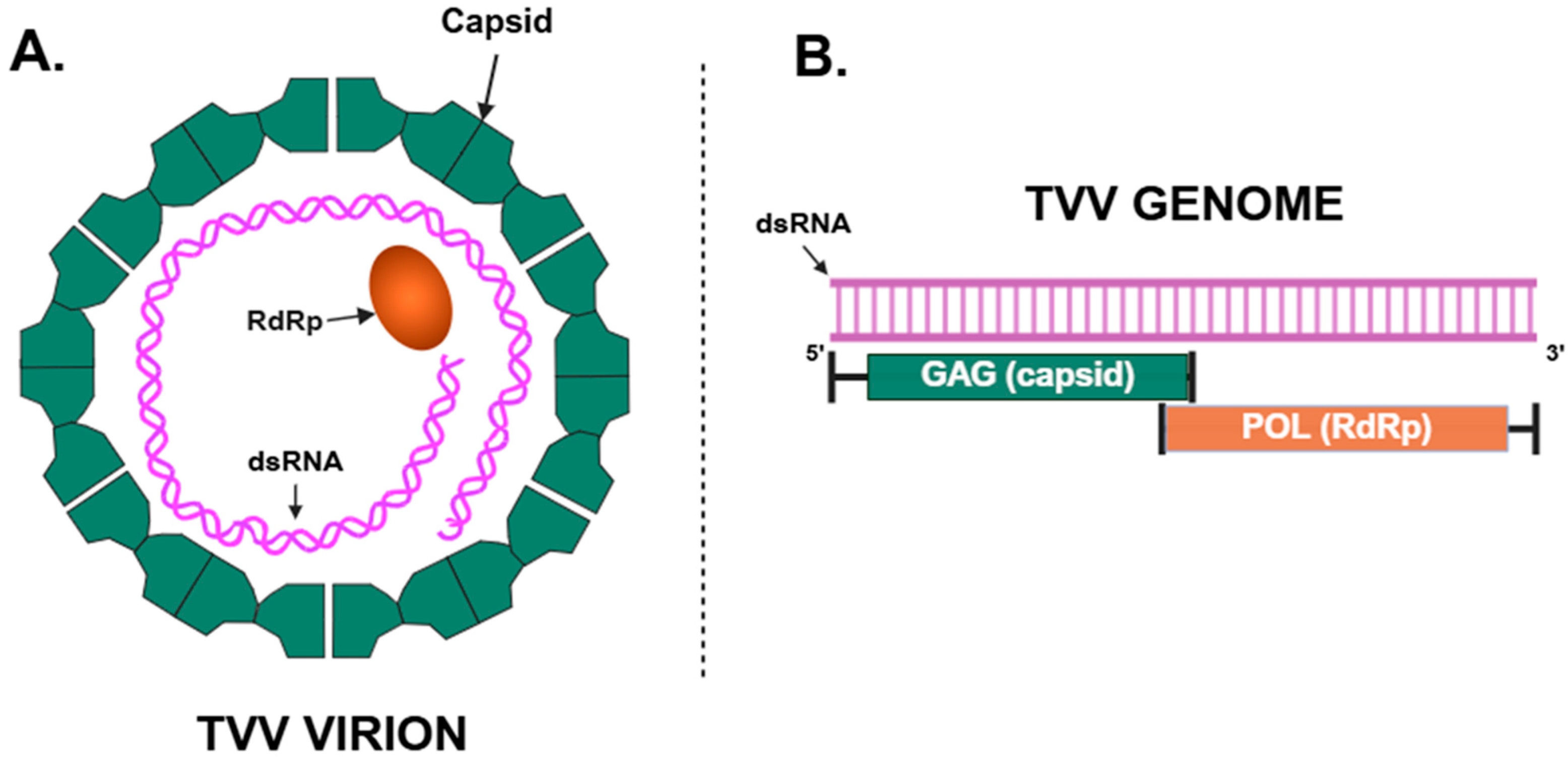
2. Genetic Structure and Transmission of TVV
3. Impact of TVV on T. vaginalis Protein Expression
3.1. Cysteine Proteases
3.2. Surface Antigens
3.3. Other Proteins
4. 5-Nitroimidazole Drug Susceptibility
5. Clinical Significance
6. Conclusions and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Rowley, J.; Vander Hoorn, S.; Korenromp, E.; Low, N.; Unemo, M.; Abu-Raddad, L.J.; Chico, R.M.; Smolak, A.; Newman, L.; Gottlieb, S.; et al. Chlamydia, gonorrhoea, trichomoniasis and syphilis: Global prevalence and incidence estimates, 2016. Bull. World Health Organ. 2019, 97, 548–562. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Liu, L.; Liu, K.; Qin, X.; Wu, J.; Jiang, L.; Shang, L. Global burden of trichomoniasis: Current status, trends, and projections (1990–2021). Front. Public. Health 2025, 13, 1530227. [Google Scholar] [CrossRef] [PubMed]
- Patel, E.U.; Gaydos, C.A.; Packman, Z.R.; Quinn, T.C.; Tobian, A.A.R. Prevalence and correlates of Trichomonas vaginalis infection among men and women in the United States. Clin. Infect. Dis. 2018, 67, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Van Gerwen, O.T.; Craig-Kuhn, M.C.; Jones, A.T.; Schroeder, J.A.; Deaver, J.; Buekens, P.; Kissinger, P.J.; Muzny, C.A. Trichomoniasis and adverse birth outcomes: A systematic review and meta-analysis. BJOG Int. J. Obstet. Gynaecol. 2021, 128, 1907–1915. [Google Scholar] [CrossRef]
- Masha, S.C.; Cools, P.; Sanders, E.J.; Vaneechoutte, M.; Crucitti, T. Trichomonas vaginalis and HIV infection acquisition: A systematic review and meta-analysis. Sex. Transm. Infect. 2019, 95, 36–42. [Google Scholar] [CrossRef]
- Allsworth, J.E.; Ratner, J.A.; Peipert, J.F. Trichomoniasis and other sexually transmitted infections: Results from the 2001–2004 National Health and Nutrition Examination Surveys. Sex. Transm. Dis. 2009, 36, 738–744. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, Y.; Lu, H.; Li, D.; Zhang, R.; Xie, X.; Guo, L.; Hao, L.; Tian, X.; Yang, Z.; et al. A systematic review of the correlation between Trichomonas vaginalis infection and infertility. Acta Trop. 2022, 236, 106693. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, D.; Li, Y.; Zhang, R.; Xie, X.; Yao, Y.; Zhao, L.; Tian, X.; Yang, Z.; Wang, S.; et al. The correlation between Trichomonas vaginalis infection and reproductive system cancer: A systematic review and meta-analysis. Infect. Agent. Cancer 2023, 18, 15. [Google Scholar] [CrossRef]
- Muzny, C.A.; George, S.; Kissinger, P.J.; Van Gerwen, O.T. Trichomoniasis and other sexually transmitted parasitic diseases in women. Clin. Obstet. Gynecol. 2025, 68, 194–205. [Google Scholar] [CrossRef]
- Workowski, K.A.; Bachmann, L.H.; Chan, P.A.; Johnston, C.M.; Muzny, C.A.; Park, I.; Reno, H.; Zenilman, J.M.; Bolan, G.A. Sexually transmitted infections treatment guidelines, 2021. MMWR Recomm. Rep. 2021, 70, 1–187. [Google Scholar]
