Diversity of Rabies Virus Variants in Insectivorous Bats (Chiroptera: Vespertilionidae and Molossidae): An Epidemiological Study in Central Argentine Patagonia
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Samples
2.3. Diagnosis and Antigenic Analysis
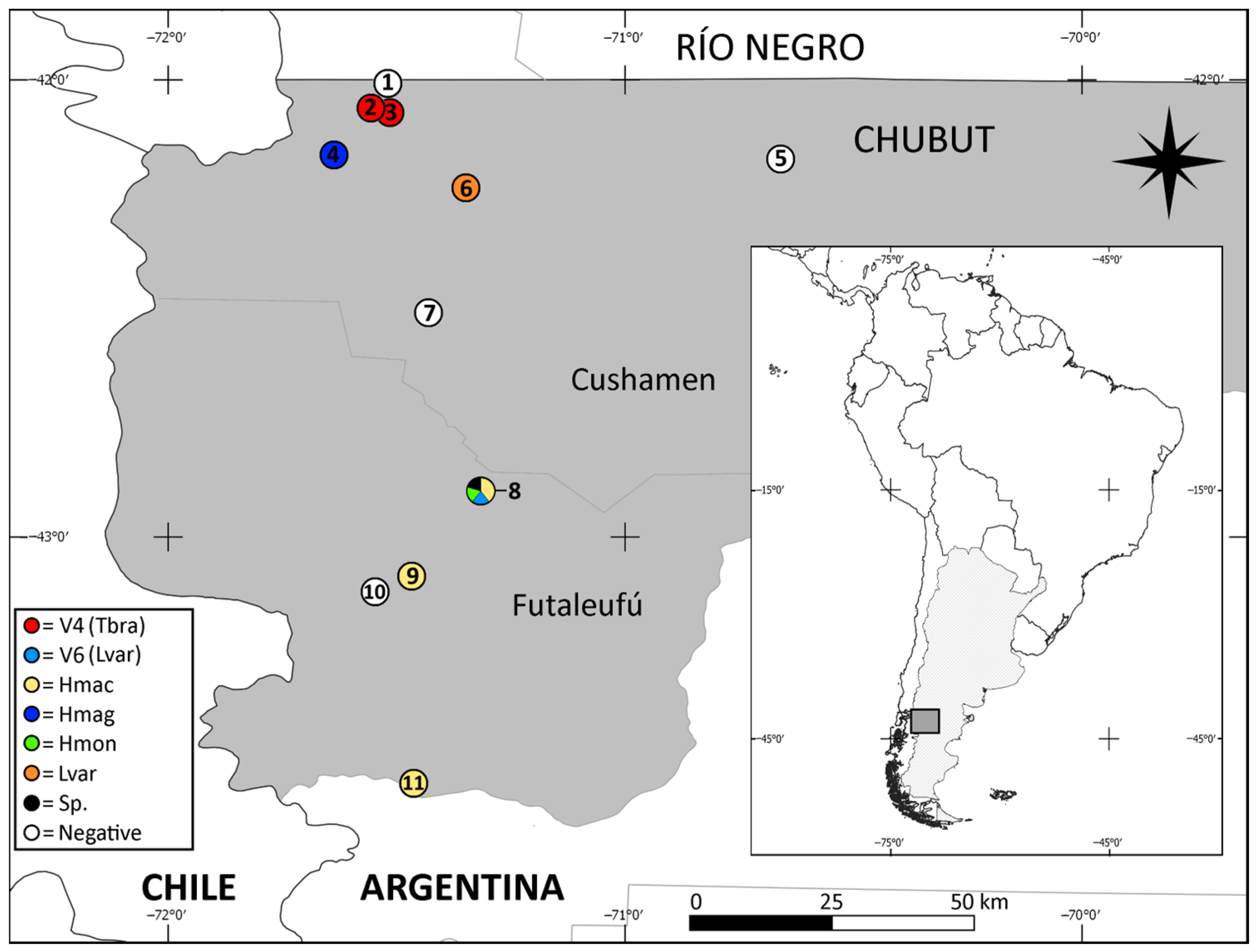
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Piñeiro, C.; Gury Dohmen, F.; Beltran, F.; Martinez, L.; Novaro, L.; Russo, S.; Palacios, G.; Cisterna, D.M. High Diversity of Rabies Viruses Associated with Insectivorous Bats in Argentina: Presence of Several Independent Enzootics. PLoS Negl. Trop. Dis. 2012, 6, e1635. [Google Scholar] [CrossRef] [PubMed]
- Caraballo, D.A.; Lombardo, M.A.; Becker, P.; Sabio, M.S.; Lema, C.; Martínez, L.M.; Beltrán, F.J.; Li, Y.; Cisterna, D.M. Evaluation of Two Real-Time, TaqMan Reverse Transcription-PCR Assays for Detection of Rabies Virus in Circulating Variants from Argentina: Influence of Sequence Variation. Viruses 2020, 13, 23. [Google Scholar] [CrossRef]
- Biscayart, C.; Casas, N.; Castillo, C.; Cisterna, D.; Ferro, N.; Giovacchini, C.; Hertlein, C.; Juárez, M.V.; Mirkin, E.; Marcos, A.; et al. Guía para la Prevención, Vigilancia y Control de la Rabia en la Argentina; Ministerio de Salud de la Nación: Buenos Aires, Argentina, 2018. [Google Scholar]
- Caraballo, D.A.; Vico, M.L.; Piccirilli, M.G.; Hirmas Riade, S.M.; Russo, S.; Martínez, G.; Beltrán, F.J.; Cisterna, D.M. Bat Rabies in the Americas: Is Myotis the Main Ancestral Spreader? Viruses 2024, 16, 1302. [Google Scholar] [CrossRef]
- Cisterna, D.; Bonaventura, R.; Caillou, S.; Pozo, O.; Andreau, M.L.; Dalla Fontana, L.; Echegoyen, C.; de Mattos, C.; de Mattos, C.; Russo, S.; et al. Antigenic and molecular characterization of rabies virus in Argentina. Virus Res. 2005, 109, 139–147. [Google Scholar] [CrossRef]
- Molina, M.I.; Reissig, E.C. La rabia en la Patagonia: Virus caninos y salud pública. Desde Patagonia. Difundiendo Saberes 2019, 16, 24. [Google Scholar]
- Firpo, S.; Piccirilli, M.G.; Urizar, R.; Vitta, N.; Hirmas Riade, S.M.; Leguizamón, C.; Vico, M.L.; Martínez, G.; Beltrán, F.J.; Cisterna, D.M. Human Rabies by Secondary Transmission in Argentina. Diseases 2022, 10, 17. [Google Scholar] [CrossRef] [PubMed]
- Delpietro, H.A.; Gury-Dhomen, F.; Larghi, O.P.; Mena-Segura, C.; Abramo, L. Monoclonal antibody characterization of rabies virus strains isolated in the River Plate Basin. J. Vet. Med. B. 1997, 44, 477–483. [Google Scholar] [CrossRef]
- World Organization for Animal Health (WOAH). Rabies (Infection with Rabies Virus and Other Lyssaviruses). In Terrestrial Manual; World Organization for Animal Health (WOAH): Paris, France, 2023. [Google Scholar]
