Insights into the Landscape of Alphavirus Receptor and Antibody Interactions
Abstract
1. Introduction
2. Alphavirus Structure and Influence on Receptors and Antibodies Binding
3. Cellular Receptors and Attachment Factors
3.1. Alphavirus Attachment Factors
3.1.1. Attachment Factors Without Structural Characterization with Alphaviruses
3.1.2. Attachment Factors Structurally Characterized with Alphaviruses
3.2. Alphavirus Receptors
3.2.1. Receptors Without Structural Characterization as Complexes with Alphaviruses
- Putative Receptors
- B.
- Bona fide receptors
3.2.2. Structurally Characterized Receptor Complexes with Alphaviruses
4. Host Cell Restriction Factors Implicated in Viral Egress
5. Antibodies Against Alphaviruses and Their Binding Mechanisms:
5.1. Structural Analyses of Alphaviruses in Complex with Fab Fragments of Neutralizing Antibodies
5.2. Pan-Arthritogenic Neutralizing Alphavirus Antibodies
5.3. Pan-Alphavirus Antibodies
6. Implications for Vaccines and Therapeutic Development
7. Conclusions and Future Directions
Funding
Conflicts of Interest
References
- Jose, J.; Snyder, J.E.; Kuhn, R.J. A structural and functional perspective of alphavirus replication and assembly. Future Microbiol. 2009, 4, 837–856. [Google Scholar] [CrossRef] [PubMed]
- Skidmore, A.M.; Bradfute, S.B. The life cycle of the alphaviruses: From an antiviral perspective. Antivir. Res. 2023, 209, 105476. [Google Scholar] [CrossRef] [PubMed]
- Sewell, D.L. Laboratory-associated infections and biosafety. Clin. Microbiol. Rev. 1995, 8, 389–405. [Google Scholar] [CrossRef] [PubMed]
- Cheng, R.H.; Kuhn, R.J.; Olson, N.H.; Rossmann, M.G.; Choi, H.K.; Smith, T.J.; Baker, T.S. Nucleocapsid and glycoprotein organization in an enveloped virus. Cell 1995, 80, 621–630. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, S.; Zhang, W.; Gabler, S.; Chipman, P.R.; Strauss, E.G.; Strauss, J.H.; Baker, T.S.; Kuhn, R.J.; Rossmann, M.G. Mapping the structure and function of the E1 and E2 glycoproteins in alphaviruses. Structure 2006, 14, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Hasan, S.S.; Sun, C.; Kim, A.S.; Watanabe, Y.; Chen, C.L.; Klose, T.; Buda, G.; Crispin, M.; Diamond, M.S.; Klimstra, W.B.; et al. Cryo-EM Structures of Eastern Equine Encephalitis Virus Reveal Mechanisms of Virus Disassembly and Antibody Neutralization. Cell Rep. 2018, 25, 3136–3147.e5. [Google Scholar] [CrossRef] [PubMed]
- Holmes, A.C.; Basore, K.; Fremont, D.H.; Diamond, M.S. A molecular understanding of alphavirus entry. PLoS Pathog. 2020, 16, e1008876. [Google Scholar] [CrossRef] [PubMed]
- Raju, S.; Adams, L.J.; Diamond, M.S. The many ways in which alphaviruses bind to cells. Trends Immunol. 2024, 45, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Ramsey, J.; Mukhopadhyay, S. Disentangling the Frames, the State of Research on the Alphavirus 6K and TF Proteins. Viruses 2017, 9, 228. [Google Scholar] [CrossRef] [PubMed]
- Snyder, J.E.; Kulcsar, K.A.; Schultz, K.L.; Riley, C.P.; Neary, J.T.; Marr, S.; Jose, J.; Griffin, D.E.; Kuhn, R.J. Functional characterization of the alphavirus TF protein. J. Virol. 2013, 87, 8511–8523. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.S.; Diamond, M.S. A molecular understanding of alphavirus entry and antibody protection. Nat. Rev. Microbiol. 2023, 21, 396–407. [Google Scholar] [CrossRef] [PubMed]
- Mateu, M.G. Introduction: The structural basis of virus function. Subcell. Biochem. 2013, 68, 3–51. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.C.; Mukhopadhyay, S.; Zlotnick, A. Geometric Defects and Icosahedral Viruses. Viruses 2018, 10, 25. [Google Scholar] [CrossRef] [PubMed]