- Mbizvo, E.M.; Msuya, S.E.; Stray-Pedersen, B.; Sundby, J.; Chirenje, Z.M.; Hussain, A. Determinants of reproductive tract infections among asymptomatic women in Harare, Zimbabwe. Cent. Afr. J. Med. 2001, 47, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Wiringa, A.E.; Ness, R.B.; Darville, T.; Beigi, R.H.; Haggerty, C.L. Trichomonas vaginalis, endometritis and sequelae among women with clinically suspected pelvic inflammatory disease. Sex. Transm. Infect. 2020, 96, 436–438. [Google Scholar] [CrossRef]
- Van Gerwen, O.T.; Camino, A.F.; Sharma, J.; Kissinger, P.J.; Muzny, C.A. Epidemiology, natural history, diagnosis, and treatment of Trichomonas vaginalis in Men. Clin. Infect. Dis. 2021, 73, 1119–1124. [Google Scholar] [CrossRef] [PubMed]
- Van Gerwen, O.T.; Opsteen, S.A.; Graves, K.J.; Muzny, C.A. Trichomoniasis. Infect. Dis. Clin. N. Am. 2023, 37, 245–265. [Google Scholar] [CrossRef]
- Kissinger, P.; Mena, L.; Levison, J.; Clark, R.A.; Gatski, M.; Henderson, H.; Schmidt, N.; Rosenthal, S.L.; Myers, L.; Martin, D.H. A randomized treatment trial: Single versus 7-day dose of metronidazole for the treatment of Trichomonas vaginalis among HIV-infected women. J. Acquir. Immune Defic. Syndr. 2010, 55, 565–571. [Google Scholar] [CrossRef] [PubMed]
- Kissinger, P.; Muzny, C.A.; Mena, L.A.; Lillis, R.A.; Schwebke, J.R.; Beauchamps, L.; Taylor, S.N.; Schmidt, N.; Myers, L.; Augostini, P.; et al. Single-dose versus 7-day-dose metronidazole for the treatment of trichomoniasis in women: An open-label, randomised controlled trial. Lancet Infect. Dis. 2018, 18, 1251–1259. [Google Scholar] [CrossRef]
- Muzny, C.A.; Schwebke, J.R.; Nyirjesy, P.; Kaufman, G.; Mena, L.A.; Lazenby, G.B.; Van Gerwen, O.T.; Graves, K.J.; Arbuckle, J.; Carter, B.A.; et al. Efficacy and safety of single oral dosing of secnidazole for trichomoniasis in women: Results of a phase 3, randomized, double-blind, placebo-controlled, delayed-treatment study. Clin. Infect. Dis. 2021, 73, e1282–e1289. [Google Scholar] [CrossRef]
- Muzny, C.A.; Van Gerwen, O.T.; Legendre, D. Secnidazole: A treatment for trichomoniasis in adolescents and adults. Expert Rev. Anti Infect. Ther. 2022, 20, 1067–1076. [Google Scholar] [CrossRef]
- Kirkcaldy, R.D.; Augostini, P.; Asbel, L.E.; Bernstein, K.T.; Kerani, R.P.; Mettenbrink, C.J.; Pathela, P.; Schwebke, J.R.; Secor, W.E.; Workowski, K.A.; et al. Trichomonas vaginalis antimicrobial drug resistance in 6 US cities, STD Surveillance Network, 2009–2010. Emerg. Infect. Dis. 2012, 18, 939–943. [Google Scholar] [CrossRef]
- Conrad, M.D.; Gorman, A.W.; Schillinger, J.A.; Fiori, P.L.; Arroyo, R.; Malla, N.; Dubey, M.L.; Gonzalez, J.; Blank, S.; Secor, W.E.; et al. Extensive genetic diversity, unique population structure and evidence of genetic exchange in the sexually transmitted parasite Trichomonas vaginalis. PLoS Negl. Trop. Dis. 2012, 6, e1573. [Google Scholar] [CrossRef]
