Inhibition of Bovine Enterovirus Infection by Magnolol via Modulating the Gut Microbiota in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Virus and Mice
2.2. Reagents
2.3. Experimental Design
2.4. Gut Microbiota Analysis
2.5. Quantitative Real-Time Polymerase Chain Reaction
2.6. Western Blot
2.7. Statistical Analysis
3. Results
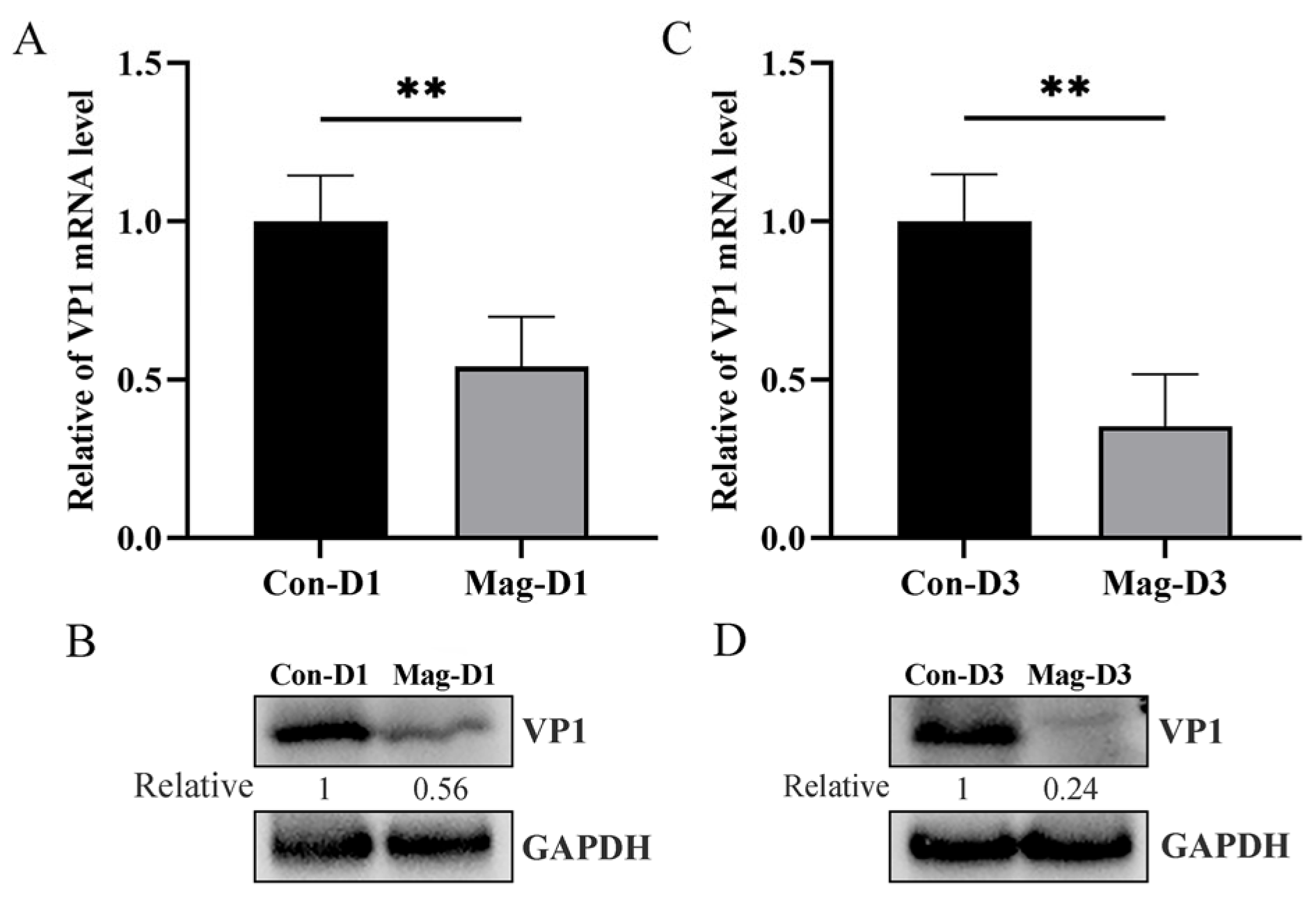