- Cao, D.; Ma, B.; Cao, Z.; Zhang, X.; Xiang, Y. Structure of Semliki Forest virus in complex with its receptor VLDLR. Cell 2023, 186, 2208–2218.e15. [Google Scholar] [CrossRef] [PubMed]
- Adams, L.J.; Raju, S.; Ma, H.; Gilliland, T.; Reed, D.S.; Klimstra, W.B.; Fremont, D.H.; Diamond, M.S. Structural and functional basis of VLDLR usage by Eastern equine encephalitis virus. Cell 2024, 187, 360–374.e19. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, O.; Holmes, A.C.; Kafai, N.M.; Adams, L.J.; Diamond, M.S. Entry receptors—The gateway to alphavirus infection. J. Clin. Investig. 2023, 133, e165307. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Kim, A.S.; Fox, J.M.; Nair, S.; Basore, K.; Klimstra, W.B.; Rimkunas, R.; Fong, R.H.; Lin, H.; Poddar, S.; et al. Mxra8 is a receptor for multiple arthritogenic alphaviruses. Nature 2018, 557, 570–574. [Google Scholar] [CrossRef] [PubMed]
- Gardner, C.L.; Ebel, G.D.; Ryman, K.D.; Klimstra, W.B. Heparan sulfate binding by natural eastern equine encephalitis viruses promotes neurovirulence. Proc. Natl. Acad. Sci. USA 2011, 108, 16026–16031. [Google Scholar] [CrossRef] [PubMed]
- Klimstra, W.B.; Nangle, E.M.; Smith, M.S.; Yurochko, A.D.; Ryman, K.D. DC-SIGN and L-SIGN can act as attachment receptors for alphaviruses and distinguish between mosquito cell- and mammalian cell-derived viruses. J. Virol. 2003, 77, 12022–12032. [Google Scholar] [CrossRef] [PubMed]
- Jemielity, S.; Wang, J.J.; Chan, Y.K.; Ahmed, A.A.; Li, W.; Monahan, S.; Bu, X.; Farzan, M.; Freeman, G.J.; Umetsu, D.T.; et al. TIM-family proteins promote infection of multiple enveloped viruses through virion-associated phosphatidylserine. PLoS Pathog. 2013, 9, e1003232. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.D.; Willment, J.A.; Whitehead, L. C-type lectins in immunity and homeostasis. Nat. Rev. Immunol. 2018, 18, 374–389. [Google Scholar] [CrossRef] [PubMed]
- Pokidysheva, E.; Zhang, Y.; Battisti, A.J.; Bator-Kelly, C.M.; Chipman, P.R.; Xiao, C.; Gregorio, G.G.; Hendrickson, W.A.; Kuhn, R.J.; Rossmann, M.G. Cryo-EM reconstruction of dengue virus in complex with the carbohydrate recognition domain of DC-SIGN. Cell 2006, 124, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Kirui, J.; Abidine, Y.; Lenman, A.; Islam, K.; Gwon, Y.D.; Lasswitz, L.; Evander, M.; Bally, M.; Gerold, G. The Phosphatidylserine Receptor TIM-1 Enhances Authentic Chikungunya Virus Cell Entry. Cells 2021, 10, 1828. [Google Scholar] [CrossRef] [PubMed]
- Morizono, K.; Xie, Y.; Olafsen, T.; Lee, B.; Dasgupta, A.; Wu, A.M.; Chen, I.S. The soluble serum protein Gas6 bridges virion envelope phosphatidylserine to the TAM receptor tyrosine kinase Axl to mediate viral entry. Cell Host Microbe 2011, 9, 286–298. [Google Scholar] [CrossRef] [PubMed]
- Heil, M.L.; Albee, A.; Strauss, J.H.; Kuhn, R.J. An amino acid substitution in the coding region of the E2 glycoprotein adapts Ross River virus to utilize heparan sulfate as an attachment moiety. J. Virol. 2001, 75, 6303–6309. [Google Scholar] [CrossRef] [PubMed]
- Kerr, P.J.; Weir, R.C.; Dalgarno, L. Ross River virus variants selected during passage in chick embryo fibroblasts: Serological, genetic, and biological changes. Virology 1993, 193, 446–449. [Google Scholar] [CrossRef] [PubMed]
- Gardner, C.L.; Choi-Nurvitadhi, J.; Sun, C.; Bayer, A.; Hritz, J.; Ryman, K.D.; Klimstra, W.B. Natural variation in the heparan sulfate binding domain of the eastern equine encephalitis virus E2 glycoprotein alters interactions with cell surfaces and virulence in mice. J. Virol. 2013, 87, 8582–8590. [Google Scholar] [CrossRef] [PubMed]
