Transmission Pathways of Zoonotic Influenza Viruses and Influencing Factors: A Systematic Review of Recent Findings
Abstract
1. Introduction
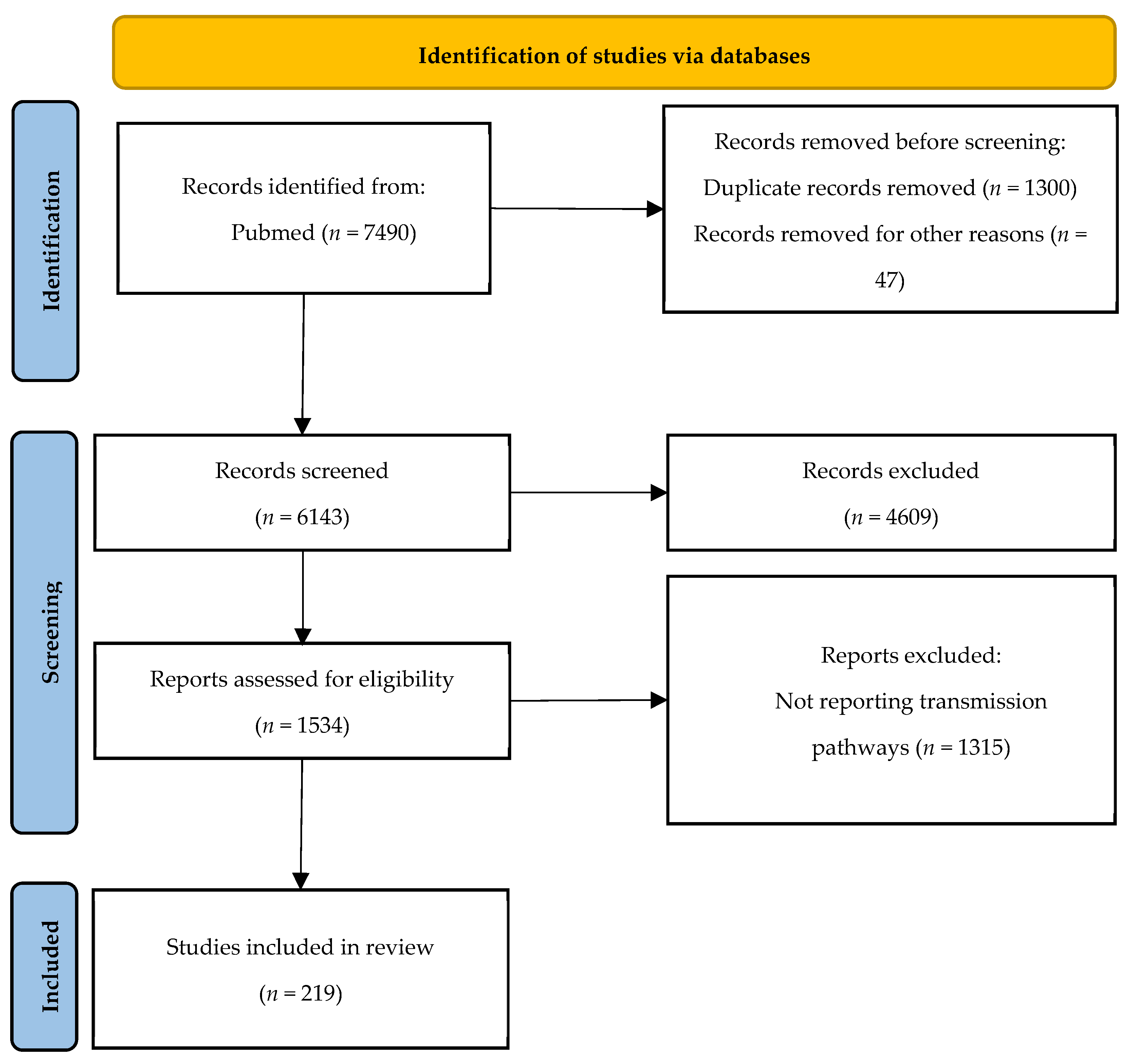
2. Materials and Methods
2.1. Search Strategy
2.2. Eligibility Criteria
2.3. Data Collection Process
3. Findings
3.1. Direct and Indirect Zoonotic Influenza Transmission Pathways Among Wild Animals
3.2. Direct and Indirect Zoonotic Influenza Transmission Pathways Between Wild and Domestic Animals
3.3. Direct and Indirect Zoonotic Influenza Transmission Pathways Among Domestic Animals
3.4. Direct and Indirect Zoonotic Influenza Transmission Pathways Between Animals and Humans
3.4.1. Transmission of Avian Influenza H7Nx Between Animals and Humans
3.4.2. Transmission of Avian Influenza H5Nx Between Animals and Humans
3.4.3. Transmission of Avian Influenza H9N2 Between Animals and Humans
3.4.4. Transmission of Swine Influenza Virus Between Animals and Humans
3.4.5. Reverse Influenza Virus Transmission from Humans to Animals
3.5. Host, Virus, and Environmental Factors Influencing Transmission
3.5.1. Environmental Factors Influencing Transmission
3.5.2. Virus Factors Influencing Transmission
3.5.3. Host Factors Influencing Transmission
3.6. Transmission of Zoonotic Influenza in Experimental Settings
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Richard, M.; de Graaf, M.; Herfst, S. Avian influenza A viruses: From zoonosis to pandemic. Future Virol. 2014, 9, 513–524. [Google Scholar] [CrossRef] [PubMed]
- Wahlgren, J. Influenza A viruses: An ecology review. Infect. Ecol. Epidemiol. 2011, 1, 6004. [Google Scholar] [CrossRef] [PubMed]
- Short, K.R.; Richard, M.; Verhagen, J.H.; van Riel, D.; Schrauwen, E.J.; van den Brand, J.M.; Mänz, B.; Bodewes, R.; Herfst, S. One health, multiple challenges: The inter-species transmission of influenza A virus. One Health 2015, 1, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Plowright, R.K.; Parrish, C.R.; McCallum, H.; Hudson, P.J.; Ko, A.I.; Graham, A.L.; Lloyd-Smith, J.O. Pathways to zoonotic spillover. Nat. Rev. Microbiol. 2017, 15, 502–510. [Google Scholar] [CrossRef]
- Esposito, M.M.; Turku, S.; Lehrfield, L.; Shoman, A. The Impact of Human Activities on Zoonotic Infection Transmissions. Animals 2023, 13, 1646. [Google Scholar] [CrossRef]
- Harvey, J.A.; Mullinax, J.M.; Runge, M.C.; Prosser, D.J. The changing dynamics of highly pathogenic avian influenza H5N1: Next steps for management & science in North America. Biol. Conserv. 2023, 282, 110041. [Google Scholar] [CrossRef]
- Kareinen, L.; Tammiranta, N.; Kauppinen, A.; Zecchin, B.; Pastori, A.; Monne, I.; Terregino, C.; Giussani, E.; Kaarto, R.; Karkamo, V.; et al. Highly pathogenic avian influenza A(H5N1) virus infections on fur farms connected to mass mortalities of black-headed gulls, Finland, July to October 2023. Eurosurveillance 2024, 29, 2400063. [Google Scholar] [CrossRef]
- Owusu, H.; Sanad, Y.M. Comprehensive Insights into Highly Pathogenic Avian Influenza H5N1 in Dairy Cattle: Transmission Dynamics, Milk-Borne Risks, Public Health Implications, Biosecurity Recommendations, and One Health Strategies for Outbreak Control. Pathogens 2025, 14, 278. [Google Scholar] [CrossRef]
- Musa, E.; Nia, Z.M.; Bragazzi, N.L.; Leung, D.; Lee, N.; Kong, J.D. Avian Influenza: Lessons from Past Outbreaks and an Inventory of Data Sources, Mathematical and AI Models, and Early Warning Systems for Forecasting and Hotspot Detection to Tackle Ongoing Outbreaks. Healthcare 2024, 12, 1959. [Google Scholar] [CrossRef]
- Gass, J.D., Jr.; Dusek, R.J.; Hall, J.S.; Hallgrimsson, G.T.; Halldórsson, H.P.; Vignisson, S.R.; Ragnarsdottir, S.B.; Jónsson, J.E.; Krauss, S.; Wong, S.S.; et al. Global dissemination of influenza A virus is driven by wild bird migration through arctic and subarctic zones. Mol. Ecol. 2023, 32, 198–213. [Google Scholar] [CrossRef]
- Role for migratory wild birds in the global spread of avian influenza H5N8. Science 2016, 354, 213–217. [CrossRef]
- Vandegrift, K.J.; Sokolow, S.H.; Daszak, P.; Kilpatrick, A.M. Ecology of avian influenza viruses in a changing world. Ann. N. Y. Acad. Sci. 2010, 1195, 113–128. [Google Scholar] [CrossRef]
- Jori, F.; Hernandez-Jover, M.; Magouras, I.; Dürr, S.; Brookes, V.J. Wildlife-livestock interactions in animal production systems: What are the biosecurity and health implications? Anim. Front. 2021, 11, 8–19. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.Y. Exploring Potential Intermediates in the Cross-Species Transmission of Influenza A Virus to Humans. Viruses 2024, 16, 1129. [Google Scholar] [CrossRef]
- Yoo, D.S.; Chun, B.C.; Kim, Y.; Lee, K.N.; Moon, O.K. Dynamics of inter-farm transmission of highly pathogenic avian influenza H5N6 integrating vehicle movements and phylogenetic information. Sci. Rep. 2021, 11, 24163. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Li, Y.; Wang, Y.; Edwards, J.; Guo, F.; Clements, A.C.A.; Huang, B.; Soares Magalhaes, R.J. The role of live poultry movement and live bird market biosecurity in the epidemiology of influenza A (H7N9): A cross-sectional observational study in four eastern China provinces. J. Infect. 2015, 71, 470–479. [Google Scholar] [CrossRef]
- Morin, C.W.; Stoner-Duncan, B.; Winker, K.; Scotch, M.; Hess, J.J.; Meschke, J.S.; Ebi, K.L.; Rabinowitz, P.M. Avian influenza virus ecology and evolution through a climatic lens. Environ. Int. 2018, 119, 241–249. [Google Scholar] [CrossRef]
- Lowen, A.C.; Steel, J. Roles of humidity and temperature in shaping influenza seasonality. J. Virol. 2014, 88, 7692–7695. [Google Scholar] [CrossRef] [PubMed]
- Siembieda, J.; Johnson, C.K.; Boyce, W.; Sandrock, C.; Cardona, C. Risk for avian influenza virus exposure at human-wildlife interface. Emerg. Infect. Dis. 2008, 14, 1151–1153. [Google Scholar] [CrossRef]
- Catalan Saenz, H.S.; Cruz-Ausejo, L. Preventive, safety and control measures against Avian Influenza A(H5N1) in occupationally exposed groups: A scoping review. One Health 2024, 19, 100766. [Google Scholar] [CrossRef]
- Zhou, L.; Liao, Q.; Dong, L.; Huai, Y.; Bai, T.; Xiang, N.; Shu, Y.; Liu, W.; Wang, S.; Qin, P.; et al. Risk factors for human illness with avian influenza A (H5N1) virus infection in China. J. Infect. Dis. 2009, 199, 1726–1734. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Jeong, S.; Cho, A.Y.; Kim, T.H.; Choi, Y.J.; Lee, H.; Song, C.S.; Nahm, S.S.; Swayne, D.E.; Lee, D.H. Caught Right on the Spot: Isolation and Characterization of Clade 2.3.4.4b H5N8 High Pathogenicity Avian Influenza Virus from a Common Pochard (Aythya ferina) Being Attacked by a Peregrine Falcon (Falco peregrinus). Avian Dis. 2024, 68, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Seo, Y.R.; Cho, A.Y.; Si, Y.J.; Lee, S.I.; Kim, D.J.; Jeong, H.; Kwon, J.H.; Song, C.S.; Lee, D.H. Evolution and Spread of Highly Pathogenic Avian Influenza A(H5N1) Clade 2.3.4.4b Virus in Wild Birds, South Korea, 2022–2023. Emerg. Infect. Dis. 2024, 30, 299–309. [Google Scholar] [CrossRef]
- Li, S.; Meng, W.; Liu, D.; Yang, Q.; Chen, L.; Dai, Q.; Ma, T.; Gao, R.; Ru, W.; Li, Y.; et al. Migratory Whooper Swans Cygnus cygnus Transmit H5N1 Virus between China and Mongolia: Combination Evidence from Satellite Tracking and Phylogenetics Analysis. Sci. Rep. 2018, 8, 7049. [Google Scholar] [CrossRef] [PubMed]
- Lickfett, T.M.; Clark, E.; Gehring, T.M.; Alm, E.W. Detection of Influenza A viruses at migratory bird stopover sites in Michigan, USA. Infect. Ecol. Epidemiol. 2018, 8, 1474709. [Google Scholar] [CrossRef]
- Torrontegi, O.; Alvarez, V.; Acevedo, P.; Gerrikagoitia, X.; Hofle, U.; Barral, M. Long-term avian influenza virus epidemiology in a small Spanish wetland ecosystem is driven by the breeding Anseriformes community. Vet. Res. 2019, 50, 4. [Google Scholar] [CrossRef]
- Bordes, L.; Vreman, S.; Heutink, R.; Roose, M.; Venema, S.; Pritz-Verschuren, S.B.E.; Rijks, J.M.; Gonzales, J.L.; Germeraad, E.A.; Engelsma, M.; et al. Highly Pathogenic Avian Influenza H5N1 Virus Infections in Wild Red Foxes (Vulpes vulpes) Show Neurotropism and Adaptive Virus Mutations. Microbiol. Spectr. 2023, 11, e02867-22. [Google Scholar] [CrossRef]
- Puryear, W.; Sawatzki, K.; Hill, N.; Foss, A.; Stone, J.J.; Doughty, L.; Walk, D.; Gilbert, K.; Murray, M.; Cox, E.; et al. Highly Pathogenic Avian Influenza A(H5N1) Virus Outbreak in New England Seals, United States. Emerg. Infect. Dis. 2023, 29, 786–791. [Google Scholar] [CrossRef]
- Uhart, M.M.; Vanstreels, R.E.T.; Nelson, M.I.; Olivera, V.; Campagna, J.; Zavattieri, V.; Lemey, P.; Campagna, C.; Falabella, V.; Rimondi, A. Epidemiological data of an influenza A/H5N1 outbreak in elephant seals in Argentina indicates mammal-to-mammal transmission. Nat. Commun. 2024, 15, 9516. [Google Scholar] [CrossRef]
- Hill, N.J.; Smith, L.M.; Muzaffar, S.B.; Nagel, J.L.; Prosser, D.J.; Sullivan, J.D.; Spragens, K.A.; DeMattos, C.A.; DeMattos, C.C.; El Sayed, L.; et al. Crossroads of highly pathogenic H5N1: Overlap between wild and domestic birds in the Black Sea-Mediterranean impacts global transmission. Virus Evol. 2021, 7, veaa093. [Google Scholar] [CrossRef]
- Bui, C.M.; Adam, D.C.; Njoto, E.; Scotch, M.; MacIntyre, C.R. Characterising routes of H5N1 and H7N9 spread in China using Bayesian phylogeographical analysis. Emerg. Microbes Infect. 2018, 7, 184. [Google Scholar] [CrossRef]
- Guinat, C.; Valenzuela Agui, C.; Vaughan, T.G.; Scire, J.; Pohlmann, A.; Staubach, C.; King, J.; Swieton, E.; Dan, A.; Cernikova, L.; et al. Disentangling the role of poultry farms and wild birds in the spread of highly pathogenic avian influenza virus in Europe. Virus Evol. 2022, 8, veac073. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.; Youk, S.; Lee, D.H. Role of wild birds in the spread of clade 2.3.4.4e H5N6 highly pathogenic avian influenza virus into South Korea and Japan. Infect. Genet. Evol. 2022, 101, 105281. [Google Scholar] [CrossRef]