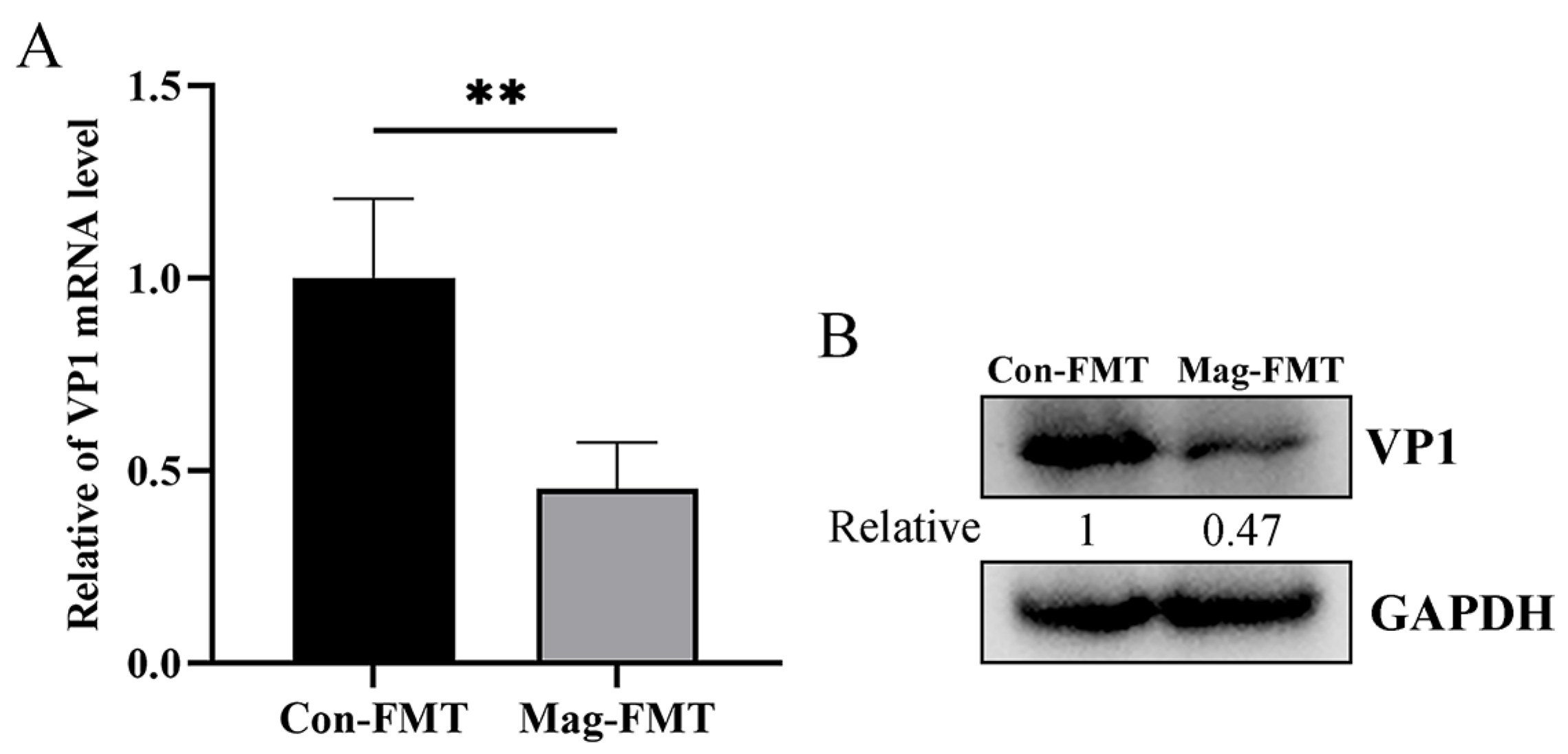
3.1. Magnolol Inhibits BEV Replication
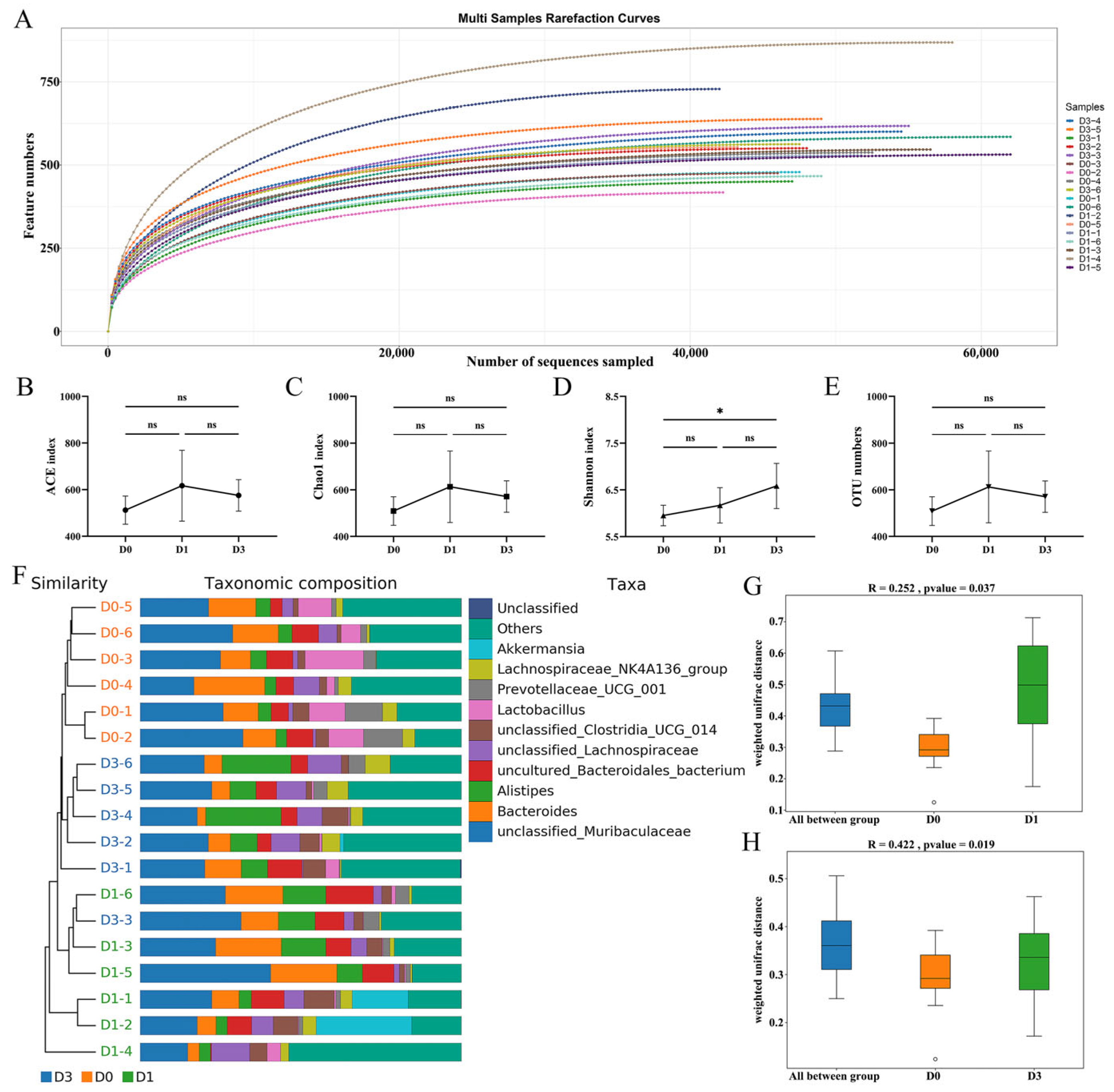
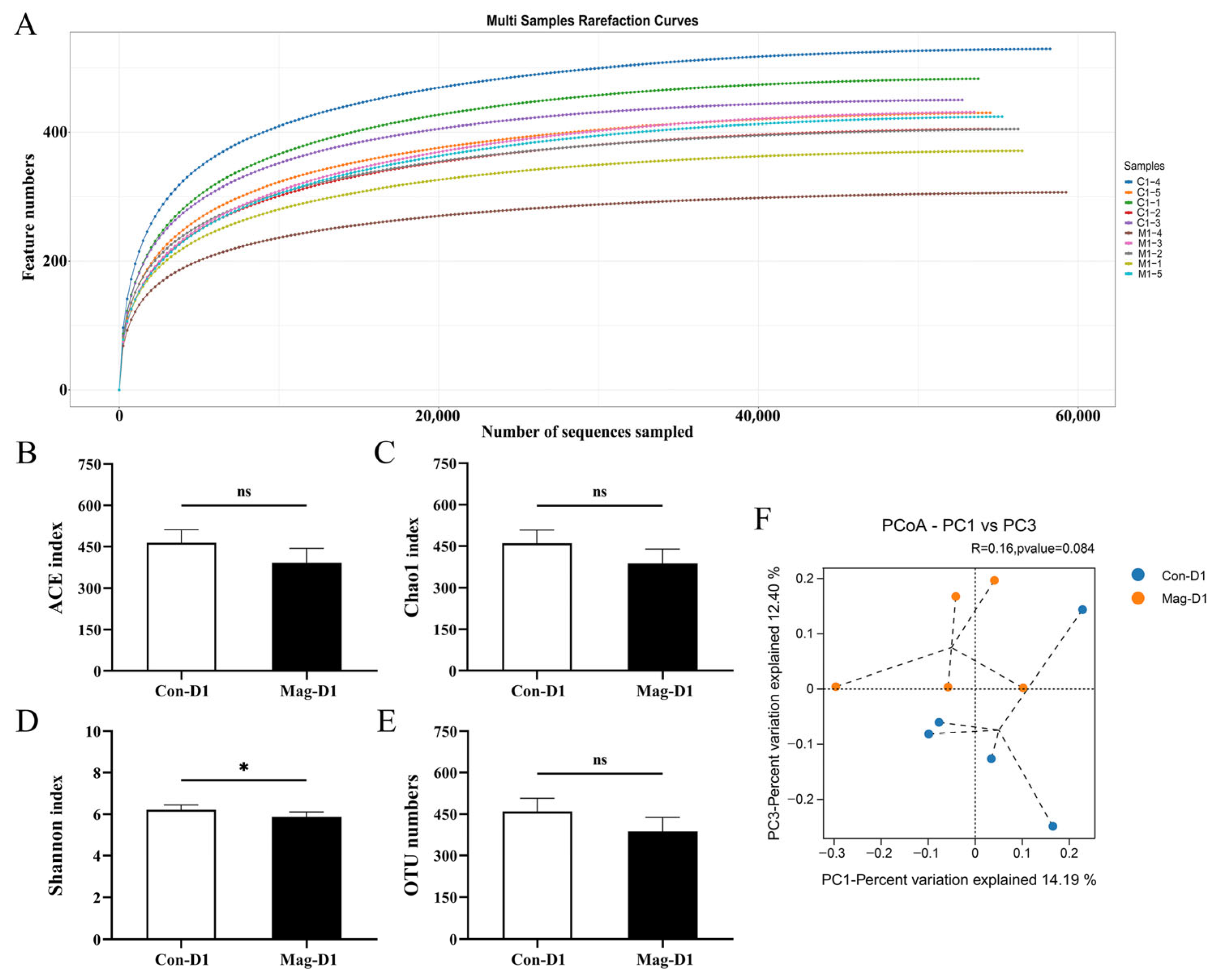
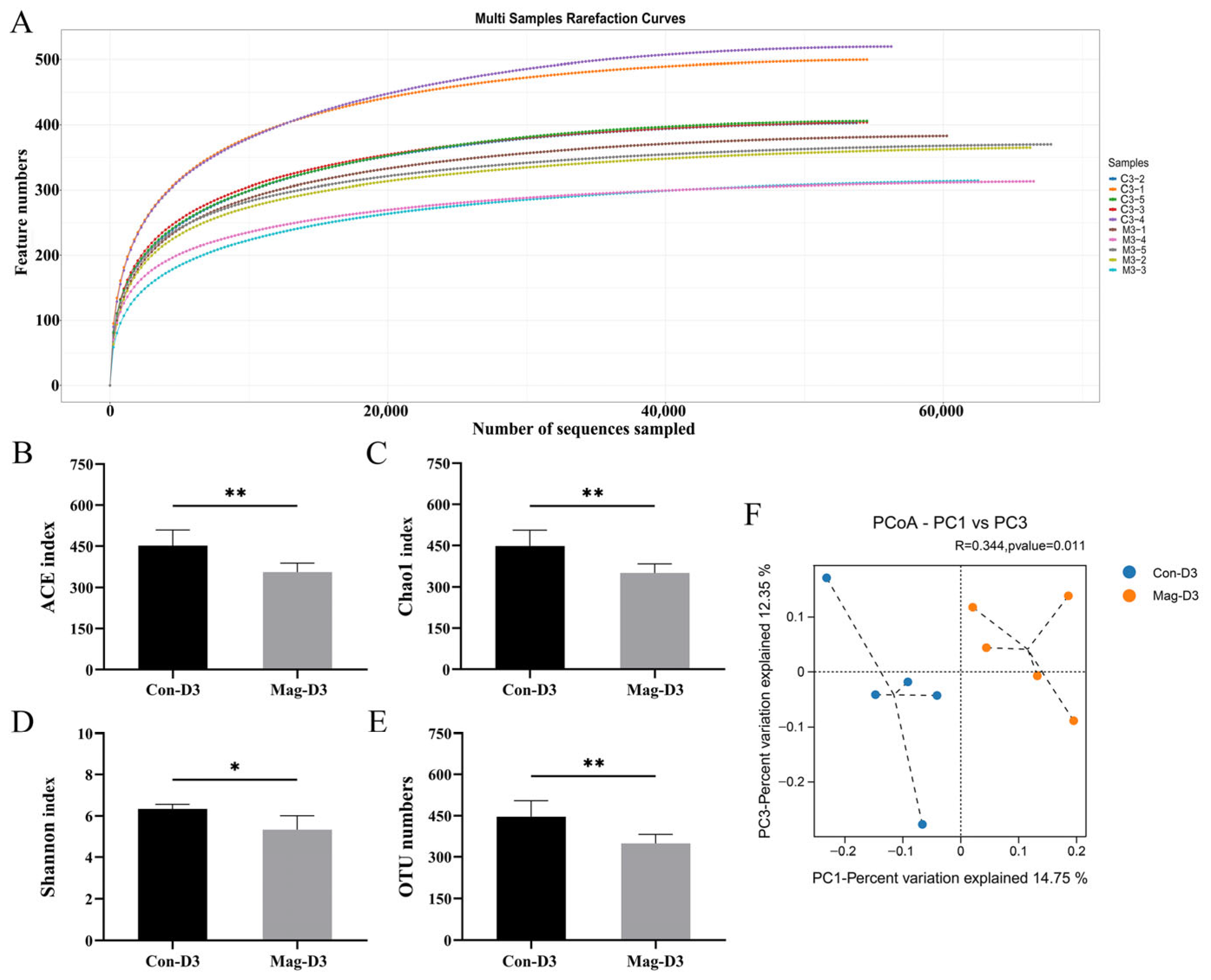