- Byrnes, A.P.; Griffin, D.E. Large-Plaque Mutants of Sindbis Virus Show Reduced Binding to Heparan Sulfate, Heightened Viremia, and Slower Clearance from the Circulation. J. Virol. 2000, 74, 644–651. [Google Scholar] [CrossRef] [PubMed]
- Byrnes, A.P.; Griffin, D.E. Binding of Sindbis Virus to Cell Surface Heparan Sulfate. J. Virol. 1998, 72, 7349–7356. [Google Scholar] [CrossRef] [PubMed]
- Bear, J.S.; Byrnes, A.P.; Griffin, D.E. Heparin-binding and patterns of virulence for two recombinant strains of Sindbis virus. Virology 2006, 347, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Heil, M.; Kuhn, R.J.; Baker, T.S. Heparin binding sites on Ross River virus revealed by electron cryo-microscopy. Virology 2005, 332, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.L.; Hasan, S.S.; Klose, T.; Sun, Y.; Buda, G.; Sun, C.; Klimstra, W.B.; Rossmann, M.G. Cryo-EM structure of eastern equine encephalitis virus in complex with heparan sulfate analogues. Proc. Natl. Acad. Sci. USA 2020, 117, 8890–8899. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Zhai, X.; Li, X.; Wang, Y.; He, W.T.; Jiang, Z.; Veit, M.; Su, S. Attenuation of Getah Virus by a Single Amino Acid Substitution at Residue 253 of the E2 Protein that Might Be Part of a New Heparan Sulfate Binding Site on Alphaviruses. J. Virol. 2022, 96, e01751-21. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.S.; Kuhn, R.J.; Strauss, E.G.; Ou, S.; Strauss, J.H. High-affinity laminin receptor is a receptor for Sindbis virus in mammalian cells. J. Virol. 1992, 66, 4992–5001. [Google Scholar] [CrossRef] [PubMed]
- Malygin, A.A.; Bondarenko, E.I.; Ivanisenko, V.A.; Protopopova, E.V.; Karpova, G.G.; Loktev, V.B. C-terminal fragment of human laminin-binding protein contains a receptor domain for venezuelan equine encephalitis and tick-borne encephalitis viruses. Biochem. Mosc. 2009, 74, 1328–1336. [Google Scholar] [CrossRef] [PubMed]
- Wintachai, P.; Wikan, N.; Kuadkitkan, A.; Jaimipuk, T.; Ubol, S.; Pulmanausahakul, R.; Auewarakul, P.; Kasinrerk, W.; Weng, W.Y.; Panyasrivanit, M.; et al. Identification of prohibitin as a Chikungunya virus receptor protein. J. Med. Virol. 2012, 84, 1757–1770. [Google Scholar] [CrossRef] [PubMed]
- De Caluwé, L.; Coppens, S.; Vereecken, K.; Daled, S.; Dhaenens, M.; Van Ostade, X.; Deforce, D.; Ariën, K.K.; Bartholomeeusen, K. The CD147 Protein Complex Is Involved in Entry of Chikungunya Virus and Related Alphaviruses in Human Cells. Front. Microbiol. 2021, 12, 615165. [Google Scholar] [CrossRef] [PubMed]
- Stichling, N.; Suomalainen, M.; Flatt, J.W.; Schmid, M.; Pacesa, M.; Hemmi, S.; Jungraithmayr, W.; Maler, M.D.; Freudenberg, M.A.; Plückthun, A.; et al. Lung macrophage scavenger receptor SR-A6 (MARCO) is an adenovirus type-specific virus entry receptor. PLoS Pathog. 2018, 14, e1006914. [Google Scholar] [CrossRef] [PubMed]
- Carpentier, K.S.; Davenport, B.J.; Haist, K.C.; McCarthy, M.K.; May, N.A.; Robison, A.; Ruckert, C.; Ebel, G.D.; Morrison, T.E. Discrete viral E2 lysine residues and scavenger receptor MARCO are required for clearance of circulating alphaviruses. Elife 2019, 8, e49163. [Google Scholar] [CrossRef] [PubMed]
- Li, F.S.; Carpentier, K.S.; Hawman, D.W.; Lucas, C.J.; Ander, S.E.; Feldmann, H.; Morrison, T.E. Species-specific MARCO-alphavirus interactions dictate chikungunya virus viremia. Cell Rep. 2023, 42, 112418. [Google Scholar] [CrossRef] [PubMed]