- McDuie, F.; Matchett, E.L.; Prosser, D.J.; Takekawa, J.Y.; Pitesky, M.E.; Lorenz, A.A.; McCuen, M.M.; Overton, C.T.; Ackerman, J.T.; De La Cruz, S.E.W.; et al. Pathways for avian influenza virus spread: GPS reveals wild waterfowl in commercial livestock facilities and connectivity with the natural wetland landscape. Transbound. Emerg. Dis. 2022, 69, 2898–2912. [Google Scholar] [CrossRef]
- Yoo, D.S.; Kang, S.I.; Lee, Y.N.; Lee, E.K.; Kim, W.Y.; Lee, Y.J. Bridging the Local Persistence and Long-Range Dispersal of Highly Pathogenic Avian Influenza Virus (HPAIv): A Case Study of HPAIv-Infected Sedentary and Migratory Wildfowls Inhabiting Infected Premises. Viruses 2022, 14, 116. [Google Scholar] [CrossRef] [PubMed]
- Root, J.J.; Ellis, J.W.; Shriner, S.A. Strength in numbers: Avian influenza A virus transmission to poultry from a flocking passerine. Transbound. Emerg. Dis. 2022, 69, e1153–e1159. [Google Scholar] [CrossRef]
- Ahmad, S.; Koh, K.; Yoo, D.; Suh, G.; Lee, J.; Lee, C.M. Impact of inland waters on highly pathogenic avian influenza outbreaks in neighboring poultry farms in South Korea. J. Vet. Sci. 2022, 23, e36. [Google Scholar] [CrossRef]
- Kwon, J.H.; Bahl, J.; Swayne, D.E.; Lee, Y.N.; Lee, Y.J.; Song, C.S.; Lee, D.H. Domestic ducks play a major role in the maintenance and spread of H5N8 highly pathogenic avian influenza viruses in South Korea. Transbound. Emerg. Dis. 2020, 67, 844–851. [Google Scholar] [CrossRef]
- Nagy, A.; Cernikova, L.; Stara, M.; Hofmannova, L.; Sedlak, K. Genotype Uniformity, Wild Bird-to-Poultry Transmissions, and Farm-to-Farm Carryover during the Spread of the Highly Pathogenic Avian Influenza H5N8 in the Czech Republic in 2021. Viruses 2022, 14, 1411. [Google Scholar] [CrossRef]
- Usui, T.; Soda, K.; Sumi, K.; Ozaki, H.; Tomioka, Y.; Ito, H.; Murase, T.; Kawamoto, T.; Miura, M.; Komatsu, M.; et al. Outbreaks of highly pathogenic avian influenza in zoo birds caused by HA clade 2.3.4.4 H5N6 subtype viruses in Japan in winter 2016. Transbound. Emerg. Dis. 2020, 67, 686–697. [Google Scholar] [CrossRef]
- Ayala, A.J.; Haas, L.K.; Williams, B.M.; Fink, S.S.; Yabsley, M.J.; Hernandez, S.M. Risky business in Georgia’s wild birds: Contact rates between wild birds and backyard chickens is influenced by supplemental feed. Epidemiol. Infect. 2022, 150, e102. [Google Scholar] [CrossRef] [PubMed]
- Willgert, K.; Meyer, A.; Tung, D.X.; Thu, N.V.; Long, P.T.; Newman, S.; Thuy, N.T.T.; Padungtod, P.; Fournie, G.; Pfeiffer, D.U.; et al. Transmission of highly pathogenic avian influenza in the nomadic free-grazing duck production system in Viet Nam. Sci. Rep. 2020, 10, 8432. [Google Scholar] [CrossRef] [PubMed]
- Modirihamedan, A.; Aghajantabar, S.; King, J.; Graaf, A.; Pohlmann, A.; Aghaiyan, L.; Ziafati Kafi, Z.; Mahfoozi, Y.; Hosseini, H.; Beer, M.; et al. Wild bird trade at live poultry markets potentiates risks of avian influenza virus introductions in Iran. Infect. Ecol. Epidemiol. 2021, 11, 1992083. [Google Scholar] [CrossRef] [PubMed]
- Agüero, M.; Monne, I.; Sánchez, A.; Zecchin, B.; Fusaro, A.; Ruano, M.J.; Del Valle Arrojo, M.; Fernández-Antonio, R.; Souto, A.M.; Tordable, P.; et al. Highly pathogenic avian influenza A(H5N1) virus infection in farmed minks, Spain, October 2022. Eurosurveillance 2023, 28, 2300001. [Google Scholar] [CrossRef]
- Li, R.; Zhang, T.; Bai, Y.; Li, H.; Wang, Y.; Bi, Y.; Chang, J.; Xu, B. Live Poultry Trading Drives China’s H7N9 Viral Evolution and Geographical Network Propagation. Front. Public Health 2018, 6, 210. [Google Scholar] [CrossRef]
- Khaw, S.W.S.; Vu, L.T.; Yulianto, D.; Meers, J.; Henning, J. Transport of Moving Duck Flocks in Indonesia and Vietnam: Management Practices That Potentially Impact Avian Pathogen Dissemination. Front. Vet. Sci. 2021, 8, 673624. [Google Scholar] [CrossRef]
- Badruzzaman, A.T.M.; Rahman, M.M.; Hasan, M.; Hossain, M.K.; Husna, A.; Hossain, F.M.A.; Giasuddin, M.; Uddin, M.J.; Islam, M.R.; Alam, J.; et al. Semi-Scavenging Poultry as Carriers of Avian Influenza Genes. Life 2022, 12, 320. [Google Scholar] [CrossRef]
- Bauzile, B.; Sicard, G.; Guinat, C.; Andraud, M.; Rose, N.; Hammami, P.; Durand, B.; Paul, M.C.; Vergne, T. Unravelling direct and indirect contact patterns between duck farms in France and their association with the 2016–2017 epidemic of Highly Pathogenic Avian Influenza (H5N8). Prev. Vet. Med. 2022, 198, 105548. [Google Scholar] [CrossRef] [PubMed]
- Sreenivasan, C.C.; Li, F.; Wang, D. Emerging Threats of Highly Pathogenic Avian Influenza A (H5N1) in US Dairy Cattle: Understanding Cross-Species Transmission Dynamics in Mammalian Hosts. Viruses 2024, 16, 1703. [Google Scholar] [CrossRef]
- Burrough, E.R.; Magstadt, D.R.; Petersen, B.; Timmermans, S.J.; Gauger, P.C.; Zhang, J.; Siepker, C.; Mainenti, M.; Li, G.; Thompson, A.C.; et al. Highly Pathogenic Avian Influenza A(H5N1) Clade 2.3.4.4b Virus Infection in Domestic Dairy Cattle and Cats, United States, 2024. Emerg. Infect. Dis. 2024, 30, 1335–1343. [Google Scholar] [CrossRef]
- Carnero, A.M.; Kitayama, K.; Diaz, D.A.; Garvich, M.; Angulo, N.; Cama, V.A.; Gilman, R.H.; Bayer, A.M. Risk for interspecies transmission of zoonotic pathogens during poultry processing and pork production in Peru: A qualitative study. Zoonoses Public Health 2018, 65, 528–539. [Google Scholar] [CrossRef]
- European Food Safety, A.; Gonzales, J.L.; Roberts, H.; Smietanka, K.; Baldinelli, F.; Ortiz-Pelaez, A.; Verdonck, F. Assessment of low pathogenic avian influenza virus transmission via raw poultry meat and raw table eggs. EFSA J. 2018, 16, e05431. [Google Scholar] [CrossRef]
- Zhao, P.; Sun, L.; Xiong, J.; Wang, C.; Chen, L.; Yang, P.; Yu, H.; Yan, Q.; Cheng, Y.; Jiang, L.; et al. Semiaquatic mammals might be intermediate hosts to spread avian influenza viruses from avian to human. Sci. Rep. 2019, 9, 11641. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, Z.B.; Cao, L.; Lu, J.Y.; Li, K.B.; Su, W.Z.; Li, T.G.; Yang, Z.C.; Wang, M. A case of human infection with highly pathogenic avian influenza A (H7N9) virus through poultry processing without protection measure. Zhonghua Liu Xing Bing Xue Za Zhi 2018, 39, 799–804. [Google Scholar] [CrossRef]
- Guo, L.; Hou, M.; Ning, R.; Li, W.; Yang, Z.; Li, H.; Chu, M.; Yu, L.; Liu, L. A family cluster of two fatal cases infected with influenza A (H7N9) virus in Kunming China, 2017. Infect. Genet. Evol. 2018, 66, 152–158. [Google Scholar] [CrossRef]
- Fournie, G.; Hog, E.; Barnett, T.; Pfeiffer, D.U.; Mangtani, P. A Systematic Review and Meta-Analysis of Practices Exposing Humans to Avian Influenza Viruses, Their Prevalence, and Rationale. Am. J. Trop. Med. Hyg. 2017, 97, 376–388. [Google Scholar] [CrossRef]
- Ke, C.; Mok, C.K.P.; Zhu, W.; Zhou, H.; He, J.; Guan, W.; Wu, J.; Song, W.; Wang, D.; Liu, J.; et al. Human Infection with Highly Pathogenic Avian Influenza A(H7N9) Virus, China. Emerg. Infect. Dis. 2017, 23, 1332–1340. [Google Scholar] [CrossRef]
- Ma, J.; Yang, N.; Gu, H.; Bai, L.; Sun, J.; Gu, S.; Gu, J. Effect of closure of live poultry markets in China on prevention and control of human infection with H7N9 avian influenza: A case study of four cities in Jiangsu Province. J. Public Health Policy 2019, 40, 436–447. [Google Scholar] [CrossRef]
- Wang, W.; Artois, J.; Wang, X.; Kucharski, A.J.; Pei, Y.; Tong, X.; Virlogeux, V.; Wu, P.; Cowling, B.J.; Gilbert, M.; et al. Effectiveness of Live Poultry Market Interventions on Human Infection with Avian Influenza A(H7N9) Virus, China. Emerg. Infect. Dis. 2020, 26, 891–901. [Google Scholar] [CrossRef]
- Chen, Y.; Cheng, J.; Xu, Z.; Hu, W.; Lu, J. Live poultry market closure and avian influenza A (H7N9) infection in cities of China, 2013–2017: An ecological study. BMC Infect. Dis. 2020, 20, 369. [Google Scholar] [CrossRef]
- Blachere, F.M.; Lindsley, W.G.; Weber, A.M.; Beezhold, D.H.; Thewlis, R.E.; Mead, K.R.; Noti, J.D. Detection of an avian lineage influenza A(H7N2) virus in air and surface samples at a New York City feline quarantine facility. Influenza Other Respir. Viruses 2018, 12, 613–622. [Google Scholar] [CrossRef]
- Oliver, I.; Roberts, J.; Brown, C.S.; Byrne, A.M.; Mellon, D.; Hansen, R.; Banyard, A.C.; James, J.; Donati, M.; Porter, R.; et al. A case of avian influenza A(H5N1) in England, January 2022. Eurosurveillance 2022, 27, 2200061. [Google Scholar] [CrossRef]
- Pardo-Roa, C.; Nelson, M.I.; Ariyama, N.; Aguayo, C.; Almonacid, L.I.; Gonzalez-Reiche, A.S.; Muñoz, G.; Ulloa, M.; Ávila, C.; Navarro, C.; et al. Cross-species and mammal-to-mammal transmission of clade 2.3.4.4b highly pathogenic avian influenza A/H5N1 with PB2 adaptations. Nat. Commun. 2025, 16, 2232. [Google Scholar] [CrossRef]
- Le, T.V.; Phan, L.T.; Ly, K.H.K.; Nguyen, L.T.; Nguyen, H.T.; Ho, N.T.T.; Trinh, T.X.; Tran Minh, N.N. Fatal avian influenza A(H5N1) infection in a 36-week pregnant woman survived by her newborn in Soc Trang Province, Vietnam, 2012. Influenza Other Respir. Viruses 2019, 13, 292–297. [Google Scholar] [CrossRef]
- Li, J.; Fang, Y.; Qiu, X.; Yu, X.; Cheng, S.; Li, N.; Sun, Z.; Ni, Z.; Wang, H. Human infection with avian-origin H5N6 influenza a virus after exposure to slaughtered poultry. Emerg. Microbes Infect. 2022, 11, 807–810. [Google Scholar] [CrossRef]
- Chauhan, R.P.; Gordon, M.L. A systematic review of influenza A virus prevalence and transmission dynamics in backyard swine populations globally. Porc. Health Manag. 2022, 8, 10. [Google Scholar] [CrossRef]
- Zhang, R.; Lei, Z.; Liu, C.; Zhu, Y.; Chen, J.; Yao, D.; Ou, X.; Ye, W.; Huang, Z.; Luo, L.; et al. Live poultry feeding and trading network and the transmission of avian influenza A(H5N6) virus in a large city in China, 2014–2015. Int. J. Infect. Dis. 2021, 108, 72–80. [Google Scholar] [CrossRef]
- WHO. Human Infection Caused by Avian Influenza A (H5)—Chile. Available online: https://www.who.int/emergencies/disease-outbreak-news/item/2023-DON453 (accessed on 10 May 2025).
