Comparative Analysis of Two Zika Virus Isolates in a Rhesus Macaque Pregnancy Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Ethics Statement
2.2. Zika Virus (ZIKV) Preparation and Cell Culture
2.3. Pregnant Dams
2.4. Viral RNA Detection
2.5. ZIKV Enzyme-Linked Immunosorbent Assay (ELISA)
2.6. Focus Reduction Neutralization (FRNT) Assays
2.7. Phenotypic Analysis of Peripheral Blood Mononuclear Cells
2.8. Ultrasound Imaging Studies
2.9. Fetal Biometry
2.10. Amniotic Fluid Index
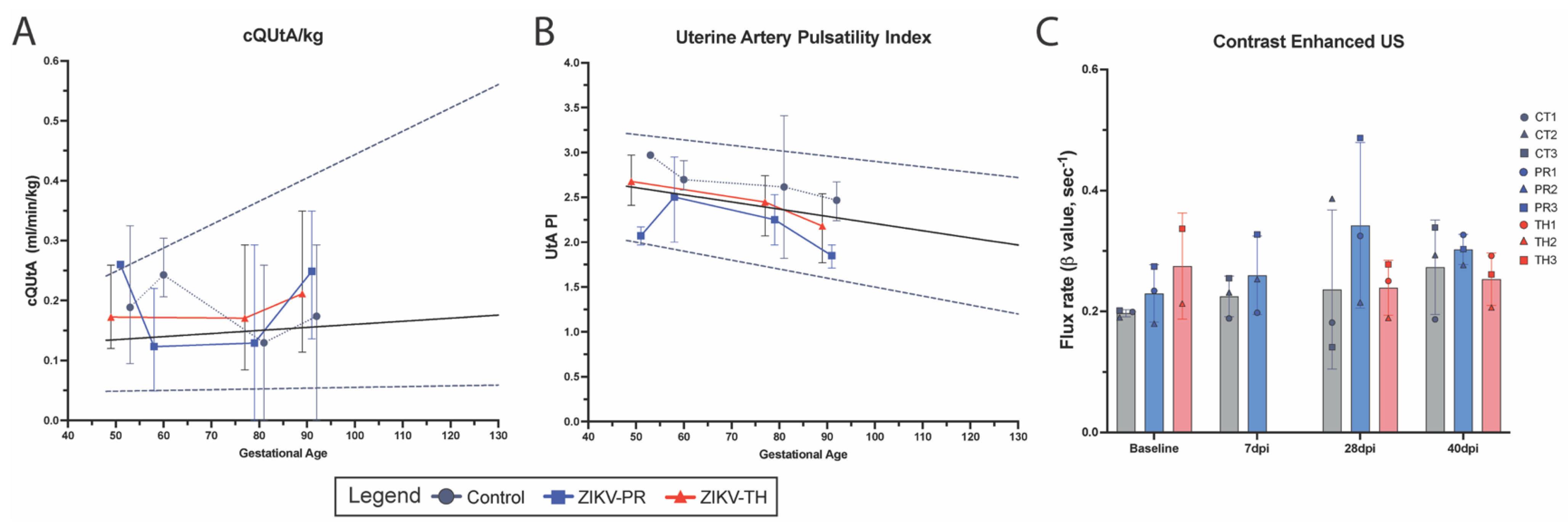
2.11. Uterine Artery Volume Blood Flow
2.12. Contrast-Enhanced Ultrasound Analysis
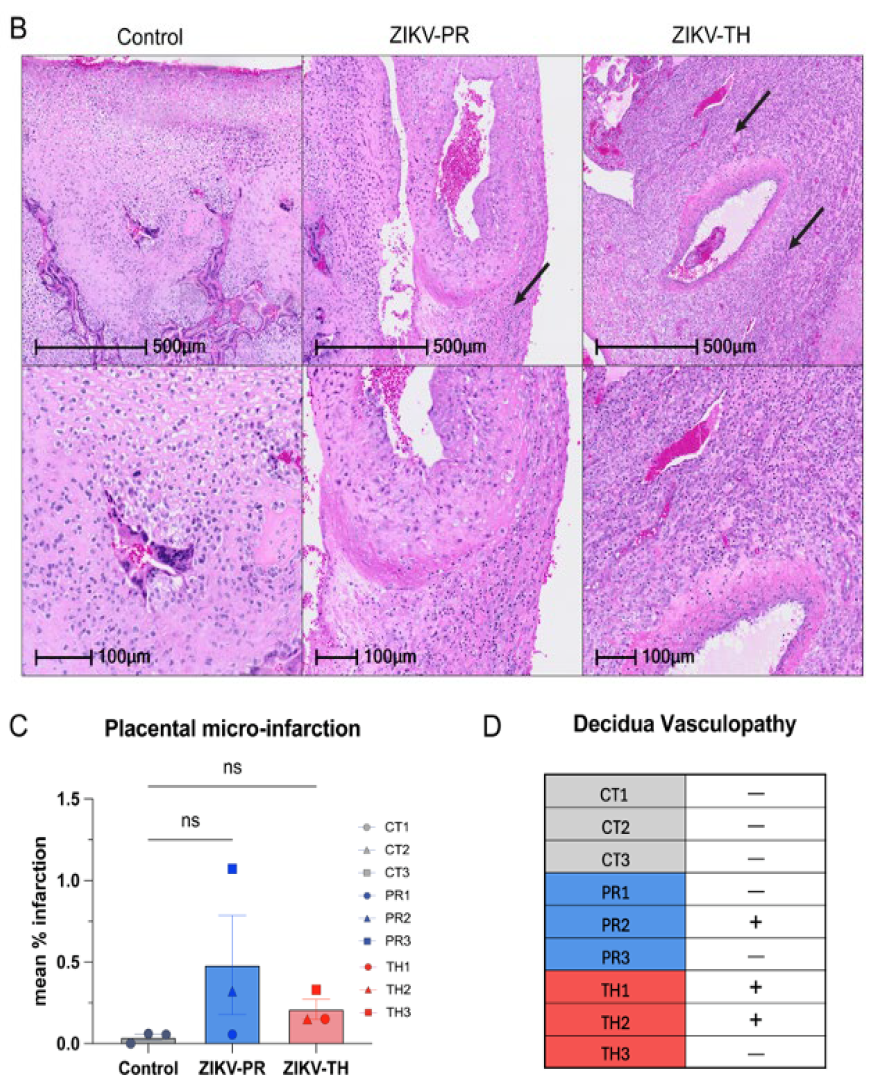
2.13. Histopathology
3. Results
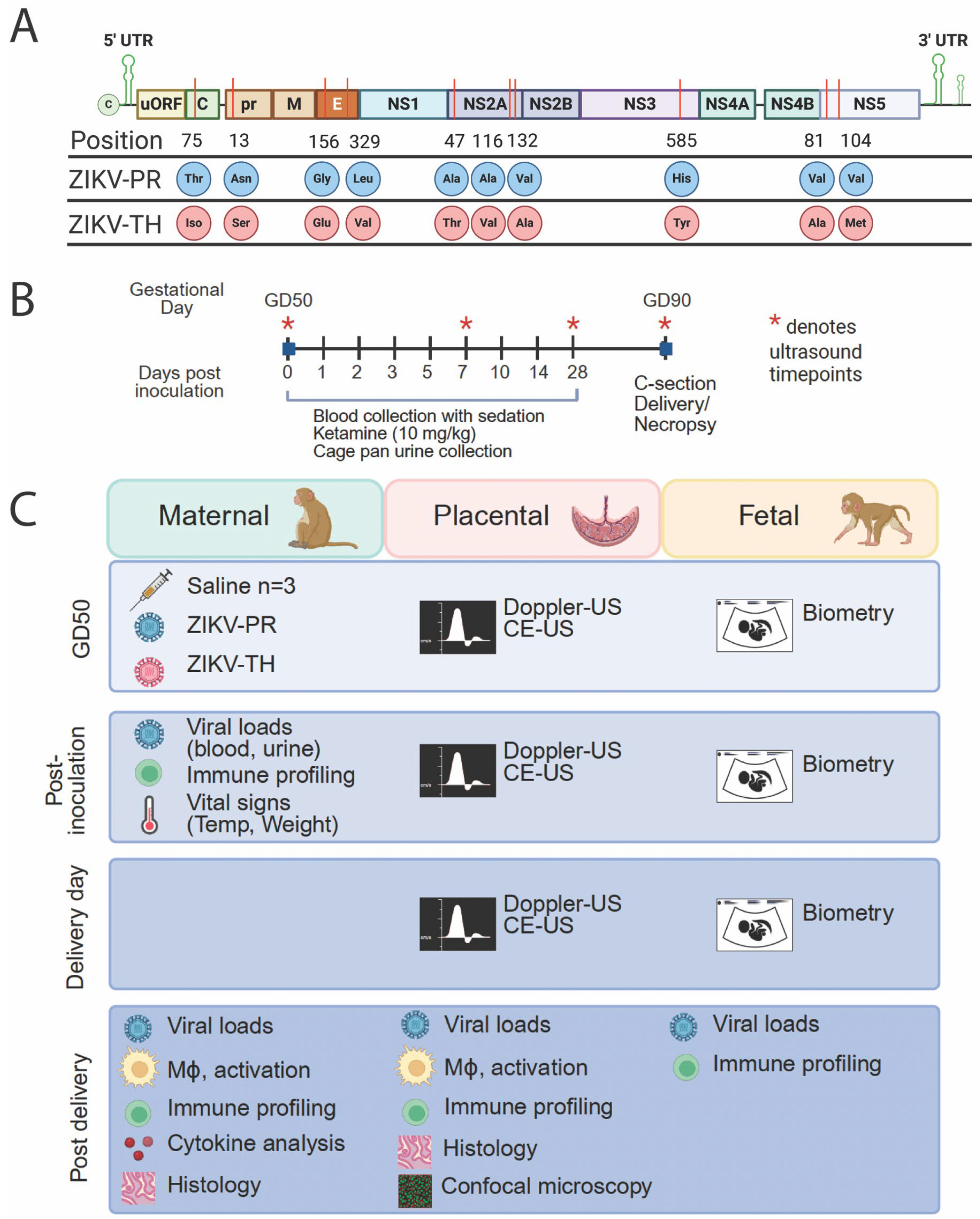
3.1. Study Design
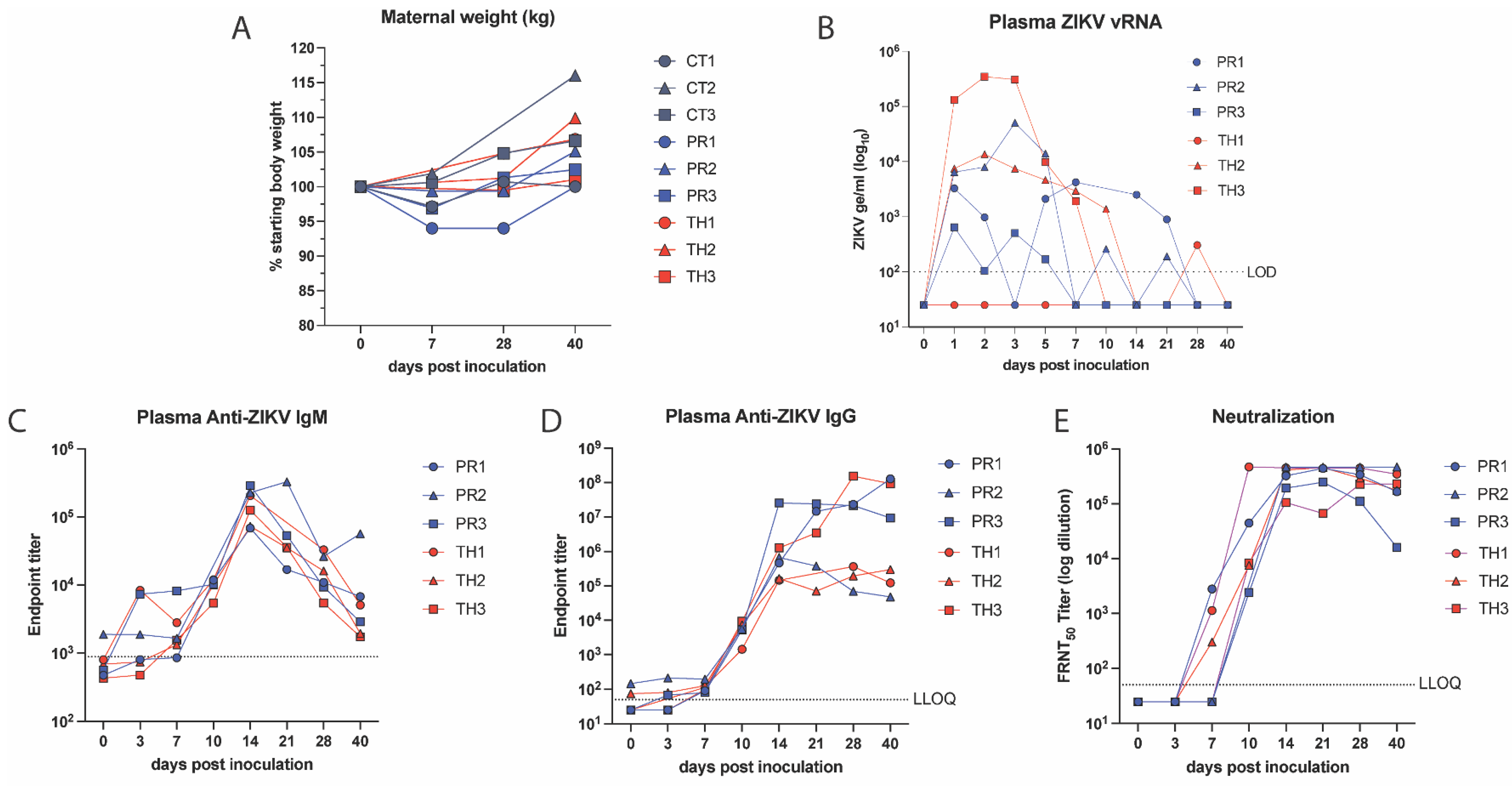
3.2. Comparison of ZIKV Strain Differences in Maternal Viral Loads and Immunity, Fetal Infection and Disease
3.3. Neutralizing Antibodies Correlate with Decreased Viral Burden
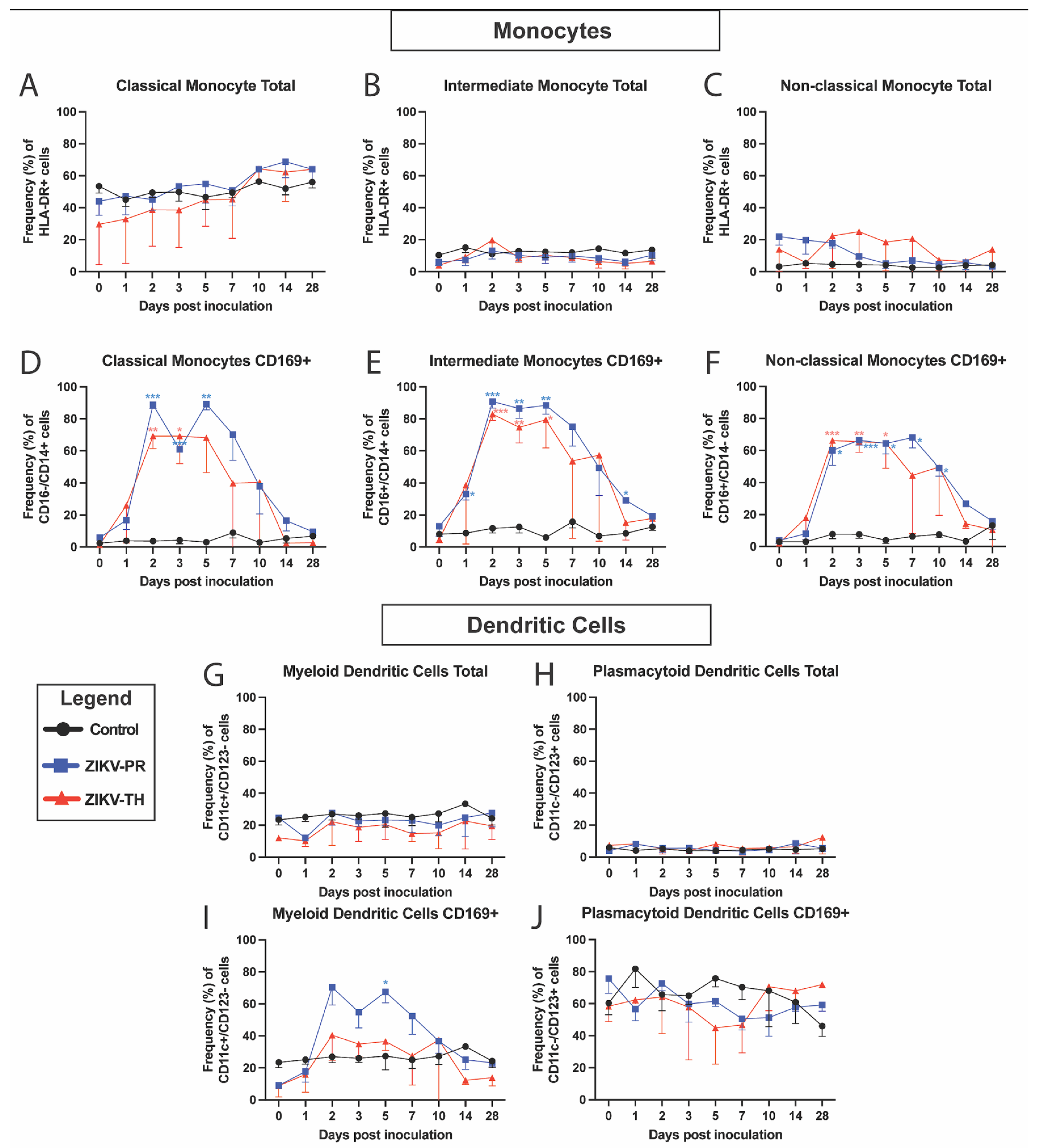
3.4. Cellular Innate Immune Signatures Following ZIKV Infection
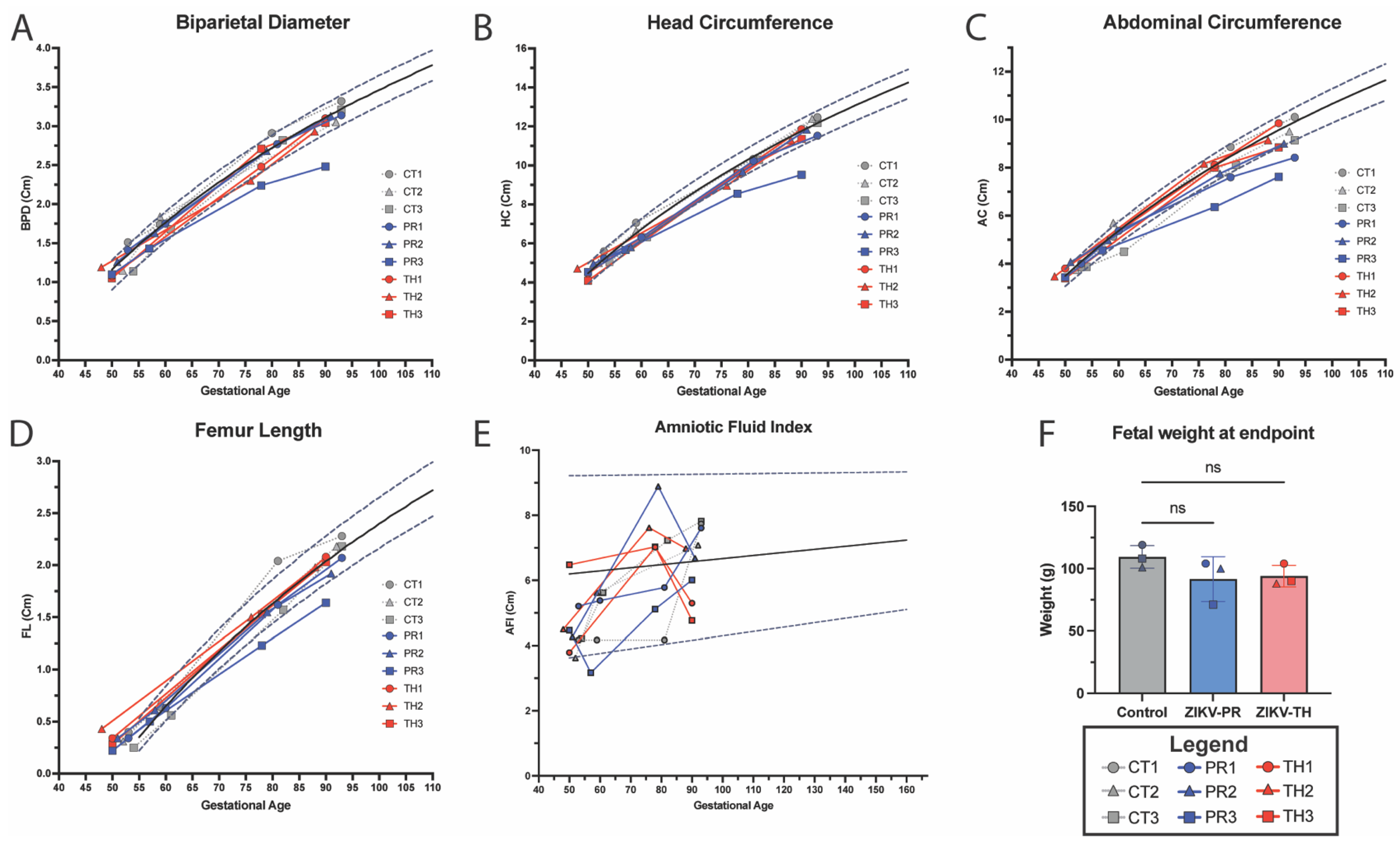
3.5. Fetal Growth Dynamics
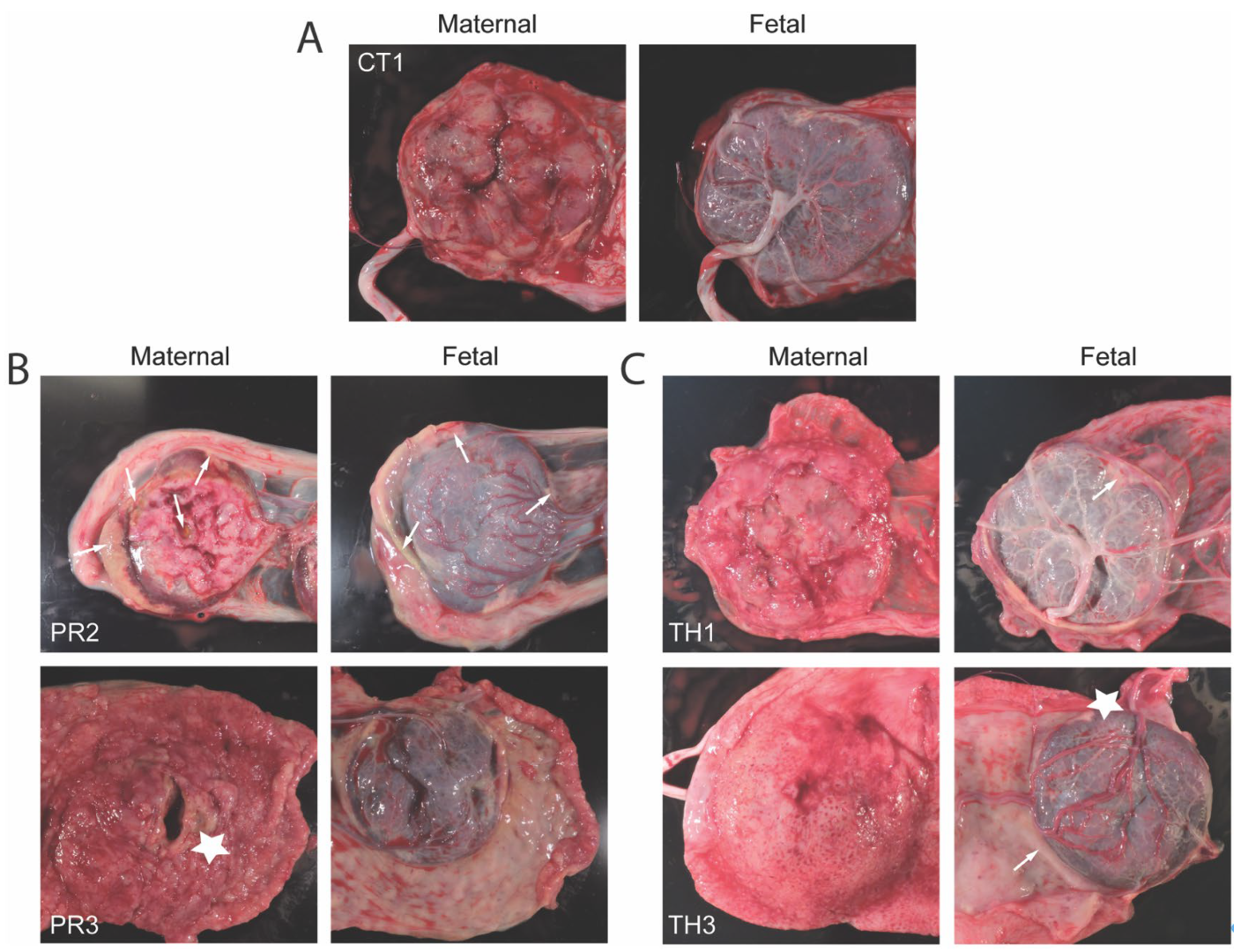
3.6. Placental Pathology Aligns with Maternal Viremia and Viral Dissemination Within the Placenta
