Age Matters: Key Contributors to Interferon Toxicity in Infants During Influenza Virus Infection
Abstract
1. Introduction
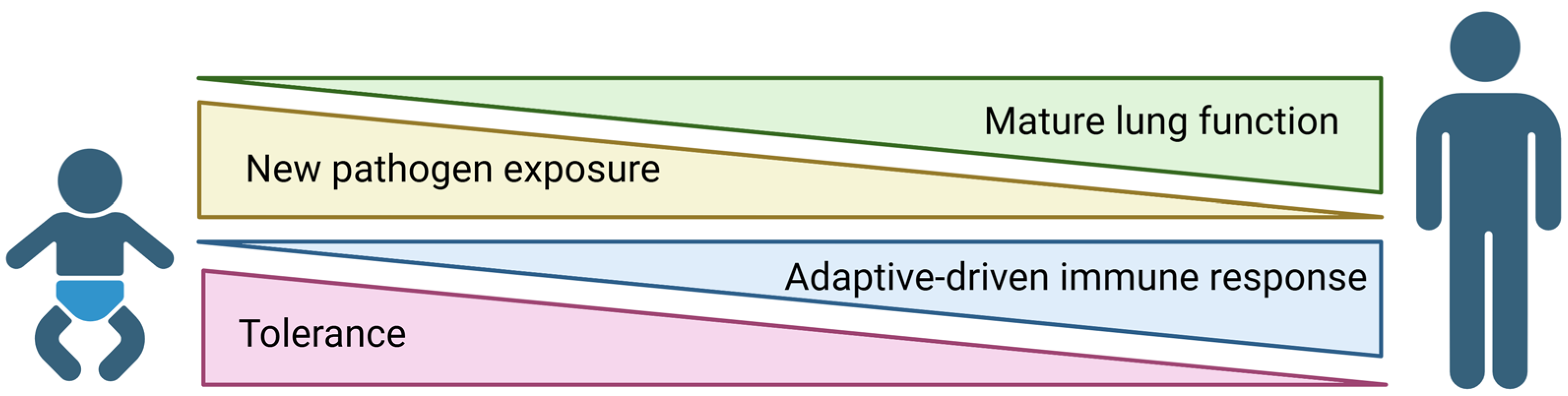
2. Age-Specific Response to Respiratory Viral Infections
2.1. Tolerance Drives Dampened Neonatal Immune Responses
2.2. Timing of IFN Signaling Impacts Severity of Respiratory Viral Infections
2.3. Developmental Regulation of the IFN Response
3. Potential Players in IFN-Mediated Viral Pathogenesis in Neonates
3.1. Inhibition of Lung Development
3.2. Dysregulation of Chemical Barrier Defenses
3.3. Disruption of the Alveolar Barrier Integrity
3.4. Exacerbation of Oxidative Stress
3.5. Modulation of Neutrophil Activity
4. Therapeutic Avenues
5. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| IV | Influenza virus |
| IFN | Interferon |
| IFN-I | Type I interferon |
| IFN-III | Type III interferon |
| IFNAR | Type I interferon receptor |
| PBMCs | Peripheral blood mononuclear cells |
| CBMCs | Cord blood mononuclear cells |
| RSV | Respiratory syncytial virus |
| SARS-CoV-2 | Severe acute respiratory syndrome coronavirus 2 |
| hpi | Hours post infection |
| LGG | Lactobacillus rhamnosus |
| ISG | Interferon-stimulated gene |
| TIIEC | Type II alveolar epithelial cell |
| TIEC | Type I alveolar epithelial cell |
| AMP | Anti-microbial peptide |
| CRAMP | Cathelicidin-related antimicrobial peptide |
| TJ | Tight junction |
| AJ | Adherens junction |
| LLI | Liquid–liquid interface |
| ALI | Air–liquid interface |
| ROS | Reactive oxygen species |
| OS | Oxidative stress |
| NAC | N-acetyl cysteine |
References
- CDC. Flu Burden. Available online: https://www.cdc.gov/flu-burden/php/about/index.html?CDC_AAref_Val=https://www.cdc.gov/flu/about/burden/index.html (accessed on 12 May 2025).
- Sommer, C.; Resch, B.; Simões, E.A. Risk factors for severe respiratory syncytial virus lower respiratory tract infection. Open Microbiol. J. 2011, 5, 144–154. [Google Scholar] [CrossRef] [PubMed]
- Halasa, N.; Zambrano, L.D.; Amarin, J.Z.; Stewart, L.S.; Newhams, M.M.; Levy, E.R.; Shein, S.L.; Carroll, C.L.; Fitzgerald, J.C.; Michaels, M.G.; et al. Infants Admitted to US Intensive Care Units for RSV Infection During the 2022 Seasonal Peak. JAMA Netw. Open 2023, 6, e2328950. [Google Scholar] [CrossRef] [PubMed]
- Curns, A.T.; Rha, B.; Lively, J.Y.; Sahni, L.C.; Englund, J.A.; Weinberg, G.A.; Halasa, N.B.; Staat, M.A.; Selvarangan, R.; Michaels, M.; et al. Respiratory Syncytial Virus-Associated Hospitalizations Among Children <5 Years Old: 2016 to 2020. Pediatrics 2024, 153, e2023062574. [Google Scholar] [CrossRef] [PubMed]
- Katze, M.G.; He, Y.; Gale, M., Jr. Viruses and interferon: A fight for supremacy. Nat. Rev. Immunol. 2002, 2, 675–687. [Google Scholar] [CrossRef] [PubMed]
- Lazear, H.M.; Schoggins, J.W.; Diamond, M.S. Shared and Distinct Functions of Type I and Type III Interferons. Immunity 2019, 50, 907–923. [Google Scholar] [CrossRef] [PubMed]
- Odendall, C.; Kagan, J.C. The unique regulation and functions of type III interferons in antiviral immunity. Curr. Opin. Virol. 2015, 12, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Wack, A.; Terczyńska-Dyla, E.; Hartmann, R. Guarding the frontiers: The biology of type III interferons. Nat. Immunol. 2015, 16, 802–809. [Google Scholar] [CrossRef] [PubMed]
- Durbin, R.K.; Kotenko, S.V.; Durbin, J.E. Interferon induction and function at the mucosal surface. Immunol. Rev. 2013, 255, 25–39. [Google Scholar] [CrossRef] [PubMed]
- Malik, G.; Zhou, Y. Innate Immune Sensing of Influenza A Virus. Viruses 2020, 12, 755. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, A.; Pillai, P.S. Innate immunity to influenza virus infection. Nat. Rev. Immunol. 2014, 14, 315–328. [Google Scholar] [CrossRef] [PubMed]
- Ioannidis, I.; Ye, F.; McNally, B.; Willette, M.; Flano, E. Toll-like receptor expression and induction of type I and type III interferons in primary airway epithelial cells. J. Virol. 2013, 87, 3261–3270. [Google Scholar] [CrossRef] [PubMed]
- Ivashkiv, L.B.; Donlin, L.T. Regulation of type I interferon responses. Nat. Rev. Immunol. 2014, 14, 36–49. [Google Scholar] [CrossRef] [PubMed]
- Crotta, S.; Davidson, S.; Mahlakoiv, T.; Desmet, C.J.; Buckwalter, M.R.; Albert, M.L.; Staeheli, P.; Wack, A. Type I and type III interferons drive redundant amplification loops to induce a transcriptional signature in influenza-infected airway epithelia. PLoS Pathog. 2013, 9, e1003773. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Hamming, O.J.; Ank, N.; Paludan, S.R.; Nielsen, A.L.; Hartmann, R. Type III interferon (IFN) induces a type I IFN-like response in a restricted subset of cells through signaling pathways involving both the Jak-STAT pathway and the mitogen-activated protein kinases. J. Virol. 2007, 81, 7749–7758. [Google Scholar] [CrossRef] [PubMed]
- Davidson, S.; McCabe, T.M.; Crotta, S.; Gad, H.H.; Hessel, E.M.; Beinke, S.; Hartmann, R.; Wack, A. IFNλ is a potent anti-influenza therapeutic without the inflammatory side effects of IFNα treatment. EMBO Mol. Med. 2016, 8, 1099–1112. [Google Scholar] [CrossRef] [PubMed]
- Galani, I.E.; Triantafyllia, V.; Eleminiadou, E.-E.; Koltsida, O.; Stavropoulos, A.; Manioudaki, M.; Thanos, D.; Doyle, S.E.; Kotenko, S.V.; Thanopoulou, K.; et al. Interferon-λ Mediates Non-redundant Front-Line Antiviral Protection against Influenza Virus Infection without Compromising Host Fitness. Immunity 2017, 46, P875–890.E6. [Google Scholar] [CrossRef] [PubMed]
- Jilg, N.; Lin, W.; Hong, J.; Schaefer, E.A.; Wolski, D.; Meixong, J.; Goto, K.; Brisac, C.; Chusri, P.; Fusco, D.N.; et al. Kinetic differences in the induction of interferon stimulated genes by interferon-α and interleukin 28B are altered by infection with hepatitis C virus. Hepatology 2014, 59, 1250–1261. [Google Scholar] [CrossRef] [PubMed]
- Davidson, S.; Crotta, S.; McCabe, T.M.; Wack, A. Pathogenic potential of interferon αβ in acute influenza infection. Nat. Commun. 2014, 5, 3864. [Google Scholar] [CrossRef] [PubMed]