- Stiles, K.M.; Kielian, M. Alphavirus entry: NRAMP leads the way. Cell Host Microbe 2011, 10, 92–93. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rose, P.P.; Hanna, S.L.; Spiridigliozzi, A.; Wannissorn, N.; Beiting, D.P.; Ross, S.R.; Hardy, R.W.; Bambina, S.A.; Heise, M.T.; Cherry, S. Natural resistance-associated macrophage protein is a cellular receptor for sindbis virus in both insect and mammalian hosts. Cell Host Microbe 2011, 10, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Clark, L.E.; Clark, S.A.; Lin, C.; Liu, J.; Coscia, A.; Nabel, K.G.; Yang, P.; Neel, D.V.; Lee, H.; Brusic, V.; et al. VLDLR and ApoER2 are receptors for multiple alphaviruses. Nature 2022, 602, 475–480. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, O.; Zimmerman, M.I.; Raju, S.; Nelson, C.A.; Errico, J.M.; Madden, E.A.; Holmes, A.C.; Hassan, A.O.; VanBlargan, L.A.; Kim, A.S.; et al. Vertebrate-class-specific binding modes of the alphavirus receptor MXRA8. Cell 2023, 186, 4818–4833. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Zhao, Z.; Chai, Y.; Jin, X.; Li, C.; Yuan, F.; Liu, S.; Gao, Z.; Wang, H.; Song, J.; et al. Molecular Basis of Arthritogenic Alphavirus Receptor MXRA8 Binding to Chikungunya Virus Envelope Protein. Cell 2019, 177, 1714–1724.e12. [Google Scholar] [CrossRef] [PubMed]
- Basore, K.; Kim, A.S.; Nelson, C.A.; Zhang, R.; Smith, B.K.; Uranga, C.; Vang, L.; Cheng, M.; Gross, M.L.; Smith, J.; et al. Cryo-EM Structure of Chikungunya Virus in Complex with the Mxra8 Receptor. Cell 2019, 177, 1725–1737.e16. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.S.; Zimmerman, O.; Fox, J.M.; Nelson, C.A.; Basore, K.; Zhang, R.; Durnell, L.; Desai, C.; Bullock, C.; Deem, S.L.; et al. An Evolutionary LD in the Mxra8 Receptor-Binding Site Confers Resistance to Alphavirus Infection and Pathogenesis. Cell Host Microbe 2020, 27, 428–440.e9. [Google Scholar] [CrossRef] [PubMed]
- Noyes, N.C.; Hampton, B.; Migliorini, M.; Strickland, D.K. Regulation of Itch and Nedd4 E3 Ligase Activity and Degradation by LRAD3. Biochemistry 2016, 55, 1204–1213. [Google Scholar] [CrossRef] [PubMed]
- Ranganathan, S.; Noyes, N.C.; Migliorini, M.; Winkles, J.A.; Battey, F.D.; Hyman, B.T.; Smith, E.; Yepes, M.; Mikhailenko, I.; Strickland, D.K. LRAD3, a novel low-density lipoprotein receptor family member that modulates amyloid precursor protein trafficking. J. Neurosci. 2011, 31, 10836–10846. [Google Scholar] [CrossRef] [PubMed]
- Jeon, H.; Blacklow, S.C. Structure and Physiologic Function of the Low-Density Lipoprotein Receptor. Annu. Rev. Biochem. 2005, 74, 535–562. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Kim, A.S.; Kafai, N.M.; Earnest, J.T.; Shah, A.P.; Case, J.B.; Basore, K.; Gilliland, T.C.; Sun, C.; Nelson, C.A.; et al. LDLRAD3 is a receptor for Venezuelan equine encephalitis virus. Nature 2020, 588, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Kafai, N.M.; Janova, H.; Cain, M.D.; Alippe, Y.; Muraro, S.; Sariol, A.; Elam-Noll, M.; Klein, R.S.; Diamond, M.S. Entry receptor LDLRAD3 is required for Venezuelan equine encephalitis virus peripheral infection and neurotropism leading to pathogenesis in mice. Cell Rep. 2023, 42, 112946. [Google Scholar] [CrossRef] [PubMed]
- Basore, K.; Ma, H.; Kafai, N.M.; Mackin, S.; Kim, A.S.; Nelson, C.A.; Diamond, M.S.; Fremont, D.H. Structure of Venezuelan equine encephalitis virus in complex with the LDLRAD3 receptor. Nature 2021, 598, 672–676. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Huang, C.; Ma, J.; Xiang, Y.; Zhang, X. Structure of Venezuelan equine encephalitis virus with its receptor LDLRAD3. Nature 2021, 598, 677–681. [Google Scholar] [CrossRef] [PubMed]
- Cao, D.; Ma, B.; Cao, Z.; Xu, X.; Zhang, X.; Xiang, Y. The receptor VLDLR binds Eastern Equine Encephalitis virus through multiple distinct modes. Nat. Commun. 2024, 15, 6866. [Google Scholar] [CrossRef] [PubMed]