- Rupprecht, C.E.; Fooks, A.R.; Abela-Ridder, B. Laboratory techniques in rabies. World Health Organ. 2018, 1, 289. [Google Scholar]
- Giménez, A.L.; Giannini, N.P.; Schiaffini, M.I.; Martin, G.M. New records of the rare Histiotus magellanicus (Chiroptera, Vespertilionidae) and other bats from Central Patagonia, Argentina. Mastozool. Neotrop. 2012, 19, 213–224. [Google Scholar]
- Díaz, M.M.; Valenzuela, A.E.J.; Sturzenbaum, S.; Barquez, R.M. New records of bats (Chiroptera) from Santa Cruz province (Argentina) and the southernmost record of Lasiurus varius (Poeppig, 1835) for Argentina. Check List 2017, 13, 397–401. [Google Scholar] [CrossRef]
- Giménez, A.L.; Schiaffini, M.I. Patagonian bats: New size limits, southernmost localities and updated distribution for Lasiurus villosissimus and Myotis dinellii (Chiroptera: Vespertilionidae). Mammalia 2019, 84, 150–161. [Google Scholar] [CrossRef]
- Barquez, R.M.; Mares, M.A.; Braun, J.K. The Bats of Argentina; Special Publications; Museum of Texas Tech University: Lubbock, TX, USA, 1999. [Google Scholar]
- Cornejo, T.A.; Arezo, M.; Gutiérrez, S.; Crowley, P.; Labanchi, J.L.; Calabro, A.; Grizmado, C.; Ochoa, A.; Herrero, E.; Talmon, G.; et al. Epidemiología, vigilancia y control de Rabia transmitida por Murciélagos Insectívoros. Cienc. Vet. 2021, 23, 1. [Google Scholar]
- Streicker, D.G.; Recuenco, S.; Valderrama, W.; Gomez Benavides, J.; Vargas, I.; Pacheco, V.; Condori Condori, R.E.; Montgomery, J.; Rupprecht, C.E.; Rohani, P.; et al. Ecological and anthropogenic drivers of rabies exposure in vampire bats: Implications for transmission and control. Proc. Biol. Sci. 2012, 279, 3384–3392. [Google Scholar] [CrossRef] [PubMed]
- De Benedictis, P.; Leopardi, S.; Markotter, W.; Velasco-Villa, A. The importance of accurate host species identification in the framework of rabies surveillance, control and elimination. Viruses 2022, 14, 492. [Google Scholar] [CrossRef]
- Mollentze, N.; Biek, R.; Streicker, D.G. The role of viral evolution in rabies host shifts and emergence. Curr. Opin. Virol. 2014, 8, 68–72. [Google Scholar] [CrossRef]
- de Thoisy, B.; Bourhy, H.; Delaval, M.; Pontier, D.; Dacheux, L.; Darcissac, E.; Donato, D.; Guidez, A.; Larrous, F.; Lavenir, R.; et al. Bioecological drivers of rabies virus circulation in a neotropical bat community. PLoS Negl. Trop. Dis. 2016, 10, e0004378. [Google Scholar] [CrossRef]
- de Sousa, L.L.F.; Guilardi, M.D.; Martins, J.O.; Silvério, B.S.; Tibo, L.H.S.; Antunes, P.D.S.; Cabral-Miranda, G.; Caldeira, D.B.; Brandão, P.E.; Souza Campos, F.; et al. Phylogenetic inferences reveal multiple intra and interhost genetic diversity among bat rabies viruses circulating in northeastern Brazil. One Health Outlook 2025, 7, 1. [Google Scholar] [CrossRef]