- Goritzka, M.; Durant, L.R.; Pereira, C.; Salek-Ardakani, S.; Openshaw, P.J.; Johansson, C. Alpha/beta interferon receptor signaling amplifies early proinflammatory cytokine production in the lung during respiratory syncytial virus infection. J. Virol. 2014, 88, 6128–6136. [Google Scholar] [CrossRef] [PubMed]
- Major, J.; Crotta, S.; Llorian, M.; McCabe, T.M.; Gad, H.H.; Priestnall, S.L.; Hartmann, R.; Wack, A. Type I and III interferons disrupt lung epithelial repair during recovery from viral infection. Science 2020, 369, 712–717. [Google Scholar] [CrossRef] [PubMed]
- Arimori, Y.; Nakamura, R.; Yamada, H.; Shibata, K.; Maeda, N.; Kase, T.; Yoshikai, Y. Type I interferon limits influenza virus-induced acute lung injury by regulation of excessive inflammation in mice. Antiviral. Res. 2013, 99, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Kumova, O.K.; Galani, I.E.; Rao, A.; Johnson, H.; Triantafyllia, V.; Matt, S.M.; Pascasio, J.; Gaskill, P.J.; Andreakos, E.; Katsikis, P.D.; et al. Severity of neonatal influenza infection is driven by type I interferon and oxidative stress. Mucosal Immunol. 2022, 15, 1309–1320. [Google Scholar] [CrossRef] [PubMed]
- Santer, D.M.; Minty, G.E.S.; Golec, D.P.; Lu, J.; May, J.; Namdar, A.; Shah, J.; Elahi, S.; Proud, D.; Joyce, M.; et al. Differential expression of interferon-lambda receptor 1 splice variants determines the magnitude of the antiviral response induced by interferon-lambda 3 in human immune cells. PLoS Pathog. 2020, 16, e1008515. [Google Scholar] [CrossRef] [PubMed]
- Jewell, N.A.; Cline, T.; Mertz, S.E.; Smirnov, S.V.; Flaño, E.; Schindler, C.; Grieves, J.L.; Durbin, R.K.; Kotenko, S.V.; Durbin, J.E. Lambda Interferon Is the Predominant Interferon Induced by Influenza A Virus Infection In Vivo. J. Virol. 2010, 84, 11515–11522. [Google Scholar] [CrossRef] [PubMed]
- Klinkhammer, J.; Schnepf, D.; Ye, L.; Schwaderlapp, M.; Gad, H.H.; Hartmann, R.; Garcin, D.; Mahlakõiv, T.; Staeheli, P. IFN-λ prevents influenza virus spread from the upper airways to the lungs and limits virus transmission. elife 2018, 7, e33354. [Google Scholar] [CrossRef] [PubMed]
- Jena, K.K.; Mambu, J.; Boehmer, D.; Sposito, B.; Millet, V.; de Sousa Casal, J.; Muendlein, H.I.; Spreafico, R.; Fenouil, R.; Spinelli, L.; et al. Type III interferons induce pyroptosis in gut epithelial cells and impair mucosal repair. Cell 2024, 187, 7533–7550.e7523. [Google Scholar] [CrossRef] [PubMed]
- Broggi, A.; Ghosh, S.; Sposito, B.; Spreafico, R.; Balzarini, F.; Lo Cascio, A.; Clementi, N.; De Santis, M.; Mancini, N.; Granucci, F.; et al. Type III interferons disrupt the lung epithelial barrier upon viral recognition. Science 2020, 369, 706–712. [Google Scholar] [CrossRef] [PubMed]
- Sebina, I.; Rashid, R.B.; Sikder, M.A.A.; Rahman, M.M.; Ahmed, T.; Radford-Smith, D.E.; Kotenko, S.V.; Hill, G.R.; Bald, T.; Phipps, S. IFN-λ Diminishes the Severity of Viral Bronchiolitis in Neonatal Mice by Limiting NADPH Oxidase–Induced PAD4-Independent NETosis. J. Immunol. 2022, 208, 2806–2816. [Google Scholar] [CrossRef] [PubMed]
- Rackaityte, E.; Halkias, J. Mechanisms of Fetal T Cell Tolerance and Immune Regulation. Front. Immunol. 2020, 11–2020, 588. [Google Scholar] [CrossRef] [PubMed]
- Carey, A.J.; Gracias, D.T.; Thayer, J.L.; Boesteanu, A.C.; Kumova, O.K.; Mueller, Y.M.; Hope, J.L.; Fraietta, J.A.; van Zessen, D.B.; Katsikis, P.D. Rapid Evolution of the CD8+ TCR Repertoire in Neonatal Mice. J. Immunol. 2016, 196, 2602–2613. [Google Scholar] [CrossRef] [PubMed]
- Fike, A.J.; Kumova, O.K.; Carey, A.J. Dissecting the defects in the neonatal CD8(+) T-cell response. J. Leukoc. Biol. 2019, 106, 1051–1061. [Google Scholar] [CrossRef] [PubMed]
- Carey, A.J.; Hope, J.L.; Mueller, Y.M.; Fike, A.J.; Kumova, O.K.; van Zessen, D.B.H.; Steegers, E.A.P.; van der Burg, M.; Katsikis, P.D. Public Clonotypes and Convergent Recombination Characterize the Naive CD8(+) T-Cell Receptor Repertoire of Extremely Preterm Neonates. Front. Immunol. 2017, 8, 1859. [Google Scholar] [CrossRef] [PubMed]
- Mold, J.E.; Venkatasubrahmanyam, S.; Burt, T.D.; Michaëlsson, J.; Rivera, J.M.; Galkina, S.A.; Weinberg, K.; Stoddart, C.A.; McCune, J.M. Fetal and Adult Hematopoietic Stem Cells Give Rise to Distinct T Cell Lineages in Humans. Science 2010, 330, 1695–1699. [Google Scholar] [CrossRef] [PubMed]
- Tsafaras, G.P.; Ntontsi, P.; Xanthou, G. Advantages and Limitations of the Neonatal Immune System. Front. Pediatr. 2020, 8, 5. [Google Scholar] [CrossRef] [PubMed]
- Forsthuber, T.; Yip, H.C.; Lehmann, P.V. Induction of TH1 and TH2 immunity in neonatal mice. Science 1996, 271, 1728–1730. [Google Scholar] [CrossRef] [PubMed]
- Lines, J.L.; Hoskins, S.; Hollifield, M.; Cauley, L.S.; Garvy, B.A. The Migration of T Cells in Response to Influenza Virus Is Altered in Neonatal Mice. J. Immunol. 2010, 185, 2980–2988. [Google Scholar] [CrossRef] [PubMed]
- Scanlon, K.M.; Snyder, Y.G.; Skerry, C.; Carbonetti, N.H. Fatal Pertussis in the Neonatal Mouse Model Is Associated with Pertussis Toxin-Mediated Pathology beyond the Airways. Infect. Immun. 2017, 85, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Cormier, S.A.; You, D.; Honnegowda, S. The use of a neonatal mouse model to study respiratory syncytial virus infections. Expert. Rev. Anti. Infect. Ther. 2010, 8, 1371–1380. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Rodriguez, B.A.; Ciabattoni, G.O.; Duerr, R.; Valero-Jimenez, A.M.; Yeung, S.T.; Crosse, K.M.; Schinlever, A.R.; Bernard-Raichon, L.; Rodriguez Galvan, J.; McGrath, M.E.; et al. A neonatal mouse model characterizes transmissibility of SARS-CoV-2 variants and reveals a role for ORF8. Nat. Commun. 2023, 14, 3026. [Google Scholar] [CrossRef] [PubMed]
- Fike, A.J.; Kumova, O.K.; Tardif, V.J.; Carey, A.J. Neonatal influenza-specific effector CTLs retain elevated CD31 levels at the site of infection and have decreased IFN-gamma production. J. Leukoc. Biol. 2019, 105, 539–549. [Google Scholar] [CrossRef] [PubMed]