- Wang, A.L.; Wang, C.C. A linear double-stranded RNA in Trichomonas vaginalis. J. Biol. Chem. 1985, 260, 3697–3702. [Google Scholar] [CrossRef]
- Wang, A.L.; Wang, C.C. The double-stranded RNA in Trichomonas vaginalis may originate from virus-like particles. Proc. Natl. Acad. Sci. USA 1986, 83, 7956–7960. [Google Scholar] [CrossRef]
- Graves, K.J.; Ghosh, A.P.; Kissinger, P.J.; Muzny, C.A. Trichomonas vaginalis virus: A review of the literature. Int. J. STD AIDS 2019, 30, 496–504. [Google Scholar] [CrossRef] [PubMed]
- Parent, K.N.; Takagi, Y.; Cardone, G.; Olson, N.H.; Ericsson, M.; Yang, M.; Lee, Y.; Asara, J.M.; Fichorova, R.N.; Baker, T.S.; et al. Structure of a protozoan virus from the human genitourinary parasite Trichomonas vaginalis. mBio 2013, 4, e00056-13. [Google Scholar] [CrossRef] [PubMed]
- Fraga, J.; Rojas, L.; Sariego, I.; Fernandez-Calienes, A.; Nunez, F.A. Species typing of Cuban Trichomonas vaginalis virus by RT-PCR, and association of TVV-2 with high parasite adhesion levels and high pathogenicity in patients. Arch. Virol. 2012, 157, 1789–1795. [Google Scholar] [CrossRef]
- Goodman, R.P.; Freret, T.S.; Kula, T.; Geller, A.M.; Talkington, M.W.; Tang-Fernandez, V.; Suciu, O.; Demidenko, A.A.; Ghabrial, S.A.; Beach, D.H.; et al. Clinical isolates of Trichomonas vaginalis concurrently infected by strains of up to four Trichomonasvirus species (Family Totiviridae). J. Virol. 2011, 85, 4258–4270. [Google Scholar] [CrossRef] [PubMed]
- Manny, A.R.; Hetzel, C.A.; Mizani, A.; Nibert, M.L. Discovery of a novel species of Trichomonasvirus in the human parasite Trichomonas vaginalis using transcriptome mining. Viruses 2022, 14, 548. [Google Scholar] [CrossRef]
- Bahadory, S.; Aminizadeh, S.; Taghipour, A.; Bokharaei-Salim, F.; Khanaliha, K.; Razizadeh, M.H.; Soleimani, A.; Beikzadeh, L.; Khatami, A. A systematic review and meta-analysis on the global status of Trichomonas vaginalis virus in Trichomonas vaginalis. Microb. Pathog. 2021, 158, 105058. [Google Scholar] [CrossRef]
- Khoshnan, A.; Alderete, J.F. Multiple double-stranded RNA segments are associated with virus particles infecting Trichomonas vaginalis. J. Virol. 1993, 67, 6950–6955. [Google Scholar] [CrossRef]
- Benchimol, M.; Chang, T.H.; Alderete, J.F. Visualization of new virus-like-particles in Trichomonas vaginalis. Tissue Cell 2002, 34, 406–415. [Google Scholar] [CrossRef]
- Benchimol, M.; Monteiro, S.; Chang, T.H.; Alderete, J.F. Virus in Trichomonas—An ultrastructural study. Parasitol. Int. 2002, 51, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Khoshnan, A.; Provenzano, D.; Alderete, J.F. Unique double-stranded RNAs associated with the Trichomonas vaginalis virus are synthesized by viral RNA-dependent RNA polymerase. J. Virol. 1994, 68, 7108–7114. [Google Scholar] [CrossRef] [PubMed]
