Abstract
Whiteflies of the Bemisia tabaci complex, along with the plant viruses they transmit, pose significant challenges to crop production worldwide. Upon infestation or infection, intimate interactions occur between plant hosts and these pests, influencing the spread and severity of pest-related epidemics in natural and agricultural ecosystems. This review explores the role of the salicylic acid (SA) signaling pathway, an essential component of plant defense, in modulating plant interactions with whiteflies and whitefly-borne viruses. We first outline the biosynthesis and signal transduction of SA. We then analyze how whitefly infestation activates the SA signaling pathway and how this defense response affects whitefly performance and preference. Next, we explore the interactions between the SA signaling pathway and whitefly-borne plant viruses, especially begomoviruses, which often activate and manipulate this pathway. We also examine how the SA signaling pathway influences plant–whitefly–virus tripartite interactions, highlighting the significant role of this defense pathway in whitefly-induced changes in plant–virus interactions and virus-induced changes in plant–whitefly interactions. Finally, we identify key areas for future research to further unravel the complexities of plant interactions with whiteflies and whitefly-borne viruses.
1. Perspectives and Overview
Phloem-feeding insects with piece-sucking mouthparts including aphids, whiteflies, leafhoppers, and planthoppers, are major pests of many crops [1]. In recent decades, whiteflies of the Bemisia tabaci species complex have emerged as global pests [2]. In particular, two cryptic species, namely Middle East–Asia Minor 1 (MEAM1) and Mediterranean (MED), have invaded numerous regions worldwide [2,3]. These two species have a broad host range and thus have become pests of many crops [4]. In addition to nutrient loss, whitefly infestation can cause plant physiological disorders and promote the growth of sooty mold [5]. Furthermore, whiteflies damage crops by transmitting plant viruses, leading to widespread epidemics of viral diseases [6,7,8].
The majority of whitefly-borne plant viruses belong to the genus Begomovirus (family Geminiviridae) [9,10]. Whiteflies are also responsible for spreading criniviruses (family Closteroviridae), ipomoviruses (family Potyviridae), torradoviruses (family Secoviridae), some carlaviruses (family Betaflexiviridae), and a polerovirus (Luteoviridae) [6,9]. Whiteflies transmit begomoviruses in a persistent, circulative manner, criniviruses, ipomoviruses, and torradoviruses in a semi-persistent manner, and carlaviruses in a non-persistent manner [9]. Over the past few decades, the spread of whiteflies has significantly contributed to the dissemination of whitefly-borne plant viruses. Notable whitefly-borne viruses, including tomato yellow leaf curl virus, African cassava mosaic virus, cotton leaf curl Multan virus, and tomato chlorosis virus, have become major disease agents worldwide [7,8].
Both insect herbivores and plant viruses, as biological stressors, interact intimately with host plants [11,12]. Upon attack, these stressors can profoundly alter plant gene expression and biochemistry, leading to changes in plant phenotype and physiology [11,12,13]. In response, plants may activate a variety of defenses mechanisms to mitigate damage. In turn, the pests may evolve strategies to evade or suppress plant defense responses, enabling their multiplication [12,13]. These interactions, particularly the interplay between host defense responses and pest activities, ultimately determine the outcome of insect infestation and virus infection [11,12]. Therefore, understanding defense mechanisms involved in plant interactions with both insect herbivores and viruses is crucial for advancing knowledge of pest epidemics and developing control strategies.
Plant hormones, such as salicylic acid (SA), play a key role in plant defense against biotic stressors [14,15,16]. Recent research has highlighted the SA signaling pathway as a key regulator in plant interactions with whitefly and whitefly-borne viruses. In this review, we first provide a brief overview of the biosynthesis and signal transduction of SA. We then explore the role of this pathway in the interactions of plants with whiteflies and whitefly-borne plant viruses, followed by an examination of its modulation of plant–whitefly–virus tripartite interactions. Finally, we identify several key issues that warrant future investigations.
2. A Brief Introduction to the SA Signaling Pathway
Most of the current knowledge regarding the plant SA signaling pathway has been derived from studies on Arabidopsis thaliana. This section summarizes the biosynthesis and signal transduction of SA to aid in understanding the following sections.
2.1. SA Biosynthesis
In plants, SA is synthesized via two primary pathways, namely the isochorismate synthase (ICS) pathway and the phenylalanine ammonia lyase (PAL) pathway [17]. The relative importance of the two pathways varies across plant species. In Arabidopsis, profiling of SA in mutants under various conditions showed that the ICS pathway plays a major role in regulating SA biosynthesis under both resting and pathogen-induced scenarios, with the PAL pathway making a minor contribution [16,18]. However, in species such as tobacco, soybean, and rice, the PAL pathway appears to be equally or even more important than the ICS pathway for SA biosynthesis [19,20,21].
In Arabidopsis, SA biosynthesis starts with the conversion of chorismate to isochorismate in plastids, catalyzed by ICS1/2 [16,22]. ICS1 plays a dominant role in this process, while the function of ICS2 may manifest only in the absence of ICS1 [22]. Isochorismate is then transported into the cytosol by Enhanced Disease Susceptibility 5 (EDS5), wherein AvrPphB Susceptible 3 (PBS3) converts isochorismate to isochorismate-9-glutamate [23]. Once synthesized, isochorismate-9-glutamate can either decompose to SA or be converted into SA by Enhanced Pseudomonas Susceptibility 1 (EPS1) [23,24,25].
In contrast to the ICS pathway, the PAL pathway is less well studied, with most research focused on plants other than Arabidopsis. Phenylalanine, synthesized from chorismate via either the arogenate or phenylpyruvate pathways [26,27], is converted by PAL enzymes into trans-cinnamic acid, which is then transformed into benzoic acid by ABNORMAL INFLORESCENCE MERISTEM1 (AIM1) [19,28]. Finally, SA is produced from benzoic acid through the action of benzoic acid 2-hydroxylase (BA2H), a member of the cytochrome P450 (CYP) superfamily [29,30].
2.2. Signal Transduction in the SA Signaling Pathway
The signal transduction of SA has been extensively studied in Arabidopsis (Figure 1) (reviewed in [14,17,31]). NPR1, a key regulator of SA signal transduction, belongs to the nonexpressor of the pathogenesis-related genes (NPR) family [17,31]. NPR1 is characterized by three functional domains, namely the Broad-complex, Tramtrack, and Bric-à-brac/poxvirus and zinc finger (BTB/POZ) domain, a central Ankyrin repeat (ANK) region and a C-terminal transactivation domain [32]. When SA level is low in plants, NPR1 exists as oligomers in the cytoplasm [33]. When SA levels increase, a redox change occurs, promoting NPR1 monomerization and its subsequent translocation into the nucleus [33]. This process is dynamically regulated, as S-nitrosylation of NPR1 promotes oligomerization, while thioredoxins facilitate the disassembly of NPR1 oligomers [34]. Once in the nucleus, NPR1 undergoes various post-translational modifications, including dephosphorylation, SUMOylation, and ubiquitination, which influence its nuclear retention, turnover, and transcription activator activity [35,36,37].