- Schittny, J.C. Development of the lung. Cell Tissue Res. 2017, 367, 427–444. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Deutsch, G.H.; Wert, S.E. Comprehensive anatomic ontologies for lung development: A comparison of alveolar formation and maturation within mouse and human lung. J. Biomed. Semant. 2019, 10, 18. [Google Scholar] [CrossRef] [PubMed]
- Kumova, O.K.; Fike, A.J.; Thayer, J.L.; Nguyen, L.T.; Mell, J.C.; Pascasio, J.; Stairiker, C.; Leon, L.G.; Katsikis, P.D.; Carey, A.J. Lung transcriptional unresponsiveness and loss of early influenza virus control in infected neonates is prevented by intranasal Lactobacillus rhamnosus GG. PLoS Pathog. 2019, 15, e1008072. [Google Scholar] [CrossRef] [PubMed]
- Wynn, J.L.; Cvijanovich, N.Z.; Allen, G.L.; Thomas, N.J.; Freishtat, R.J.; Anas, N.; Meyer, K.; Checchia, P.A.; Lin, R.; Shanley, T.P.; et al. The Influence of Developmental Age on the Early Transcriptomic Response of Children with Septic Shock. Mol. Med. 2011, 17, 1146–1156. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Tang, W.; He, L.; Li, M.; Lin, X.; Hu, A.; Huang, X.; Wu, Z.; Wu, Z.; Chen, S.; et al. Engineered probiotic Escherichia coli elicits immediate and long-term protection against influenza A virus in mice. Nat. Commun. 2024, 15, 6802. [Google Scholar] [CrossRef] [PubMed]
- Valentin, C.; Brito Rodrigues, P.; Verce, M.; Delbauve, S.; La Palombara, L.; Demaret, F.; Allard, J.; Salmon, I.; Cani, P.D.; Köhler, A.; et al. Maternal probiotic exposure enhances CD8 T cell protective neonatal immunity and modulates offspring metabolome to control influenza virus infection. Gut Microbes 2025, 17, 2442526. [Google Scholar] [CrossRef] [PubMed]
- Ng, C.T.; Mendoza, J.L.; Garcia, K.C.; Oldstone, M.B.A. Alpha and Beta Type 1 Interferon Signaling: Passage for Diverse Biologic Outcomes. Cell 2016, 164, 349–352. [Google Scholar] [CrossRef] [PubMed]
- Jaks, E.; Gavutis, M.; Uzé, G.; Martal, J.; Piehler, J. Differential Receptor Subunit Affinities of Type I Interferons Govern Differential Signal Activation. J. Mol. Biol. 2007, 366, 525–539. [Google Scholar] [CrossRef] [PubMed]
- Lavoie, T.B.; Kalie, E.; Crisafulli-Cabatu, S.; Abramovich, R.; DiGioia, G.; Moolchan, K.; Pestka, S.; Schreiber, G. Binding and activity of all human alpha interferon subtypes. Cytokine 2011, 56, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Dijkman, R.; Verma Abhishek, K.; Selvaraj, M.; Ghimire, R.; Gad Hans, H.; Hartmann, R.; More, S.; Perlman, S.; Thiel, V.; Channappanavar, R. Effective Interferon Lambda Treatment Regimen To Control Lethal MERS-CoV Infection in Mice. J. Virol. 2022, 96, e0036422. [Google Scholar] [CrossRef] [PubMed]
- Holt, P.G.; Jones, C.A. The development of the immune system during pregnancy and early life. Allergy 2000, 55, 688–697. [Google Scholar] [CrossRef] [PubMed]
- Cao, R.G.; Smith, B.; Bunsow, E.; Heinonen, S.; Mertz, S.; Ye, F.; Best, V.; Klis, F.V.D.; Smits, G.; Mejias, A.; et al. Interferon gene expression as marker of immune maturation and response to vaccination. Open Forum Infect. Dis. 2017, 4, S61. [Google Scholar] [CrossRef]
- Holt, P.G.; Mok, D.; Panda, D.; Renn, L.; Fabozzi, G.; deKlerk, N.H.; Kusel, M.M.H.; Serralha, M.; Hollams, E.M.; Holt, B.J.; et al. Developmental regulation of type 1 and type 3 interferon production and risk for infant infections and asthma development. J. Allergy Clin. Immunol. 2019, 143, P1176–1182.E5. [Google Scholar] [CrossRef] [PubMed]
- Thwaites, R.S.; Coates, M.; Ito, K.; Ghazaly, M.; Feather, C.; Abdulla, F.; Tunstall, T.; Jain, P.; Cass, L.; Rapeport, G.; et al. Reduced Nasal Viral Load and IFN Responses in Infants with Respiratory Syncytial Virus Bronchiolitis and Respiratory Failure. Am. J. Respir. Crit. Care Med. 2018, 198, 1074–1084. [Google Scholar] [CrossRef] [PubMed]
- Taveras, J.; Garcia-Maurino, C.; Moore-Clingenpeel, M.; Xu, Z.; Mertz, S.; Ye, F.; Chen, P.; Cohen, S.H.; Cohen, D.; Peeples, M.E.; et al. Type III Interferons, Viral Loads, Age, and Disease Severity in Young Children With Respiratory Syncytial Virus Infection. J. Infect. Dis. 2022, 227, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Selvaggi, C.; Pierangeli, A.; Fabiani, M.; Spano, L.; Nicolai, A.; Papoff, P.; Moretti, C.; Midulla, F.; Antonelli, G.; Scagnolari, C. Interferon lambda 1–3 expression in infants hospitalized for RSV or HRV associated bronchiolitis. J. Infect. 2014, 68, 467–477. [Google Scholar] [CrossRef] [PubMed]
- Voigt, E.A.; Yin, J. Kinetic Differences and Synergistic Antiviral Effects Between Type I and Type III Interferon Signaling Indicate Pathway Independence. J. Interferon Cytokine Res. 2015, 35, 734–747. [Google Scholar] [CrossRef] [PubMed]
- Ng, C.T.; Sullivan, B.M.; Teijaro, J.R.; Sheehan, K.C.F.; Schreiber, R.D.; Oldstone, M.B.A. Blockade of Interferon Beta, but Not Interferon Alpha, Signaling Controls Persistent Viral Infection. Cell Host Microbe 2015, 17, 653–661. [Google Scholar] [CrossRef] [PubMed]
- Hiti, L.; Markovič, T.; Lainscak, M.; Farkaš Lainščak, J.; Pal, E.; Mlinarič-Raščan, I. The immunopathogenesis of a cytokine storm: The key mechanisms underlying severe COVID-19. Cytokine Growth Factor Rev. 2025, 82, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Contoli, M.; Papi, A.; Tomassetti, L.; Rizzo, P.; Vieceli Dalla Sega, F.; Fortini, F.; Torsani, F.; Morandi, L.; Ronzoni, L.; Zucchetti, O.; et al. Blood Interferon-α Levels and Severity, Outcomes, and Inflammatory Profiles in Hospitalized COVID-19 Patients. Front. Immunol. 2021, 12, 648004. [Google Scholar] [CrossRef] [PubMed]
- Hadjadj, J.; Yatim, N.; Barnabei, L.; Corneau, A.; Boussier, J.; Smith, N.; Péré, H.; Charbit, B.; Bondet, V.; Chenevier-Gobeaux, C.; et al. Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients. Science 2020, 369, 718–724. [Google Scholar] [CrossRef] [PubMed]