- Benchimol, M.; Chang, T.H.; Alderete, J.F. Trichomonas vaginalis: Observation of coexistence of multiple viruses in the same isolate. FEMS Microbiol. Lett. 2002, 215, 197–201. [Google Scholar] [CrossRef]
- Liu, H.W.; Chu, Y.D.; Tai, J.H. Characterization of Trichomonas vaginalis virus proteins in the pathogenic protozoan T. vaginalis. Arch. Virol. 1998, 143, 963–970. [Google Scholar] [CrossRef]
- Vanacova, S.; Tachezy, J.; Kulda, J.; Flegr, J. Characterization of trichomonad species and strains by PCR fingerprinting. J. Eukaryot. Microbiol. 1997, 44, 545–552. [Google Scholar] [CrossRef]
- Ong, S.C.; Cheng, W.H.; Ku, F.M.; Tsai, C.Y.; Huang, P.J.; Lee, C.C.; Yeh, Y.M.; Rada, P.; Hrdy, I.; Narayanasamy, R.K.; et al. Identification of endosymbiotic virus in small extracellular vesicles derived from Trichomonas vaginalis. Genes 2022, 13, 531. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, H.M.; Marcet, R.; Sarracent, J. Biological roles of cysteine proteinases in the pathogenesis of Trichomonas vaginalis. Parasite 2014, 21, 54. [Google Scholar] [CrossRef]
- Carlton, J.M.; Hirt, R.P.; Silva, J.C.; Delcher, A.L.; Schatz, M.; Zhao, Q.; Wortman, J.R.; Bidwell, S.L.; Alsmark, U.C.; Besteiro, S.; et al. Draft genome sequence of the sexually transmitted pathogen Trichomonas vaginalis. Science 2007, 315, 207–212. [Google Scholar] [CrossRef]
- Arroyo, R.; Cardenas-Guerra, R.E.; Figueroa-Angulo, E.E.; Puente-Rivera, J.; Zamudio-Prieto, O.; Ortega-Lopez, J. Trichomonas vaginalis cysteine proteinases: Iron response in gene expression and proteolytic activity. Biomed. Res. Int. 2015, 2015, 946787. [Google Scholar] [CrossRef]
- Miranda-Ozuna, J.F.T.; Rivera-Rivas, L.A.; Cardenas-Guerra, R.E.; Hernandez-Garcia, M.S.; Rodriguez-Cruz, S.; Gonzalez-Robles, A.; Chavez-Munguia, B.; Arroyo, R. Glucose-restriction increases Trichomonas vaginalis cellular damage towards HeLa cells and proteolytic activity of cysteine proteinases (CPs), such as TvCP2. Parasitology 2019, 146, 1156–1166. [Google Scholar] [CrossRef]
- Rivera-Rivas, L.A.; Arroyo, R. Iron restriction increases the expression of a cytotoxic cysteine proteinase TvCP2 by a novel mechanism of tvcp2 mRNA alternative polyadenylation in Trichomonas vaginalis. Biochim. Biophys. Acta Gene Regul. Mech. 2023, 1866, 194935. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Rivas, L.A.; Lorenzo-Benito, S.; Sanchez-Rodriguez, D.B.; Miranda-Ozuna, J.F.; Euceda-Padilla, E.A.; Ortega-Lopez, J.; Chavez-Munguia, B.; Lagunes-Guillen, A.; Velazquez-Valassi, B.; Jasso-Villazul, L.; et al. The effect of iron on Trichomonas vaginalis TvCP2: A cysteine proteinase found in vaginal secretions of trichomoniasis patients. Parasitology 2020, 147, 760–774. [Google Scholar] [CrossRef] [PubMed]