- INDEC. Unidades Geoestadísticas. Cartografía y Códigos Geográficos del Sistema Estadístico Nacional. Buenos Aires, Argentina. Available online: https://www.indec.gob.ar/ (accessed on 22 January 2025).
- Barquez, R.M.; Díaz, M.M.; Montani, M.E.; Pérez, M.J. Nueva Guía de los Murciélagos de Argentina; Special Publication N°3; PCMA (Programa de Conservación de los Murciélagos de Argentina): Tucumán, Argentina, 2020. [Google Scholar]
- Barboza, C.M.; Lima, J.S.; Gomes, B.F.; Garcia, J.G.; Pardo Souza, T.D.C.; Zamudio, R.M.; Rodrigues Chierato, M.E.; de Novaes Oliveira, R.; Junior, P.C.; da Silva, M.C.C.; et al. Activity of BatIFIT5 in different species of healthy and naturally infected with rabies virus bats. Vet. Ital. 2024, 60, 2. [Google Scholar]
- Underwood, W.; Anthony, R. AVMA Guidelines for the Euthanasia of Animals: 2020 Edition; American Veterinary Medical Association: Schaumburg, IL, USA, 2020. [Google Scholar]
- Novaes, R.L.M.; Almeida, B.; Cláudio, V.C.; Costa-Neto, S.F.; Couto, A.L.G.; Schmidt, E.; Bertocchi, N.A.; Oliveira Costa, J.; Ferreira, C.F.; de Oliveira, A.M.R.; et al. Rabies virus circulation in a highly diverse bat assemblage from a high-risk area for zoonoses outbreaks in the Brazilian Amazon. Acta Trop. 2024, 257, 107309. [Google Scholar] [CrossRef]
- Rico-Chavéz, O.; Flores-Pérez, N.; Martínez-Pérez, K.U.; Villalobos-Segura, M.D.C.; Ávila-Flores, R. Bats, Pathogen Diversity and Rabies in a Changing Neotropic Landscape. In Ecology of Wildlife Diseases in the Neotropics; Acosta-Jamett, G., Chaves, A., Eds.; Springer: Cham, Switzerland, 2024; pp. 185–212. [Google Scholar]
- Dohmen, F.G.; Kovacs, E.; Prestrera, N.E.; Beltrán, F.J. Evaluation of a rapid immunochromatographic diagnostic test (RIDT) for diagnosis of rabies in samples from Argentina. J. Infect. Dev. Ctries. 2018, 12, 415–421. [Google Scholar] [CrossRef]
- Fleming, T.H. Bat Migration. In Encyclopedia of Animal Behavior, 2nd ed.; Choe, J.C., Ed.; Elsevier Academic Press: Cambridge, MA, USA, 2019; pp. 605–610. [Google Scholar]
- Rojas-Herrera, K.; Villalobos, F.; Guillén-Servent, A.; Solari, S.; Rojas-Soto, O. Seasonal distribution analysis of five lasiurine bat species: Clues to migration patterns and behavior. Mammalia 2023, 87, 499–510. [Google Scholar] [CrossRef]
- Pearson, O.P.; Pearson, A.K. Reproduction of bats in southern Argentina. In Advances in Neotropical Mammalogy; Redford, K., Eisenberg, J.F., Eds.; Sandhill Crane Press: Gainesville, FL, USA, 1989; pp. 549–566. [Google Scholar]