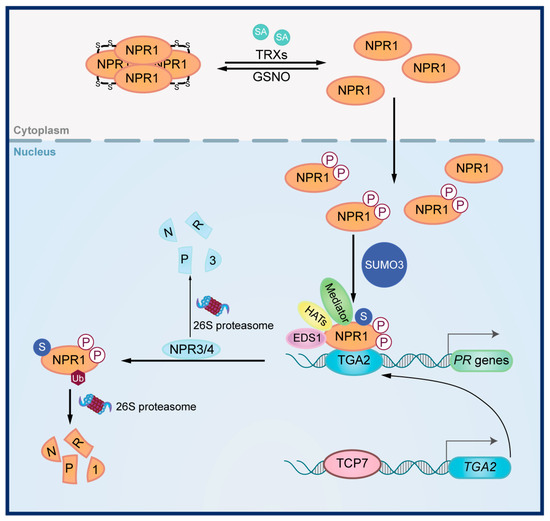
Figure 1.
Signal transduction in the SA signaling pathway. When SA level is low, NPR1 exists as oligomers in the cytoplasm. Upon the rise in SA levels, thioredoxins (TRXs) promote NPR1 monomerization and its subsequent translocation into the nucleus. NPR1 undergoes phosphorylation and then SUMOylation. Phosphorylated and SUMOylated NPR1 forms a complex with the transcription factor TGACG-binding TF2 (TGA2), ENHANCED DISEASE SUSCEPTIBILITY 1 (EDS1), histone acetyltransferases (HATs), and Mediator, thereby activating the expression of PR genes. TCP7 drives the expression of TGA2. NPR1 activity is modulated by NPR3/4, which promotes NPR1 ubiquitination and in turn degradation.
Several proteins, including TGACG-binding TF (TGA), ENHANCED DISEASE SUSCEPTIBILITY 1 (EDS1) and NPR3/4 are involved in the regulation of SA signal transduction by NPR1. Upon the rise in SA level, NPR1 binds to TGA transcription factors to promote the transcription of downstream SA-responsive genes [38,39]. This process is further modulated by various proteins that interact with NPR1 and TGA, including histone acetyltransferases (HATs), Mediator proteins, and EDS1 [40,41,42,43]. As paralogs of NPR1, NPR3 and NPR4 negatively regulate SA signal transduction by acting as adaptors for the Cullin 3 ubiquitin E3 ligase, promoting NPR1 degradation and serving as transcription repressors of TGA factors [44,45].
3. The Role of the SA Signaling Pathway in Plant–Whitefly Interactions
3.1. The Response of the Plant SA Signaling Pathway to Whitefly Infestation
In the past two decades, extensive research has been conducted on plant–whitefly interactions [46]. These studies show that whitefly feeding significantly modulates the plant SA signaling pathway (Table 1). In 26 out of 28 cases studies, whitefly infestation either increased SA content (13 cases), activated the expression of SA-related genes (9 cases), or both (4 cases).

Table 1.
Response of the plant SA signaling pathway to whitefly infestation.
Research on various whitefly species and developmental stages have been conducted. The most studied whitefly species is Bemisia tabaci MEAM1 (23 out of 26 cases where the species of whitefly was specified), followed by MED (3 cases), and in 2 cases, the identity of whitefly species was unspecified. As for the developmental stage, the majority of studies (20 cases) focused on adult whiteflies, followed by nymph (6 cases), egg (1 case), and mixed stages (adult, nymph, and egg) (1 case). Notably, in two cases where MEAM1 and MED adults were used, no significant impact on SA content or SA-related gene expression was observed (Table 1).
Plants of various species have been examined in relation to whitefly infestation. The Solanaceae family is the most frequently studied (23 cases), followed by Brassicaceae (3 cases), and then Leguminosae (2 cases). Within the Solanaceae family, tobacco (Nicotiana tabacum) is the most commonly investigated (11 cases), followed by tomato (Solanum lycopersicum, 7 cases), pepper (Capsicum annuum, 4 cases), and Nicotiana benthamiana (1 case). In the Brassicaceae family, only the model plant A. thaliana has been examined (3 cases). In the Leguminosae family, Lima bean (Phaseolus lunatus) and soybean (Glycine max) have been studied. Notably, pepper plants were used in the 2 cases wherein whitefly infestation did not affect the SA signaling pathway (Table 1).
3.2. Effects of SA Signaling Pathway on Whitefly Performance and Preference
Since the initial discovery that whitefly infestation activates the SA signaling pathway, the effects of the SA signaling pathway on whitefly biology, including performance and preference, have been extensively explored. Early studies suggested that the SA signaling pathway negatively regulates plant defenses against whitefly by suppressing the effectual jasmonate (JA)-mediated defenses. However, more recent research has indicated that the SA signaling pathway also plays a role in enhancing plant resistance to whiteflies. Moreover, JA seems to be more potent in conferring plant defenses against whitefly, suggesting that in earlier studies the effect of upregulated SA signaling pathway may be masked by downregulated JA signaling pathway. As for whitefly preference, recent studies have shown that the SA signaling pathway negatively regulates plant attractiveness to whiteflies.
The first study on the interaction between plant SA signaling and whiteflies, conducted by Zarate et al. (2007), showed that whitefly nymphs induce SA signaling pathway while suppressing the JA signaling pathway [47]. Experiments with various A. thaliana mutants demonstrated that impaired SA accumulation or signaling led to delayed nymphal development, indicating that the SA signaling pathway negatively regulates plant defenses against whitefly nymphs [47]. This was confirmed in a subsequent study by Zhang et al. (2013), who also showed that the SA signaling pathway suppresses JA-mediated defenses, which are crucial for plant resistance to whiteflies [52]. Similarly, Xu et al. (2019) found that whitefly adult infestation induces SA accumulation, which in turn suppresses the effectual JA-mediated defenses [57].
Two recent studies have reevaluated the role of the SA signaling pathway in affecting whitefly performance on plants. By manipulating the SA signaling pathway with exogenous application, mutagenesis and transgene techniques, Liu et al. (2024) showed that the activation of the SA signaling pathway negatively modulates the survival and fecundity of whitefly adults on plants [62]. Additionally, Song et al. (2024) found that the SA signaling pathway contributes to plant defense against whitefly eggs, as whitefly eggs exhibited higher hatching rate on plants with lower SA levels [64].
Although limited case studies are available, it appears that the SA signaling pathway negatively modulates plant attractiveness to whiteflies. Using tomato plants, Shi et al. (2016) and Jafarbeigi et al. (2020) showed that exogenous SA application repels whitefly settling [52,53]. Furthermore, whiteflies significantly preferred SA-deficient plants over wild-type plants [62,64,70,71,72].