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gregory, C.J.; Oduyebo, T.; Brault, A.C.; Brooks, J.T.; Chung, K.W.; Hills, S.; Kuehnert, M.J.; Mead, P.; Meaney-Delman, D.; Rabe, I.; et al. Modes of Transmission of Zika Virus. J. Infect. Dis. 2017, 216, S875–S883. [Google Scholar] [CrossRef] [PubMed]
- Gong, Z.; Gao, Y.; Han, G.Z. Zika Virus: Two or Three Lineages? Trends Microbiol. 2016, 24, 521–522. [Google Scholar] [CrossRef] [PubMed]
- Haddow, A.D.; Schuh, A.J.; Yasuda, C.Y.; Kasper, M.R.; Heang, V.; Huy, R.; Guzman, H.; Tesh, R.B.; Weaver, S.C. Genetic characterization of Zika virus strains: Geographic expansion of the Asian lineage. PLoS Negl. Trop. Dis. 2012, 6, e1477. [Google Scholar] [CrossRef]
- Lanciotti, R.S.; Lambert, A.J.; Holodniy, M.; Saavedra, S.; Signor Ldel, C. Phylogeny of Zika Virus in Western Hemisphere, 2015. Emerg. Infect. Dis. 2016, 22, 933–935. [Google Scholar] [CrossRef]
- Musso, D.; Gubler, D.J. Zika Virus. Clin. Microbiol. Rev. 2016, 29, 487–524. [Google Scholar] [CrossRef]
- Brasil, P.; Pereira, J.P., Jr.; Moreira, M.E.; Ribeiro Nogueira, R.M.; Damasceno, L.; Wakimoto, M.; Rabello, R.S.; Valderramos, S.G.; Halai, U.A.; Salles, T.S.; et al. Zika Virus Infection in Pregnant Women in Rio de Janeiro. N. Engl. J. Med. 2016, 375, 2321–2334. [Google Scholar] [CrossRef]