- Zhai, X.; Li, X.; Veit, M.; Wang, N.; Wang, Y.; Merits, A.; Jiang, Z.; Qin, Y.; Zhang, X.; Qi, K.; et al. LDLR is used as a cell entry receptor by multiple alphaviruses. Nat. Commun. 2024, 15, 622. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Plante, J.A.; Lin, C.; Basu, H.; Plung, J.S.; Fan, X.; Boeckers, J.M.; Oros, J.; Buck, T.K.; Anekal, P.V.; et al. Shifts in receptors during submergence of an encephalitic arbovirus. Nature 2024, 632, 614–621. [Google Scholar] [CrossRef] [PubMed]
- Pancho, A.; Aerts, T.; Mitsogiannis, M.D.; Seuntjens, E. Protocadherins at the Crossroad of Signaling Pathways. Front. Mol. Neurosci. 2020, 13, 117. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhao, L.X.; Li, Z.Q.; Wang, S.Y.; Xu, Z.S.; Wang, Y.Y. PCDH10 is a neuronal receptor for western equine encephalitis virus. Cell Res. 2024, 34, 802–805. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Li, W.; Oros, J.; Plung, J.S.; Plante, J.A.; Basu, H.; Nagappan-Chettiar, S.; Boeckers, J.M.; Tjang, L.V.; Mann, C.J.; et al. Molecular basis for shifted receptor recognition by an encephalitic arbovirus. Cell 2025, 188, 2957–2973. [Google Scholar] [CrossRef] [PubMed]
- Ooi, Y.S.; Dubé, M.; Kielian, M. BST2/tetherin inhibition of alphavirus exit. Viruses 2015, 7, 2147–2167. [Google Scholar] [CrossRef] [PubMed]
- Martinez, M.G.; Snapp, E.L.; Perumal, G.S.; Macaluso, F.P.; Kielian, M.; Doms, R.W. Imaging the alphavirus exit pathway. J. Virol. 2014, 88, 6922–6933. [Google Scholar] [CrossRef] [PubMed]
- Perez-Caballero, D.; Zang, T.; Ebrahimi, A.; McNatt, M.W.; Gregory, D.A.; Johnson, M.C.; Bieniasz, P.D. Tetherin inhibits HIV-1 release by directly tethering virions to cells. Cell 2009, 139, 499–511. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhao, K.; Wang, S.; Du, J. Multi-functional BST2/tetherin against HIV-1, other viruses and LINE-1. Front. Cell. Infect. Microbiol. 2022, 12, 979091. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.H.; Maric, M.; Madison, M.N.; Maury, W.; Roller, R.J.; Okeoma, C.M. BST-2/tetherin-mediated restriction of chikungunya (CHIKV) VLP budding is counteracted by CHIKV non-structural protein 1 (nsP1). Virology 2013, 438, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, K.; Skasko, M.; Deerinck, T.J.; Crum, J.; Ellisman, M.H.; Guatelli, J. Direct restriction of virus release and incorporation of the interferon-induced protein BST-2 into HIV-1 particles. PLoS Pathog. 2010, 6, e1000701. [Google Scholar] [CrossRef] [PubMed]
- Ballista, J.M.R.; Hoover, A.J.; Noble, J.T.; Acciani, M.D.; Miazgowicz, K.L.; Harrison, S.A.; Tabscott, G.A.L.; Duncan, A.; Barnes, D.N.; Jimenez, A.R.; et al. Chikungunya virus release is reduced by TIM-1 receptors through binding of envelope phosphatidylserine. J. Virol. 2024, 98, e0077524. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Ablan, S.D.; Miao, C.; Zheng, Y.M.; Fuller, M.S.; Rennert, P.D.; Maury, W.; Johnson, M.C.; Freed, E.O.; Liu, S.L. TIM-family proteins inhibit HIV-1 release. Proc. Natl. Acad. Sci. USA 2014, 111, E3699–E3707. [Google Scholar] [CrossRef] [PubMed]