4. Interactions of Plants with Whitefly-Borne Viruses
4.1. The Response of the SA Signaling Pathway to Whitefly-Borne Viruses
Whitefly-borne plant viruses include mostly begomoviruses (Geminiviridae), although a few viruses from 5 other families have been recorded [9]. To date, 14 case studies have been reported on the response of the plant SA signaling pathway to the infection of whitefly-borne viruses, all being begomoviruses (Table 2). In 13 out of these 14 studies, begomovirus infection activates the SA signaling pathway. Specifically, in 8 studies, begomoviruses induce the expression of SA-related genes, while in 5 cases infection results in both increased SA content and the activation of SA-related gene expression.

Table 2.
Response of the SA signaling pathway to whitefly-borne viruses.
Nine begomovirus–plant pathosystems have been examined. The most studied system is tomato yellow leaf curl virus (TYLCV)–tomato (Solanum lycopersicum) pathosystem with 6 studies. Other pathosystems have each been reported only once, including cabbage leaf curl virus in A. thaliana, tomato yellow leaf curl Sardinia virus in tomato, mungbean yellow mosaic India virus in V. mungo, euphorbia mosaic virus in pepper (C. annuum), tomato leaf curl Palampur virus in tomato, Sri Lankan cassava mosaic virus in N. benthamiana, tomato leaf curl New Delhi virus in potato (S. tuberosum), and tobacco curly shoot virus in N. benthamiana. The only exception, where virus infection decreases the expression of SA-related genes (PAL), occurs in the tomato yellow leaf curl Sardinia virus–tomato system.
4.2. Manipulation of the SA Signaling Pathways by Viral Proteins
The SA signaling pathway plays a crucial role in plant antiviral defense [85,86] and has been shown to contribute to immunity against begomoviruses [55,65,86]. Consequently, several studies have explored how viral proteins encoded by whitefly-borne begomoviruses manipulate the SA signaling pathway.
Four studies have examined this manipulation, with viral proteins inducing the SA signaling pathway in one case and suppressing it in three cases (Table 3) (Figure 2). Specifically, when the AV2 protein of tomato leaf curl Palampur virus is expressed in N. benthamiana, the expression of three SA-related genes increases significantly [77]. Conversely, the C4 protein of tomato yellow leaf curl virus negatively regulates the SA signaling pathway by targeting SA biosynthesis in chloroplast, leading to impaired SA biosynthesis [87]. The βC1 protein encoded by the betasatellite associated with tobacco curly shoot virus and the C2 protein encoded by TYLCV suppress the expression of SA-downstream genes by targeting NPR3 and TGA2, respectively [65,88]. NPR3 and TGA2 are key regulators of SA signal transduction in plants [17], with βC1 inhibiting NPR3 proteasomal degradation and C2 inhibiting TGA2 transcription.

Table 3.
Manipulation of the SA signaling pathway by viral proteins encoded by whitefly-borne viruses.
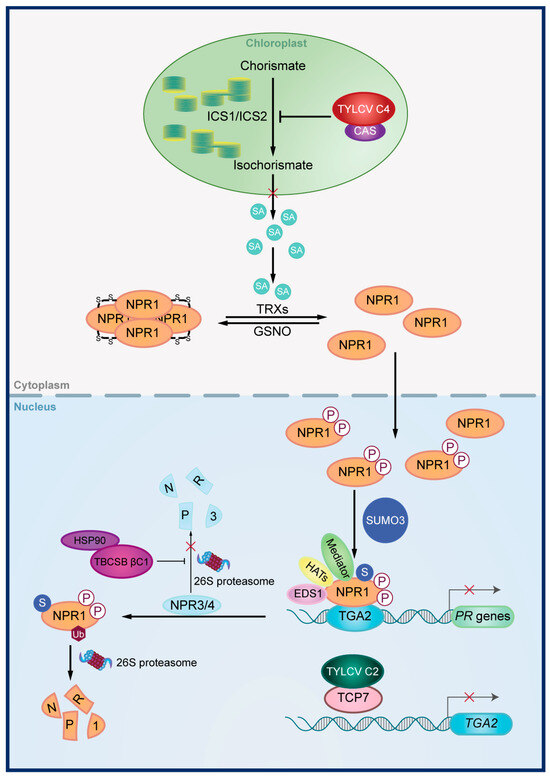
Figure 2.
Disruption of the SA signaling pathway by viral proteins. The C4 protein of tomato yellow leaf curl virus (TYLCV) negatively regulates the SA signaling pathway by targeting CAS in chloroplast, leading to impaired SA biosynthesis. The βC1 protein encoded by tobacco curly shoot betasatellite (TbCSB) and the C2 protein encoded by TYLCV suppress the expression of SA-downstream genes by targeting NPR3 and TGA2, respectively. TbCSB βC1 inhibits NPR3 degradation by interacting with HSP90 and TYLCV C2 inhibits TGA2 transcription by targeting TCP7.
5. Plant–Whitefly–Virus Tripartite Interactions
5.1. Effects of Virus-Induced Plant SA Signaling Pathway on Whitefly Performance
Two studies have investigated the effects of virus-induced SA signaling pathway in plants on whitefly performance. Su et al. (2015) showed that TYLCV infection in tomato plants increases SA content and the expression of SA-related genes, while suppressing the expression of JA-related genes [80]. Concurrently, whitefly development is accelerated and fecundity is increased on virus-infected plants. Similarly, Cui et al. (2019) demonstrated that the increased whitefly population growth on TYLCV-infected tomato plants is associated with enhanced SA content, upregulated SA-related genes, and downregulated JA-dependent defenses [50]. These studies suggest that enhanced whitefly performance on virus-infected plants is linked to virus-induced activation of the SA signaling pathway and suppression of the JA signaling pathway.
5.2. Effects of Whitefly-Induced Plant SA Signaling Pathway on Virus Infection
Two studies have shown that whitefly infestation induced activation of the SA signaling pathway can suppress the infection of whitefly-borne begomovirus. Li et al. (2017) found that whitefly infestation on tobacco and tomato seedlings activates the expression of SA-related genes, leading to callose deposition that inhibits subsequent TYLCV infection [56]. A follow-up study by Zhang et al. (2024) showed that whitefly infestation on tobacco and tomato plants induces substantial SA accumulation, which enhances plant antiviral resistance and reduces begomovirus infection [65].
6. Prospects for Future Research
While significant progress has been made in understanding the role of the SA signaling pathway in plant–whitefly, plant–begomovirus, as well as plant–whitefly–virus tripartite interactions, many key questions remain to be addressed. Future research should focus on the following areas: (1) How has the induction of the plant SA signaling pathway evolved in response to whitefly infestation? (2) What are the whitefly and plant factors that mediate the induction of the SA signaling pathway by whitefly? (3) How are SA signaling pathways activated and mitigated by whitefly-borne viruses? (4) How does the SA signaling pathway modulate the tripartite interactions between whiteflies, viruses, and plants? Addressing these questions will unravel the key molecular mechanisms governing plant interactions with both pests, aiding the development of novel control strategies.