- Cameron Mark, J.; Ran, L.; Xu, L.; Danesh, A.; Bermejo-Martin Jesus, F.; Cameron Cheryl, M.; Muller Matthew, P.; Gold Wayne, L.; Richardson Susan, E.; Poutanen Susan, M.; et al. Interferon-Mediated Immunopathological Events Are Associated with Atypical Innate and Adaptive Immune Responses in Patients with Severe Acute Respiratory Syndrome. J. Virol. 2007, 81, 8692–8706. [Google Scholar] [CrossRef] [PubMed]
- Nehar-Belaid, D.; Mejías, A.; Xu, Z.; Marches, R.; Yerrabelli, R.; Chen, G.; Mertz, S.; Ye, F.; Sánchez, P.J.; Tsang, J.S.; et al. SARS-CoV-2 induced immune perturbations in infants vary with disease severity and differ from adults’ responses. Nat. Commun. 2025, 16, 4562. [Google Scholar] [CrossRef] [PubMed]
- Maher, S.G.; Sheikh, F.; Scarzello, A.J.; Romero-Weaver, A.L.; Baker, D.P.; Donnelly, R.P.; Gamero, A.M. IFN-α and IFN-λ differ in their antiproliferative effects and duration of JAK/STAT signaling activity. Cancer Biol. Ther. 2008, 7, 1109–1115. [Google Scholar] [CrossRef] [PubMed]
- Bekisz, J.; Baron, S.; Balinsky, C.; Morrow, A.; Zoon, K.C. Antiproliferative Properties of Type I and Type II Interferon. Pharmaceuticals 2010, 3, 994–1015. [Google Scholar] [CrossRef] [PubMed]
- Onufer, A.P.; Mell, J.C.; Cort, L.; Rao, A.; Mdluli, N.V.; Carey, A.J. Influenza virus-induced type I interferons disrupt alveolar epithelial repair and tight junction integrity in the developing lung. Mucosal Immunol. 2025, 18, 607–619. [Google Scholar] [CrossRef] [PubMed]
- Fehrenbach, H. Alveolar epithelial type II cell: Defender of the alveolus revisited. Respir. Res. 2001, 2, 33–46. [Google Scholar] [CrossRef] [PubMed]
- Nikolić, M.Z.; Sun, D.; Rawlins, E.L. Human lung development: Recent progress and new challenges. Development 2018, 145, dev163485. [Google Scholar] [CrossRef] [PubMed]
- Chroneos, Z.C.; Sever-Chroneos, Z.; Shepherd, V.L. Pulmonary surfactant: An immunological perspective. Cell. Physiol. Biochem. 2010, 25, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Mallampalli, R.K. The Role of Surfactant in Lung Disease and Host Defense against Pulmonary Infections. Ann. Am. Thorac. Soc. 2015, 12, 765–774. [Google Scholar] [CrossRef] [PubMed]
- Olajuyin, A.M.; Zhang, X.; Ji, H.-L. Alveolar type 2 progenitor cells for lung injury repair. Cell Death Discov. 2019, 5, 63. [Google Scholar] [CrossRef] [PubMed]
- Sisson, T.H.; Mendez, M.; Choi, K.; Subbotina, N.; Courey, A.; Cunningham, A.; Dave, A.; Engelhardt, J.F.; Liu, X.; White, E.S.; et al. Targeted injury of type II alveolar epithelial cells induces pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2010, 181, 254–263. [Google Scholar] [CrossRef] [PubMed]
- Weinheimer, V.K.; Becher, A.; Tonnies, M.; Holland, G.; Knepper, J.; Bauer, T.T.; Schneider, P.; Neudecker, J.; Ruckert, J.C.; Szymanski, K.; et al. Influenza A viruses target type II pneumocytes in the human lung. J. Infect. Dis. 2012, 206, 1685–1694. [Google Scholar] [CrossRef] [PubMed]
- Nabhan, A.N.; Brownfield, D.G.; Harbury, P.B.; Krasnow, M.A.; Desai, T.J. Single-cell Wnt signaling niches maintain stemness of alveolar type 2 cells. Science 2018, 359, 1118–1123. [Google Scholar] [CrossRef] [PubMed]
- Frank, D.B.; Peng, T.; Zepp, J.A.; Snitow, M.; Vincent, T.L.; Penkala, I.J.; Cui, Z.; Herriges, M.J.; Morley, M.P.; Zhou, S.; et al. Emergence of a Wave of Wnt Signaling that Regulates Lung Alveologenesis by Controlling Epithelial Self-Renewal and Differentiation. Cell Rep. 2016, 17, 2312–2325. [Google Scholar] [CrossRef] [PubMed]
- Aros, C.J.; Pantoja, C.J.; Gomperts, B.N. Wnt signaling in lung development, regeneration, and disease progression. Commun. Biol. 2021, 4, 601. [Google Scholar] [CrossRef] [PubMed]
- Abdelwahab, E.M.M.; Rapp, J.; Feller, D.; Csongei, V.; Pal, S.; Bartis, D.; Thickett, D.R.; Pongracz, J.E. Wnt signaling regulates trans-differentiation of stem cell like type 2 alveolar epithelial cells to type 1 epithelial cells. Respir. Res. 2019, 20, 204. [Google Scholar] [CrossRef] [PubMed]
- Zacharias, W.J.; Frank, D.B.; Zepp, J.A.; Morley, M.P.; Alkhaleel, F.A.; Kong, J.; Zhou, S.; Cantu, E.; Morrisey, E.E. Regeneration of the lung alveolus by an evolutionarily conserved epithelial progenitor. Nature 2018, 555, 251–255. [Google Scholar] [CrossRef] [PubMed]
- Nabhan, A.N.; Webster, J.D.; Adams, J.J.; Blazer, L.; Everrett, C.; Eidenschenk, C.; Arlantico, A.; Fleming, I.; Brightbill, H.D.; Wolters, P.J.; et al. Targeted alveolar regeneration with Frizzled-specific agonists. Cell 2023, 186, 2995–3012.e2915. [Google Scholar] [CrossRef] [PubMed]
- More, S.; Yang, X.; Zhu, Z.; Bamunuarachchi, G.; Guo, Y.; Huang, C.; Bailey, K.; Metcalf, J.P.; Liu, L. Regulation of influenza virus replication by Wnt/β-catenin signaling. PLoS ONE 2018, 13, e0191010. [Google Scholar] [CrossRef] [PubMed]
- Bai, M.; Li, W.; Yu, N.; Zhang, H.; Long, F.; Zeng, A. The crosstalk between β-catenin signaling and type I, type II and type III interferons in lung cancer cells. Am. J. Transl. Res. 2017, 9, 2788–2797. [Google Scholar] [PubMed]
- Hancock, A.S.; Stairiker, C.J.; Boesteanu, A.C.; Monzon-Casanova, E.; Lukasiak, S.; Mueller, Y.M.; Stubbs, A.P.; Garcia-Sastre, A.; Turner, M.; Katsikis, P.D. Transcriptome Analysis of Infected and Bystander Type 2 Alveolar Epithelial Cells during Influenza A Virus Infection Reveals In Vivo Wnt Pathway Downregulation. J. Virol. 2018, 92, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Mangoni, M.L.; McDermott, A.M.; Zasloff, M. Antimicrobial peptides and wound healing: Biological and therapeutic considerations. Exp. Dermatol. 2016, 25, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Lei, J.; Sun, L.; Huang, S.; Zhu, C.; Li, P.; He, J.; Mackey, V.; Coy, D.H.; He, Q. The antimicrobial peptides and their potential clinical applications. Am. J. Transl. Res. 2019, 11, 3919–3931. [Google Scholar] [PubMed]
- Starner, T.D.; Agerberth, B.; Gudmundsson, G.H.; McCray, P.B., Jr. Expression and Activity of β-Defensins and LL-37 in the Developing Human Lung1. J. Immunol. 2005, 174, 1608–1615. [Google Scholar] [CrossRef] [PubMed]