- Kesari, A.S.; Sharkey, C.M.; Sanders, D.A. Role of heparan sulfate in entry and exit of Ross River virus glycoprotein-pseudotyped retroviral vectors. Virology 2019, 529, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Therkelsen, M.D.; Klose, T.; Vago, F.; Jiang, W.; Rossmann, M.G.; Kuhn, R.J. Flaviviruses have imperfect icosahedral symmetry. Proc. Natl. Acad. Sci. USA 2018, 115, 11608–11612. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, R.; Paredes, A.; Brown, D.T. Sindbis virus conformational changes induced by a neutralizing anti-E1 monoclonal antibody. J. Virol. 2008, 82, 5750–5760. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hunt, A.R.; Bowen, R.A.; Frederickson, S.; Maruyama, T.; Roehrig, J.T.; Blair, C.D. Treatment of mice with human monoclonal antibody 24h after lethal aerosol challenge with virulent Venezuelan equine encephalitis virus prevents disease but not infection. Virology 2011, 414, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Hunt, A.R.; Frederickson, S.; Hinkel, C.; Bowdish, K.S.; Roehrig, J.T. A humanized murine monoclonal antibody protects mice either before or after challenge with virulent Venezuelan equine encephalomyelitis virus. J. Gen. Virol. 2006, 87 Pt 9, 2467–2476. [Google Scholar] [CrossRef] [PubMed]
- Hunt, A.R.; Frederickson, S.; Maruyama, T.; Roehrig, J.T.; Blair, C.D. The first human epitope map of the alphaviral E1 and E2 proteins reveals a new E2 epitope with significant virus neutralizing activity. PLoS Negl. Trop. Dis. 2010, 4, e739. [Google Scholar] [CrossRef] [PubMed]
- Roehrig, J.T.; Mathews, J.H. The neutralization site on the E2 glycoprotein of Venezuelan equine encephalomyelitis (TC-83) virus is composed of multiple conformationally stable epitopes. Virology 1985, 142, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Roehrig, J.T.; Day, J.W.; Kinney, R.M. Antigenic analysis of the surface glycoproteins of a Venezuelan equine encephalomyelitis virus (TC-83) using monoclonal antibodies. Virology 1982, 118, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Roehrig, J.T.; Hunt, A.R.; Chang, G.J.; Sheik, B.; Bolin, R.A.; Tsai, T.F.; Trent, D.W. Identification of monoclonal antibodies capable of differentiating antigenic varieties of eastern equine encephalitis viruses. Am. J. Trop. Med. Hyg. 1990, 42, 394–398. [Google Scholar] [CrossRef] [PubMed]
- Torres-Ruesta, A.; Chee, R.S.L.; Ng, L.F. Insights into Antibody-Mediated Alphavirus Immunity and Vaccine Development Landscape. Microorganisms 2021, 9, 899. [Google Scholar] [CrossRef] [PubMed]
- Williamson, L.E.; Reeder, K.M.; Bailey, K.; Tran, M.H.; Roy, V.; Fouch, M.E.; Kose, N.; Trivette, A.; Nargi, R.S.; Winkler, E.S.; et al. Therapeutic alphavirus cross-reactive E1 human antibodies inhibit viral egress. Cell 2021, 184, 4430–4446. [Google Scholar] [CrossRef] [PubMed]
- Sutton, M.S.; Pletnev, S.; Callahan, V.; Ko, S.; Tsybovsky, Y.; Bylund, T.; Casner, R.G.; Cerutti, G.; Gardner, C.L.; Guirguis, V.; et al. Vaccine elicitation and structural basis for antibody protection against alphaviruses. Cell 2023, 186, 2672–2689.e25. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Xiang, Y.; Akahata, W.; Holdaway, H.; Pal, P.; Zhang, X.; Diamond, M.S.; Nabel, G.J.; Rossmann, M.G. Structural analyses at pseudo atomic resolution of Chikungunya virus and antibodies show mechanisms of neutralization. eLife 2013, 2, e00435. [Google Scholar] [CrossRef] [PubMed]
- Powell, L.A.; Fox, J.M.; Kose, N.; Kim, A.S.; Majedi, M.; Bombardi, R.; Carnahan, R.H.; Slaughter, J.C.; Morrison, T.E.; Diamond, M.S.; et al. Human monoclonal antibodies against Ross River virus target epitopes within the E2 protein and protect against disease. PLoS Pathog. 2020, 16, e1008517. [Google Scholar] [CrossRef] [PubMed]
- Earnest, J.T.; Basore, K.; Roy, V.; Bailey, A.L.; Wang, D.; Alter, G.; Fremont, D.H.; Diamond, M.S. Neutralizing antibodies against Mayaro virus require Fc effector functions for protective activity. J. Exp. Med. 2019, 216, 2282–2301. [Google Scholar] [CrossRef] [PubMed]