6.1. The Evolution of Whitefly Interaction with Plant SA Signaling Pathway
Based on the review of the case studies, whitefly infestation generally activates the SA signaling pathway (Table 1). Earlier studies suggested that while whiteflies induce the SA signaling pathway, they suppress the more defense-effective JA signaling pathway [47]. Subsequent work confirmed that whiteflies suppress the JA signaling by activating SA signaling, leading to the hypothesis that the activation of SA signaling pathway helps whiteflies suppress plant defenses [52]. However, recent findings by Liu et al. (2024) indicate that SA signaling pathway may allow plants to balance growth and defense, as JA is more effective in promoting plant defense but causes stronger growth inhibition [62].
The evolutionary dynamics between plants and whiteflies need to be examined in terms of mutual fitness. We propose studying plant species/variety with varying levels of SA signaling pathway and whitefly species with different abilities to induce this pathway. Long-term experiments that assess the fitness of both organisms will offer insights into the optimal conditions for their coexistence. While plants with various levels of SA signaling are readily available (e.g., Arabidopsis mutants), whiteflies with different abilities to induce SA signaling are more difficult to obtain. However, genome-editing tools, such as CRISPR/cas9, or using different whitefly species may offer viable solutions [2].
6.2. Whitefly and Plant Factors Mediating the Induction of the SA Signaling Pathway
While many studies show that whitefly infestation activates the SA signaling pathway, the specific whitefly and plant factors involved are understudied. Whiteflies cause mechanical damage to plant tissues, secrete saliva into plants, lay eggs that extract nutrients, and produce honeydew during feeding. Recent research indicates that these factors, particularly salivary effectors, eggs, and honeydew, play roles in activating SA signaling pathways [57,64,89]. More investigations are warranted to determine the roles of these and possibly other whitefly-associated factors. One issue of particular relevance is the relatively minor mechanical damage caused by whitefly feeding. In contrast to insect herbivores with chewing mouthparts that cause extensive damage to plants, whiteflies penetrate into plant tissue with their tiny stylets. Insects with chewing mouthparts often induce JA signaling pathways, while whiteflies primarily induce SA signaling pathways [90]. Empirical investigations should compare how different feeding behaviors influence signaling pathway activation.
For plant factors, Xu et al. (2019) found that a whitefly salivary effector Bt56 induces SA accumulation by targeting plant NTH202, a negative regulator of SA accumulation in plants [57]. Song et al. (2024) found that whitefly eggs induce the expression of two WRKY70 genes, leading to the expression of SA-downstream genes [64]. Further investigations of plant factors mediating the induction of SA signaling pathway may harness sophisticated research tools such as Arabidopsis mutants. Specifically, the activation level of SA signaling pathways in wild-type and a series of mutant Arabidopsis plants may be analyzed upon whitefly infestation. In this way, the set of plant factors that are required in the induction of SA signaling by whitefly feeding, salivary effectors, eggs, and honeydew can be identified and subjected to further functional characterization.
6.3. The Mechanisms Underlying the Induction and Mitigation of Plant SA Signaling Pathways by Whitefly-Borne Viruses
Whitefly-borne begomoviruses have been shown to induce SA accumulation or the expression of SA-related genes. As for the viral proteins mediating the induction, there is only one report, wherein the AV2 encoded by tomato leaf curl Palampur virus induces the expression of SA-related genes in N. benthamiana plants [77]. Hence, more efforts are warranted to determine the viral and plant factors and the mechanisms underlying the induction of the SA signaling pathway by whitefly-borne begomoviruses. We propose that the viral factors should be determined first and then use them as probes to identify plant factors. Viral factors such as proteins and nucleic acids can be functionally characterized in plants with transgene and ectopic expression technique. Protein–protein or protein–nucleic acid interaction assays may then be harnessed to identify plant factors and to examine the mechanisms underlying virus-induced activation of SA signaling pathway.
Begomoviruses may also suppress SA signaling pathways to promote their infection [91]. Known viral proteins, such as C4 and C2 of TYLCV and βC1 encoded in the betasatellite of tobacco curly shoot virus, target key components of the SA signaling pathway, and the mechanisms by which C4, C2, and βC1 suppress SA signaling pathways have been fairly well deciphered [65,87,88]. More research is needed to identify additional viral suppressors of SA signaling pathway, and to uncover the mechanisms underlying the suppression of the SA signaling pathway by viral factors.
6.4. The Role of SA Signaling Pathways in Plant–Whitefly–Virus Tripartite Interactions and Beyond
As both whiteflies and whitefly-borne viruses share host plants, changes in plant physiology induced by one pest can affect the other. The role of SA signaling pathways in these tripartite interactions has been explored, particularly regarding how SA signaling pathways mediate virus-induced changes in plant–whitefly interactions and vice versa. While some aspects of the SA signaling pathway’s role are well understood, its exact impact on virus-induced changes in whitefly behavior and performance remains unresolved. Further research should investigate how virus infection modulates the SA signaling pathway and its impact on whitefly behavior and performance.
Beyond the primary interactions, plant–whitefly–virus associations also involve other organisms, such as bacterial and fungal pathogens, non-vector herbivores, and rhizosphere microbes. The modulation of plant SA signaling pathway by whitefly, virus, or both may impact these organisms, influencing the broader plant-associated community. Further research into how the SA signaling pathway shapes these interactions at the community and ecosystem levels will enhance our understanding of the ecological dynamics in both natural and agricultural ecosystems.
Author Contributions
S.-X.Z., S.-D.W. and L.-L.P. performed the literature searches and drafted the manuscript and tables. Y.-Q.L. and L.-L.P. revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
Financial support for this study was provided by the Zhejiang Provincial Natural Science Foundation of China (LQ23C140007) and the earmarked fund for China Agriculture Research System (CARS-23-C05).
Acknowledgments
We thank Shu-Sheng Liu (Zhejiang University, China) for his insightful comments on an earlier version of the manuscript.
Conflicts of Interest
The authors declare no competing interests.