- Battersby, A.J.; Khara, J.; Wright, V.J.; Levy, O.; Kampmann, B. Antimicrobial Proteins and Peptides in Early Life: Ontogeny and Translational Opportunities. Front. Immunol. 2016, 7, 309. [Google Scholar] [CrossRef] [PubMed]
- Marissen, J.; Reichert, L.; Härtel, C.; Fortmann, M.I.; Faust, K.; Msanga, D.; Harder, J.; Zemlin, M.; Gomez de Agüero, M.; Masjosthusmann, K.; et al. Antimicrobial Peptides (AMPs) and the Microbiome in Preterm Infants: Consequences and Opportunities for Future Therapeutics. Int. J. Mol. Sci. 2024, 25, 6684. [Google Scholar] [CrossRef] [PubMed]
- Lokken-Toyli, K.L.; de Steenhuijsen Piters, W.A.A.; Zangari, T.; Martel, R.; Kuipers, K.; Shopsin, B.; Loomis, C.; Bogaert, D.; Weiser, J.N. Decreased production of epithelial-derived antimicrobial molecules at mucosal barriers during early life. Mucosal Immunol. 2021, 14, 1358–1368. [Google Scholar] [CrossRef] [PubMed]
- Barlow, P.G.; Beaumont, P.E.; Cosseau, C.; Mackellar, A.; Wilkinson, T.S.; Hancock, R.E.; Haslett, C.; Govan, J.R.; Simpson, A.J.; Davidson, D.J. The human cathelicidin LL-37 preferentially promotes apoptosis of infected airway epithelium. Am. J. Respir. Cell Mol. Biol. 2010, 43, 692–702. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Cherryholmes, G.; Shively, J.E. Neutrophil secondary necrosis is induced by LL-37 derived from cathelicidin. J. Leukoc. Biol. 2008, 84, 780–788. [Google Scholar] [CrossRef] [PubMed]
- Aarbiou, J.; Tjabringa, G.S.; Verhoosel, R.M.; Ninaber, D.K.; White, S.R.; Peltenburg, L.T.C.; Rabe, K.F.; Hiemstra, P.S. Mechanisms of cell death induced by the neutrophil antimicrobial peptides α-defensins and LL-37. Inflamm. Res. 2006, 55, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Pouwels, S.D.; Hesse, L.; Wu, X.; Allam, V.; van Oldeniel, D.; Bhiekharie, L.J.; Phipps, S.; Oliver, B.G.; Gosens, R.; Sukkar, M.B.; et al. LL-37 and HMGB1 induce alveolar damage and reduce lung tissue regeneration via RAGE. Am. J. Physiol. Lung Cell. Mol. Physiol. 2021, 321, L641–L652. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.S.; Ugwu, N.; Onufer, A.P.; Kumova, O.; Carey, A.J. Cathelicidin-related antimicrobial peptide (CRAMP) is toxic during neonatal murine influenza virus infection. J. Immunol. 2025, 245, 1022–1031. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.-j.; Sen, G.L.; Ward, N.L.; Johnston, A.; Chun, K.; Chen, Y.; Adase, C.; Sanford, J.A.; Gao, N.; Chensee, M.; et al. Antimicrobial Peptide LL37 and MAVS Signaling Drive Interferon-β Production by Epidermal Keratinocytes during Skin Injury. Immunity 2016, 45, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Brune, K.; Frank, J.; Schwingshackl, A.; Finigan, J.; Sidhaye, V.K. Pulmonary epithelial barrier function: Some new players and mechanisms. Am. J. Physiol. Lung Cell Mol. Physiol. 2015, 308, L731–L745. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, S.; Comstock, A.T.; Sajjan, U.S. Barrier function of airway tract epithelium. Tissue Barriers 2013, 1, e24997. [Google Scholar] [CrossRef] [PubMed]
- Short, K.R.; Kroeze, E.; Fouchier, R.A.M.; Kuiken, T. Pathogenesis of influenza-induced acute respiratory distress syndrome. Lancet Infect. Dis. 2014, 14, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Herrero, R.; Sanchez, G.; Lorente, J.A. New insights into the mechanisms of pulmonary edema in acute lung injury. Ann. Transl. Med. 2018, 6, 32. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, A.; Donaghey, T.C.; Konduru, N.V.; Pyrgiotakis, G.; Van Winkle, L.S.; Zhang, Z.; Edwards, P.; Bustamante, J.M.; Brain, J.D.; Demokritou, P. Age-Dependent Translocation of Gold Nanoparticles across the Air-Blood Barrier. ACS Nano 2019, 13, 10095–10102. [Google Scholar] [CrossRef] [PubMed]
- Wittekindt, O.H. Tight junctions in pulmonary epithelia during lung inflammation. Pflug. Arch. Eur. J. Physiol. 2017, 469, 135–147. [Google Scholar] [CrossRef] [PubMed]
- Coyne, C.B.; Gambling, T.M.; Boucher, R.C.; Carson, J.L.; Johnson, L.G. Role of claudin interactions in airway tight junctional permeability. Am. J. Physiol. Lung Cell. Mol. Physiol. 2003, 285, L1166–L1178. [Google Scholar] [CrossRef] [PubMed]
- Lochbaum, R.; Schilpp, C.; Nonnenmacher, L.; Frick, M.; Dietl, P.; Wittekindt, O.H. Retinoic acid signalling adjusts tight junction permeability in response to air-liquid interface conditions. Cell. Signal. 2020, 65, 109421. [Google Scholar] [CrossRef] [PubMed]
- Linfield, D.T.; Raduka, A.; Aghapour, M.; Rezaee, F. Airway tight junctions as targets of viral infections. Tissue Barriers 2021, 9, 1883965. [Google Scholar] [CrossRef] [PubMed]
- Short, K.R.; Kasper, J.; van der Aa, S.; Andeweg, A.C.; Zaaraoui-Boutahar, F.; Goeijenbier, M.; Richard, M.; Herold, S.; Becker, C.; Scott, D.P.; et al. Influenza virus damages the alveolar barrier by disrupting epithelial cell tight junctions. Eur. Respir. J. 2016, 47, 954–966. [Google Scholar] [CrossRef] [PubMed]
- Smallcombe, C.C.; Linfield, D.T.; Harford, T.J.; Bokun, V.; Ivanov, A.I.; Piedimonte, G.; Rezaee, F. Disruption of the airway epithelial barrier in a murine model of respiratory syncytial virus infection. Am. J. Physiol. Lung Cell. Mol. Physiol. 2019, 316, L358–L368. [Google Scholar] [CrossRef] [PubMed]
- Pharo, E.A.; Williams, S.M.; Boyd, V.; Sundaramoorthy, V.; Durr, P.A.; Baker, M.L. Host-Pathogen Responses to Pandemic Influenza H1N1pdm09 in a Human Respiratory Airway Model. Viruses 2020, 12, 679. [Google Scholar] [CrossRef] [PubMed]
- Vaswani, C.M.; Varkouhi, A.K.; Gupta, S.; Ektesabi, A.M.; Tsoporis, J.N.; Yousef, S.; Plant, P.J.; da Silva, A.L.; Cen, Y.; Tseng, Y.-C.; et al. Preventing occludin tight-junction disruption via inhibition of microRNA-193b-5p attenuates viral load and influenza-induced lung injury. Mol. Ther. 2023, 31, 2681–2701. [Google Scholar] [CrossRef] [PubMed]
- Brierley, M.M.; Marchington, K.L.; Jurisica, I.; Fish, E.N. Identification of GAS-dependent interferon-sensitive target genes whose transcription is STAT2-dependent but ISGF3-independent. FEBS J. 2006, 273, 1569–1581. [Google Scholar] [CrossRef] [PubMed]