- He, D.; Pengtao, G.; Ju, Y.; Jianhua, L.; He, L.; Guocai, Z.; Xichen, Z. Differential protein expressions in virus-infected and uninfected Trichomonas vaginalis. Korean J. Parasitol. 2017, 55, 121–128. [Google Scholar] [CrossRef]
- Provenzano, D.; Khoshnan, A.; Alderete, J.F. Involvement of dsRNA virus in the protein composition and growth kinetics of host Trichomonas vaginalis. Arch. Virol. 1997, 142, 939–952. [Google Scholar] [CrossRef] [PubMed]
- Alderete, J.F. Iron modulates phenotypic variation and phosphorylation of P270 in double-stranded RNA virus-infected Trichomonas vaginalis. Infect. Immun. 1999, 67, 4298–4302. [Google Scholar] [CrossRef]
- Zimmann, N.; Rada, P.; Zarsky, V.; Smutna, T.; Zahonova, K.; Dacks, J.; Harant, K.; Hrdy, I.; Tachezy, J. Proteomic analysis of Trichomonas vaginalis phagolysosome, lysosomal targeting, and unconventional secretion of cysteine peptidases. Mol. Cell Proteom. 2022, 21, 100174. [Google Scholar] [CrossRef]
- Musatovova, O.; Alderete, J.F. The Trichomonas vaginalis phenotypically varying P270 immunogen is highly conserved except for numbers of repeated elements. Microb. Pathog. 1999, 27, 93–104. [Google Scholar]
- Musatovova, O.; Alderete, J.F. Molecular analysis of the gene encoding the immunodominant phenotypically varying P270 protein of Trichomonas vaginalis. Microb. Pathog. 1998, 24, 223–239. [Google Scholar] [CrossRef]
- Dailey, D.C.; Alderete, J.F. The phenotypically variable surface protein of Trichomonas vaginalis has a single, tandemly repeated immunodominant epitope. Infect. Immun. 1991, 59, 2083–2088. [Google Scholar] [CrossRef]
- Alderete, J.F. Localization of the phenotypically varying P270 protein on dsRNA virus-positive and negative Trichomonas vaginalis isolates. Am. J. Biomed. Sci. Res. 2021, 14, 199–209. [Google Scholar] [CrossRef]
- Wang, A.; Wang, C.C.; Alderete, J.F. Trichomonas vaginalis phenotypic variation occurs only among trichomonads infected with the double-stranded RNA virus. J. Exp. Med. 1987, 166, 142–150. [Google Scholar] [CrossRef]
- Khoshnan, A.; Alderete, J.F. Trichomonas vaginalis with a double-stranded RNA virus has upregulated levels of phenotypically variable immunogen mRNA. J. Virol. 1994, 68, 4035–4038. [Google Scholar] [CrossRef]
- Alderete, J.F.; Kasmala, L.; Metcalfe, E.; Garza, G.E. Phenotypic variation and diversity among Trichomonas vaginalis isolates and correlation of phenotype with trichomonal virulence determinants. Infect. Immun. 1986, 53, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Stafkova, J.; Rada, P.; Meloni, D.; Zarsky, V.; Smutna, T.; Zimmann, N.; Harant, K.; Pompach, P.; Hrdy, I.; Tachezy, J. Dynamic secretome of Trichomonas vaginalis: Case study of beta-amylases. Mol. Cell Proteom. 2018, 17, 304–320. [Google Scholar] [CrossRef]