3.2. Richness and Diversity of the Microbiota After BEV Infection
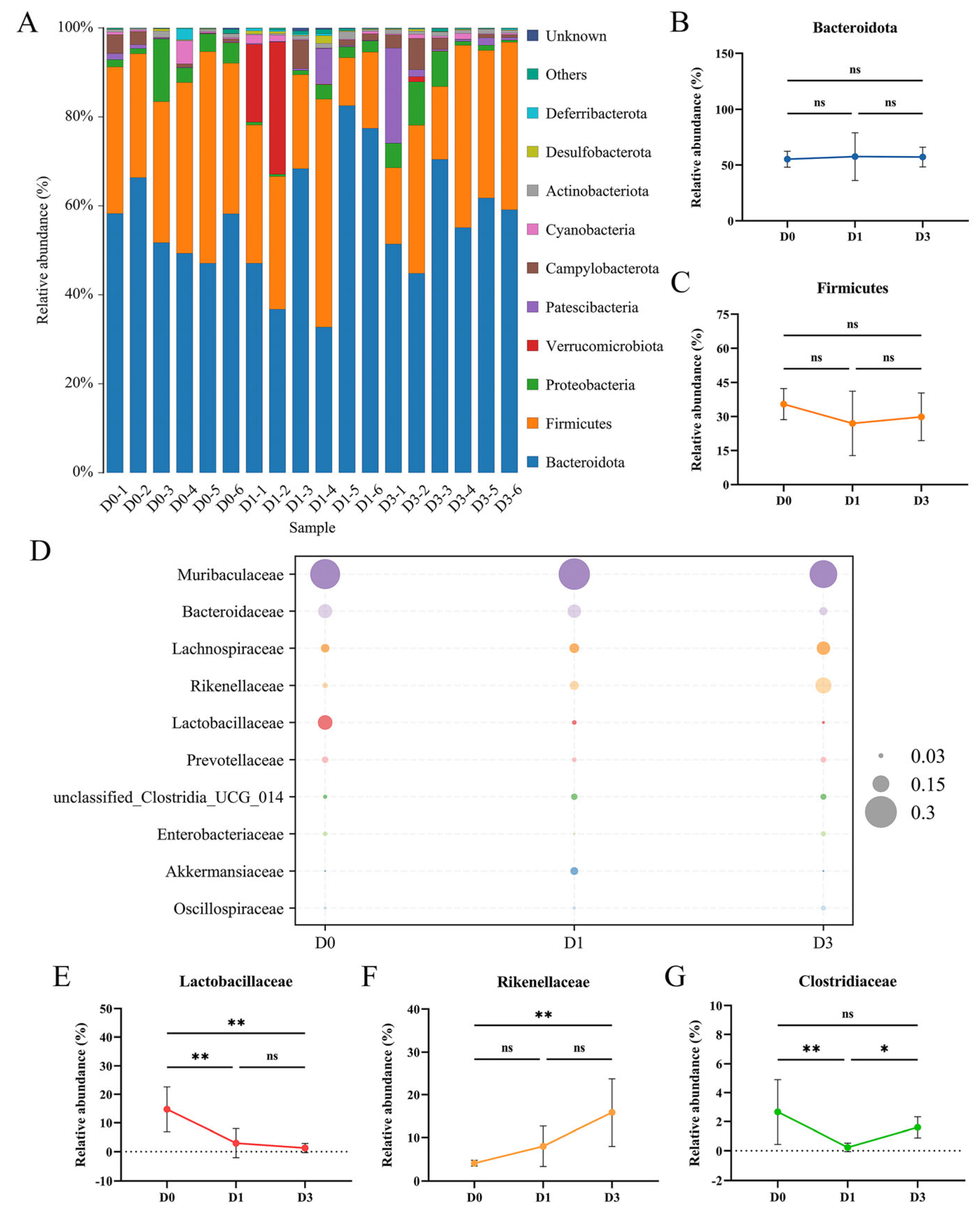
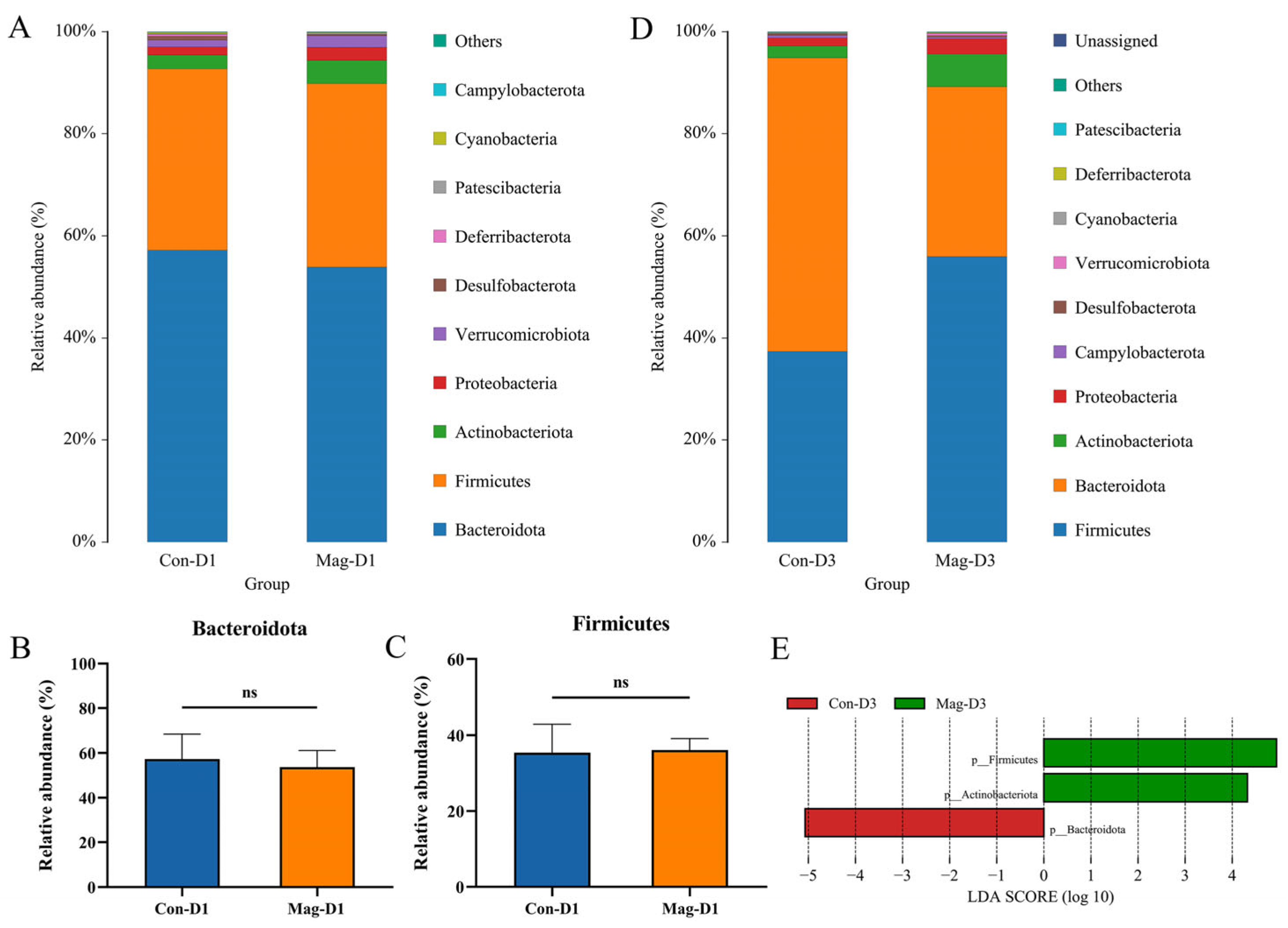