- Uyeki, T.M.; Milton, S.; Hamid, C.A.; Webb, C.R.; Presley, S.M.; Shetty, V.; Rollo, S.N.; Martinez, D.L.; Rai, S.; Gonzales, E.R.; et al. Highly Pathogenic Avian Influenza A(H5N1) Virus Infection in a Dairy Farm Worker. N. Engl. J. Med. 2024, 390, 2028–2029. [Google Scholar] [CrossRef]
- Carnaccini, S.; Perez, D.R. H9 Influenza Viruses: An Emerging Challenge. Cold Spring Harb. Perspect. Med. 2020, 10, a038588. [Google Scholar] [CrossRef]
- WHO. Risk Assessments and Summaries of Influenza at the Human-Animal Interface. Available online: https://www.who.int/teams/global-influenza-programme/avian-influenza/monthly-risk-assessment-summary (accessed on 10 May 2025).
- Jin, Y.; Cui, H.; Jiang, L.; Zhang, C.; Li, J.; Cheng, H.; Chen, Z.; Zheng, J.; Zhang, Y.; Fu, Y.; et al. Evidence for human infection with avian influenza A(H9N2) virus via environmental transmission inside live poultry market in Xiamen, China. J. Med. Virol. 2023, 95, e28242. [Google Scholar] [CrossRef]
- Wu, Y.; Lin, J.; Yang, S.; Xie, Y.; Wang, M.; Chen, X.; Zhu, Y.; Luo, L.; Shi, W. The molecular characteristics of avian influenza viruses (H9N2) derived from air samples in live poultry markets. Infect. Genet. Evol. 2018, 60, 191–196. [Google Scholar] [CrossRef]
- Guo, Y.; Li, J.; Cheng, X. Discovery of men infected by avian influenza A (H9N2) virus. Zhonghua Shi Yan He Lin Chuang Bing Du Xue Za Zhi 1999, 13, 105–108. [Google Scholar]
- Butt, K.M.; Smith, G.J.; Chen, H.; Zhang, L.J.; Leung, Y.H.; Xu, K.M.; Lim, W.; Webster, R.G.; Yuen, K.Y.; Peiris, J.S.; et al. Human infection with an avian H9N2 influenza A virus in Hong Kong in 2003. J. Clin. Microbiol. 2005, 43, 5760–5767. [Google Scholar] [CrossRef]
- Li, X.; Tian, B.; Jianfang, Z.; Yongkun, C.; Xiaodan, L.; Wenfei, Z.; Yan, L.; Jing, T.; Junfeng, G.; Tao, C.; et al. A comprehensive retrospective study of the seroprevalence of H9N2 avian influenza viruses in occupationally exposed populations in China. PLoS ONE 2017, 12, e0178328. [Google Scholar] [CrossRef]
- Quan, C.; Wang, Q.; Zhang, J.; Zhao, M.; Dai, Q.; Huang, T.; Zhang, Z.; Mao, S.; Nie, Y.; Liu, J.; et al. Avian Influenza A Viruses among Occupationally Exposed Populations, China, 2014–2016. Emerg. Infect. Dis. 2019, 25, 2215–2225. [Google Scholar] [CrossRef]
- Pu, J.; Yin, Y.; Liu, J.; Wang, X.; Zhou, Y.; Wang, Z.; Sun, Y.; Sun, H.; Li, F.; Song, J.; et al. Reassortment with dominant chicken H9N2 influenza virus contributed to the fifth H7N9 virus human epidemic. J. Virol. 2021, 95, jvi.01578-20. [Google Scholar] [CrossRef]
- El Sayes, M.; Kandeil, A.; Moatasim, Y.; El Taweel, A.; Rubrum, A.; Kutkat, O.; Kamel, M.N.; Badra, R.; Barakat, A.B.; McKenzie, P.P.; et al. Insights into Genetic Characteristics and Virological Features of Endemic Avian Influenza A (H9N2) Viruses in Egypt from 2017–2021. Viruses 2022, 14, 1484. [Google Scholar] [CrossRef]
- Gu, M.; Xu, L.; Wang, X.; Liu, X. Current situation of H9N2 subtype avian influenza in China. Vet. Res. 2017, 48, 49. [Google Scholar] [CrossRef]
- Dürrwald, R.; Wedde, M.; Biere, B.; Oh, D.Y.; Heßler-Klee, M.; Geidel, C.; Volmer, R.; Hauri, A.M.; Gerst, K.; Thürmer, A.; et al. Zoonotic infection with swine A/H1(av)N1 influenza virus in a child, Germany, June 2020. Eurosurveillance 2020, 25, 2001638. [Google Scholar] [CrossRef]
- McBride, D.S.; Nolting, J.M.; Nelson, S.W.; Spurck, M.M.; Bliss, N.T.; Kenah, E.; Trock, S.C.; Bowman, A.S. Shortening Duration of Swine Exhibitions to Reduce Risk for Zoonotic Transmission of Influenza A Virus. Emerg. Infect. Dis. 2022, 28, 2035–2042. [Google Scholar] [CrossRef]
- Nelson, M.I.; Perofsky, A.; McBride, D.S.; Rambo-Martin, B.L.; Wilson, M.M.; Barnes, J.R.; van Bakel, H.; Khan, Z.; Dutta, J.; Nolting, J.M.; et al. A Heterogeneous Swine Show Circuit Drives Zoonotic Transmission of Influenza A Viruses in the United States. J. Virol. 2020, 94, jvi.01453-20. [Google Scholar] [CrossRef]
- Lauterbach, S.E.; Wright, C.M.; Zentkovich, M.M.; Nelson, S.W.; Lorbach, J.N.; Bliss, N.T.; Nolting, J.M.; Pierson, R.M.; King, M.D.; Bowman, A.S. Detection of influenza A virus from agricultural fair environment: Air and surfaces. Prev. Vet. Med. 2018, 153, 24–29. [Google Scholar] [CrossRef]
- Lopez-Moreno, G.; Davies, P.; Yang, M.; Culhane, M.R.; Corzo, C.A.; Li, C.; Rendahl, A.; Torremorell, M. Evidence of influenza A infection and risk of transmission between pigs and farmworkers. Zoonoses Public Health 2022, 69, 560–571. [Google Scholar] [CrossRef]
- Bliss, N.; Stull, J.W.; Moeller, S.J.; Rajala-Schultz, P.J.; Bowman, A.S. Movement patterns of exhibition swine and associations of influenza A virus infection with swine management practices. J. Am. Vet. Med. Assoc. 2017, 251, 706–713. [Google Scholar] [CrossRef]
- McBride, D.S.; Perofsky, A.C.; Nolting, J.M.; Nelson, M.I.; Bowman, A.S. Tracing the Source of Influenza A Virus Zoonoses in Interconnected Circuits of Swine Exhibitions. J. Infect. Dis. 2021, 224, 458–468. [Google Scholar] [CrossRef]
- Usui, T.; Ueda, M.; Azumano, A.; Nomura, M.; Arima, T.; Murata, K.; Ito, T.; Yamaguchi, T. A cluster epidemic of influenza A(H1N1)pdm09 virus infection in four captive cheetahs (Acinonyx jubatus). Zoonoses Public Health 2021, 68, 239–246. [Google Scholar] [CrossRef]
- Sharma, A.; Zeller, M.A.; Souza, C.K.; Anderson, T.K.; Vincent, A.L.; Harmon, K.; Li, G.; Zhang, J.; Gauger, P.C. Characterization of a 2016–2017 Human Seasonal H3 Influenza A Virus Spillover Now Endemic to U.S. Swine. mSphere 2022, 7, e0080921. [Google Scholar] [CrossRef]
- Liu, T.; Jiang, Y.; Ouyang, R. Swine-spread severe influenza-associated pneumonia: A case report and literature review. Zhong Nan Da Xue Xue Bao Yi Xue Ban 2018, 43, 1266–1271. [Google Scholar] [CrossRef]
- Ayim-Akonor, M.; Mertens, E.; May, J.; Harder, T. Exposure of domestic swine to influenza A viruses in Ghana suggests unidirectional, reverse zoonotic transmission at the human-animal interface. Zoonoses Public Health 2020, 67, 697–707. [Google Scholar] [CrossRef]
- Kuroda, M.; Usui, T.; Shibata, C.; Nishigaki, H.; Yamaguchi, T. Possible bidirectional human-swine and subsequent human-human transmission of influenza virus A(H1N1)/2009 in Japan. Zoonoses Public Health 2022, 69, 721–728. [Google Scholar] [CrossRef]
- Adeola, O.A.; Olugasa, B.O.; Emikpe, B.O.; Folitse, R.D. Syndromic survey and molecular analysis of influenza viruses at the human-swine interface in two West African cosmopolitan cities suggest the possibility of bidirectional interspecies transmission. Zoonoses Public Health 2019, 66, 232–247. [Google Scholar] [CrossRef]
- Rajao, D.S.; Vincent, A.L.; Perez, D.R. Adaptation of Human Influenza Viruses to Swine. Front. Vet. Sci. 2018, 5, 347. [Google Scholar] [CrossRef]
- Yamaguchi, E.; Hayama, Y.; Murato, Y.; Sawai, K.; Kondo, S.; Yamamoto, T. A case-control study of the infection risk of H5N8 highly pathogenic avian influenza in Japan during the winter of 2020–2021. Res. Vet. Sci. 2024, 168, 105149. [Google Scholar] [CrossRef]
- Das Gupta, S.; Barua, B.; Fournie, G.; Hoque, M.A.; Henning, J. Village and farm-level risk factors for avian influenza infection on backyard chicken farms in Bangladesh. Sci. Rep. 2022, 12, 13009. [Google Scholar] [CrossRef]
- Islam, A.; Amin, E.; Munro, S.; Hossain, M.E.; Islam, S.; Hassan, M.M.; Al Mamun, A.; Samad, M.A.; Shirin, T.; Rahman, M.Z.; et al. Potential risk zones and climatic factors influencing the occurrence and persistence of avian influenza viruses in the environment of live bird markets in Bangladesh. One Health 2023, 17, 100644. [Google Scholar] [CrossRef]
- Wei, J.; Zhou, J.; Cheng, K.; Wu, J.; Zhong, Z.; Song, Y.; Ke, C.; Yen, H.L.; Li, Y. Assessing the risk of downwind spread of avian influenza virus via airborne particles from an urban wholesale poultry market. Build. Environ. 2018, 127, 120–126. [Google Scholar] [CrossRef]
- Hall, J.S.; Dusek, R.J.; Nashold, S.W.; TeSlaa, J.L.; Allen, R.B.; Grear, D.A. Avian influenza virus prevalence in marine birds is dependent on ocean temperatures. Ecol. Appl. 2020, 30, e02040. [Google Scholar] [CrossRef]
- Ahrens, A.K.; Selinka, H.C.; Mettenleiter, T.C.; Beer, M.; Harder, T.C. Exploring surface water as a transmission medium of avian influenza viruses—Systematic infection studies in mallards. Emerg. Microbes Infect. 2022, 11, 1250–1261. [Google Scholar] [CrossRef]
- Sealy, J.E.; Fournie, G.; Trang, P.H.; Dang, N.H.; Sadeyen, J.R.; Thanh, T.L.; van Doorn, H.R.; Bryant, J.E.; Iqbal, M. Poultry trading behaviours in Vietnamese live bird markets as risk factors for avian influenza infection in chickens. Transbound. Emerg. Dis. 2019, 66, 2507–2516. [Google Scholar] [CrossRef]
- Sayeed, M.A.; Smallwood, C.; Imam, T.; Mahmud, R.; Hasan, R.B.; Hasan, M.; Anwer, M.S.; Rashid, M.H.; Hoque, M.A. Assessment of hygienic conditions of live bird markets on avian influenza in Chittagong metro, Bangladesh. Prev. Vet. Med. 2017, 142, 7–15. [Google Scholar] [CrossRef]
- Hog, E.; Fournie, G.; Hoque, M.A.; Mahmud, R.; Pfeiffer, D.U.; Barnett, T. Avian Influenza Risk Environment: Live Bird Commodity Chains in Chattogram, Bangladesh. Front. Vet. Sci. 2021, 8, 694753. [Google Scholar] [CrossRef]