- Guerrero Saldivia, S.E.; Unnikrishnan, S.; Chavarria, Y.Y.; Akindele, A.O.; Jalkh, A.P.; Eastmond, A.K.; Shetty, C.; Rizvi, S.; Sharaf, J.; Williams, K.D.; et al. Zika Virus: A Systematic Review of Teratogenesis, Congenital Anomalies, and Child Mortality. Cureus 2023, 15, e34735. [Google Scholar] [CrossRef]
- Chepkorir, E.; Tchouassi, D.P.; Konongoi, S.L.; Lutomiah, J.; Tigoi, C.; Irura, Z.; Eyase, F.; Venter, M.; Sang, R. Serological evidence of Flavivirus circulation in human populations in Northern Kenya: An assessment of disease risk 2016–2017. Virol. J. 2019, 16, 65. [Google Scholar] [CrossRef]
- World Health Organization. Zika Epidemiology Update—February; WHO: Geneva, Switzerland, 2022.
- Faria, N.R.; Azevedo, R.; Kraemer, M.U.G.; Souza, R.; Cunha, M.S.; Hill, S.C.; Thézé, J.; Bonsall, M.B.; Bowden, T.A.; Rissanen, I.; et al. Zika virus in the Americas: Early epidemiological and genetic findings. Science 2016, 352, 345–349. [Google Scholar] [CrossRef]
- Khongwichit, S.; Wikan, N.; Auewarakul, P.; Smith, D.R. Zika virus in Thailand. Microbes Infect. 2018, 20, 670–675. [Google Scholar] [CrossRef]