- Wray, C.; Mao, Y.; Pan, J.; Chandrasena, A.; Piasta, F.; Frank, J.A. Claudin-4 augments alveolar epithelial barrier function and is induced in acute lung injury. Am. J. Physiol. Lung Cell Mol. Physiol. 2009, 297, L219–L227. [Google Scholar] [CrossRef] [PubMed]
- Rokkam, D.; LaFemina, M.J.; Lee, J.W.; Matthay, M.A.; Frank, J.A. Claudin-4 Levels Are Associated with Intact Alveolar Fluid Clearance in Human Lungs. Am. J. Pathol. 2011, 179, 1081–1087. [Google Scholar] [CrossRef] [PubMed]
- Jia, D.; Rahbar, R.; Fish, E.N. Interferon-Inducible Stat2 Activation of JUND and CLDN4: Mediators of IFN Responses. J. Interferon Cytokine Res. 2007, 27, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Lokken-Toyli, K.L.; Aggarwal, S.D.; Bee, G.C.W.; de Steenhuijsen Piters, W.A.A.; Wu, C.; Chen, K.Z.M.; Loomis, C.; Bogaert, D.; Weiser, J.N. Impaired upper respiratory tract barrier function during postnatal development predisposes to invasive pneumococcal disease. PLoS Pathog. 2024, 20, e1012111. [Google Scholar] [CrossRef] [PubMed]
- CDC. Pneumococcal Disease Surveillance and Trends. Available online: https://www.cdc.gov/pneumococcal/php/surveillance/index.html#:~:text=Global%20trends,deaths%20occur%20in%20developing%20countries (accessed on 1 June 2025).
- Van Itallie, C.M.; Anderson, J.M. Claudin interactions in and out of the tight junction. Tissue Barriers 2013, 1, e25247. [Google Scholar] [CrossRef] [PubMed]
- Ruan, T.; Sun, J.; Liu, W.; Prinz, R.A.; Peng, D.; Liu, X.; Xu, X. H1N1 Influenza Virus Cross-Activates Gli1 to Disrupt the Intercellular Junctions of Alveolar Epithelial Cells. Cell Rep. 2020, 31, 107801. [Google Scholar] [CrossRef] [PubMed]
- Ruan, T.; Sun, Y.; Zhang, J.; Sun, J.; Liu, W.; Prinz, R.A.; Peng, D.; Liu, X.; Xu, X. H5N1 infection impairs the alveolar epithelial barrier through intercellular junction proteins via Itch-mediated proteasomal degradation. Commun. Biol. 2022, 5, 186. [Google Scholar] [CrossRef] [PubMed]
- Mazzon, E.; Cuzzocrea, S. Role of TNF-alpha in lung tight junction alteration in mouse model of acute lung inflammation. Respir. Res. 2007, 8, 75. [Google Scholar] [CrossRef] [PubMed]
- George, S.T.; Lai, J.; Ma, J.; Stacey, H.D.; Miller, M.S.; Mullarkey, C.E. Neutrophils and Influenza: A Thin Line between Helpful and Harmful. Vaccines 2021, 9, 597. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Wang, R.; Zou, W.; Sun, X.; Liu, X.; Zhao, L.; Wang, S.; Jin, M. The Influenza Virus H5N1 Infection Can Induce ROS Production for Viral Replication and Host Cell Death in A549 Cells Modulated by Human Cu/Zn Superoxide Dismutase (SOD1) Overexpression. Viruses 2016, 8, 13. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Cao, Z.; Wang, Z.; Guo, J.; Wen, J. Reactive oxygen species associated immunoregulation post influenza virus infection. Front. Immunol. 2022, 13, 927593. [Google Scholar] [CrossRef] [PubMed]
- Amatore, D.; Sgarbanti, R.; Aquilano, K.; Baldelli, S.; Limongi, D.; Civitelli, L.; Nencioni, L.; Garaci, E.; Ciriolo, M.R.; Palamara, A.T. Influenza virus replication in lung epithelial cells depends on redox-sensitive pathways activated by NOX4-derived ROS. Cell Microbiol. 2015, 17, 131–145. [Google Scholar] [CrossRef] [PubMed]
- Nencioni, L.; Iuvara, A.; Aquilano, K.; Ciriolo, M.R.; Cozzolino, F.; Rotilio, G.; Garaci, E.; Palamara, A.T. Influenza A virus replication is dependent on an antioxidant pathway that involves GSH and Bcl-2. FASEB J. 2003, 17, 758–760. [Google Scholar] [CrossRef] [PubMed]
- Zughaier, S.M.; Shafer, W.M.; Stephens, D.S. Antimicrobial peptides and endotoxin inhibit cytokine and nitric oxide release but amplify respiratory burst response in human and murine macrophages. Cell. Microbiol. 2005, 7, 1251–1262. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Niyonsaba, F.; Ushio, H.; Nagaoka, I.; Ikeda, S.; Okumura, K.; Ogawa, H. Cathelicidin LL-37 induces the generation of reactive oxygen species and release of human alpha-defensins from neutrophils. Br. J. Dermatol. 2007, 157, 1124–1131. [Google Scholar] [CrossRef] [PubMed]
- Glennon-Alty, L.; Moots, R.J.; Edwards, S.W.; Wright, H.L. Type I interferon regulates cytokine-delayed neutrophil apoptosis, reactive oxygen species production and chemokine expression. Clin. Exp. Immunol. 2021, 203, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-Y.; Choi, G.-E.; Yoo, H.J.; Kim, H.S. Interferon Potentiates Toll-Like Receptor-Induced Prostaglandin D2 Production through Positive Feedback Regulation between Signal Transducer and Activators of Transcription 1 and Reactive Oxygen Species. Front. Immunol. 2017, 8, 1720. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, A.; Hegazy, A.N.; Deigendesch, N.; Kosack, L.; Cupovic, J.; Kandasamy, R.K.; Hildebrandt, A.; Merkler, D.; Kuhl, A.A.; Vilagos, B.; et al. Superoxide Dismutase 1 Protects Hepatocytes from Type I Interferon-Driven Oxidative Damage. Immunity 2015, 43, 974–986. [Google Scholar] [CrossRef] [PubMed]
- Snelgrove, R.J.; Edwards, L.; Rae, A.J.; Hussell, T. An absence of reactive oxygen species improves the resolution of lung influenza infection. Eur. J. Immunol. 2006, 36, 1364–1373. [Google Scholar] [CrossRef] [PubMed]
- To, E.E.; Erlich, J.R.; Liong, F.; Liong, S.; Luong, R.; Oseghale, O.; Miles, M.A.; Papagianis, P.C.; Quinn, K.M.; Bozinovski, S.; et al. Therapeutic Targeting of Endosome and Mitochondrial Reactive Oxygen Species Protects Mice From Influenza Virus Morbidity. Front. Pharmacol. 2022, 13, 870156. [Google Scholar] [CrossRef] [PubMed]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef] [PubMed]
- Berkelhamer, S.K.; Farrow, K.N. Developmental regulation of antioxidant enzymes and their impact on neonatal lung disease. Antioxid. Redox Signal. 2014, 21, 1837–1848. [Google Scholar] [CrossRef] [PubMed]
- Cannavò, L.; Perrone, S.; Viola, V.; Marseglia, L.; Di Rosa, G.; Gitto, E. Oxidative Stress and Respiratory Diseases in Preterm Newborns. Int. J. Mol. Sci. 2021, 22, 12504. [Google Scholar] [CrossRef] [PubMed]