- Liu, T.; Zhu, G.H.; Zhang, B.; Song, T.; Kang, M.; Lu, J.; Zhao, Y.Q.; Huang, Z.; Huang, Y.L.; Wang, X.J.; et al. The effects of closure to live poultry markets on Avian influenza A (H7N9) epidemics in China. Zhonghua Liu Xing Bing Xue Za Zhi 2017, 38, 1716–1718. [Google Scholar] [CrossRef]
- Wang, Z.; Li, H.; Li, Y.; Wu, Z.; Ai, H.; Zhang, M.; Rong, L.; Blinov, M.L.; Tong, Q.; Liu, L.; et al. Mixed selling of different poultry species facilitates emergence of public-health-threating avian influenza viruses. Emerg. Microbes Infect. 2023, 12, 2214255. [Google Scholar] [CrossRef]
- Hassan, M.M.; Hoque, M.A.; Ujvari, B.; Klaassen, M. Live bird markets in Bangladesh as a potentially important source for Avian Influenza Virus transmission. Prev. Vet. Med. 2018, 156, 22–27. [Google Scholar] [CrossRef]
- Hu, M.; Jin, Y.; Zhou, J.; Huang, Z.; Li, B.; Zhou, W.; Ren, H.; Yue, J.; Liang, L. Genetic Characteristic and Global Transmission of Influenza A H9N2 Virus. Front. Microbiol. 2017, 8, 2611. [Google Scholar] [CrossRef]
- Ma, H.X.; Wang, R.L.; Nie, Y.F.; Su, J.; Li, D.X.; Li, Y.; Du, Y.H.; Wei, H.Y.; Li, X.L.; Wang, Z.; et al. Distribution of Avian Influenza A Viruses in Poultry-Related Environment and Its Association with Human Infection in Henan, 2016 to 2017. Biomed. Environ. Sci. 2019, 32, 797–803. [Google Scholar] [CrossRef]
- Peacock, T.P.; Barclay, W.S. Mink farming poses risks for future viral pandemics. Proc. Natl. Acad. Sci. USA 2023, 120, e2303408120. [Google Scholar] [CrossRef]
- Larsen, H.D.; Fonager, J.; Lomholt, F.K.; Dalby, T.; Benedetti, G.; Kristensen, B.; Urth, T.R.; Rasmussen, M.; Lassaunière, R.; Rasmussen, T.B. Preliminary report of an outbreak of SARS-CoV-2 in mink and mink farmers associated with community spread, Denmark, June to November 2020. Eurosurveillance 2021, 26, 2100009. [Google Scholar] [CrossRef]
- Stevenson, P. Links between industrial livestock production, disease including zoonoses and antimicrobial resistance. Anim. Res. One Health 2023, 1, 137–144. [Google Scholar] [CrossRef]
- Sun, X.; Belser, J.A.; Maines, T.R. Adaptation of H9N2 Influenza Viruses to Mammalian Hosts: A Review of Molecular Markers. Viruses 2020, 12, 541. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, K.; Li, B.; Chen, Y.; Qiu, Z.; Xing, J.; Huang, J.; Hu, C.; Huang, Y.; Li, H.; et al. A risk marker of tribasic hemagglutinin cleavage site in influenza A (H9N2) virus. Commun. Biol. 2021, 4, 71. [Google Scholar] [CrossRef]
- He, W.T.; Wang, L.; Zhao, Y.; Wang, N.; Li, G.; Veit, M.; Bi, Y.; Gao, G.F.; Su, S. Adaption and parallel evolution of human-isolated H5 avian influenza viruses. J. Infect. 2020, 80, 630–638. [Google Scholar] [CrossRef]
- Cáceres, C.J.; Rajao, D.S.; Perez, D.R. Airborne Transmission of Avian Origin H9N2 Influenza A Viruses in Mammals. Viruses 2021, 13, 1919. [Google Scholar] [CrossRef]
- Kim, S.H. Challenge for One Health: Co-Circulation of Zoonotic H5N1 and H9N2 Avian Influenza Viruses in Egypt. Viruses 2018, 10, 121. [Google Scholar] [CrossRef]
- Song, H.; Qi, J.; Xiao, H.; Bi, Y.; Zhang, W.; Xu, Y.; Wang, F.; Shi, Y.; Gao, G.F. Avian-to-Human Receptor-Binding Adaptation by Influenza A Virus Hemagglutinin H4. Cell Rep. 2017, 20, 1201–1214. [Google Scholar] [CrossRef]
- Meng, F.; Yang, H.; Qu, Z.; Chen, Y.; Zhang, Y.; Zhang, Y.; Liu, L.; Zeng, X.; Li, C.; Kawaoka, Y.; et al. A Eurasian avian-like H1N1 swine influenza reassortant virus became pathogenic and highly transmissible due to mutations in its PA gene. Proc. Natl. Acad. Sci. USA 2022, 119, e2203919119. [Google Scholar] [CrossRef]
- Zhang, H.; Li, H.; Wang, W.; Wang, Y.; Han, G.Z.; Chen, H.; Wang, X. A unique feature of swine ANP32A provides susceptibility to avian influenza virus infection in pigs. PLoS Pathog. 2020, 16, e1008330. [Google Scholar] [CrossRef]
- Pinto, R.M.; Bakshi, S.; Lytras, S.; Zakaria, M.K.; Swingler, S.; Worrell, J.C.; Herder, V.; Hargrave, K.E.; Varjak, M.; Cameron-Ruiz, N.; et al. BTN3A3 evasion promotes the zoonotic potential of influenza A viruses. Nature 2023, 619, 338–347. [Google Scholar] [CrossRef]
- Gaide, N.; Filaire, F.; Bertran, K.; Crispo, M.; Dirat, M.; Secula, A.; Foret-Lucas, C.; Payre, B.; Perlas, A.; Cantero, G.; et al. The feather epithelium contributes to the dissemination and ecology of clade 2.3.4.4b H5 high pathogenicity avian influenza viruses in ducks. Emerg. Microbes Infect. 2023, 12, 2272644. [Google Scholar] [CrossRef]
- Ellis, J.W.; Root, J.J.; McCurdy, L.M.; Bentler, K.T.; Barrett, N.L.; VanDalen, K.K.; Dirsmith, K.L.; Shriner, S.A. Avian influenza A virus susceptibility, infection, transmission, and antibody kinetics in European starlings. PLoS Pathog. 2021, 17, e1009879. [Google Scholar] [CrossRef]
- James, J.; Billington, E.; Warren, C.J.; De Sliva, D.; Di Genova, C.; Airey, M.; Meyer, S.M.; Lewis, T.; Peers-Dent, J.; Thomas, S.S.; et al. Clade 2.3.4.4b H5N1 high pathogenicity avian influenza virus (HPAIV) from the 2021/22 epizootic is highly duck adapted and poorly adapted to chickens. J. Gen. Virol. 2023, 104, 001852. [Google Scholar] [CrossRef]
- Rohaim, M.A.; El Naggar, R.F.; Madbouly, Y.; AbdelSabour, M.A.; Ahmed, K.A.; Munir, M. Comparative infectivity and transmissibility studies of wild-bird and chicken-origin highly pathogenic avian influenza viruses H5N8 in chickens. Comp. Immunol. Microbiol. Infect. Dis. 2021, 74, 101594. [Google Scholar] [CrossRef] [PubMed]
- Beerens, N.; Germeraad, E.A.; Venema, S.; Verheij, E.; Pritz-Verschuren, S.B.E.; Gonzales, J.L. Comparative pathogenicity and environmental transmission of recent highly pathogenic avian influenza H5 viruses. Emerg. Microbes Infect. 2021, 10, 97–108. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, K.; Ye, X.; Wang, W.; Wu, W.; Wang, X.; Guan, Y.; He, Z.; Wang, Y.; Jiao, P. Comparative Pathogenicity and Transmissibility of the H7N9 Highly Pathogenic Avian Influenza Virus and the H7N9 Low Pathogenic Avian Influenza Virus in Chickens. Viruses 2019, 11, 1047. [Google Scholar] [CrossRef]
- Takadate, Y.; Tsunekuni, R.; Kumagai, A.; Mine, J.; Kikutani, Y.; Sakuma, S.; Miyazawa, K.; Uchida, Y. Different Infectivity and Transmissibility of H5N8 and H5N1 High Pathogenicity Avian Influenza Viruses Isolated from Chickens in Japan in the 2021/2022 Season. Viruses 2023, 15, 265. [Google Scholar] [CrossRef]
- Kwon, J.H.; Noh, J.Y.; Jeong, J.H.; Jeong, S.; Lee, S.H.; Kim, Y.J.; Yuk, S.S.; Lee, D.H.; Bae, Y.C.; Park, S.C.; et al. Different pathogenicity of two strains of clade 2.3.4.4c H5N6 highly pathogenic avian influenza viruses bearing different PA and NS gene in domestic ducks. Virology 2019, 530, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.H.; Bertran, K.; Lee, D.H.; Criado, M.F.; Killmaster, L.; Pantin-Jackwood, M.J.; Swayne, D.E. Diverse infectivity, transmissibility, and pathobiology of clade 2.3.4.4 H5Nx highly pathogenic avian influenza viruses in chickens. Emerg. Microbes Infect. 2023, 12, 2218945. [Google Scholar] [CrossRef] [PubMed]
- Slomka, M.J.; Puranik, A.; Mahmood, S.; Thomas, S.S.; Seekings, A.H.; Byrne, A.M.P.; Nunez, A.; Bianco, C.; Mollett, B.C.; Watson, S.; et al. Ducks Are Susceptible to Infection with a Range of Doses of H5N8 Highly Pathogenic Avian Influenza Virus (2016, Clade 2.3.4.4b) and Are Largely Resistant to Virus-Specific Mortality, but Efficiently Transmit Infection to Contact Turkeys. Avian Dis. 2019, 63, 172–180. [Google Scholar] [CrossRef]
- Liu, K.; Ding, P.; Pei, Y.; Gao, R.; Han, W.; Zheng, H.; Ji, Z.; Cai, M.; Gu, J.; Li, X.; et al. Emergence of a novel reassortant avian influenza virus (H10N3) in Eastern China with high pathogenicity and respiratory droplet transmissibility to mammals. Sci. China Life Sci. 2022, 65, 1024–1035. [Google Scholar] [CrossRef]
- Pepin, K.M.; Leach, C.B.; Barrett, N.L.; Ellis, J.W.; VanDalen, K.K.; Webb, C.T.; Shriner, S.A. Environmental transmission of influenza A virus in mallards. mBio 2023, 14, e0086223. [Google Scholar] [CrossRef]
- Verma, A.K.; Kumar, M.; Murugkar, H.V.; Nagarajan, S.; Tosh, C.; Namdeo, P.; Singh, R.; Mishra, S.; Kombiah, S.; Dhanapal, S.; et al. Experimental Infection and In-Contact Transmission of H9N2 Avian Influenza Virus in Crows. Pathogens 2022, 11, 304. [Google Scholar] [CrossRef] [PubMed]
- Sakuma, S.; Tanikawa, T.; Tsunekuni, R.; Mine, J.; Kumagai, A.; Miyazawa, K.; Takadate, Y.; Uchida, Y. Experimental Infection of Chickens with H5N8 High Pathogenicity Avian Influenza Viruses Isolated in Japan in the Winter of 2020–2021. Viruses 2023, 15, 2293. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.H.; Lee, D.H.; Swayne, D.E.; Noh, J.Y.; Yuk, S.S.; Jeong, S.; Lee, S.H.; Woo, C.; Shin, J.H.; Song, C.S. Experimental infection of H5N1 and H5N8 highly pathogenic avian influenza viruses in Northern Pintail (Anas acuta). Transbound. Emerg. Dis. 2018, 65, 1367–1371. [Google Scholar] [CrossRef]
- Kang, H.M.; Lee, E.K.; Song, B.M.; Heo, G.B.; Jung, J.; Jang, I.; Bae, Y.C.; Jung, S.C.; Lee, Y.J. Experimental infection of mandarin duck with highly pathogenic avian influenza A (H5N8 and H5N1) viruses. Vet. Microbiol. 2017, 198, 59–63. [Google Scholar] [CrossRef]