- Long, F.; Fong, R.H.; Austin, S.K.; Chen, Z.; Klose, T.; Fokine, A.; Liu, Y.; Porta, J.; Sapparapu, G.; Akahata, W.; et al. Cryo-EM structures elucidate neutralizing mechanisms of anti-chikungunya human monoclonal antibodies with therapeutic activity. Proc. Natl. Acad. Sci. USA 2015, 112, 13898–13903. [Google Scholar] [CrossRef] [PubMed]
- Quiroz, J.A.; Malonis, R.J.; Thackray, L.B.; Cohen, C.A.; Pallesen, J.; Jangra, R.K.; Brown, R.S.; Hofmann, D.; Holtsberg, F.W.; Shulenin, S.; et al. Human monoclonal antibodies against chikungunya virus target multiple distinct epitopes in the E1 and E2 glycoproteins. PLoS Pathog. 2019, 15, e1008061. [Google Scholar] [CrossRef] [PubMed]
- Burke, C.W.; Froude, J.W.; Rossi, F.; White, C.E.; Moyer, C.L.; Ennis, J.; Pitt, M.L.; Streatfield, S.; Jones, R.M.; Musiychuk, K.; et al. Therapeutic monoclonal antibody treatment protects nonhuman primates from severe Venezuelan equine encephalitis virus disease after aerosol exposure. PLoS Pathog. 2019, 15, e1008157. [Google Scholar] [CrossRef] [PubMed]
- Williamson, L.E.; Bandyopadhyay, A.; Bailey, K.; Sirohi, D.; Klose, T.; Julander, J.G.; Kuhn, R.J.; Crowe, J.E. Structural constraints link differences in neutralization potency of human anti-Eastern equine encephalitis virus monoclonal antibodies. Proc. Natl. Acad. Sci. USA 2023, 120, e2213690120. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Zhang, X.; Dejnirattisai, W.; Dai, X.; Gong, D.; Wongwiwat, W.; Duquerroy, S.; Rouvinski, A.; Vaney, M.C.; Guardado-Calvo, P.; et al. The epitope arrangement on flavivirus particles contributes to Mab C10’s extraordinary neutralization breadth across Zika and dengue viruses. Cell 2021, 184, 6052–6066.e18. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Loy, T.; Ng, T.S.; Lim, X.N.; Chew, S.V.; Tan, T.Y.; Xu, M.; Kostyuchenko, V.A.; Tukijan, F.; Shi, J.; et al. A Human Antibody Neutralizes Different Flaviviruses by Using Different Mechanisms. Cell Rep. 2020, 31, 107584. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.F.; Fox, J.M.; Earnest, J.T.; Ng, T.S.; Kim, A.S.; Fibriansah, G.; Kostyuchenko, V.A.; Shi, J.; Shu, B.; Diamond, M.S.; et al. Structural basis of Chikungunya virus inhibition by monoclonal antibodies. Proc. Natl. Acad. Sci. USA 2020, 117, 27637–27645. [Google Scholar] [CrossRef] [PubMed]
- Hasan, S.S.; Miller, A.; Sapparapu, G.; Fernandez, E.; Klose, T.; Long, F.; Fokine, A.; Porta, J.C.; Jiang, W.; Diamond, M.S.; et al. A human antibody against Zika virus crosslinks the E protein to prevent infection. Nat. Commun. 2017, 8, 14722. [Google Scholar] [CrossRef] [PubMed]
- Sevvana, M.; Kuhn, R.J. Mapping the diverse structural landscape of the flavivirus antibody repertoire. Curr. Opin. Virol. 2020, 45, 51–64. [Google Scholar] [CrossRef] [PubMed]
- Fox, J.M.; Long, F.; Edeling, M.A.; Lin, H.; Duijl-Richter, M.K.S.; Fong, R.H.; Kahle, K.M.; Smit, J.M.; Jin, J.; Simmons, G.; et al. Broadly Neutralizing Alphavirus Antibodies Bind an Epitope on E2 and Inhibit Entry and Egress. Cell 2015, 163, 1095–1107. [Google Scholar] [CrossRef] [PubMed]
- Powell, L.A.; Miller, A.; Fox, J.M.; Kose, N.; Klose, T.; Kim, A.S.; Bombardi, R.; Tennekoon, R.N.; Silva, A.; Carnahan, R.H.; et al. Human mAbs Broadly Protect against Arthritogenic Alphaviruses by Recognizing Conserved Elements of the Mxra8 Receptor-Binding Site. Cell Host Microbe 2020, 28, 699–711.e7. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.S.; Kafai, N.M.; Winkler, E.S.; Gilliland, T.C.; Cottle, E.L.; Earnest, J.T.; Jethva, P.N.; Kaplonek, P.; Shah, A.P.; Fong, R.H.; et al. Pan-protective anti-alphavirus human antibodies target a conserved E1 protein epitope. Cell 2021, 184, 4414–4429. [Google Scholar] [CrossRef] [PubMed]