- Buathong, R.; Hermann, L.; Thaisomboonsuk, B.; Rutvisuttinunt, W.; Klungthong, C.; Chinnawirotpisan, P.; Manasatienkij, W.; Nisalak, A.; Fernandez, S.; Yoon, I.K.; et al. Detection of Zika Virus Infection in Thailand, 2012–2014. Am. J. Trop. Med. Hyg. 2015, 93, 380–383. [Google Scholar] [CrossRef] [PubMed]
- Ruchusatsawat, K.; Wongjaroen, P.; Posanacharoen, A.; Rodriguez-Barraquer, I.; Sangkitporn, S.; Cummings, D.A.T.; Salje, H. Long-term circulation of Zika virus in Thailand: An observational study. Lancet Infect. Dis. 2019, 19, 439–446. [Google Scholar] [CrossRef]
- Nitatpattana, N.; Chaiyo, K.; Rajakam, S.; Poolam, K.; Chansiprasert, K.; Pesirikan, N.; Buree, S.; Rodpai, E.; Yoksan, S. Complete Genome Sequence of a Zika Virus Strain Isolated from the Serum of an Infected Patient in Thailand in 2006. Genome Announc. 2018, 6. [Google Scholar] [CrossRef]
- Vector-borne Disease Division/Risk Communication Office. The Department of Disease Control Reminds People to Protect Themselves and Reduce Zika Virus Infection, and Emphasizes that Hotel and Accommodation Operators Should Reduce the Risk to Customers and Manage the Environment to Prevent the Disease-Carrying Mosquitoes. Available online: https://ddc.moph.go.th/brc/news.php?news=39794&deptcode= (accessed on 11 November 2024).