- Abolnik, C.; Stutchbury, S.; Hartman, M.J. Experimental infection of racing pigeons (Columba livia domestica) with highly pathogenic Clade 2.3.4.4 sub-group B H5N8 avian influenza virus. Vet. Microbiol. 2018, 227, 127–132. [Google Scholar] [CrossRef]
- Kwon, J.H.; Noh, Y.K.; Lee, D.H.; Yuk, S.S.; Erdene-Ochir, T.O.; Noh, J.Y.; Hong, W.T.; Jeong, J.H.; Jeong, S.; Gwon, G.B.; et al. Experimental infection with highly pathogenic H5N8 avian influenza viruses in the Mandarin duck (Aix galericulata) and domestic pigeon (Columba livia domestica). Vet. Microbiol. 2017, 203, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Zhang, Y.; Chen, L.; Zhang, B.; Zhang, M.; Wang, J.; Jiang, Y.; Yang, C.; Jiang, T. Genetic Characteristics and Pathogenicity Analysis in Chickens and Mice of Three H9N2 Avian Influenza Viruses. Viruses 2019, 11, 1127. [Google Scholar] [CrossRef]
- He, Z.; Wang, X.; Lin, Y.; Feng, S.; Huang, X.; Zhao, L.; Zhang, J.; Ding, Y.; Li, W.; Yuan, R.; et al. Genetic characteristics of waterfowl-origin H5N6 highly pathogenic avian influenza viruses and their pathogenesis in ducks and chickens. Front. Microbiol. 2023, 14, 1211355. [Google Scholar] [CrossRef]
- Scheibner, D.; Breithaupt, A.; Luttermann, C.; Blaurock, C.; Mettenleiter, T.C.; Abdelwhab, E.M. Genetic Determinants for Virulence and Transmission of the Panzootic Avian Influenza Virus H5N8 Clade 2.3.4.4 in Pekin Ducks. J. Virol. 2022, 96, e0014922. [Google Scholar] [CrossRef]
- Mostafa, A.; Blaurock, C.; Scheibner, D.; Muller, C.; Blohm, U.; Schafer, A.; Gischke, M.; Salaheldin, A.H.; Nooh, H.Z.; Ali, M.A.; et al. Genetic incompatibilities and reduced transmission in chickens may limit the evolution of reassortants between H9N2 and panzootic H5N8 clade 2.3.4.4 avian influenza virus showing high virulence for mammals. Virus Evol. 2020, 6, veaa077. [Google Scholar] [CrossRef]
- Lv, X.; Tian, J.; Li, X.; Bai, X.; Li, Y.; Li, M.; An, Q.; Song, X.; Xu, Y.; Sun, H.; et al. H10Nx avian influenza viruses detected in wild birds in China pose potential threat to mammals. One Health 2023, 16, 100515. [Google Scholar] [CrossRef] [PubMed]
- Jiao, P.; Song, Y.; Huang, J.; Xiang, C.; Cui, J.; Wu, S.; Qu, N.; Wang, N.; Ouyang, G.; Liao, M. H7N9 Avian Influenza Virus Is Efficiently Transmissible and Induces an Antibody Response in Chickens. Front. Immunol. 2018, 9, 789. [Google Scholar] [CrossRef]
- Bosco-Lauth, A.; Rodriguez, A.; Maison, R.M.; Porter, S.M.; Root, J.J. H7N9 influenza A virus transmission in a multispecies barnyard model. Virology 2023, 582, 100–105. [Google Scholar] [CrossRef]
- Seiler, P.; Kercher, L.; Feeroz, M.M.; Shanmuganatham, K.; Jones-Engel, L.; Turner, J.; Walker, D.; Alam, S.M.R.; Hasan, M.K.; Akhtar, S.; et al. H9N2 influenza viruses from Bangladesh: Transmission in chicken and New World quail. Influenza Other Respir. Viruses 2018, 12, 814–817. [Google Scholar] [CrossRef]
- Naiqing, X.; Tang, X.; Wang, X.; Cai, M.; Liu, X.; Lu, X.; Hu, S.; Gu, M.; Hu, J.; Gao, R.; et al. Hemagglutinin affects replication, stability and airborne transmission of the H9N2 subtype avian influenza virus. Virology 2024, 589, 109926. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.K.; Kumar, M.; Murugkar, H.V.; Nagarajan, S.; Tosh, C.; Namdeo, P.; Singh, R.; Mishra, S.; Senthilkumar, D.; Singh, V.P.; et al. Highly pathogenic avian influenza (H5N1) infection in crows through ingestion of infected crow carcasses. Microb. Pathog. 2023, 183, 106330. [Google Scholar] [CrossRef]
- Ducatez, M.; Sonnberg, S.; Crumpton, J.C.; Rubrum, A.; Phommachanh, P.; Douangngeun, B.; Peiris, M.; Guan, Y.; Webster, R.; Webby, R. Highly pathogenic avian influenza H5N1 clade 2.3.2.1 and clade 2.3.4 viruses do not induce a clade-specific phenotype in mallard ducks. J. Gen. Virol. 2017, 98, 1232–1244. [Google Scholar] [CrossRef]
- Seekings, A.H.; Warren, C.J.; Thomas, S.S.; Mahmood, S.; James, J.; Byrne, A.M.P.; Watson, S.; Bianco, C.; Nunez, A.; Brown, I.H.; et al. Highly pathogenic avian influenza virus H5N6 (clade 2.3.4.4b) has a preferable host tropism for waterfowl reflected in its inefficient transmission to terrestrial poultry. Virology 2021, 559, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Huang, J.; Huang, Q.; Zhang, J.; Liu, J.; Xue, Q.; Li, W.; Liao, M.; Jiao, P. Host Innate Immune Response of Geese Infected with Clade 2.3.4.4 H5N6 Highly Pathogenic Avian Influenza Viruses. Microorganisms 2020, 8, 224. [Google Scholar] [CrossRef]
- Verhagen, J.H.; Eriksson, P.; Leijten, L.; Blixt, O.; Olsen, B.; Waldenstrom, J.; Ellstrom, P.; Kuiken, T. Host Range of Influenza A Virus H1 to H16 in Eurasian Ducks Based on Tissue and Receptor Binding Studies. J. Virol. 2021, 95, jvi.01873-20. [Google Scholar] [CrossRef]
- Wang, C.; Wang, Z.; Ren, X.; Wang, L.; Li, C.; Sun, Y.; Wang, M.; Tong, Q.; Sun, H.; Pu, J. Infection of chicken H9N2 influenza viruses in different species of domestic ducks. Vet. Microbiol. 2019, 233, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Pantin-Jackwood, M.J.; Costa-Hurtado, M.; Bertran, K.; DeJesus, E.; Smith, D.; Swayne, D.E. Infectivity, transmission and pathogenicity of H5 highly pathogenic avian influenza clade 2.3.4.4 (H5N8 and H5N2) United States index viruses in Pekin ducks and Chinese geese. Vet. Res. 2017, 48, 33. [Google Scholar] [CrossRef] [PubMed]
- Evseev, D.; Magor, K.E. Innate Immune Responses to Avian Influenza Viruses in Ducks and Chickens. Vet. Sci. 2019, 6, 5. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Xu, X.; Wang, C.; Bing, G.; Sun, H.; Pu, J.; Liu, J.; Sun, Y. Isolation and characterization of H4N6 avian influenza viruses from mallard ducks in Beijing, China. PLoS ONE 2017, 12, e0184437. [Google Scholar] [CrossRef]
- Leyson, C.; Youk, S.S.; Smith, D.; Dimitrov, K.; Lee, D.H.; Larsen, L.E.; Swayne, D.E.; Pantin-Jackwood, M.J. Pathogenicity and genomic changes of a 2016 European H5N8 highly pathogenic avian influenza virus (clade 2.3.4.4) in experimentally infected mallards and chickens. Virology 2019, 537, 172–185. [Google Scholar] [CrossRef]
- Xiang, B.; Liang, J.; You, R.; Han, L.; Mei, K.; Chen, L.; Chen, R.; Zhang, Y.; Dai, X.; Gao, P.; et al. Pathogenicity and transmissibility of a highly pathogenic avian influenza virus H5N6 isolated from a domestic goose in Southern China. Vet. Microbiol. 2017, 212, 16–21. [Google Scholar] [CrossRef]
- Liu, K.; Gao, R.; Wang, X.; Han, W.; Ji, Z.; Zheng, H.; Gu, M.; Hu, J.; Liu, X.; Hu, S.; et al. Pathogenicity and transmissibility of clade 2.3.4.4 highly pathogenic avian influenza virus subtype H5N6 in pigeons. Vet. Microbiol. 2020, 247, 108776. [Google Scholar] [CrossRef]
- Roy Chowdhury, I.; Yeddula, S.G.R.; Kim, S.H. Pathogenicity and Transmissibility of North American H7 Low Pathogenic Avian Influenza Viruses in Chickens and Turkeys. Viruses 2019, 11, 163. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Shen, X.; Yuan, R.; Xiang, B.; Fang, Z.; Murphy, R.W.; Liao, M.; Shen, Y.; Ren, T. Pathogenicity and transmissibility of three avian influenza A (H5N6) viruses isolated from wild birds. J. Infect. 2018, 76, 286–294. [Google Scholar] [CrossRef]
- Soda, K.; Tomioka, Y.; Usui, T.; Ozaki, H.; Yamaguchi, T.; Ito, T. Pathogenicity of H5 highly pathogenic avian influenza virus in rooks (Corvus frugilegus). Avian Pathol. 2020, 49, 261–267. [Google Scholar] [CrossRef]
- El-Shall, N.A.; Abd El Naby, W.S.H.; Hussein, E.G.S.; Yonis, A.E.; Sedeik, M.E. Pathogenicity of H5N8 avian influenza virus in chickens and in duck breeds and the role of MX1 and IFN-alpha in infection outcome and transmission to contact birds. Comp. Immunol. Microbiol. Infect. Dis. 2023, 100, 102039. [Google Scholar] [CrossRef] [PubMed]
- Park, M.J.; Cha, R.M.; Kye, S.J.; Lee, Y.N.; Kim, N.Y.; Baek, Y.G.; Heo, G.B.; Sagong, M.; Lee, K.N.; Lee, Y.J.; et al. Pathogenicity of H5N8 High Pathogenicity Avian Influenza Virus in Chickens and Ducks from South Korea in 2020–2021. Viruses 2021, 13, 1903. [Google Scholar] [CrossRef]
- Tarasiuk, K.; Kycko, A.; Knitter, M.; Swieton, E.; Wyrostek, K.; Domanska-Blicharz, K.; Bocian, L.; Meissner, W.; Smietanka, K. Pathogenicity of highly pathogenic avian influenza H5N8 subtype for herring gulls (Larus argentatus): Impact of homo- and heterosubtypic immunity on the outcome of infection. Vet. Res. 2022, 53, 108. [Google Scholar] [CrossRef] [PubMed]
- Yehia, N.; Erfan, A.M.; Adel, A.; El-Tayeb, A.; Hassan, W.M.M.; Samy, A.; Abd El-Hack, M.E.; El-Saadony, M.T.; El-Tarabily, K.A.; Ahmed, K.A. Pathogenicity of three genetically distinct and highly pathogenic Egyptian H5N8 avian influenza viruses in chickens. Poult. Sci. 2022, 101, 101662. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Li, W.; Wu, W.; Liu, Z.; He, Z.; Chen, Z.; Zhao, B.; Wu, S.; Yang, C.; Qu, X.; et al. Phylogeny, Pathogenicity, Transmission, and Host Immune Responses of Four H5N6 Avian Influenza Viruses in Chickens and Mice. Viruses 2019, 11, 1048. [Google Scholar] [CrossRef]