- Sorrentino, L.; Toscanelli, W.; Fracella, M.; De Angelis, M.; Frasca, F.; Scagnolari, C.; Petrarca, L.; Nenna, R.; Midulla, F.; Palamara, A.T.; et al. NRF2 Antioxidant Response and Interferon-Stimulated Genes Are Differentially Expressed in Respiratory-Syncytial-Virus- and Rhinovirus-Infected Hospitalized Children. Pathogens 2023, 12, 577. [Google Scholar] [CrossRef] [PubMed]
- Kosmider, B.; Messier, E.M.; Janssen, W.J.; Nahreini, P.; Wang, J.; Hartshorn, K.L.; Mason, R.J. Nrf2 protects human alveolar epithelial cells against injury induced by influenza A virus. Respir. Res. 2012, 13, 43. [Google Scholar] [CrossRef] [PubMed]
- Akaike, T.; Noguchi, Y.; Ijiri, S.; Setoguchi, K.; Suga, M.; Zheng, Y.M.; Dietzschold, B.; Maeda, H. Pathogenesis of influenza virus-induced pneumonia: Involvement of both nitric oxide and oxygen radicals. Proc. Natl. Acad. Sci. USA 1996, 93, 2448–2453. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.-K.; Minakuchi, M.; Wuputra, K.; Ku, C.-C.; Pan, J.-B.; Kuo, K.-K.; Lin, Y.-C.; Saito, S.; Lin, C.-S.; Yokoyama, K.K. Redox control in the pathophysiology of influenza virus infection. BMC Microbiol. 2020, 20, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Chen, F.; Liu, T.; Chen, F.; Liu, S.; Yang, J. The role of oxidative stress in influenza virus infection. Microbes Infect. 2017, 19, 580–586. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.M.; Auten, R.L. Maturation of the antioxidant system and the effects on preterm birth. Semin. Fetal Neonatal Med. 2010, 15, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Fernandes-Silva, H.; Araújo-Silva, H.; Correia-Pinto, J.; Moura, R.S. Retinoic Acid: A Key Regulator of Lung Development. Biomolecules 2020, 10, 152. [Google Scholar] [CrossRef] [PubMed]
- Tyson, J.E.; Wright, L.L.; Oh, W.; Kennedy, K.A.; Mele, L.; Ehrenkranz, R.A.; Stoll, B.J.; Lemons, J.A.; Stevenson, D.K.; Bauer, C.R.; et al. Vitamin A Supplementation for Extremely-Low-Birth-Weight Infants. N. Engl. J. Med. 1999, 340, 1962–1968. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Baybutt, R.C.; Molteni, A. Vitamin A Deficiency Injures Lung and Liver Parenchyma and Impairs Function of Rat Type II Pneumocytes. J. Nutr. 2000, 130, 1159–1165. [Google Scholar] [CrossRef] [PubMed]
- Auten, R.L.; O’Reilly, M.A.; Oury, T.D.; Nozik-Grayck, E.; Whorton, M.H. Transgenic extracellular superoxide dismutase protects postnatal alveolar epithelial proliferation and development during hyperoxia. Am. J. Physiol. Lung Cell. Mol. Physiol. 2006, 290, L32–L40. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.N.; Suliman, H.B.; Folz, R.J.; Nozik-Grayck, E.; Golson, M.L.; Mason, S.N.; Auten, R.L. Extracellular Superoxide Dismutase Protects Lung Development in Hyperoxia-exposed Newborn Mice. Am. J. Respir. Crit. Care Med. 2003, 167, 400–405. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Penkert, R.R.; Smith, A.P.; Hrincius, E.R.; McCullers, J.A.; Vogel, P.; Smith, A.M.; Hurwitz, J.L. Effect of Vitamin A Deficiency in Dysregulating Immune Responses to Influenza Virus and Increasing Mortality Rates After Bacterial Coinfections. J. Infect. Dis. 2021, 223, 1806–1816. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.A. Neutrophils, host defense, and inflammation: A double-edged sword. J. Leukoc. Biol. 1994, 56, 672–686. [Google Scholar] [CrossRef] [PubMed]
- Sebina, I.; Phipps, S. The Contribution of Neutrophils to the Pathogenesis of RSV Bronchiolitis. Viruses 2020, 12, 808. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.M.; Shojaei, M.; Teoh, S.; Meyers, A.; Ho, J.; Ball, T.B.; Keynan, Y.; Pisipati, A.; Kumar, A.; Eisen, D.P.; et al. Neutrophils-related host factors associated with severe disease and fatality in patients with influenza infection. Nat. Commun. 2019, 10, 3422. [Google Scholar] [CrossRef] [PubMed]
- Pylaeva, E.; Lang, S.; Jablonska, J. The Essential Role of Type I Interferons in Differentiation and Activation of Tumor-Associated Neutrophils. Front. Immunol. 2016, 7, 629. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Wu, X.; Zhang, S.; Deng, C.; Zhao, L.; Wang, M.; Wu, Q.; Yang, H.; Zhou, J.; Peng, L.; et al. The potential roles of type I interferon activated neutrophils and neutrophil extracellular traps (NETs) in the pathogenesis of primary Sjögren’s syndrome. Arthritis Res. Ther. 2022, 24, 170. [Google Scholar] [CrossRef] [PubMed]
- Espinosa, V.; Dutta, O.; McElrath, C.; Du, P.; Chang, Y.-J.; Cicciarelli, B.; Pitler, A.; Whitehead, I.; Obar, J.J.; Durbin, J.E.; et al. Type III interferon is a critical regulator of innate antifungal immunity. Sci. Immunol. 2017, 2, eaan5357. [Google Scholar] [CrossRef] [PubMed]
- Broggi, A.; Tan, Y.; Granucci, F.; Zanoni, I. IFN-λ suppresses intestinal inflammation by non-translational regulation of neutrophil function. Nat. Immunol. 2017, 18, 1084–1093. [Google Scholar] [CrossRef] [PubMed]
- Vlahos, R.; Stambas, J.; Selemidis, S. Suppressing production of reactive oxygen species (ROS) for influenza A virus therapy. Trends Pharmacol. Sci. 2012, 33, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Vlahos, R.; Stambas, J.; Bozinovski, S.; Broughton, B.R.S.; Drummond, G.R.; Selemidis, S. Inhibition of Nox2 Oxidase Activity Ameliorates Influenza A Virus-Induced Lung Inflammation. PLoS Pathoge. 2011, 7, e1001271. [Google Scholar] [CrossRef] [PubMed]
- Filipi, M.; Jack, S. Interferons in the Treatment of Multiple Sclerosis: A Clinical Efficacy, Safety, and Tolerability Update. Int. J. MS Care 2020, 22, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Krupp, L.B.; Pohl, D.; Ghezzi, A.; Boyko, A.; Tenembaum, S.; Chen, L.; Aycardi, E.; Banwell, B. Subcutaneous interferon β-1a in pediatric patients with multiple sclerosis: Regional differences in clinical features, disease management, and treatment outcomes in an international retrospective study. J. Neurol. Sci. 2016, 363, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Portnoy, J.; Hicks, R.; Pacheco, F.; Olson, L. Pilot study of recombinant interferon alpha-2a for treatment of infants with bronchiolitis induced by respiratory syncytial virus. Antimicrob. Agents Chemother. 1988, 32, 589–591. [Google Scholar] [CrossRef] [PubMed]