- Nabi, G.; Wang, Y.; Lü, L.; Jiang, C.; Ahmad, S.; Wu, Y.; Li, D. Bats and birds as viral reservoirs: A physiological and ecological perspective. Sci. Total Environ. 2021, 754, 142372. [Google Scholar] [CrossRef] [PubMed]
| Sample | Species | Sex | Age Range | Locality | Origin | Antigenic Variant |
|---|---|---|---|---|---|---|
| 006/22 | H. macrotus | ♂ | Adult | Trevelin | Urban | * |
| 008/23 | H. macrotus | ♂ | Juvenile | Corcovado | Rural | * |
| 012/23 | H. macrotus | ♀ | Adult | Esquel | Urban | * |
| 021/24 | T. brasiliensis | ♀ | Adult | Paraje Currumahuida | Rural | V4 |
| 024/24 | L. varius | ♀ | Adult | Esquel | Urban | V6 |
| 025/24 | T. brasiliensis | ♂ | Adult | Paraje Currumahuida | Rural | V4 |
| 026/24 | H. montanus | ♀ | Adult | Esquel | Urban | * |
| 029/24 | H. magellanicus | ♂ | Juvenile | Lago Puelo | Rural | * |
| 030/24 | L. varius | ♂ | Adult | Epuyén | Rural | * |
| 034/24 | Unidentified | - | - | Esquel | Urban | * |
| 035/24 | H. macrotus | ♀ | Juvenile | Esquel | Urban | * |
| 060/24 | T. brasiliensis | ♂ | Adult | El Hoyo | Urban | V4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giménez, A.L.; Zabalza, M.J.; Novaro, L.P.; Centurion, G.A.; Barrios-Benito, M.Y.; Moncá, I.; Chaar Letourneau, F.; Casanovas, R.; Russo, S.E. Diversity of Rabies Virus Variants in Insectivorous Bats (Chiroptera: Vespertilionidae and Molossidae): An Epidemiological Study in Central Argentine Patagonia. Viruses 2025, 17, 788. https://doi.org/10.3390/v17060788
Giménez AL, Zabalza MJ, Novaro LP, Centurion GA, Barrios-Benito MY, Moncá I, Chaar Letourneau F, Casanovas R, Russo SE. Diversity of Rabies Virus Variants in Insectivorous Bats (Chiroptera: Vespertilionidae and Molossidae): An Epidemiological Study in Central Argentine Patagonia. Viruses. 2025; 17(6):788. https://doi.org/10.3390/v17060788
Chicago/Turabian StyleGiménez, Analía L., Marcelo J. Zabalza, Laura P. Novaro, Gabriela A. Centurion, Melanie Y. Barrios-Benito, Ivana Moncá, Fabricio Chaar Letourneau, Román Casanovas, and Susana E. Russo. 2025. "Diversity of Rabies Virus Variants in Insectivorous Bats (Chiroptera: Vespertilionidae and Molossidae): An Epidemiological Study in Central Argentine Patagonia" Viruses 17, no. 6: 788. https://doi.org/10.3390/v17060788
APA StyleGiménez, A. L., Zabalza, M. J., Novaro, L. P., Centurion, G. A., Barrios-Benito, M. Y., Moncá, I., Chaar Letourneau, F., Casanovas, R., & Russo, S. E. (2025). Diversity of Rabies Virus Variants in Insectivorous Bats (Chiroptera: Vespertilionidae and Molossidae): An Epidemiological Study in Central Argentine Patagonia. Viruses, 17(6), 788. https://doi.org/10.3390/v17060788