- Zhu, Y.C.; Zhang, B.; Sun, Z.H.; Wang, X.J.; Fan, X.H.; Gao, L.X.; Liang, Y.; Chen, X.Y.; Zhang, Z.F. Replication and Pathology of Duck Influenza Virus Subtype H9N2 in Chukar. Biomed. Environ. Sci. 2018, 31, 306–310. [Google Scholar] [CrossRef]
- Jeong, S.; Kwon, J.H.; Lee, S.H.; Kim, Y.J.; Jeong, J.H.; Park, J.E.; Jheong, W.H.; Lee, D.H.; Song, C.S. Subclinical Infection and Transmission of Clade 2.3.4.4 H5N6 Highly Pathogenic Avian Influenza Virus in Mandarin Duck (Aix galericulata) and Domestic Pigeon (Columbia livia domestica). Viruses 2021, 13, 1069. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Wu, S.; Wu, W.; Liang, Y.; Zhuang, H.; Ye, Z.; Qu, X.; Liao, M.; Jiao, P. The Biological Characteristics of Novel H5N6 Highly Pathogenic Avian Influenza Virus and Its Pathogenesis in Ducks. Front. Microbiol. 2021, 12, 628545. [Google Scholar] [CrossRef]
- Pantin-Jackwood, M.J.; Stephens, C.B.; Bertran, K.; Swayne, D.E.; Spackman, E. The pathogenesis of H7N8 low and highly pathogenic avian influenza viruses from the United States 2016 outbreak in chickens, turkeys and mallards. PLoS ONE 2017, 12, e0177265. [Google Scholar] [CrossRef]
- Mo, J.; Youk, S.; Pantin-Jackwood, M.J.; Suarez, D.L.; Lee, D.H.; Killian, M.L.; Bergeson, N.H.; Spackman, E. The pathogenicity and transmission of live bird market H2N2 avian influenza viruses in chickens, Pekin ducks, and guinea fowl. Vet. Microbiol. 2021, 260, 109180. [Google Scholar] [CrossRef]
- Puranik, A.; Slomka, M.J.; Warren, C.J.; Thomas, S.S.; Mahmood, S.; Byrne, A.M.P.; Ramsay, A.M.; Skinner, P.; Watson, S.; Everett, H.E.; et al. Transmission dynamics between infected waterfowl and terrestrial poultry: Differences between the transmission and tropism of H5N8 highly pathogenic avian influenza virus (clade 2.3.4.4a) among ducks, chickens and turkeys. Virology 2020, 541, 113–123. [Google Scholar] [CrossRef]
- Root, J.J.; Shriner, S.A.; Ellis, J.W.; VanDalen, K.K.; Franklin, A.B. Transmission of H6N2 wild bird-origin influenza A virus among multiple bird species in a stacked-cage setting. Arch. Virol. 2017, 162, 2617–2624. [Google Scholar] [CrossRef] [PubMed]
- Raj, S.; Astill, J.; Alqazlan, N.; Boodhoo, N.; Hodgins, D.C.; Nagy, E.; Mubareka, S.; Karimi, K.; Sharif, S. Transmission of H9N2 Low Pathogenicity Avian Influenza Virus (LPAIV) in a Challenge-Transmission Model. Vaccines 2022, 10, 1040. [Google Scholar] [CrossRef]
- Kobayashi, D.; Hiono, T.; Ichii, O.; Nishihara, S.; Takase-Yoden, S.; Yamamoto, K.; Kawashima, H.; Isoda, N.; Sakoda, Y. Turkeys possess diverse Siaalpha2-3Gal glycans that facilitate their dual susceptibility to avian influenza viruses isolated from ducks and chickens. Virus Res. 2022, 315, 198771. [Google Scholar] [CrossRef] [PubMed]
- Criado, M.F.; Moresco, K.A.; Stallknecht, D.E.; Swayne, D.E. Low-pathogenicity influenza viruses replicate differently in laughing gulls and mallards. Influenza Other Respir. Viruses 2021, 15, 701–706. [Google Scholar] [CrossRef] [PubMed]
- Graaf, A.; Ulrich, R.; Maksimov, P.; Scheibner, D.; Koethe, S.; Abdelwhab, E.M.; Mettenleiter, T.C.; Beer, M.; Harder, T. A viral race for primacy: Co-infection of a natural pair of low and highly pathogenic H7N7 avian influenza viruses in chickens and embryonated chicken eggs. Emerg. Microbes Infect. 2018, 7, 204. [Google Scholar] [CrossRef]
- Bertran, K.; Balzli, C.; Kwon, Y.K.; Tumpey, T.M.; Clark, A.; Swayne, D.E. Airborne Transmission of Highly Pathogenic Influenza Virus during Processing of Infected Poultry. Emerg. Infect. Dis. 2017, 23, 1806–1814. [Google Scholar] [CrossRef]
- Naguib, M.M.; Eriksson, P.; Jax, E.; Wille, M.; Lindskog, C.; Brojer, C.; Krambrich, J.; Waldenstrom, J.; Kraus, R.H.S.; Larson, G.; et al. A Comparison of Host Responses to Infection with Wild-Type Avian Influenza Viruses in Chickens and Tufted Ducks. Microbiol. Spectr. 2023, 11, e0258622. [Google Scholar] [CrossRef]
- Grund, C.; Hoffmann, D.; Ulrich, R.; Naguib, M.; Schinkothe, J.; Hoffmann, B.; Harder, T.; Saenger, S.; Zscheppang, K.; Tonnies, M.; et al. A novel European H5N8 influenza A virus has increased virulence in ducks but low zoonotic potential. Emerg. Microbes Infect. 2018, 7, 132. [Google Scholar] [CrossRef]
- Cui, P.; Shi, J.; Yan, C.; Wang, C.; Zhang, Y.; Zhang, Y.; Xing, X.; Chen, Y.; Zhang, J.; Liu, L.; et al. Analysis of avian influenza A (H3N8) viruses in poultry and their zoonotic potential, China, September 2021 to May 2022. Eurosurveillance 2023, 28, 2200871. [Google Scholar] [CrossRef]
- Skog, E.; Nykvist, M.; Naguib, M.M.; Wille, M.; Brojer, C.; Agarwal, V.; Ellstrom, P.; Westman, G.; Lundkvist, A.; Jarhult, J.D. An oseltamivir-resistant avian H1N1 influenza A virus can transmit from mallards to chickens similarly to a wild-type strain: Implications for the risk of resistance transmission to humans. J. Gen. Virol. 2023, 104, 001835. [Google Scholar] [CrossRef] [PubMed]
- Oh, K.H.; Mo, J.S.; Bae, Y.J.; Lee, S.B.; Lai, V.D.; Wang, S.J.; Mo, I.P. Amino acid substitutions in low pathogenic avian influenza virus strains isolated from wild birds in Korea. Virus Genes 2018, 54, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Wan, Z.; Zhao, Z.; Sang, J.; Jiang, W.; Chen, J.; Tang, T.; Li, Y.; Kan, Q.; Shao, H.; Zhang, J.; et al. Amino Acid Variation at Hemagglutinin Position 193 Impacts the Properties of H9N2 Avian Influenza Virus. J. Virol. 2023, 97, e0137922. [Google Scholar] [CrossRef]
- Swieton, E.; Tarasiuk, K.; Olszewska-Tomczyk, M.; Iwan, E.; Smietanka, K. A Turkey-origin H9N2 Avian Influenza Virus Shows Low Pathogenicity but Different Within-Host Diversity in Experimentally Infected Turkeys, Quail and Ducks. Viruses 2020, 12, 319. [Google Scholar] [CrossRef] [PubMed]
- Bhat, S.; James, J.; Sadeyen, J.R.; Mahmood, S.; Everest, H.J.; Chang, P.; Walsh, S.K.; Byrne, A.M.P.; Mollett, B.; Lean, F.; et al. Coinfection of Chickens with H9N2 and H7N9 Avian Influenza Viruses Leads to Emergence of Reassortant H9N9 Virus with Increased Fitness for Poultry and a Zoonotic Potential. J. Virol. 2022, 96, e0185621. [Google Scholar] [CrossRef]
- Kwon, H.I.; Kim, E.H.; Kim, Y.I.; Park, S.J.; Si, Y.J.; Lee, I.W.; Nguyen, H.D.; Yu, K.M.; Yu, M.A.; Jung, J.H.; et al. Comparison of the pathogenic potential of highly pathogenic avian influenza (HPAI) H5N6, and H5N8 viruses isolated in South Korea during the 2016–2017 winter season. Emerg. Microbes Infect. 2018, 7, 29. [Google Scholar] [CrossRef]
- Richard, M.; Herfst, S.; van den Brand, J.M.A.; de Meulder, D.; Lexmond, P.; Bestebroer, T.M.; Fouchier, R.A.M. Mutations Driving Airborne Transmission of A/H5N1 Virus in Mammals Cause Substantial Attenuation in Chickens only when combined. Sci. Rep. 2017, 7, 7187. [Google Scholar] [CrossRef]
- Naguib, M.M.; Ulrich, R.; Kasbohm, E.; Eng, C.L.P.; Hoffmann, D.; Grund, C.; Beer, M.; Harder, T.C. Natural Reassortants of Potentially Zoonotic Avian Influenza Viruses H5N1 and H9N2 from Egypt Display Distinct Pathogenic Phenotypes in Experimentally Infected Chickens and Ferrets. J. Virol. 2017, 91, jvi.01300-17. [Google Scholar] [CrossRef]
- Kayed, A.E.; Kutkat, O.; Kandeil, A.; Moatasim, Y.; El Taweel, A.; El Sayes, M.; El-Shesheny, R.; Aboulhoda, B.E.; Abdeltawab, N.F.; Kayali, G.; et al. Comparative pathogenic potential of avian influenza H7N3 viruses isolated from wild birds in Egypt and their sensitivity to commercial antiviral drugs. Arch. Virol. 2023, 168, 82. [Google Scholar] [CrossRef]
- Yudhawati, R.; Prasetya, R.R.; Dewantari, J.R.; Nastri, A.M.; Rahardjo, K.; Novianti, A.N.; Amin, M.; Rantam, F.A.; Poetranto, E.D.; Wulandari, L.; et al. Comparison of Virulence and Lethality in Mice for Avian Influenza Viruses of Two A/H5N1 and One A/H3N6 Isolated from Poultry During Year 2013–2014 in Indonesia. Jpn. J. Infect. Dis. 2020, 73, 336–342. [Google Scholar] [CrossRef]
- Xie, R.; Wang, W.; Gao, Y.; Liu, W.; Yue, B.; Liu, S.; Fan, W.; Song, S.; Yan, L. Evolution and mammalian adaptation of H3 and H10 subtype avian influenza viruses in wild birds in Yancheng Wetland of China. Vet. Microbiol. 2023, 279, 109669. [Google Scholar] [CrossRef] [PubMed]
- El-Shesheny, R.; Feeroz, M.M.; Krauss, S.; Vogel, P.; McKenzie, P.; Webby, R.J.; Webster, R.G. Replication and pathogenic potential of influenza A virus subtypes H3, H7, and H15 from free-range ducks in Bangladesh in mammals. Emerg. Microbes Infect. 2018, 7, 70. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Lei, H.; Song, W.; Shin, S.C.; Wang, J.; Xiao, B.; Kocer, Z.A.; Song, M.S.; Webster, R.; Webby, R.J.; et al. Amino acids in the polymerase complex of shorebird-isolated H1N1 influenza virus impact replication and host-virus interactions in mammalian models. Emerg. Microbes Infect. 2024, 13, 2332652. [Google Scholar] [CrossRef]
- Zhang, C.; Zhao, Z.; Guo, Z.; Zhang, J.; Li, J.; Yang, Y.; Lu, S.; Wang, Z.; Zhi, M.; Fu, Y.; et al. Amino Acid Substitutions Associated with Avian H5N6 Influenza A Virus Adaptation to Mice. Front. Microbiol. 2017, 8, 1763. [Google Scholar] [CrossRef] [PubMed]