- Chipps, B.E.; Sullivan, W.F.; Portnoy, J.M. Alpha-2A-interferon for treatment of bronchiolitis caused by respiratory syncytial virus. Pediatr. Infect. Dis. J. 1993, 12, 653–658. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Shi, M.; Deng, Q.; Liu, W.; Li, Q.; Ye, P.; Yu, X.; Zhang, B.; Xu, Y.; Li, X.; et al. A multi-center randomized prospective study on the treatment of infant bronchiolitis with interferon α1b nebulization. PLoS ONE 2020, 15, e0228391. [Google Scholar] [CrossRef]
- He, L.; Yang, L.; Zhang, H.; Luo, Q. Efficacy and safety of interferon on neonates with respiratory syncytial virus pneumonia. Exp. Ther. Med. 2020, 20, 220. [Google Scholar] [CrossRef] [PubMed]
- Hall, C.B.; Douglas, R.G.; Simons, R.L.; Geiman, J.M. Interferon production in children with respiratory syncytial, influenza, and parainfluenza virus infections. J. Pediatr. 1978, 93, 28–32. [Google Scholar] [CrossRef] [PubMed]
- Loncharich, M.F.; Robertson, I. Anifrolumab in systemic lupus erythematosus. Drugs Today 2023, 59, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Furie, R.; Khamashta, M.; Merrill, J.T.; Werth, V.P.; Kalunian, K.; Brohawn, P.; Illei, G.G.; Drappa, J.; Wang, L.; Yoo, S.; et al. Anifrolumab, an Anti–Interferon-α Receptor Monoclonal Antibody, in Moderate-to-Severe Systemic Lupus Erythematosus. Arthritis Rheumatol. 2017, 69, 376–386. [Google Scholar] [CrossRef] [PubMed]
- Chronopoulou, S.; Tsochantaridis, I. Interferon Lambda: The Next Frontier in Antiviral Therapy? Pharmaceuticals 2025, 18, 785. [Google Scholar] [CrossRef] [PubMed]
- Santer, D.M.; Li, D.; Ghosheh, Y.; Zahoor, M.A.; Prajapati, D.; Hansen, B.E.; Tyrrell, D.L.J.; Feld, J.J.; Gehring, A.J. Interferon-λ treatment accelerates SARS-CoV-2 clearance despite age-related delays in the induction of T cell immunity. Nat. Commun. 2022, 13, 6992. [Google Scholar] [CrossRef] [PubMed]
- Reis, G.; Silva, E.A.S.M.; Silva, D.C.M.; Thabane, L.; Campos, V.H.S.; Ferreira, T.S.; Santos, C.V.Q.; Nogueira, A.M.R.; Almeida, A.P.F.G.; Savassi, L.C.M.; et al. Early Treatment with Pegylated Interferon Lambda for COVID-19. N. Engl. J. Med. 2023, 388, 518–528. [Google Scholar] [CrossRef] [PubMed]
- Freeman, J.; Baglino, S.; Friborg, J.; Kraft, Z.; Gray, T.; Hill, M.; McPhee, F.; Hillson, J.; Lopez-Talavera, J.C.; Wind-Rotolo, M. Pegylated interferons Lambda-1a and alfa-2a display different gene induction and cytokine and chemokine release profiles in whole blood, human hepatocytes and peripheral blood mononuclear cells. J. Viral Hepat. 2014, 21, e1–e9. [Google Scholar] [CrossRef] [PubMed]
- Feld, J.J.; Kandel, C.; Biondi, M.J.; Kozak, R.A.; Zahoor, M.A.; Lemieux, C.; Borgia, S.M.; Boggild, A.K.; Powis, J.; McCready, J.; et al. Peginterferon lambda for the treatment of outpatients with COVID-19: A phase 2, placebo-controlled randomised trial. Lancet Respir. Med. 2021, 9, 498–510. [Google Scholar] [CrossRef] [PubMed]
- Chow, C.W.; Herrera Abreu, M.T.; Suzuki, T.; Downey, G.P. Oxidative stress and acute lung injury. Am. J. Respir. Cell Mol. Biol. 2003, 29, 427–431. [Google Scholar] [CrossRef] [PubMed]
- Mata, M.; Morcillo, E.; Gimeno, C.; Cortijo, J. N-acetyl-L-cysteine (NAC) inhibit mucin synthesis and pro-inflammatory mediators in alveolar type II epithelial cells infected with influenza virus A and B and with respiratory syncytial virus (RSV). Biochem. Pharmacol. 2011, 82, 548–555. [Google Scholar] [CrossRef] [PubMed]
- Geiler, J.; Michaelis, M.; Naczk, P.; Leutz, A.; Langer, K.; Doerr, H.W.; Cinatl, J., Jr. N-acetyl-L-cysteine (NAC) inhibits virus replication and expression of pro-inflammatory molecules in A549 cells infected with highly pathogenic H5N1 influenza A virus. Biochem. Pharmacol. 2010, 79, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.H.; Li, C.H.; Wang, C.L.; Xu, M.J.; Xu, T.; Wei, D.; Liu, B.J.; Wang, G.H.; Tian, S.F. N-acetyl-l-cystine (NAC) protects against H9N2 swine influenza virus-induced acute lung injury. Int. Immunopharmacol. 2014, 22, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Garozzo, A.; Tempera, G.; Ungheri, D.; Timpanaro, R.; Castro, A. N-acetylcysteine synergizes with oseltamivir in protecting mice from lethal influenza infection. Int. J. Immunopathol. Pharmacol. 2007, 20, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Ghezzi, P.; Ungheri, D. Synergistic combination of N-acetylcysteine and ribavirin to protect from lethal influenza viral infection in a mouse model. Int. J. Immunopathol. Pharmacol. 2004, 17, 99–102. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, D.D.; Wiest, D.B.; Mulvihill, D.M.; Hlavacek, A.M.; Majstoravich, S.J.; Brown, T.R.; Taylor, J.J.; Buckley, J.R.; Turner, R.P.; Rollins, L.G.; et al. Fetal and Neonatal Effects of N-Acetylcysteine When Used for Neuroprotection in Maternal Chorioamnionitis. J. Pediatr. 2016, 168, 67–76.e6. [Google Scholar] [CrossRef] [PubMed]
- Poggi, C.; Dani, C. Antioxidant Strategies and Respiratory Disease of the Preterm Newborn: An Update. Oxidative Med. Cell. Longev. 2014, 2014, 721043. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Onufer, A.P.; Carey, A.J. Age Matters: Key Contributors to Interferon Toxicity in Infants During Influenza Virus Infection. Viruses 2025, 17, 1002. https://doi.org/10.3390/v17071002
Onufer AP, Carey AJ. Age Matters: Key Contributors to Interferon Toxicity in Infants During Influenza Virus Infection. Viruses. 2025; 17(7):1002. https://doi.org/10.3390/v17071002
Chicago/Turabian StyleOnufer, Abigail P., and Alison J. Carey. 2025. "Age Matters: Key Contributors to Interferon Toxicity in Infants During Influenza Virus Infection" Viruses 17, no. 7: 1002. https://doi.org/10.3390/v17071002
APA StyleOnufer, A. P., & Carey, A. J. (2025). Age Matters: Key Contributors to Interferon Toxicity in Infants During Influenza Virus Infection. Viruses, 17(7), 1002. https://doi.org/10.3390/v17071002