References
- Walling, L.L. The myriad plant responses to herbivores. J. Plant Growth Regul. 2000, 19, 195–216. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.L.; Lei, T.; Wang, X.W.; Cameron, S.; Navas-Castillo, J.; Liu, Y.Q.; Maruthi, M.N.; Omongo, C.A.; Delatte, H.; Lee, Y.K.; et al. A comprehensive framework for the delimitation of species within the Bemisia tabaci cryptic complex, a global pest-species group. Insect Sci. 2025, 32, 321–342. [Google Scholar] [CrossRef]
- Hu, J.; DeBarro, P.J.; Zhao, H.; Wang, J.; Nardi, F.; Liu, S.S. An extensive field survey combined with a phylogenetic analysis reveals rapid and widespread invasion of two alien whiteflies in China. PLoS ONE 2011, 6, e16061. [Google Scholar] [CrossRef]
- Malka, O.; Santos-Garcia, D.; Feldmesser, E.; Sharon, E.; Krause-Sakate, R.; Delatte, H.; van Brunschot, S.; Patel, M.; Visendi, P.; Mugerwa, H.; et al. Species-complex diversification and host-plant associations in Bemisia tabaci: A plant defence, detoxification perspective revealed by RNA-Seq analyses. Mol. Ecol. 2018, 27, 4241–4256. [Google Scholar] [CrossRef]
- Oliveira, M.R.V.; Henneberry, T.J.; Anderson, P. History, current status, and collaborative research projects for Bemisia tabaci. Crop Prot. 2001, 20, 709–723. [Google Scholar] [CrossRef]
- Navas-Castillo, J.; Fiallo-Olivé, E.; Sánchez-Campos, S. Emerging virus diseases transmitted by whiteflies. Annu. Rev. Phytopathol. 2011, 49, 219–248. [Google Scholar] [CrossRef]
- Fiallo-Olivé, E.; Navas-Castillo, J. Tomato chlorosis virus, a promiscuous virus with multiple host plants and whitefly vectors. Ann. Appl. Biol. 2023, 182, 29–36. [Google Scholar] [CrossRef]
- Fiallo-Olivé, E.; Navas-Castillo, J. Begomoviruses: What is the secret(s) of their success? Trends Plant Sci. 2023, 28, 715–727. [Google Scholar] [CrossRef] [PubMed]
- Fiallo-Olivé, E.; Pan, L.L.; Liu, S.S.; Navas-Castillo, J. Transmission of begomoviruses and other whitefly-borne viruses: Dependence on the vector species. Phytopathology 2020, 110, 10–17. [Google Scholar] [CrossRef]
- Wang, X.W.; Blanc, S. Insect transmission of plant single-stranded DNA viruses. Annu. Rev. Entomol. 2021, 66, 389–405. [Google Scholar] [CrossRef]
- Mithöfer, A.; Boland, W. Plant defense against herbivores: Chemical aspects. Annu. Rev. Plant Biol. 2012, 63, 431–450. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Zhang, L.L.; Zhang, X.; Wu, X.J.; Fang, R.X. Plant defense networks against insect-borne pathogens. Trends Plant Sci. 2021, 26, 272–287. [Google Scholar] [CrossRef]
- Ge, L.H.; Zhou, X.P.; Li, F.F. Plant-virus arms race beyond RNA interference. Trends Plant Sci. 2024, 29, 16–19. [Google Scholar] [CrossRef]
- Pieterse, C.M.J.; Van der Does, D.; Zamioudis, C.; Leon-Reyes, A.; Van Wees, S.C. Hormonal modulation of plant immunity. Annu. Rev. Cell Dev. Biol. 2012, 28, 489–521. [Google Scholar] [CrossRef]
- Chivasa, S.; Murphy, A.M.; Naylor, M.; Carr, J.P. Salicylic acid interferes with tobacco mosaic virus replication via a novel salicylhydroxamic acid-sensitive mechanism. Plant Cell 1997, 9, 547–557. [Google Scholar] [CrossRef] [PubMed]
- Wildermuth, M.C.; Dewdney, J.; Wu, G.; Ausubel, F.M. Isochorismate synthase is required to synthesize salicylic acid for plant defence. Nature 2001, 414, 562–565. [Google Scholar] [CrossRef]
- Peng, Y.J.; Yang, J.F.; Li, X.; Zhang, Y.L. Salicylic acid: Biosynthesis and signaling. Annu. Rev. Plant Biol. 2021, 72, 761–791. [Google Scholar] [CrossRef]
- Huang, J.; Gu, M.; Lai, Z.; Fan, B.F.; Shi, K.; Zhou, Y.H.; Yu, J.Q.; Chen, Z.X. Functional analysis of the Arabidopsis PAL gene family in plant growth, development, and response to environmental stress. Plant Physiol. 2010, 153, 1526–1538. [Google Scholar] [CrossRef] [PubMed]
- Yalpani, N.; León, J.; Lawton, M.A.; Raskin, I. Pathway of salicylic acid biosynthesis in healthy and virus-inoculated tobacco. Plant Physiol. 1993, 103, 315–321. [Google Scholar] [CrossRef]
- Shine, M.B.; Yang, J.W.; El-Habbak, M.; Nagyabhyru, P.; Fu, D.Q.; Navarre, D.; Ghabrial, S.; Kachroo, P.; Kachroo, A. Cooperative functioning between phenylalanine ammonia lyase and isochorismate synthase activities contributes to salicylic acid biosynthesis in soybean. New Phytol. 2016, 212, 627–636. [Google Scholar] [CrossRef]
- Wang, Z.Q.; Yang, G.Q.; Zhang, D.D.; Li, G.X.; Qiu, J.L.; Wu, J. Isochorismate synthase is required for phylloquinone, but not salicylic acid biosynthesis in rice. aBIOTECH 2024, 5, 488–496. [Google Scholar] [CrossRef] [PubMed]
- Garcion, C.; Lohmann, A.; Lamodière, E.; Catinot, J.; Buchala, A.; Doermann, P.; Métraux, J.P. Characterization and biological function of the ISOCHORISMATE SYNTHASE2 gene of Arabidopsis. Plant Physiol. 2008, 147, 1279–1287. [Google Scholar] [CrossRef]
- Rekhter, D.; Lüdke, D.; Ding, Y.; Feussner, K.; Zienkiewicz, K.; Lipka, V.; Wiermer, M.; Zhang, Y.L.; Feussner, I. Isochorismate-derived biosynthesis of the plant stress hormone salicylic acid. Science 2019, 365, 498–502. [Google Scholar] [CrossRef] [PubMed]
- Torrens-Spence, M.P.; Bobokalonova, A.; Carballo, V.; Glinkerman, C.M.; Pluskal, T.; Shen, A.; Weng, J.K. PBS3 and EPS1 complete salicylic acid biosynthesis from isochorismate in Arabidopsis. Mol. Plant 2019, 12, 1577–1586. [Google Scholar] [CrossRef]
- Torrens-Spence, M.P.; Matos, J.O.; Li, T.J.; Kastner, D.W.; Kim, C.Y.; Wang, Z.Q.; Glinkerman, C.M.; Sherk, J.; Kulik, H.J.; Wang, Y.; et al. Mechanistic basis for the emergence of EPS1 as a catalyst in salicylic acid biosynthesis of Brassicaceae. Nat. Commun. 2024, 15, 10356. [Google Scholar] [CrossRef]