- Belser, J.A.; Pulit-Penaloza, J.A.; Sun, X.; Brock, N.; Pappas, C.; Creager, H.M.; Zeng, H.; Tumpey, T.M.; Maines, T.R. A Novel A(H7N2) Influenza Virus Isolated from a Veterinarian Caring for Cats in a New York City Animal Shelter Causes Mild Disease and Transmits Poorly in the Ferret Model. J. Virol. 2017, 91, jvi.00672-17. [Google Scholar] [CrossRef]
- Fu, X.; Huang, Y.; Fang, B.; Liu, Y.; Cai, M.; Zhong, R.; Huang, J.; Wenbao, Q.; Tian, Y.; Zhang, G. Evidence of H10N8 influenza virus infection among swine in southern China and its infectivity and transmissibility in swine. Emerg. Microbes Infect. 2020, 9, 88–94. [Google Scholar] [CrossRef]
- Stadejek, W.; Chiers, K.; Van Reeth, K. Infectivity and transmissibility of an avian H3N1 influenza virus in pigs. Vet. Res. 2023, 54, 4. [Google Scholar] [CrossRef]
- Abente, E.J.; Kitikoon, P.; Lager, K.M.; Gauger, P.C.; Anderson, T.K.; Vincent, A.L. A highly pathogenic avian-derived influenza virus H5N1 with 2009 pandemic H1N1 internal genes demonstrates increased replication and transmission in pigs. J. Gen. Virol. 2017, 98, 18–30. [Google Scholar] [CrossRef]
- Powell, J.D.; Abente, E.J.; Torchetti, M.K.; Killian, M.L.; Vincent, A.L. An avian influenza virus A(H7N9) reassortant that recently emerged in the United States with low pathogenic phenotype does not efficiently infect swine. Influenza Other Respir. Viruses 2019, 13, 288–291. [Google Scholar] [CrossRef]
- Kaplan, B.S.; Torchetti, M.K.; Lager, K.M.; Webby, R.J.; Vincent, A.L. Absence of clinical disease and contact transmission of HPAI H5NX clade 2.3.4.4 from North America in experimentally infected pigs. Influenza Other Respir. Viruses 2017, 11, 464–470. [Google Scholar] [CrossRef]
- Danzy, S.; Lowen, A.C.; Steel, J. A quantitative approach to assess influenza A virus fitness and transmission in guinea pigs. J. Virol. 2021, 95, jvi.02320-20. [Google Scholar] [CrossRef] [PubMed]
- Dreier, C.; Resa-Infante, P.; Thiele, S.; Stanelle-Bertram, S.; Walendy-Gnirss, K.; Speiseder, T.; Preuss, A.; Muller, Z.; Klenk, H.D.; Stech, J.; et al. Mutations in the H7 HA and PB1 genes of avian influenza a viruses increase viral pathogenicity and contact transmission in guinea pigs. Emerg. Microbes Infect. 2019, 8, 1324–1336. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, Y.; Jin, S.; Zhang, Y.; Sun, L.; Hu, X.; Zhao, M.; Li, F.; Wang, T.; Sun, W.; et al. PB1 S524G mutation of wild bird-origin H3N8 influenza A virus enhances virulence and fitness for transmission in mammals. Emerg. Microbes Infect. 2021, 10, 1038–1051. [Google Scholar] [CrossRef]
- Lyoo, K.S.; Na, W.; Phan, L.V.; Yoon, S.W.; Yeom, M.; Song, D.; Jeong, D.G. Experimental infection of clade 1.1.2 (H5N1), clade 2.3.2.1c (H5N1) and clade 2.3.4.4 (H5N6) highly pathogenic avian influenza viruses in dogs. Transbound. Emerg. Dis. 2017, 64, 1669–1675. [Google Scholar] [CrossRef]
- Yuk, S.S.; Lee, D.H.; Park, J.K.; Tseren-Ochir, E.O.; Kwon, J.H.; Noh, J.Y.; Song, C.S. Experimental infection of dogs with highly pathogenic avian influenza virus (H5N8). J. Vet. Sci. 2017, 18, 381–384. [Google Scholar] [CrossRef]
- Liang, J.; Li, Q.; Cai, L.; Yuan, Q.; Chen, L.; Lin, Q.; Xiao, C.; Xiang, B.; Ren, T. Adaptation of Two Wild Bird-Origin H3N8 Avian Influenza Viruses to Mammalian Hosts. Viruses 2022, 14, 1097. [Google Scholar] [CrossRef]
- Zhao, Z.; Liu, L.; Guo, Z.; Zhang, C.; Wang, Z.; Wen, G.; Zhang, W.; Shang, Y.; Zhang, T.; Jiao, Z.; et al. A Novel Reassortant Avian H7N6 Influenza Virus Is Transmissible in Guinea Pigs via Respiratory Droplets. Front. Microbiol. 2019, 10, 18. [Google Scholar] [CrossRef]
- Zhu, H.; Damdinjav, B.; Gonzalez, G.; Patrono, L.V.; Ramirez-Mendoza, H.; Amat, J.A.R.; Crispell, J.; Parr, Y.A.; Hammond, T.A.; Shiilegdamba, E.; et al. Absence of adaptive evolution is the main barrier against influenza emergence in horses in Asia despite frequent virus interspecies transmission from wild birds. PLoS Pathog. 2019, 15, e1007531. [Google Scholar] [CrossRef] [PubMed]
- Imai, M.; Watanabe, T.; Kiso, M.; Nakajima, N.; Yamayoshi, S.; Iwatsuki-Horimoto, K.; Hatta, M.; Yamada, S.; Ito, M.; Sakai-Tagawa, Y.; et al. A Highly Pathogenic Avian H7N9 Influenza Virus Isolated from A Human Is Lethal in Some Ferrets Infected via Respiratory Droplets. Cell Host Microbe 2017, 22, 615–626.e618. [Google Scholar] [CrossRef]
- Herfst, S.; Begeman, L.; Spronken, M.I.; Poen, M.J.; Eggink, D.; de Meulder, D.; Lexmond, P.; Bestebroer, T.M.; Koopmans, M.P.G.; Kuiken, T.; et al. A Dutch highly pathogenic H5N6 avian influenza virus showed remarkable tropism for extra-respiratory organs and caused severe disease but was not transmissible via air in the ferret model. mSphere 2023, 8, e0020023. [Google Scholar] [CrossRef]
- Braun, K.M.; Haddock Iii, L.A.; Crooks, C.M.; Barry, G.L.; Lalli, J.; Neumann, G.; Watanabe, T.; Imai, M.; Yamayoshi, S.; Ito, M.; et al. Avian H7N9 influenza viruses are evolutionarily constrained by stochastic processes during replication and transmission in mammals. Virus Evol. 2023, 9, vead004. [Google Scholar] [CrossRef] [PubMed]
- Pulit-Penaloza, J.A.; Jones, J.; Sun, X.; Jang, Y.; Thor, S.; Belser, J.A.; Zanders, N.; Creager, H.M.; Ridenour, C.; Wang, L.; et al. Antigenically Diverse Swine Origin H1N1 Variant Influenza Viruses Exhibit Differential Ferret Pathogenesis and Transmission Phenotypes. J. Virol. 2018, 92, jvi.00095-18. [Google Scholar] [CrossRef]
- Lin, C.Y.; Chia, M.Y.; Chen, P.L.; Yeh, C.T.; Cheng, M.C.; Su, I.J.; Lee, M.S. Assessment of pathogenicity and antigenicity of American lineage influenza H5N2 viruses in Taiwan. Virology 2017, 508, 159–163. [Google Scholar] [CrossRef] [PubMed]
- Guan, M.; Hall, J.S.; Zhang, X.; Dusek, R.J.; Olivier, A.K.; Liu, L.; Li, L.; Krauss, S.; Danner, A.; Li, T.; et al. Aerosol Transmission of Gull-Origin Iceland Subtype H10N7 Influenza A Virus in Ferrets. J. Virol. 2019, 93, jvi.00282-19. [Google Scholar] [CrossRef]
- Kaplan, B.S.; Kimble, J.B.; Chang, J.; Anderson, T.K.; Gauger, P.C.; Janas-Martindale, A.; Killian, M.L.; Bowman, A.S.; Vincent, A.L. Aerosol Transmission from Infected Swine to Ferrets of an H3N2 Virus Collected from an Agricultural Fair and Associated with Human Variant Infections. J. Virol. 2020, 94, jvi.01009-20. [Google Scholar] [CrossRef]
- Chen, P.; Jin, Z.; Peng, L.; Zheng, Z.; Cheung, Y.M.; Guan, J.; Chen, L.; Huang, Y.; Fan, X.; Zhang, Z.; et al. Characterization of an Emergent Chicken H3N8 Influenza Virus in Southern China: A Potential Threat to Public Health. J. Virol. 2023, 97, e0043423. [Google Scholar] [CrossRef]
- Pulit-Penaloza, J.A.; Brock, N.; Pappas, C.; Sun, X.; Belser, J.A.; Zeng, H.; Tumpey, T.M.; Maines, T.R. Characterization of highly pathogenic avian influenza H5Nx viruses in the ferret model. Sci. Rep. 2020, 10, 12700. [Google Scholar] [CrossRef] [PubMed]
- Kutter, J.S.; Linster, M.; de Meulder, D.; Bestebroer, T.M.; Lexmond, P.; Rosu, M.E.; Richard, M.; de Vries, R.P.; Fouchier, R.A.M.; Herfst, S. Continued adaptation of A/H2N2 viruses during pandemic circulation in humans. J. Gen. Virol. 2023, 104, 001881. [Google Scholar] [CrossRef]
- El-Shesheny, R.; Franks, J.; Turner, J.; Seiler, P.; Walker, D.; Friedman, K.; Mukherjee, N.; Kercher, L.; Hasan, M.K.; Feeroz, M.M.; et al. Continued Evolution of H5Nx Avian Influenza Viruses in Bangladeshi Live Poultry Markets: Pathogenic Potential in Poultry and Mammalian Models. J. Virol. 2020, 94, jvi.01141-20. [Google Scholar] [CrossRef]
- Peng, W.; Bouwman, K.M.; McBride, R.; Grant, O.C.; Woods, R.J.; Verheije, M.H.; Paulson, J.C.; de Vries, R.P. Enhanced Human-Type Receptor Binding by Ferret-Transmissible H5N1 with a K193T Mutation. J. Virol. 2018, 92, jvi.02016-17. [Google Scholar] [CrossRef]
- Pearce, M.B.; Pappas, C.; Gustin, K.M.; Davis, C.T.; Pantin-Jackwood, M.J.; Swayne, D.E.; Maines, T.R.; Belser, J.A.; Tumpey, T.M. Enhanced virulence of clade 2.3.2.1 highly pathogenic avian influenza A H5N1 viruses in ferrets. Virology 2017, 502, 114–122. [Google Scholar] [CrossRef]
- Shi, J.; Deng, G.; Kong, H.; Gu, C.; Ma, S.; Yin, X.; Zeng, X.; Cui, P.; Chen, Y.; Yang, H.; et al. H7N9 virulent mutants detected in chickens in China pose an increased threat to humans. Cell Res. 2017, 27, 1409–1421. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, Y.; Jin, S.; Wang, T.; Sun, W.; Zhang, Y.; Li, F.; Zhao, M.; Sun, L.; Hu, X.; et al. H9N2 influenza virus spillover into wild birds from poultry in China bind to human-type receptors and transmit in mammals via respiratory droplets. Transbound. Emerg. Dis. 2022, 69, 669–684. [Google Scholar] [CrossRef] [PubMed]
- Herfst, S.; Zhang, J.; Richard, M.; McBride, R.; Lexmond, P.; Bestebroer, T.M.; Spronken, M.I.J.; de Meulder, D.; van den Brand, J.M.; Rosu, M.E.; et al. Hemagglutinin Traits Determine Transmission of Avian A/H10N7 Influenza Virus between Mammals. Cell Host Microbe 2020, 28, 602–613.E7. [Google Scholar] [CrossRef]
- Gaymard, A.; Frobert, E.; Lina, B.; Escuret, V. Human infections caused by A (H7N9) influenza virus: Emergence, evolution and pandemic risk. Virologie 2017, 21, 255–265. [Google Scholar]
- Nunez, I.A.; Jang, H.; Huang, Y.; Kelvin, A.; Ross, T.M. Influenza virus immune imprinting dictates the clinical outcomes in ferrets challenged with highly pathogenic avian influenza virus H5N1. Front. Vet. Sci. 2023, 10, 1286758. [Google Scholar] [CrossRef] [PubMed]