- Khongwichit, S.; Chuchaona, W.; Vongpunsawad, S.; Poovorawan, Y. Molecular epidemiology, clinical analysis, and genetic characterization of Zika virus infections in Thailand (2020–2023). Sci. Rep. 2023, 13, 21030. [Google Scholar] [CrossRef]
- Jitsatja, A.; Ramphan, S.; Promma, P.; Kuadkitkan, A.; Wikan, N.; Uiprasertkul, M.; Phatihattakorn, C.; Smith, D.R. Comparative analysis of a Thai congenital-Zika-syndrome-associated virus with a Thai Zika-fever-associated virus. Arch. Virol. 2020, 165, 1791–1801. [Google Scholar] [CrossRef]
- Yuan, L.; Huang, X.Y.; Liu, Z.Y.; Zhang, F.; Zhu, X.L.; Yu, J.Y.; Ji, X.; Xu, Y.P.; Li, G.; Li, C.; et al. A single mutation in the prM protein of Zika virus contributes to fetal microcephaly. Science 2017, 358, 933–936. [Google Scholar] [CrossRef]
- Regla-Nava, J.A.; Wang, Y.T.; Fontes-Garfias, C.R.; Liu, Y.; Syed, T.; Susantono, M.; Gonzalez, A.; Viramontes, K.M.; Verma, S.K.; Kim, K.; et al. A Zika virus mutation enhances transmission potential and confers escape from protective dengue virus immunity. Cell Rep. 2022, 39, 110655. [Google Scholar] [CrossRef]
- Li, M.; Brokaw, A.; Furuta, A.M.; Coler, B.; Obregon-Perko, V.; Chahroudi, A.; Wang, H.Y.; Permar, S.R.; Hotchkiss, C.E.; Golos, T.G.; et al. Non-human Primate Models to Investigate Mechanisms of Infection-Associated Fetal and Pediatric Injury, Teratogenesis and Stillbirth. Front. Genet. 2021, 12, 680342. [Google Scholar] [CrossRef]
- Grigsby, P.L. Animal Models to Study Placental Development and Function throughout Normal and Dysfunctional Human Pregnancy. Semin. Reprod. Med. 2016, 34, 11–16. [Google Scholar] [CrossRef]
- Li, A.; Coffey, L.L.; Mohr, E.L.; Raper, J.; Chahroudi, A.; Ausderau, K.K.; Aliota, M.T.; Friedrich, T.C.; Mitzey, A.M.; Koenig, M.R.; et al. Role of non-human primate models in accelerating research and developing countermeasures against Zika virus infection. Lancet Microbe 2025, 101030. [Google Scholar] [CrossRef]
- Krabbe, N.P.; Razo, E.; Abraham, H.J.; Spanton, R.V.; Shi, Y.; Bhattacharya, S.; Bohm, E.K.; Pritchard, J.C.; Weiler, A.M.; Mitzey, A.M.; et al. Control of maternal Zika virus infection during pregnancy is associated with lower antibody titers in a macaque model. Front. Immunol. 2023, 14, 1267638. [Google Scholar] [CrossRef] [PubMed]
- Coffey, L.L.; Keesler, R.I.; Pesavento, P.A.; Woolard, K.; Singapuri, A.; Watanabe, J.; Cruzen, C.; Christe, K.L.; Usachenko, J.; Yee, J.; et al. Intraamniotic Zika virus inoculation of pregnant rhesus macaques produces fetal neurologic disease. Nat. Commun. 2018, 9, 2414. [Google Scholar] [CrossRef] [PubMed]
- Adams Waldorf, K.M.; Nelson, B.R.; Stencel-Baerenwald, J.E.; Studholme, C.; Kapur, R.P.; Armistead, B.; Walker, C.L.; Merillat, S.; Vornhagen, J.; Tisoncik-Go, J.; et al. Congenital Zika virus infection as a silent pathology with loss of neurogenic output in the fetal brain. Nat. Med. 2018, 24, 368–374. [Google Scholar] [CrossRef]
- Osuna, C.E.; Lim, S.Y.; Deleage, C.; Griffin, B.D.; Stein, D.; Schroeder, L.T.; Omange, R.W.; Best, K.; Luo, M.; Hraber, P.T.; et al. Zika viral dynamics and shedding in rhesus and cynomolgus macaques. Nat. Med. 2016, 22, 1448–1455. [Google Scholar] [CrossRef]
- Koide, F.; Goebel, S.; Snyder, B.; Walters, K.B.; Gast, A.; Hagelin, K.; Kalkeri, R.; Rayner, J. Development of a Zika Virus Infection Model in Cynomolgus Macaques. Front. Microbiol. 2016, 7, 2028. [Google Scholar] [CrossRef]
- Gurung, S.; Reuter, D.; Norris, A.; Dubois, M.; Maxted, M.; Singleton, K.; Castillo-Castrejon, M.; Papin, J.F.; Myers, D.A. Early and mid-gestation Zika virus (ZIKV) infection in the olive baboon (Papio anubis) leads to fetal CNS pathology by term gestation. PLoS Pathog. 2022, 18, e1010386. [Google Scholar] [CrossRef]
- Carroll, T.; Lo, M.; Lanteri, M.; Dutra, J.; Zarbock, K.; Silveira, P.; Rourke, T.; Ma, Z.M.; Fritts, L.; O’Connor, S.; et al. Zika virus preferentially replicates in the female reproductive tract after vaginal inoculation of rhesus macaques. PLoS Pathog. 2017, 13, e1006537. [Google Scholar] [CrossRef]
- Dudley, D.M.; Van Rompay, K.K.; Coffey, L.L.; Ardeshir, A.; Keesler, R.I.; Bliss-Moreau, E.; Grigsby, P.L.; Steinbach, R.J.; Hirsch, A.J.; MacAllister, R.P.; et al. Miscarriage and stillbirth following maternal Zika virus infection in nonhuman primates. Nat. Med. 2018, 24, 1104–1107. [Google Scholar] [CrossRef]
- Sousa, A.Q.; Cavalcante, D.I.M.; Franco, L.M.; Araújo, F.M.C.; Sousa, E.T.; Valença-Junior, J.T.; Rolim, D.B.; Melo, M.E.L.; Sindeaux, P.D.T.; Araújo, M.T.F.; et al. Postmortem Findings for 7 Neonates with Congenital Zika Virus Infection. Emerg. Infect. Dis. 2017, 23, 1164–1167. [Google Scholar] [CrossRef]
- Melo, A.S.; Aguiar, R.S.; Amorim, M.M.; Arruda, M.B.; Melo, F.O.; Ribeiro, S.T.; Batista, A.G.; Ferreira, T.; Dos Santos, M.P.; Sampaio, V.V.; et al. Congenital Zika Virus Infection: Beyond Neonatal Microcephaly. JAMA Neurol. 2016, 73, 1407–1416. [Google Scholar] [CrossRef]
- Oliveira Melo, A.S.; Malinger, G.; Ximenes, R.; Szejnfeld, P.O.; Alves Sampaio, S.; Bispo de Filippis, A.M. Zika virus intrauterine infection causes fetal brain abnormality and microcephaly: Tip of the iceberg? Ultrasound Obstet. Gynecol. 2016, 47, 6–7. [Google Scholar] [CrossRef] [PubMed]
- Silasi, M.; Cardenas, I.; Kwon, J.Y.; Racicot, K.; Aldo, P.; Mor, G. Viral infections during pregnancy. Am. J. Reprod. Immunol. 2015, 73, 199–213. [Google Scholar] [CrossRef] [PubMed]
- Rosado, L.E.P.; Martelli, C.M.T.; Brickley, E.B.; Gomes, M.B.F.; de Toledo Lima, T.; da Costa, P.S.S.; de Ávila, M.P.; Viggiano, M.B.; do Amaral, W.N.; de Rezende Feres, V.C.; et al. Risk of adverse pregnancy and infant outcomes associated with prenatal Zika virus infection: A post-epidemic cohort in Central-West Brazil. Sci. Rep. 2023, 13, 7335. [Google Scholar] [CrossRef]
- D’Mello, R.J.; Lo, J.O.; Hagen, O.L.; Castro, J.N.; Graham, J.A.; Frias, A.E.; Roberts, V.H.J. Ultrasound evaluation of normal rhesus macaque fetal biometry and uteroplacental hemodynamics. Am. J. Primatol. 2023, 85, e23504. [Google Scholar] [CrossRef]