- Rada, P.; Kellerova, P.; Verner, Z.; Tachezy, J. Investigation of the secretory pathway in Trichomonas vaginalis argues against a moonlighting function of hydrogenosomal enzymes. J. Eukaryot. Microbiol. 2019, 66, 899–910. [Google Scholar] [CrossRef] [PubMed]
- Twu, O.; de Miguel, N.; Lustig, G.; Stevens, G.C.; Vashisht, A.A.; Wohlschlegel, J.A.; Johnson, P.J. Trichomonas vaginalis exosomes deliver cargo to host cells and mediate hostratioparasite interactions. PLoS Pathog. 2013, 9, e1003482. [Google Scholar] [CrossRef]
- Kochanowsky, J.A.; Mira, P.M.; Elikaee, S.; Muratore, K.; Rai, A.K.; Riestra, A.M.; Johnson, P.J. Trichomonas vaginalis extracellular vesicles up-regulate and directly transfer adherence factors promoting host cell colonization. Proc. Natl. Acad. Sci. USA 2024, 121, e2401159121. [Google Scholar] [CrossRef]
- Rada, P.; Hrdy, I.; Zdrha, A.; Narayanasamy, R.K.; Smutna, T.; Horackova, J.; Harant, K.; Benes, V.; Ong, S.C.; Tsai, C.Y.; et al. Double-Stranded RNA viruses are released from Trichomonas vaginalis inside small extracellular vesicles and modulate the exosomal cargo. Front. Microbiol. 2022, 13, 893692. [Google Scholar] [CrossRef] [PubMed]
- Nievas, Y.R.; Coceres, V.M.; Midlej, V.; de Souza, W.; Benchimol, M.; Pereira-Neves, A.; Vashisht, A.A.; Wohlschlegel, J.A.; Johnson, P.J.; de Miguel, N. Membrane-shed vesicles from the parasite Trichomonas vaginalis: Characterization and their association with cell interaction. Cell. Mol. Life Sci. 2018, 75, 2211–2226. [Google Scholar] [CrossRef]
- Santana de Andrade, J.C.; Benchimol, M.; de Souza, W. Stimulation of microvesicle secretion in Trichomonas vaginalis. Exp. Parasitol. 2024, 259, 108722. [Google Scholar] [CrossRef]
- Artuyants, A.; Campos, T.L.; Rai, A.K.; Johnson, P.J.; Dauros-Singorenko, P.; Phillips, A.; Simoes-Barbosa, A. Extracellular vesicles produced by the protozoan parasite Trichomonas vaginalis contain a preferential cargo of tRNA-derived small RNAs. Int. J. Parasitol. 2020, 50, 1145–1155. [Google Scholar] [CrossRef] [PubMed]
- Flegr, J.; Cerkasov, J.; Kulda, J.; Tachezy, J.; Stokrov, J. The dsRNA of Trichomonas vaginalis is associated with virus like particles and does not correlate with metronidazole resistance. Folia Microbiol. 1987, 32, 345–348. [Google Scholar] [CrossRef]
- Snipes, L.J.; Gamard, P.M.; Narcisi, E.M.; Beard, C.B.; Lehmann, T.; Secor, W.E. Molecular epidemiology of metronidazole resistance in a population of Trichomonas vaginalis clinical isolates. J. Clin. Microbiol. 2000, 38, 3004–3009. [Google Scholar] [CrossRef]
- Hampl, V.; Vanacova, S.; Kulda, J.; Flegr, J. Concordance between genetic relatedness and phenotypic similarities of Trichomonas vaginalis strains. BMC Evol. Biol. 2001, 1, 11. [Google Scholar] [CrossRef]