- Belser, J.A.; Brock, N.; Sun, X.; Jones, J.; Zanders, N.; Hodges, E.; Pulit-Penaloza, J.A.; Wentworth, D.; Tumpey, T.M.; Davis, T.; et al. Mammalian Pathogenesis and Transmission of Avian Influenza A(H7N9) Viruses, Tennessee, USA, 2017. Emerg. Infect. Dis. 2018, 24, 149–152. [Google Scholar] [CrossRef]
- Belser, J.A.; Sun, X.; Brock, N.; Pulit-Penaloza, J.A.; Jones, J.; Zanders, N.; Davis, C.T.; Tumpey, T.M.; Maines, T.R. Mammalian pathogenicity and transmissibility of low pathogenic avian influenza H7N1 and H7N3 viruses isolated from North America in 2018. Emerg. Microbes Infect. 2020, 9, 1037–1045. [Google Scholar] [CrossRef]
- Zanin, M.; Le, T.B.; Na, W.; Kang, J.A.; Kwon, H.J.; Hwang, J.; Ga, E.H.; Wong, S.S.; Cho, H.J.; Song, D.; et al. Potential for transmission of naturally mutated H10N1 avian influenza virus to mammalian hosts and causing severe pulmonary disease. Front. Microbiol. 2023, 14, 1256090. [Google Scholar] [CrossRef]
- Lee, J.; Wang, L.; Palinski, R.; Walsh, T.; He, D.; Li, Y.; Wu, R.; Lang, Y.; Sunwoo, S.Y.; Richt, J.A.; et al. Comparison of Pathogenicity and Transmissibility of Influenza B and D Viruses in Pigs. Viruses 2019, 11, 905. [Google Scholar] [CrossRef]
- Kaplan, B.S.; Falkenberg, S.; Dassanayake, R.; Neill, J.; Velayudhan, B.; Li, F.; Vincent, A.L. Virus strain influenced the interspecies transmission of influenza D virus between calves and pigs. Transbound. Emerg. Dis. 2021, 68, 3396–3404. [Google Scholar] [CrossRef] [PubMed]
- Salem, E.; Hagglund, S.; Cassard, H.; Corre, T.; Naslund, K.; Foret, C.; Gauthier, D.; Pinard, A.; Delverdier, M.; Zohari, S.; et al. Pathogenesis, Host Innate Immune Response, and Aerosol Transmission of Influenza D Virus in Cattle. J. Virol. 2019, 93, jvi.01853-18. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Na, W.; Kang, A.; Yeom, M.; Yuk, H.; Moon, H.; Kim, S.J.; Kim, H.W.; Kim, J.K.; Pang, M.; et al. Comparison of the virulence of three H3N2 canine influenza virus isolates from Korea and China in mouse and Guinea pig models. BMC Vet. Res. 2018, 14, 149. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Su, R.; Gu, Y.; Yu, Y.; Li, S.; Sun, H.; Pan, L.; Cui, X.; Zhu, X.; Yang, Q.; et al. Molecular Characteristics, Antigenicity, Pathogenicity, and Zoonotic Potential of a H3N2 Canine Influenza Virus Currently Circulating in South China. Front. Microbiol. 2021, 12, 628979. [Google Scholar] [CrossRef]
- Pulit-Penaloza, J.A.; Simpson, N.; Yang, H.; Creager, H.M.; Jones, J.; Carney, P.; Belser, J.A.; Yang, G.; Chang, J.; Zeng, H.; et al. Assessment of Molecular, Antigenic, and Pathological Features of Canine Influenza A(H3N2) Viruses That Emerged in the United States. J. Infect. Dis. 2017, 216, S499–S507. [Google Scholar] [CrossRef]
- Lee, I.W.; Kim, Y.I.; Lim, G.J.; Kwon, H.I.; Si, Y.J.; Park, S.J.; Kim, E.H.; Kim, S.M.; Nguyen, H.D.; Song, M.S.; et al. Comparison of the virulence and transmissibility of canine H3N2 influenza viruses and characterization of their canine adaptation factors. Emerg. Microbes Infect. 2018, 7, 17. [Google Scholar] [CrossRef]
- Zhou, P.; Chen, B.; Hu, X.; Xiao, X.; Liu, R.; Li, S. Domestic poultry are not susceptible to avian-origin H3N2 subtype canine in fl uenza A virus. Vet. Microbiol. 2022, 272, 109501. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, H.; Chen, Y.; Tao, S.; Liu, L.; Kong, H.; Ma, S.; Meng, F.; Suzuki, Y.; Qiao, C.; et al. A Single-Amino-Acid Substitution at Position 225 in Hemagglutinin Alters the Transmissibility of Eurasian Avian-Like H1N1 Swine Influenza Virus in Guinea Pigs. J. Virol. 2017, 91, JVI.00800-17. [Google Scholar] [CrossRef]
- Meng, F.; Chen, Y.; Song, Z.; Zhong, Q.; Zhang, Y.; Qiao, C.; Yan, C.; Kong, H.; Liu, L.; Li, C.; et al. Continued evolution of the Eurasian avian-like H1N1 swine influenza viruses in China. Sci. China Life Sci. 2023, 66, 269–282. [Google Scholar] [CrossRef]
- Hu, M.; Yang, G.; DeBeauchamp, J.; Crumpton, J.C.; Kim, H.; Li, L.; Wan, X.F.; Kercher, L.; Bowman, A.S.; Webster, R.G.; et al. HA stabilization promotes replication and transmission of swine H1N1 gamma influenza viruses in ferrets. Elife 2020, 9, e56236. [Google Scholar] [CrossRef]
- Kimble, J.B.; Souza, C.K.; Anderson, T.K.; Arendsee, Z.W.; Hufnagel, D.E.; Young, K.M.; Lewis, N.S.; Davis, C.T.; Thor, S.; Vincent Baker, A.L. Interspecies Transmission from Pigs to Ferrets of Antigenically Distinct Swine H1 Influenza A Viruses with Reduced Reactivity to Candidate Vaccine Virus Antisera as Measures of Relative Zoonotic Risk. Viruses 2022, 14, 2398. [Google Scholar] [CrossRef] [PubMed]
- Everett, H.E.; Nash, B.; Londt, B.Z.; Kelly, M.D.; Coward, V.; Nunez, A.; van Diemen, P.M.; Brown, I.H.; Brookes, S.M. Interspecies Transmission of Reassortant Swine Influenza A Virus Containing Genes from Swine Influenza A(H1N1)pdm09 and A(H1N2) Viruses. Emerg. Infect. Dis. 2020, 26, 273–281. [Google Scholar] [CrossRef] [PubMed]
- van Diemen, P.M.; Byrne, A.M.P.; Ramsay, A.M.; Watson, S.; Nunez, A.; Moreno, A.V.; Chiapponi, C.; Foni, E.; Brown, I.H.; Brookes, S.M.; et al. Interspecies Transmission of Swine Influenza A Viruses and Human Seasonal Vaccine-Mediated Protection Investigated in Ferret Model. Emerg. Infect. Dis. 2023, 29, 1798–1807. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Belser, J.A.; Pulit-Penaloza, J.A.; Brock, N.; Pappas, C.; Zanders, N.; Jang, Y.; Jones, J.; Tumpey, T.M.; Davis, C.T.; et al. Pathogenesis and transmission assessment of three swine-origin influenza A(H3N2) viruses with zoonotic risk to humans isolated in the U.S from 2017–2020. J. Infect. Dis. 2023, 229, 1107–1111. [Google Scholar] [CrossRef]
- Pulit-Penaloza, J.A.; Brock, N.; Jones, J.; Belser, J.A.; Jang, Y.; Sun, X.; Thor, S.; Pappas, C.; Zanders, N.; Tumpey, T.M.; et al. Pathogenesis and transmission of human seasonal and swine-origin A(H1) influenza viruses in the ferret model. Emerg. Microbes Infect. 2022, 11, 1452–1459. [Google Scholar] [CrossRef]
- Sun, H.; Kaplan, B.S.; Guan, M.; Zhang, G.; Ye, J.; Long, L.P.; Blackmon, S.; Yang, C.K.; Chiang, M.J.; Xie, H.; et al. Pathogenicity and transmission of a swine influenza A(H6N6) virus. Emerg. Microbes Infect. 2017, 6, e17. [Google Scholar] [CrossRef]
- Hu, M.; Jones, J.C.; Banoth, B.; Ojha, C.R.; Crumpton, J.C.; Kercher, L.; Webster, R.G.; Webby, R.J.; Russell, C.J. Swine H1N1 Influenza Virus Variants with Enhanced Polymerase Activity and HA Stability Promote Airborne Transmission in Ferrets. J. Virol. 2022, 96, e0010022. [Google Scholar] [CrossRef]
- Bravo-Vasquez, N.; Karlsson, E.A.; Jimenez-Bluhm, P.; Meliopoulos, V.; Kaplan, B.; Marvin, S.; Cortez, V.; Freiden, P.; Beck, M.A.; Hamilton-West, C.; et al. Swine Influenza Virus (H1N2) Characterization and Transmission in Ferrets, Chile. Emerg. Infect. Dis. 2017, 23, 241–251. [Google Scholar] [CrossRef]
- Pulit-Penaloza, J.A.; Belser, J.A.; Tumpey, T.M.; Maines, T.R. Swine-Origin H1 Influenza Viruses Isolated from Humans Exhibit Sustained Infectivity in an Aerosol State. Appl. Environ. Microbiol. 2019, 85, e00210-19. [Google Scholar] [CrossRef]
- Souza, C.K.; Kimble, J.B.; Anderson, T.K.; Arendsee, Z.W.; Hufnagel, D.E.; Young, K.M.; Gauger, P.C.; Lewis, N.S.; Davis, C.T.; Thor, S.; et al. Swine-to-Ferret Transmission of Antigenically Drifted Contemporary Swine H3N2 Influenza A Virus Is an Indicator of Zoonotic Risk to Humans. Viruses 2023, 15, 331. [Google Scholar] [CrossRef]
- Zhu, J.; Jiang, Z.; Liu, J. The matrix gene of pdm/09 H1N1 contributes to the pathogenicity and transmissibility of SIV in mammals. Vet. Microbiol. 2021, 255, 109039. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Badra, R.; Zhang, W.; Tam, J.S.L.; Webby, R.; van der Werf, S.; Nikisins, S.; Cullinane, A.; Gharaibeh, S.; Njouom, R.; Peiris, M.; et al. Transmission Pathways of Zoonotic Influenza Viruses and Influencing Factors: A Systematic Review of Recent Findings. Viruses 2025, 17, 857. https://doi.org/10.3390/v17060857
Badra R, Zhang W, Tam JSL, Webby R, van der Werf S, Nikisins S, Cullinane A, Gharaibeh S, Njouom R, Peiris M, et al. Transmission Pathways of Zoonotic Influenza Viruses and Influencing Factors: A Systematic Review of Recent Findings. Viruses. 2025; 17(6):857. https://doi.org/10.3390/v17060857
Chicago/Turabian StyleBadra, Rebecca, Wenqing Zhang, John S. L. Tam, Richard Webby, Sylvie van der Werf, Sergejs Nikisins, Ann Cullinane, Saad Gharaibeh, Richard Njouom, Malik Peiris, and et al. 2025. "Transmission Pathways of Zoonotic Influenza Viruses and Influencing Factors: A Systematic Review of Recent Findings" Viruses 17, no. 6: 857. https://doi.org/10.3390/v17060857
APA StyleBadra, R., Zhang, W., Tam, J. S. L., Webby, R., van der Werf, S., Nikisins, S., Cullinane, A., Gharaibeh, S., Njouom, R., Peiris, M., Kayali, G., & Heraud, J.-M. (2025). Transmission Pathways of Zoonotic Influenza Viruses and Influencing Factors: A Systematic Review of Recent Findings. Viruses, 17(6), 857. https://doi.org/10.3390/v17060857