- Steinbach, R.J.; Haese, N.N.; Smith, J.L.; Colgin, L.M.A.; MacAllister, R.P.; Greene, J.M.; Parkins, C.J.; Kempton, J.B.; Porsov, E.; Wang, X.; et al. A neonatal nonhuman primate model of gestational Zika virus infection with evidence of microencephaly, seizures and cardiomyopathy. PLoS ONE 2020, 15, e0227676. [Google Scholar] [CrossRef]
- Hirsch, A.J.; Roberts, V.H.J.; Grigsby, P.L.; Haese, N.; Schabel, M.C.; Wang, X.; Lo, J.O.; Liu, Z.; Kroenke, C.D.; Smith, J.L.; et al. Zika virus infection in pregnant rhesus macaques causes placental dysfunction and immunopathology. Nat. Commun. 2018, 9, 263. [Google Scholar] [CrossRef]
- Hoen, B.; Schaub, B.; Funk, A.L.; Ardillon, V.; Boullard, M.; Cabié, A.; Callier, C.; Carles, G.; Cassadou, S.; Césaire, R.; et al. Pregnancy Outcomes after ZIKV Infection in French Territories in the Americas. N. Engl. J. Med. 2018, 378, 985–994. [Google Scholar] [CrossRef]
- Martinot, A.J.; Abbink, P.; Afacan, O.; Prohl, A.K.; Bronson, R.; Hecht, J.L.; Borducchi, E.N.; Larocca, R.A.; Peterson, R.L.; Rinaldi, W.; et al. Fetal Neuropathology in Zika Virus-Infected Pregnant Female Rhesus Monkeys. Cell 2018, 173, 1111–1122.e1110. [Google Scholar] [CrossRef]
- Rosinski, J.R.; Raasch, L.E.; Barros Tiburcio, P.; Breitbach, M.E.; Shepherd, P.M.; Yamamoto, K.; Razo, E.; Krabbe, N.P.; Bliss, M.I.; Richardson, A.D.; et al. Frequent first-trimester pregnancy loss in rhesus macaques infected with African-lineage Zika virus. PLoS Pathog. 2023, 19, e1011282. [Google Scholar] [CrossRef]
- Seferovic, M.; Sánchez-San Martín, C.; Tardif, S.D.; Rutherford, J.; Castro, E.C.C.; Li, T.; Hodara, V.L.; Parodi, L.M.; Giavedoni, L.; Layne-Colon, D.; et al. Experimental Zika Virus Infection in the Pregnant Common Marmoset Induces Spontaneous Fetal Loss and Neurodevelopmental Abnormalities. Sci. Rep. 2018, 8, 6851. [Google Scholar] [CrossRef]
- Nguyen, S.M.; Antony, K.M.; Dudley, D.M.; Kohn, S.; Simmons, H.A.; Wolfe, B.; Salamat, M.S.; Teixeira, L.B.C.; Wiepz, G.J.; Thoong, T.H.; et al. Highly efficient maternal-fetal Zika virus transmission in pregnant rhesus macaques. PLoS Pathog. 2017, 13, e1006378. [Google Scholar] [CrossRef] [PubMed]
- Gurung, S.; Reuter, N.; Preno, A.; Dubaut, J.; Nadeau, H.; Hyatt, K.; Singleton, K.; Martin, A.; Parks, W.T.; Papin, J.F.; et al. Zika virus infection at mid-gestation results in fetal cerebral cortical injury and fetal death in the olive baboon. PLoS Pathog. 2019, 15, e1007507. [Google Scholar] [CrossRef] [PubMed]
- Aubry, F.; Jacobs, S.; Darmuzey, M.; Lequime, S.; Delang, L.; Fontaine, A.; Jupatanakul, N.; Miot, E.F.; Dabo, S.; Manet, C.; et al. Recent African strains of Zika virus display higher transmissibility and fetal pathogenicity than Asian strains. Nat. Commun. 2021, 12, 916. [Google Scholar] [CrossRef]
- Mohr, E.L.; Block, L.N.; Newman, C.M.; Stewart, L.M.; Koenig, M.; Semler, M.; Breitbach, M.E.; Teixeira, L.B.C.; Zeng, X.; Weiler, A.M.; et al. Ocular and uteroplacental pathology in a macaque pregnancy with congenital Zika virus infection. PLoS ONE 2018, 13, e0190617. [Google Scholar] [CrossRef]
- Percie du Sert, N.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. PLoS Biol. 2020, 18, e3000410. [Google Scholar] [CrossRef]
- Wongsurawat, T.; Athipanyasilp, N.; Jenjaroenpun, P.; Jun, S.R.; Kaewnapan, B.; Wassenaar, T.M.; Leelahakorn, N.; Angkasekwinai, N.; Kantakamalakul, W.; Ussery, D.W.; et al. Case of Microcephaly after Congenital Infection with Asian Lineage Zika Virus, Thailand. Emerg. Infect. Dis. 2018, 24, 1758–1761. [Google Scholar] [CrossRef]
- Hirsch, A.J.; Smith, J.L.; Haese, N.N.; Broeckel, R.M.; Parkins, C.J.; Kreklywich, C.; DeFilippis, V.R.; Denton, M.; Smith, P.P.; Messer, W.B.; et al. Zika Virus infection of rhesus macaques leads to viral persistence in multiple tissues. PLoS Pathog. 2017, 13, e1006219. [Google Scholar] [CrossRef]
- Henchal, E.A.; McCown, J.M.; Burke, D.S.; Seguin, M.C.; Brandt, W.E. Epitopic analysis of antigenic determinants on the surface of dengue-2 virions using monoclonal antibodies. Am. J. Trop. Med. Hyg. 1985, 34, 162–169. [Google Scholar] [CrossRef]
- Weber, W.C.; Labriola, C.S.; Kreklywich, C.N.; Ray, K.; Haese, N.N.; Andoh, T.F.; Denton, M.; Medica, S.; Streblow, M.M.; Smith, P.P.; et al. Mayaro virus pathogenesis and immunity in rhesus macaques. PLoS Negl. Trop. Dis. 2023, 17, e0011742. [Google Scholar] [CrossRef]
- Sonner, J.M.; Werner, D.F.; Elsen, F.P.; Xing, Y.; Liao, M.; Harris, R.A.; Harrison, N.L.; Fanselow, M.S.; Eger, E.I., 2nd; Homanics, G.E. Effect of isoflurane and other potent inhaled anesthetics on minimum alveolar concentration, learning, and the righting reflex in mice engineered to express alpha1 gamma-aminobutyric acid type A receptors unresponsive to isoflurane. Anesthesiology 2007, 106, 107–113. [Google Scholar] [CrossRef]
- Kennedy, G.L., Jr.; Smith, S.H.; Keplinger, M.L.; Calandra, J.C. Reproductive and teratologic studies with isoflurane. Drug Chem. Toxicol. 1977, 1, 75–88. [Google Scholar] [CrossRef] [PubMed]
- Reighard, C.; Junaid, S.; Jackson, W.M.; Arif, A.; Waddington, H.; Whitehouse, A.J.O.; Ing, C. Anesthetic Exposure During Childhood and Neurodevelopmental Outcomes: A Systematic Review and Meta-analysis. JAMA Network Open 2022, 5, e2217427. [Google Scholar] [CrossRef] [PubMed]
- Olutoye, O.A.; Baker, B.W.; Belfort, M.A.; Olutoye, O.O. Food and Drug Administration warning on anesthesia and brain development: Implications for obstetric and fetal surgery. Am. J. Obstet. Gynecol. 2018, 218, 98–102. [Google Scholar] [CrossRef]
- Sarris, I.; Ioannou, C.; Dighe, M.; Mitidieri, A.; Oberto, M.; Qingqing, W.; Shah, J.; Sohoni, S.; Al Zidjali, W.; Hoch, L.; et al. Standardization of fetal ultrasound biometry measurements: Improving the quality and consistency of measurements. Ultrasound Obstet. Gynecol. 2011, 38, 681–687. [Google Scholar] [CrossRef]
- American College of Obstetricians and Gynecologists. Ultrasound in Pregnancy. Practice Bulletin No. 175. Obstet. Gynecol. 2016, 128, e241–e256. [Google Scholar] [CrossRef]