- Tsetsarkin, K.A.; Vanlandingham, D.L.; McGee, C.E.; Higgs, S. A single mutation in chikungunya virus affects vector specificity and epidemic potential. PLoS Pathog. 2007, 3, e201. [Google Scholar] [CrossRef] [PubMed]
- Powers, A.M.; Williamson, L.E.; Carnahan, R.H.; Crowe Jr, J.E.; Hyde, J.L.; Jonsson, C.B.; Nasar, F.; Weaver, S.C. Developing a Prototype Pathogen Plan and Research Priorities for the Alphaviruses. J. Infect. Dis. 2023, 228, S414–S426. [Google Scholar] [CrossRef] [PubMed]
- Walton, T.E.; Alvarez, O.; Buckwalter, R.M.; Johnson, K.M. Experimental Infection of Horses with Enzootic and Epizootic Strains of Venezuelan Equine Encephalomyelitis Virus. J. Infect. Dis. 1973, 128, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, D.N.; Heppner, D.G.; Gardner, S.N.; Jaing, C.; Dupuy, L.C.; Schmaljohn, C.S.; Carlton, K. Current Strategic Thinking for the Development of a Trivalent Alphavirus Vaccine for Human Use. Am. Soc. Trop. Med. Hyg. 2014, 91, 442–450. [Google Scholar] [CrossRef] [PubMed]
- Hasan, S.S.; Dey, D.; Singh, S.; Martin, M. The Structural Biology of Eastern Equine Encephalitis Virus, an Emerging Viral Threat. Pathogens 2021, 10, 973. [Google Scholar] [CrossRef] [PubMed]
- Kafai, N.M.; Williamson, L.E.; Binshtein, E.; Sukupolvi-Petty, S.; Gardner, C.L.; Liu, J.; Mackin, S.; Kim, A.S.; Kose, N.; Carnahan, R.H.; et al. Neutralizing antibodies protect mice against Venezuelan equine encephalitis virus aerosol challenge. J. Exp. Med. 2022, 219, e20212532. [Google Scholar] [CrossRef] [PubMed]




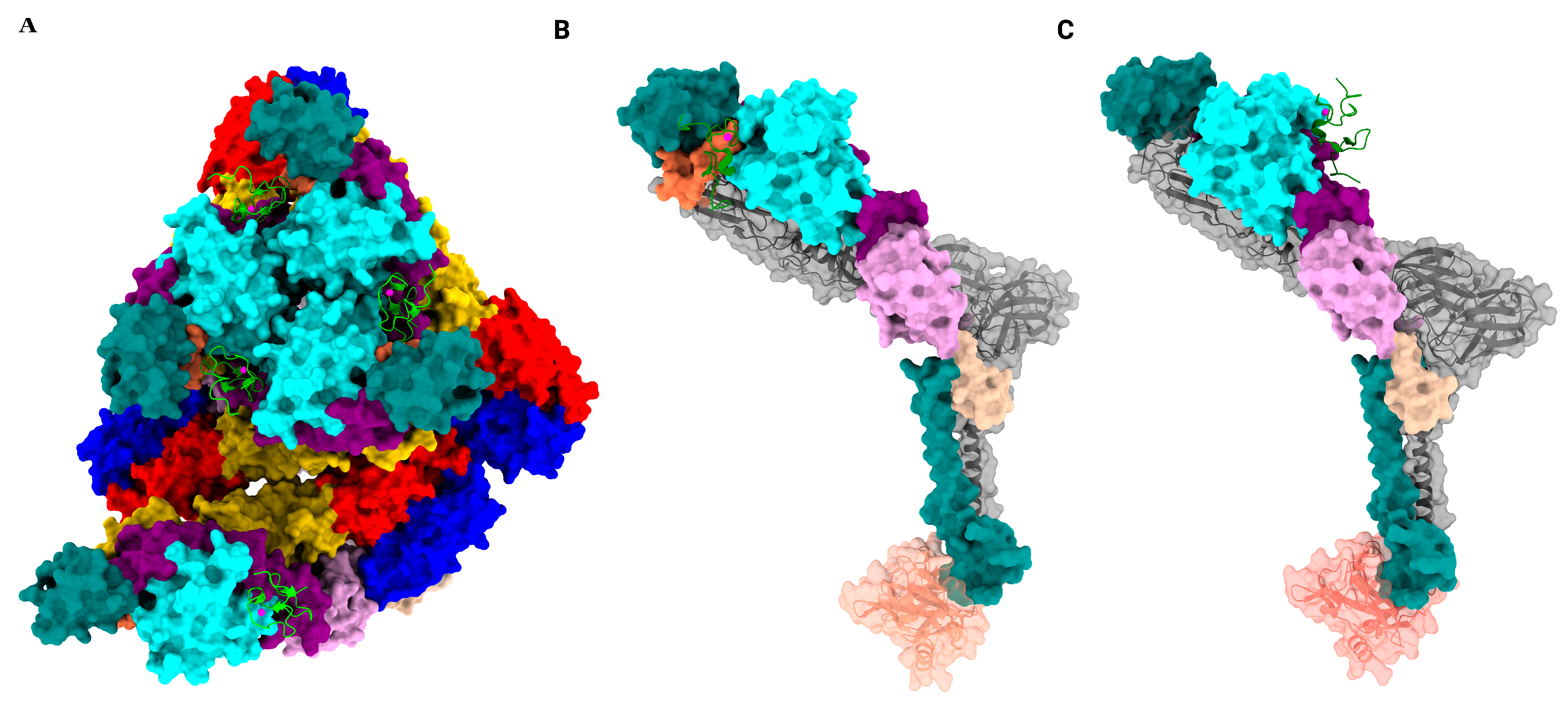

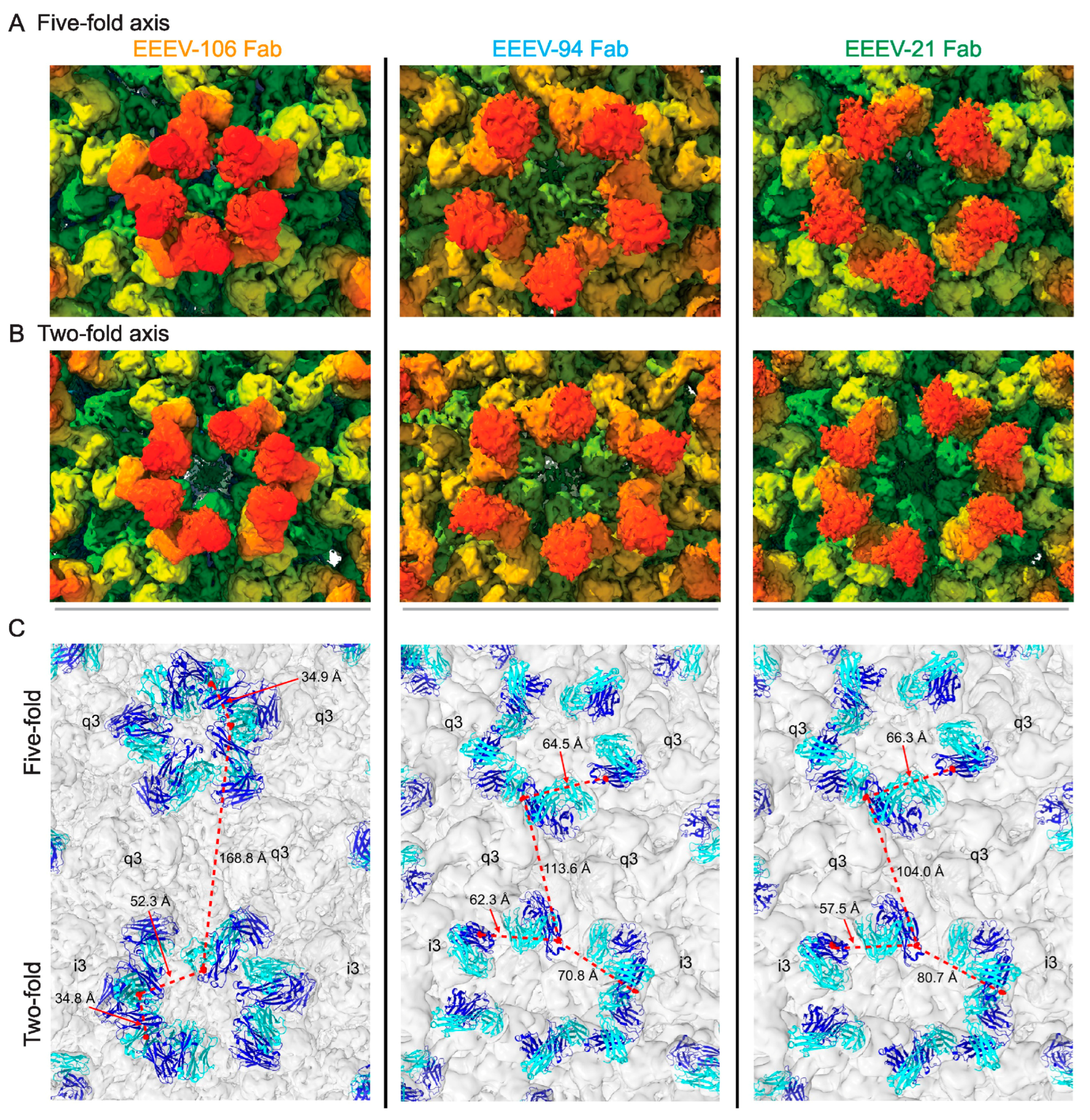
| i. | How does the interplay of different attachment factors and receptors influence tissue tropism and viral entry? |
| ii. | Do other endocytic pathways play a role in alphavirus entry, and how do host factors influence the outcome? |
| iii. | Since SFV and EEEV bind VLDLR in two different modes, are there other alphavirus-receptor pairs that display different binding modes or engage multiple receptors? |
| iv. | Alphaviruses infect a wide range of hosts by engaging different receptors. Are there common receptors utilized by some alphaviruses in both mammalian and insect hosts that can be targeted? |
| v. | Since no pan-alpha neutralizing antibodies against both the encephalitic and arthritogenic viruses have yet been isolated, is it possible to utilize the structures of alphaviruses in complex with Fab fragments of pan-alpha non-neutralizing antibodies along with computational tools to develop neutralizing versions of these types of antibodies while the search continues for such antibodies from both human and murine sources? |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poudyal, S.; Bandyopadhyay, A.; Kuhn, R.J. Insights into the Landscape of Alphavirus Receptor and Antibody Interactions. Viruses 2025, 17, 1019. https://doi.org/10.3390/v17071019
Poudyal S, Bandyopadhyay A, Kuhn RJ. Insights into the Landscape of Alphavirus Receptor and Antibody Interactions. Viruses. 2025; 17(7):1019. https://doi.org/10.3390/v17071019
Chicago/Turabian StylePoudyal, Shishir, Abhishek Bandyopadhyay, and Richard J. Kuhn. 2025. "Insights into the Landscape of Alphavirus Receptor and Antibody Interactions" Viruses 17, no. 7: 1019. https://doi.org/10.3390/v17071019
APA StylePoudyal, S., Bandyopadhyay, A., & Kuhn, R. J. (2025). Insights into the Landscape of Alphavirus Receptor and Antibody Interactions. Viruses, 17(7), 1019. https://doi.org/10.3390/v17071019