- Maeda, H.; Shasany, A.K.; Schnepp, J.; Orlova, I.; Taguchi, G.; Cooper, B.R.; Rhodes, D.; Pichersky, E.; Dudareva, N. RNAi suppression of Arogenate Dehydratase1 reveals that phenylalanine is synthesized predominantly via the arogenate pathway in petunia petals. Plant Cell 2010, 22, 832–849. [Google Scholar] [CrossRef]
- Qian, Y.C.; Lynch, J.H.; Guo, L.Y.; Yang, Y.; Schmelz, E.A.; Pichersky, E.; Maeda, H. Completion of the cytosolic post-chorismate phenylalanine biosynthetic pathway in plants. Nat. Commun. 2019, 10, 15. [Google Scholar] [CrossRef]
- Bussell, J.D.; Reichelt, M.; Wiszniewski, A.A.; Gershenzon, J.; Pichersky, E.; Maeda, H. Peroxisomal ATP-binding cassette transporter COMATOSE and the multifunctional protein abnormal INFLORESCENCE MERISTEM are required for the production of benzoylated metabolites in Arabidopsis seeds. Plant Physiol. 2014, 164, 48–54. [Google Scholar] [CrossRef] [PubMed]
- León, J.; Yalpani, N.; Raskin, I.; Lawton, M.A. Induction of benzoic acid 2-hydroxylase in virus-inoculated tobacco. Plant Physiol. 1993, 103, 323–328. [Google Scholar] [CrossRef]
- León, J.; Shulaev, V.; Yalpani, N.; Lawton, M.A.; Raskin, I. Benzoic acid 2-hydroxylase, a soluble oxygenase from tobacco, catalyzes salicylic acid biosynthesis. Proc. Natl. Acad. Sci. USA 1995, 92, 10413–10417. [Google Scholar] [CrossRef]
- Zavaliev, R.; Dong, X. NPR1, a key immune regulator for plant survival under biotic and abiotic stresses. Mol. Cell 2024, 84, 131–141. [Google Scholar] [CrossRef]
- Cao, H.; Glazebrook, J.; Clarke, J.D.; Volko, S.M.; Dong, X. The Arabidopsis NPR1 gene that controls systemic acquired resistance encodes a novel protein containing ankyrin repeats. Cell 1997, 88, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Mou, Z.; Fan, W.; Dong, X. Inducers of plant systemic acquired resistance regulate NPR1 function through redox changes. Cell 2003, 113, 935–944. [Google Scholar] [CrossRef] [PubMed]
- Tada, Y.; Spoel, S.H.; Pajerowska-Mukhtar, K.; Mou, Z.; Song, J.; Durrant, W.E.; Wang, D.; Dong, X.; Parker, J.E. Plant immunity requires conformational changes of NPR1 via S-nitrosylation and thioredoxins. Science 2008, 321, 952–956. [Google Scholar] [CrossRef] [PubMed]
- Spoel, S.H.; Mou, Z.; Tada, Y.; Trujillo, M.; Chen, Z.; Parker, J.E.; Dong, X. Proteasome-mediated turnover of the transcription coactivator NPR1 plays dual roles in regulating plant immunity. Cell 2009, 137, 860–872. [Google Scholar] [CrossRef]
- Saleh, A.; Withers, J.; Mohan, R.; Durrant, W.E.; Dong, X.; Després, C.; Parker, J.E.; Jones, J.D. Posttranslational modifications of the master transcriptional regulator NPR1 enable dynamic but tight control of plant immune responses. Cell Host Microbe 2015, 18, 169–182. [Google Scholar] [CrossRef]
- Skelly, M.J.; Furniss, J.J.; Grey, H.; Sadanandom, A.; Spoel, S.H. Dynamic ubiquitination determines transcriptional activity of the plant immune coactivator NPR1. eLife 2019, 8, e47005. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Fan, W.; Kinkema, M.; Li, X.; Dong, X. Interaction of NPR1 with basic leucine zipper protein transcription factors that bind sequences required for salicylic acid induction of the PR-1 gene. Proc. Natl. Acad. Sci. USA 1999, 96, 6523–6528. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Tessaro, M.J.; Lassner, M.; Li, X. Knockout analysis of Arabidopsis transcription factors TGA2, TGA5, and TGA6 reveals their redundant and essential roles in systemic acquired resistance. Plant Cell 2003, 15, 2647–2653. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, C.; Zhang, Y.; Li, X.; Dong, X. The Arabidopsis Mediator complex subunit16 positively regulates salicylate-mediated systemic acquired resistance and jasmonate/ethylene-induced defense pathways. Plant Cell 2012, 24, 4294–4309. [Google Scholar] [CrossRef]
- Zhang, X.; Yao, J.; Zhang, Y.; Li, X.; Dong, X. The Arabidopsis Mediator complex subunits MED14/SWP and MED16/SFR6/IEN1 differentially regulate defense gene expression in plant immune responses. Plant J. 2013, 75, 484–497. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Choi, S.M.; Kang, M.J.; Yun, S.H.; Kwon, D.J.; Noh, Y.S.; Noh, B. Salicylic acid-induced transcriptional reprogramming by the HAC–NPR1–TGA histone acetyltransferase complex in Arabidopsis. Nucleic Acids Res. 2018, 46, 11712–11725. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Li, M.; Qi, G.; Zhao, M.; Liu, L.; Liu, L.; Palmer, I.A.; Gassmann, W.; Fu, Z.Q. Two interacting transcriptional coactivators cooperatively control plant immune responses. Sci. Adv. 2021, 7, eabl7173. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.Q.; Yan, S.; Saleh, A.; Wang, W.; Liao, H.; Wang, X.; Dong, X. NPR3 and NPR4 are receptors for the immune signal salicylic acid in plants. Nature 2012, 486, 228–232. [Google Scholar] [CrossRef]
- Ding, Y.; Sun, T.; Ao, K.; Wang, D.; Zhang, Y.; Li, Y.; Dong, X. Opposite roles of salicylic acid receptors NPR1 and NPR3/NPR4 in transcriptional regulation of plant immunity. Cell 2018, 173, 1454–1467. [Google Scholar] [CrossRef]
- Morin, S.; Atkinson, P.W.; Walling, L.L. Whitefly-plant interactions: An integrated molecular perspective. Annu. Rev. Entomol. 2024, 69, 503–525. [Google Scholar] [CrossRef]
- Zarate, S.I.; Kempema, L.A.; Walling, L.L. Silverleaf whitefly induces salicylic acid defenses and suppresses effectual jasmonic acid defenses. Plant Physiol. 2007, 143, 866–875. [Google Scholar] [CrossRef]
- Zhang, P.J.; Zheng, S.J.; van Loon, J.J.; Boland, W.; David, A.; Mumm, R.; Dicke, M. Whiteflies interfere with indirect plant defense against spider mites in Lima bean. Proc. Natl. Acad. Sci. USA 2009, 106, 21202–21207. [Google Scholar] [CrossRef]