- Frias, A.E.; Morgan, T.K.; Evans, A.E.; Rasanen, J.; Oh, K.Y.; Thornburg, K.L.; Grove, K.L. Maternal high-fat diet disturbs uteroplacental hemodynamics and increases the frequency of stillbirth in a nonhuman primate model of excess nutrition. Endocrinology 2011, 152, 2456–2464. [Google Scholar] [CrossRef]
- Lo, J.O.; Schabel, M.C.; Roberts, V.H.; Morgan, T.K.; Rasanen, J.P.; Kroenke, C.D.; Shoemaker, S.R.; Spindel, E.R.; Frias, A.E. Vitamin C supplementation ameliorates the adverse effects of nicotine on placental hemodynamics and histology in nonhuman primates. Am. J. Obstet. Gynecol. 2015, 212, 370.e1–370.e8. [Google Scholar] [CrossRef]
- Roberts, V.H.; Lo, J.O.; Salati, J.A.; Lewandowski, K.S.; Lindner, J.R.; Morgan, T.K.; Frias, A.E. Quantitative assessment of placental perfusion by contrast-enhanced ultrasound in macaques and human subjects. Am. J. Obstet. Gynecol. 2016, 214, 369.e1–369.e8. [Google Scholar] [CrossRef]
- Barry, P.A.; Lockridge, K.M.; Salamat, S.; Tinling, S.P.; Yue, Y.; Zhou, S.S.; Gospe, S.M., Jr.; Britt, W.J.; Tarantal, A.F. Nonhuman primate models of intrauterine cytomegalovirus infection. ILAR J. 2006, 47, 49–64. [Google Scholar] [CrossRef]
- Pomar, L.; Lambert, V.; Matheus, S.; Pomar, C.; Hcini, N.; Carles, G.; Rousset, D.; Vouga, M.; Panchaud, A.; Baud, D. Prolonged Maternal Zika Viremia as a Marker of Adverse Perinatal Outcomes. Emerg. Infect. Dis. 2021, 27, 490–498. [Google Scholar] [CrossRef]
- Aid, M.; Abbink, P.; Larocca, R.A.; Boyd, M.; Nityanandam, R.; Nanayakkara, O.; Martinot, A.J.; Moseley, E.T.; Blass, E.; Borducchi, E.N.; et al. Zika Virus Persistence in the Central Nervous System and Lymph Nodes of Rhesus Monkeys. Cell 2017, 169, 610–620.e614. [Google Scholar] [CrossRef] [PubMed]
- Singh, T.; Hwang, K.K.; Miller, A.S.; Jones, R.L.; Lopez, C.A.; Dulson, S.J.; Giuberti, C.; Gladden, M.A.; Miller, I.; Webster, H.S.; et al. A Zika virus-specific IgM elicited in pregnancy exhibits ultrapotent neutralization. Cell 2022, 185, 4826–4840.e4817. [Google Scholar] [CrossRef] [PubMed]
- Barzon, L.; Percivalle, E.; Pacenti, M.; Rovida, F.; Zavattoni, M.; Del Bravo, P.; Cattelan, A.M.; Palù, G.; Baldanti, F. Virus and Antibody Dynamics in Travelers With Acute Zika Virus Infection. Clin. Infect. Dis. 2018, 66, 1173–1180. [Google Scholar] [CrossRef]
- Porras-García, A.; Villanueva-García, D.; Arnaud-Rios, R.; García-Lemus, N.; Castillo-Romero, A.; Mejía-Flores, M.; Contreras, L.E.; Hernández-Castillo, L.; Jiménez-Hernández, E.; Mejía-Aranguré, J.M.; et al. Intrauterine Transmission of Zika and Vertical Transfer of Neutralizing Antibodies Detected Immediately at Birth in Oaxaca, Mexico: An Analysis in the Context of Microcephaly. Microorganisms 2024, 12, 423. [Google Scholar] [CrossRef]
- Rossi, S.L.; Tesh, R.B.; Azar, S.R.; Muruato, A.E.; Hanley, K.A.; Auguste, A.J.; Langsjoen, R.M.; Paessler, S.; Vasilakis, N.; Weaver, S.C. Characterization of a Novel Murine Model to Study Zika Virus. Am. J. Trop. Med. Hyg. 2016, 94, 1362–1369. [Google Scholar] [CrossRef]
- Jagger, B.W.; Miner, J.J.; Cao, B.; Arora, N.; Smith, A.M.; Kovacs, A.; Mysorekar, I.U.; Coyne, C.B.; Diamond, M.S. Gestational Stage and IFN-λ Signaling Regulate ZIKV Infection In Utero. Cell Host Microbe 2017, 22, 366–376.e363. [Google Scholar] [CrossRef]
- Nazerai, L.; Schøller, A.S.; Bassi, M.R.; Buus, S.; Stryhn, A.; Christensen, J.P.; Thomsen, A.R. Effector CD8 T Cell-Dependent Zika Virus Control in the CNS: A Matter of Time and Numbers. Front. Immunol. 2020, 11, 1977. [Google Scholar] [CrossRef]
- Cimini, E.; Castilletti, C.; Sacchi, A.; Casetti, R.; Bordoni, V.; Romanelli, A.; Turchi, F.; Martini, F.; Tumino, N.; Nicastri, E.; et al. Human Zika infection induces a reduction of IFN-γ producing CD4 T-cells and a parallel expansion of effector Vδ2 T-cells. Sci. Rep. 2017, 7, 6313. [Google Scholar] [CrossRef]
- El Sahly, H.M.; Gorchakov, R.; Lai, L.; Natrajan, M.S.; Patel, S.M.; Atmar, R.L.; Keitel, W.A.; Hoft, D.F.; Barrett, J.; Bailey, J.; et al. Clinical, Virologic, and Immunologic Characteristics of Zika Virus Infection in a Cohort of US Patients: Prolonged RNA Detection in Whole Blood. Open Forum Infect. Dis. 2019, 6, ofy352. [Google Scholar] [CrossRef]
- Moreno, G.K.; Newman, C.M.; Koenig, M.R.; Mohns, M.S.; Weiler, A.M.; Rybarczyk, S.; Weisgrau, K.L.; Vosler, L.J.; Pomplun, N.; Schultz-Darken, N.; et al. Long-Term Protection of Rhesus Macaques from Zika Virus Reinfection. J. Virol. 2020, 94, 10–128. [Google Scholar] [CrossRef]
- Haese, N.N.; Roberts, V.H.J.; Chen, A.; Streblow, D.N.; Morgan, T.K.; Hirsch, A.J. Nonhuman Primate Models of Zika Virus Infection and Disease during Pregnancy. Viruses 2021, 13, 88. [Google Scholar] [CrossRef] [PubMed]
- Peleg, D.; Kennedy, C.M.; Hunter, S.K. Intrauterine growth restriction: Identification and management. Am. Fam. Physician 1998, 58, 453–466. [Google Scholar] [PubMed]
- Kuadkitkan, A.; Wikan, N.; Sornjai, W.; Smith, D.R. Zika virus and microcephaly in Southeast Asia: A cause for concern? J. Infect. Public Health 2020, 13, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Koenig, M.R.; Mitzey, A.M.; Morgan, T.K.; Zeng, X.; Simmons, H.A.; Mejia, A.; Leyva Jaimes, F.; Keding, L.T.; Crooks, C.M.; Weiler, A.M.; et al. Infection of the maternal-fetal interface and vertical transmission following low-dose inoculation of pregnant rhesus macaques (Macaca mulatta) with an African-lineage Zika virus. PLoS ONE 2023, 18, e0284964. [Google Scholar] [CrossRef]
- Koenig, M.R.; Mitzey, A.M.; Zeng, X.; Reyes, L.; Simmons, H.A.; Morgan, T.K.; Bohm, E.K.; Pritchard, J.C.; Schmidt, J.A.; Ren, E.; et al. Vertical transmission of African-lineage Zika virus through the fetal membranes in a rhesus macaque (Macaca mulatta) model. PLoS Pathog. 2023, 19, e1011274. [Google Scholar] [CrossRef]
- Raasch, L.E.; Yamamoto, K.; Newman, C.M.; Rosinski, J.R.; Shepherd, P.M.; Razo, E.; Crooks, C.M.; Bliss, M.I.; Breitbach, M.E.; Sneed, E.L.; et al. Fetal loss in pregnant rhesus macaques infected with high-dose African-lineage Zika virus. PLoS Negl. Trop. Dis. 2022, 16, e0010623. [Google Scholar] [CrossRef]