3.3. BEV Infection Affects the Composition of the Gut Microbiota at the Phylum and Family Levels
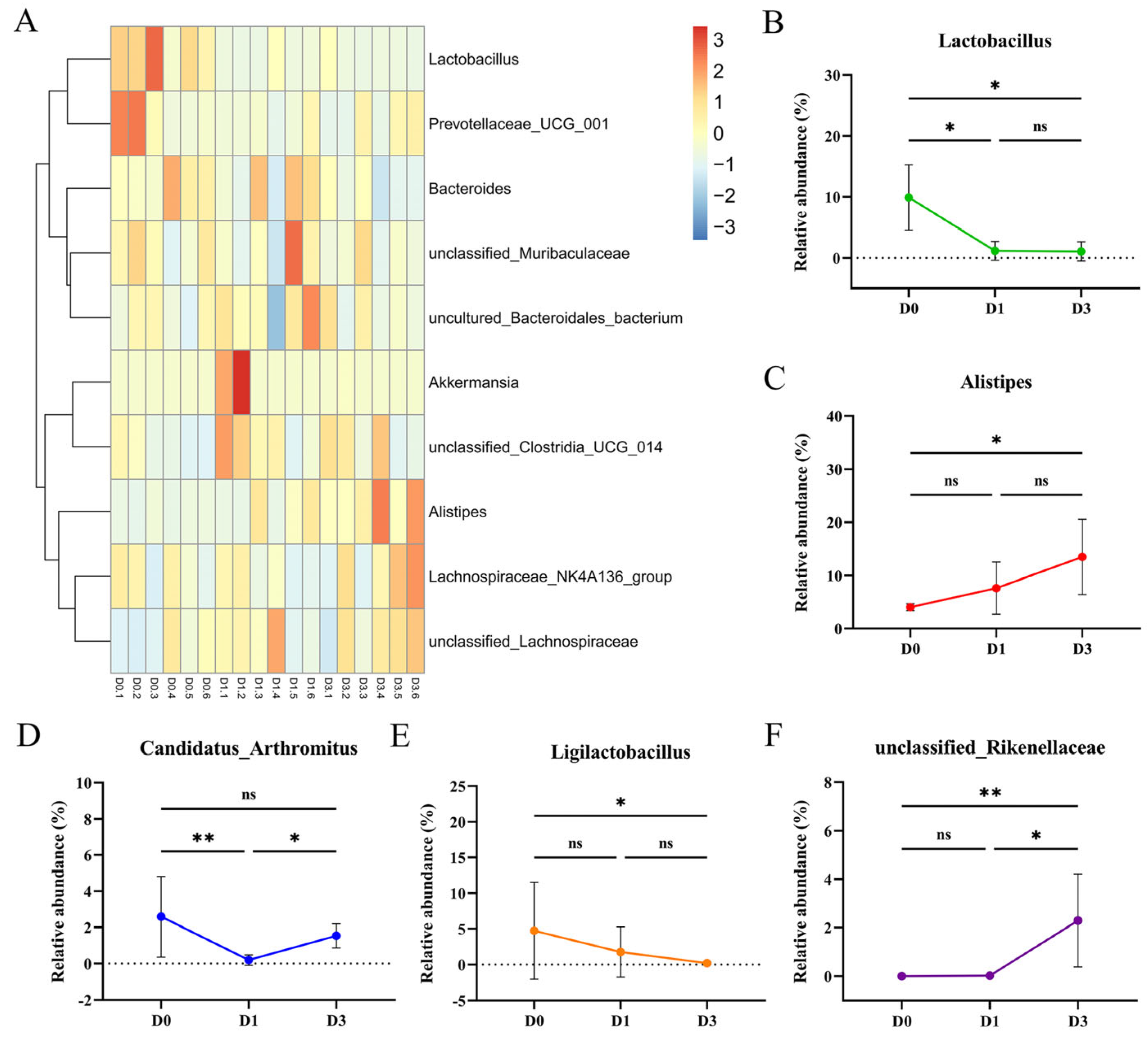
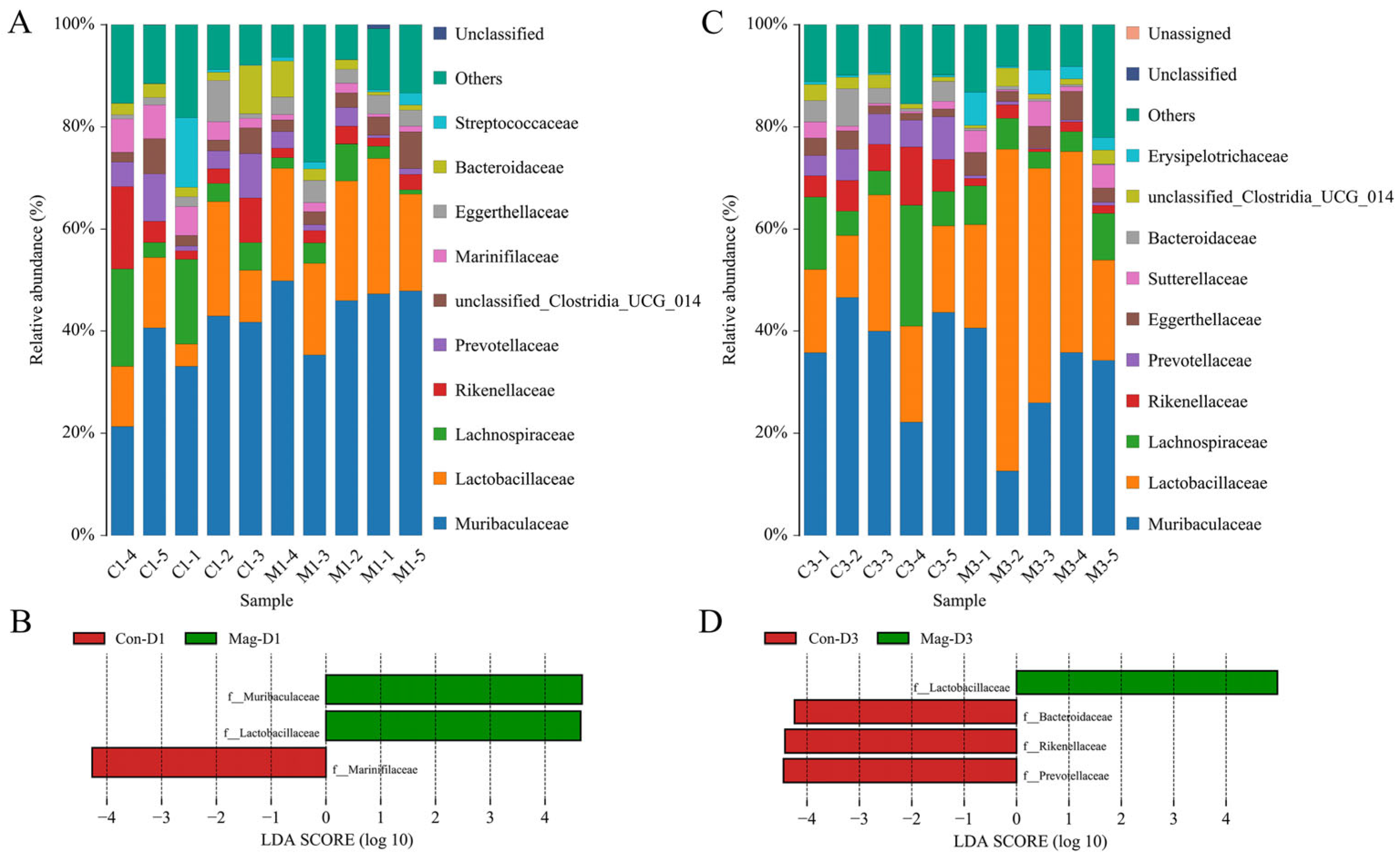
3.4. BEV Infection Changes the