- Puthoff, D.P.; Holzer, F.M.; Perring, T.M.; Walling, L.L. Tomato pathogenesis-related protein genes are expressed in response to Trialeurodes vaporariorum and Bemisia tabaci biotype B feeding. J. Chem. Ecol. 2010, 36, 1271–1285. [Google Scholar] [CrossRef]
- Cui, H.Y.; Sun, Y.C.; Zhao, Z.H.; Zhang, Y.J. The combined effect of elevated O3 levels and TYLCV infection increases the fitness of Bemisia tabaci Mediterranean on tomato plants. Environ. Entomol. 2019, 48, 1425–1433. [Google Scholar] [CrossRef]
- Zhang, P.J.; Xu, C.X.; Zhang, J.M.; Lu, Y.B.; Wei, J.N.; Liu, Y.Q.; David, A.; Boland, W.; Turlings, T.C.J. Phloem-feeding whiteflies can fool their host plants, but not their parasitoids. Funct. Ecol. 2013, 27, 1304–1312. [Google Scholar] [CrossRef]
- Zhang, P.J.; Li, W.D.; Huang, F.; Zhang, J.M.; Xu, F.C.; Lu, Y.B. Feeding by whiteflies suppresses downstream jasmonic acid signaling by eliciting salicylic acid signaling. J. Chem. Ecol. 2013, 39, 612–619. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xue, M.; Zhao, H.P. Species-specific effects on salicylic acid content and subsequent Myzus persicae (Sulzer) performance by three phloem-sucking insects infesting Nicotiana tabacum L. Arthropod-Plant Interact. 2015, 9, 383–391. [Google Scholar] [CrossRef]
- Zhao, H.P.; Zhang, X.; Xue, M.; Zhang, X. Feeding of whitefly on tobacco decreases aphid performance via increased salicylate signaling. PLoS ONE 2015, 10, e0138584. [Google Scholar] [CrossRef]
- Vieira, S.S.; Lourenço, A.L.; da Graça, J.P.; Janegitz, T.; Salvador, M.C.; de Oliveira, M.C.N.; Hoffmann-Campo, C.B. Biological aspects of Bemisia tabaci biotype B and the chemical causes of resistance in soybean genotypes. Arthropod-Plant Interact. 2016, 10, 525–534. [Google Scholar] [CrossRef]
- Li, P.; Shu, Y.N.; Fu, S.; Liu, Y.Q.; Zhou, X.P.; Liu, S.S.; Wang, X.W. Vector and nonvector insect feeding reduces subsequent plant susceptibility to virus transmission. New Phytol. 2017, 215, 699–710. [Google Scholar] [CrossRef]
- Xu, H.X.; Qian, L.X.; Wang, X.W.; Shao, R.X.; Hong, Y.; Liu, S.S.; Wang, X.W. A salivary effector enables whitefly to feed on host plants by eliciting salicylic acid-signaling pathway. Proc. Natl. Acad. Sci. USA 2019, 116, 490–495. [Google Scholar] [CrossRef]
- Zhang, P.J.; Wei, J.N.; Zhao, C.; Zhang, Y.F.; Li, C.Y.; Liu, S.S.; Dicke, M.; Yu, X.P.; Turlings, T.C.J. Airborne host-plant manipulation by whiteflies via an inducible blend of plant volatiles. Proc. Natl. Acad. Sci. USA 2019, 116, 7387–7396. [Google Scholar] [CrossRef]
- Silva, D.B.; Jiménez, A.; Urbaneja, A.; Pérez-Hedo, M.; Bento, J.M. Changes in plant responses induced by an arthropod influence the colonization behavior of a subsequent herbivore. Pest. Manag. Sci. 2021, 77, 4168–4180. [Google Scholar] [CrossRef]
- Li, Y.; Qu, C.; Yan, X.Y.; Sun, X.; Yin, Z.Y.; Zhao, H.P. Effect of feeding stage and density of whiteflies on subsequent aphid performance on tobacco plants. Agronomy 2022, 12, 1025. [Google Scholar] [CrossRef]
- Hu, J.; Sun, G.; Yang, Y.; Jiao, X.; Chen, Z.; Zhang, Y. Pepper previously infested by MED facilitates settling and oviposition by MEAM1 of the Bemisia tabaci species complex. J. Pest. Sci. 2023, 96, 1019–1034. [Google Scholar] [CrossRef]
- Liu, Y.X.; Han, W.H.; Wang, J.X.; Zhang, Y.L.; Li, Y.; Li, M.; Liu, S.S.; Wang, X.W. Differential induction of JA/SA determines plant defense against successive leaf-chewing and phloem-feeding insects. J. Pest. Sci. 2025, 98, 1085–1100. [Google Scholar] [CrossRef]
- Wu, H.; Han, W.H.; Liang, K.L.; Wang, J.X.; Zhang, F.B.; Ji, S.X.; Wang, X.W. Using salicylic acid-responsive promoters to drive the expression of jasmonic acid-regulated genes enhances plant resistance to whiteflies. Pest Manag Sci. 2024. [Google Scholar] [CrossRef]
- Song, H.D.; Zhang, F.B.; Ji, S.X.; Zhang, Y.L.; Li, Y.; Liu, L.; Liu, S.S.; Wang, X.W. The SA-WRKY70-PR-Callose axis mediates plant defense against whitefly eggs. Int. J. Mol. Sci. 2024, 25, 12076. [Google Scholar] [CrossRef]
- Zhang, J.R.; Liu, Y.M.; Li, D.; Wu, Y.J.; Zhao, S.X.; Wang, X.W.; Liu, S.S.; Walling, L.L.; Pan, L.L. Viral proteins resolve the virus-vector conundrum during hemipteran-mediated transmission by subverting salicylic acid signaling pathway. Nat. Commun. 2024, 15, 9448. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.B.; Pan, H.P.; Xie, W.; Jiao, X.G.; Fang, Y.; Chen, G.; Yang, X.; Wu, Q.J.; Wang, S.L.; Zhang, Y.J. Three-way interactions between the tomato plant, tomato yellow leaf curl virus, and Bemisia tabaci (Hemiptera: Aleyrodidae) facilitate virus spread. J. Econ. Entomol. 2014, 107, 920–926. [Google Scholar] [CrossRef]
- Rodríguez-Álvarez, C.I.; López-Climent, M.F.; Gómez-Cadenas, A.; Kaloshian, I.; Nombela, G. Salicylic acid is required for Mi-1-mediated resistance of tomato to whitefly Bemisia tabaci, but not for basal defense to this insect pest. Bull. Entomol. Res. 2015, 105, 574–582. [Google Scholar] [CrossRef]
- Yang, J.W.; Yi, H.S.; Kim, H.; Lee, B.; Lee, S.; Ghim, S.Y.; Ryu, C.M. Whitefly infestation of pepper plants elicits defence responses against bacterial pathogens in leaves and roots and changes the below-ground microflora. J. Ecol. 2011, 99, 46–56. [Google Scholar] [CrossRef]
- Park, Y.S.; Ryu, C.M. Understanding cross-communication between aboveground and belowground tissues via transcriptome analysis of a sucking insect whitefly-infested pepper plants. Biochem. Biophys. Res. Commun. 2014, 443, 272–277. [Google Scholar] [CrossRef]
- Shi, X.B.; Chen, G.; Tian, L.X.; Zhang, Y.L.; Li, Y.; Liu, L.; Liu, S.S.; Wang, X.W. The salicylic acid-mediated release of plant volatiles affects the host choice of Bemisia tabaci. Int. J. Mol. Sci. 2016, 17, 1048. [Google Scholar] [CrossRef]