- Rayner, J.O.; Kalkeri, R.; Goebel, S.; Cai, Z.; Green, B.; Lin, S.; Snyder, B.; Hagelin, K.; Walters, K.B.; Koide, F. Comparative Pathogenesis of Asian and African-Lineage Zika Virus in Indian Rhesus Macaque’s and Development of a Non-Human Primate Model Suitable for the Evaluation of New Drugs and Vaccines. Viruses 2018, 10, 229. [Google Scholar] [CrossRef]
- Newman, C.M.; Tarantal, A.F.; Martinez, M.L.; Simmons, H.A.; Morgan, T.K.; Zeng, X.; Rosinski, J.R.; Bliss, M.I.; Bohm, E.K.; Dudley, D.M.; et al. Early Embryonic Loss Following Intravaginal Zika Virus Challenge in Rhesus Macaques. Front. Immunol. 2021, 12, 686437. [Google Scholar] [CrossRef]
- O’Connor, M.A.; Tisoncik-Go, J.; Lewis, T.B.; Miller, C.J.; Bratt, D.; Moats, C.R.; Edlefsen, P.T.; Smedley, J.; Klatt, N.R.; Gale, M., Jr.; et al. Early cellular innate immune responses drive Zika viral persistence and tissue tropism in pigtail macaques. Nat. Commun. 2018, 9, 3371. [Google Scholar] [CrossRef]
- Silveira, E.L.V.; Rogers, K.A.; Gumber, S.; Amancha, P.; Xiao, P.; Woollard, S.M.; Byrareddy, S.N.; Teixeira, M.M.; Villinger, F. Immune Cell Dynamics in Rhesus Macaques Infected with a Brazilian Strain of Zika Virus. J. Immunol. 2017, 199, 1003–1011. [Google Scholar] [CrossRef]
- Haese, N.N.; Smith, H.; Onwuzu, K.; Kreklywich, C.N.; Smith, J.L.; Denton, M.; Kreklywich, N.; Streblow, A.D.; Frias, A.E.; Morgan, T.K.; et al. Differential Type 1 IFN Gene Expression in CD14+ Placenta Cells Elicited by Zika Virus Infection During Pregnancy. Front. Virol. 2021, 1, 783407. [Google Scholar] [CrossRef]

| ZIKV-PR | ZIKV-TH | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PR1-D | PR1-F | PR2-D | PR2-F | PR3-D | PR3-F | TH1-D | TH1-F | TH2-D | TH2-F | TH3-D | TH3-F | ||
| % vRNA+ tissues | 3/62 (4.8%) | 1/43 (2%) | 10/63 (16%) | 2/43 (4.7%) | 9/63 (14%) | 0/49 (0%) | 3/67 (4.5%) | 0/48 (0%) | 6/63 (9.5%) | 3/55 (5%) | 5/49 (10%) | 1/36 (2.7%) | |
| Lymph tissue | Axillary LN | 3.76 | — | 3.78 | — | 6.20 | — | 5.52 | — | 5.68 | — | ||
| Inguinal LN | — | 2.90 | 2.78 | — | 5.84 | — | — | — | — | — | 4.24 | ||
| Mesenteric LN | — | — | 3.66 | — | 5.72 | — | 4.49 | — | 4.07 | — | — | — | |
| Thymus | — | — | — | — | — | — | — | — | — | — | 3.31 | — | |
| Digestive system | Stomach | — | — | — | — | — | — | — | — | — | — | 3.57 | — |
| Duodenum | — | — | — | 2.99 | — | — | — | — | — | — | — | ||
| Colon | — | — | — | — | — | — | — | — | — | — | — | 4.34 | |
| Musculoskeletal | Biceps Brachii | — | — | — | — | — | 3.18 | — | — | ||||
| Elbow | — | 3.49 | — | — | — | — | — | — | |||||
| Finger | — | 3.55 | 5.54 | 4.63 | — | 3.77 | — | — | |||||
| Hand/Wrist | — | — | 3.93 | — | 5.65 | — | — | — | — | — | — | ||
| Quadriceps muscle | — | — | — | — | — | 2.91 | — | 3.03 | — | ||||
| Hamstring | 2.87 | — | — | — | — | — | 3.36 | — | — | ||||
| Foot/Ankle | — | — | — | — | 4.55 | — | — | — | — | — | — | ||
| Genito-urinary | Uterus | — | — | — | N/A | — | N/A | — | — | 4.77 | — | — | |
| Vagina | — | — | — | — | — | 3.11 | |||||||
| Urethra | 2.95 | — | — | — | — | — | — | — | — | — | — | ||
| Nervous tissue | |||||||||||||
| PNS | Brachial Plexus | — | — | — | 3.00 | — | — | — | — | — | — | — | |
| Trigeminal Nerve | — | 3.47 | 5.10 | — | — | ||||||||
| Femoral Nerve | — | 2.90 | — | — | — | ||||||||
| Sciatic Nerve | 2.73 | 5.03 | — | — | — | — | — | ||||||
| CNS | Pituitary Gland | — | — | — | — | — | — | — | — | — | — | ||
| Frontal Lobe | — | — | — | — | — | — | 3.71 | — | |||||
| Occipital lobe | — | — | — | — | — | — | 2.99 | — | — | ||||
| Brain Stem | — | 2.61 | — | — | — | — | |||||||
| Endocrine | Arendal Glands | — | — | — | — | 4.76 | — | — | — | — | — | — | — |
| ZIKV-PR | ZIKV-TH | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PR1 | PR2 | PR3 | TH1 | TH2 | TH3 | |||||||
| Placental Disc | 1° | 2° | 1° | 2° | 1° | 2° | 1° | 2° | 1° | 2° | 1° | 2° |
| Cotyledon # | ||||||||||||
| 1 | — | — | 3.58 | — | — | — | — | — | — | — | — | 3.58 |
| 2 | — | — | — | — | — | — | — | — | — | — | 3.64 | — |
| 3 | — | — | 1.74 | 2.72 | — | — | — | — | — | — | 5.22 | 5.16 |
| 4 | — | — | — | — | — | — | — | — | — | — | — | 3.58 |
| 5 | 1.07 | — | — | — | — | — | — | — | — | 5.90 | ||
| 6 | — | — | 2.08 | 2.19 | — | — | — | — | 2.56 | 3.87 | ||
| 7 | — | — | — | 2.10 | — | 3.29 | — | — | — | |||
| 8 | — | — | — | — | — | — | — | 4.15 | ||||
| 9 | — | — | 1.93 | — | — | 5.04 | ||||||
| 10 | — | 2.87 | — | |||||||||
| 11 | — | 2.09 | — | |||||||||
| 12 | — | |||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jaeger, H.K.; Smith, J.L.; Parkins, C.J.; Haese, N.N.; Kreklywich, C.N.; Denton, M.; Labriola, C.S.; Axthelm, M.K.; Barber-Axthelm, A.; Chun, K.; et al. Comparative Analysis of Two Zika Virus Isolates in a Rhesus Macaque Pregnancy Model. Viruses 2025, 17, 762. https://doi.org/10.3390/v17060762
Jaeger HK, Smith JL, Parkins CJ, Haese NN, Kreklywich CN, Denton M, Labriola CS, Axthelm MK, Barber-Axthelm A, Chun K, et al. Comparative Analysis of Two Zika Virus Isolates in a Rhesus Macaque Pregnancy Model. Viruses. 2025; 17(6):762. https://doi.org/10.3390/v17060762
Chicago/Turabian StyleJaeger, Hannah K., Jessica L. Smith, Christopher J. Parkins, Nicole N. Haese, Craig N. Kreklywich, Michael Denton, Caralyn S. Labriola, Michael K. Axthelm, Aaron Barber-Axthelm, Kim Chun, and et al. 2025. "Comparative Analysis of Two Zika Virus Isolates in a Rhesus Macaque Pregnancy Model" Viruses 17, no. 6: 762. https://doi.org/10.3390/v17060762
APA StyleJaeger, H. K., Smith, J. L., Parkins, C. J., Haese, N. N., Kreklywich, C. N., Denton, M., Labriola, C. S., Axthelm, M. K., Barber-Axthelm, A., Chun, K., Swanson, T., D’Mello, R. J., Morgan, T. K., Smith, D. R., Lo, J. O., Hirsch, A. J., Roberts, V. H. J., & Streblow, D. N. (2025). Comparative Analysis of Two Zika Virus Isolates in a Rhesus Macaque Pregnancy Model. Viruses, 17(6), 762. https://doi.org/10.3390/v17060762