- Malla, N.; Kaul, P.; Sehgal, R.; Gupta, I. The presence of dsRNA virus in Trichomonas vaginalis isolates from symptomatic and asymptomatic Indian women and its correlation with in vitro metronidazole sensitivity. Indian. J. Med. Microbiol. 2011, 29, 152–157. [Google Scholar] [CrossRef] [PubMed]
- da Luz Becker, D.; dos Santos, O.; Frasson, A.P.; de Vargas Rigo, G.; Macedo, A.J.; Tasca, T. High rates of double-stranded RNA viruses and Mycoplasma hominis in Trichomonas vaginalis clinical isolates in South Brazil. Infect. Genet. Evol. 2015, 34, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Wendel, K.A.; Rompalo, A.M.; Erbelding, E.J.; Chang, T.H.; Alderete, J.F. Double-stranded RNA viral infection of Trichomonas vaginalis infecting patients attending a sexually transmitted diseases clinic. J. Infect. Dis. 2002, 186, 558–561. [Google Scholar] [CrossRef]
- Fraga, J.; Rojas, L.; Sariego, I.; Fernandez-Calienes, A.; Nunez, F.A. Double-Stranded RNA viral infection of Trichomonas vaginalis and association with clinical presentation. Acta Protozool. 2007, 46, 93–98. [Google Scholar]
- Fraga, J.; Rojas, L.; Sariego, I.; Fernandez-Calienes, A. Double-stranded RNA viral infection of Trichomonas vaginalis and correlation with genetic polymorphism of isolates. Exp. Parasitol. 2011, 127, 593–599. [Google Scholar] [CrossRef]
- El-Gayar, E.K.; Mokhtar, A.B.; Hassan, W.A. Molecular characterization of double-stranded RNA virus in Trichomonas vaginalis Egyptian isolates and its association with pathogenicity. Parasitol. Res. 2016, 115, 4027–4036. [Google Scholar] [CrossRef]
- Jehee, I.; van der Veer, C.; Himschoot, M.; Hermans, M.; Bruisten, S. Direct detection of Trichomonas vaginalis virus in Trichomonas vaginalis positive clinical samples from the Netherlands. J. Virol. Methods 2017, 250, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Fichorova, R.N.; Lee, Y.; Yamamoto, H.S.; Takagi, Y.; Hayes, G.R.; Goodman, R.P.; Chepa-Lotrea, X.; Buck, O.R.; Murray, R.; Kula, T.; et al. Endobiont viruses sensed by the human host—Beyond conventional antiparasitic therapy. PLoS ONE 2012, 7, e48418. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Graves, K.J.; Novak, J.; Muzny, C.A. Trichomonas vaginalis Virus: Current Insights and Emerging Perspectives. Viruses 2025, 17, 898. https://doi.org/10.3390/v17070898
Graves KJ, Novak J, Muzny CA. Trichomonas vaginalis Virus: Current Insights and Emerging Perspectives. Viruses. 2025; 17(7):898. https://doi.org/10.3390/v17070898
Chicago/Turabian StyleGraves, Keonte J., Jan Novak, and Christina A. Muzny. 2025. "Trichomonas vaginalis Virus: Current Insights and Emerging Perspectives" Viruses 17, no. 7: 898. https://doi.org/10.3390/v17070898
APA StyleGraves, K. J., Novak, J., & Muzny, C. A. (2025). Trichomonas vaginalis Virus: Current Insights and Emerging Perspectives. Viruses, 17(7), 898. https://doi.org/10.3390/v17070898