- Jafarbeigi, F.; Samih, M.A.; Alaei, H.; Shirani, H. Induced tomato resistance against Bemisia tabaci triggered by salicylic acid, β-aminobutyric acid, and Trichoderma. Neotrop. Entomol. 2020, 49, 456–467. [Google Scholar] [CrossRef]
- Ueda, H.; Kugimiya, S.; Tabata, J.; Noh, Y.S.; Noh, B. Accumulation of salicylic acid in tomato plant under biological stress affects oviposition preference of Bemisia tabaci. J. Plant Interact. 2018, 14, 73–78. [Google Scholar] [CrossRef]
- Ascencio-Ibáñez, J.T.; Sozzani, R.; Lee, T.J.; Chu, T.M.; Wolfinger, R.D.; Cella, R.; Hanley-Bowdoin, L. Global analysis of Arabidopsis gene expression uncovers a complex array of changes impacting pathogen response and cell cycle during geminivirus infection. Plant Physiol. 2018, 148, 436–454. [Google Scholar] [CrossRef]
- Luna-Rivero, M.S.; Hernández-Zepeda, C.; Villanueva-Alonzo, H.; Minero-García, Y.; Castell-González, S.E.; Moreno-Valenzuela, O.A. Expression of genes involved in the salicylic acid pathway in type h1 thioredoxin transiently silenced pepper plants during a begomovirus compatible interaction. Mol. Genet. Genom. 2016, 291, 819–830. [Google Scholar] [CrossRef] [PubMed]
- Kundu, A.; Patel, A.; Paul, S.; Pal, A. Transcript dynamics at early stages of molecular interactions of MYMIV with resistant and susceptible genotypes of the leguminous host, Vigna mungo. PLoS ONE 2015, 10, e0124687. [Google Scholar] [CrossRef] [PubMed]
- Jeevalatha, A.; Siddappa, S.; Kumar, R.; Tiwari, R.K.; Lal, M.K.; Sharma, S.; Chakrabarti, S.K.; Singh, B.P. RNA-seq analysis reveals an early defense response to tomato leaf curl New Delhi virus in potato cultivar Kufri Bahar. Funct. Integr. Genom. 2023, 23, 215. [Google Scholar] [CrossRef] [PubMed]
- Roshan, P.; Kulshreshtha, A.; Kumar, S.; Sharma, R.; Kumar, V.; Kumar, M.; Kumar, S.; Kumar, A.; Ranjan, A.; Sharma, Y.K.; et al. AV2 protein of Tomato Leaf Curl Palampur Virus promotes systemic necrosis in Nicotiana benthamiana and interacts with host catalase2. Sci. Rep. 2018, 8, 1273. [Google Scholar] [CrossRef]
- Miozzi, L.; Napoli, C.; Sardo, L.; Accotto, G.P. Transcriptomics of the interaction between the monopartite phloem-limited geminivirus Tomato Yellow Leaf Curl Sardinia Virus and Solanum lycopersicum highlights a role for plant hormones, autophagy, and plant immune system fine tuning during infection. PLoS ONE 2014, 9, e89951. [Google Scholar] [CrossRef]
- Sade, D.; Sade, N.; Shriki, O.; Lerner, S.; Gebremedhin, A.; Karavani, A.; Brotman, Y.; Osorio, S.; Fernie, A.R.; Willmitzer, L.; et al. Water balance, hormone homeostasis, and sugar signaling are all involved in tomato resistance to Tomato yellow leaf curl virus. Plant Physiol. 2014, 165, 1684–1697. [Google Scholar] [CrossRef]
- Su, Q.; Preisser, E.L.; Zhou, X.M.; Li, Y.; Wang, X.W. Manipulation of host quality and defense by a plant virus improves performance of whitefly vectors. J. Econ. Entomol. 2015, 108, 11–19. [Google Scholar] [CrossRef]
- Zingariello, E.; Larocca, M.; Rossano, R.; Crescenzi, A.; Fanigliulo, A.; Viggiano, A. Comparative analysis of induction of Pr-1 protein between acibenzolar-S-methyl treatment and virus infection by TSWV and TYLCV in Solanum lycopersicum L. Acta Hortic. 2015, 1069, 265–270. [Google Scholar] [CrossRef]
- Cui, H.; Sun, Y.; Chen, F.; Zhang, Y.; Ge, F. Elevated O3 and TYLCV infection reduce the suitability of tomato as a host for the whitefly Bemisia tabaci. Int. J. Mol. Sci. 2016, 17, 1964. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Zhang, Z.; Liu, Y.; Sun, S.; Wang, H.; Geng, X. Phenotype and signaling pathway analysis to explore the interaction between tomato plants and TYLCV in different organs. Plant Sci. 2024, 339, 111955. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Zhang, X.; Yao, X.; Zhang, P.; Fang, R.; Ye, J. A 7-amino-acid motif of Rep protein essential for virulence is critical for triggering host defense against Sri Lankan cassava mosaic virus. Mol. Plant Microbe Interact. 2020, 33, 78–86. [Google Scholar] [CrossRef]
- Zhao, S.S.; Li, Y. Current understanding of the interplays between host hormones and plant viral infections. PLoS Pathog. 2021, 17, e1009242. [Google Scholar] [CrossRef]
- Li, T.; Huang, Y.; Xu, Z.S.; Li, Y.; Liu, L.; Wang, X.W. Salicylic acid-induced differential resistance to the tomato yellow leaf curl virus among resistant and susceptible tomato cultivars. BMC Plant Biol. 2019, 19, 173. [Google Scholar] [CrossRef]
- Medina-Puche, L.; Tan, H.; Dogra, V.; Van den Ackerveken, G.; Bouwmeester, H.J.; Kant, M.R. A defense pathway linking plasma membrane and chloroplasts and co-opted by pathogens. Cell 2020, 182, 1109–1124. [Google Scholar] [CrossRef]
- Li, P.; Zhang, L.; Yan, X.T.; Liu, Y.Q.; Liu, L.; Liu, S.S.; Wang, X.W. Suppression of TGA2-mediated salicylic acid defence by tomato yellow leaf curl virus C2 via disruption of TCP7-like transcription factor activity in tobacco. Plant Cell Environ. 2025, 48, 4039–4050. [Google Scholar] [CrossRef]
- VanDoorn, A.; de Vries, M.; Kant, M.R.; Schuurink, R.C. Whiteflies glycosylate salicylic acid and secrete the conjugate via their honeydew. J. Chem. Ecol. 2015, 41, 52–58. [Google Scholar] [CrossRef]
- Walling, L.L. Avoiding effective defenses: Strategies employed by phloem-feeding insects. Plant Physiol. 2008, 146, 859–866. [Google Scholar] [CrossRef]
- Peng, W.; Sheng, S.; Liu, K.R.; Rong, P.; Na, L.; Hu, B.; Wang, L.M.; Wang, H.H.; Afzal, A.J.; Geng, X.Q. Physiological and transcriptomic analyses revealed gene networks involved in heightened resistance against tomato yellow leaf curl virus infection in salicylic acid and jasmonic acid treated tomato plants. Front. Microbiol. 2022, 13, 970139. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).