Composition of the Gut Microbiota at the Genus Level
3.5. Magnolol Treatment Alters the Diversity and Richness of the Microbiota
3.6. Magnolol Treatment Affects the Microbiota Composition at the Phylum and Family Levels
3.7. Microbiota Composition at the Genus Level Following Magnolol Treatment
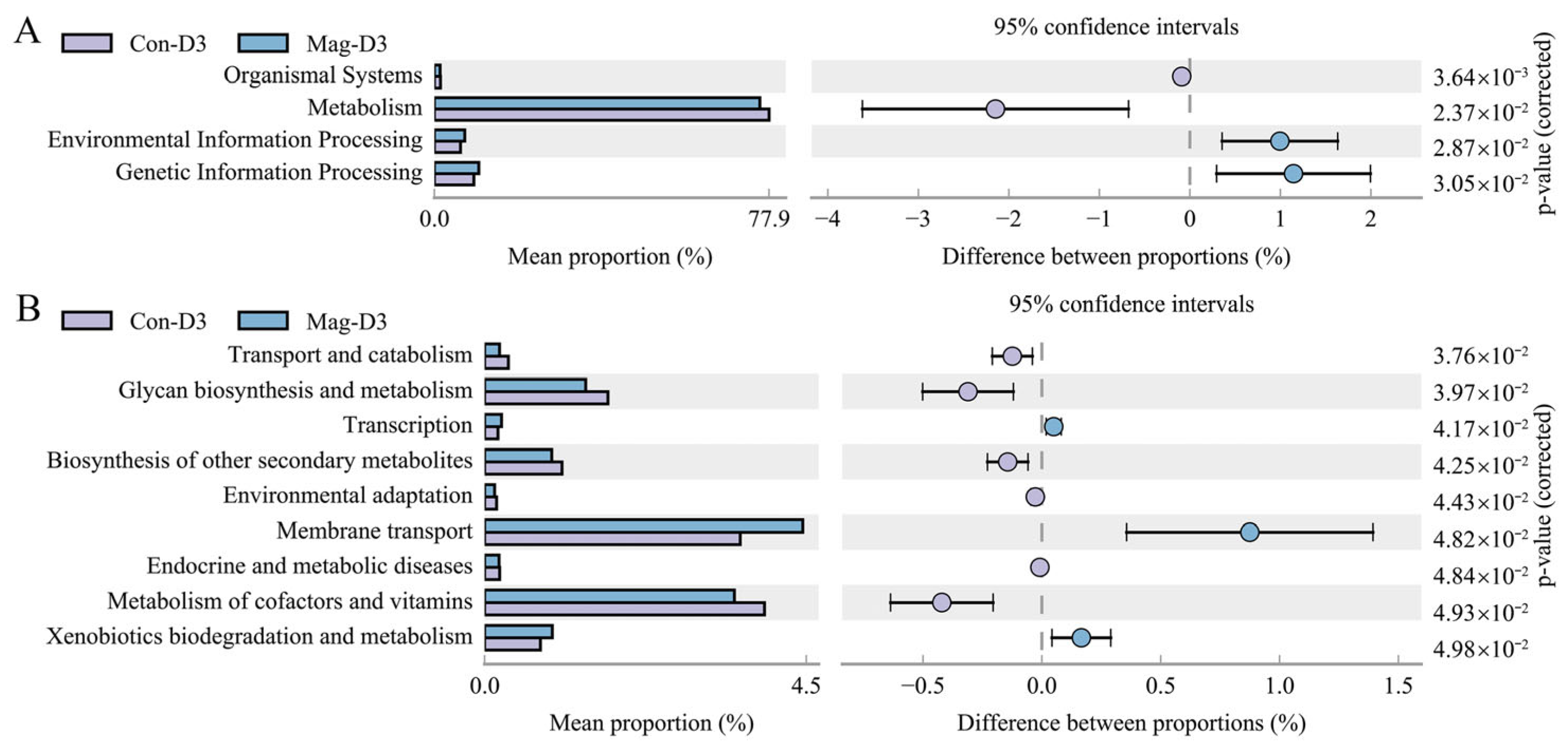
3.8. Functional Alterations in the Microbiota Induced by Magnolol Treatment
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, J.; Meng, W.; Zeng, H.; Wang, J.; Liu, S.; Jiang, Q.; Chen, Z.; Ma, Z.; Wang, Z.; Li, S.; et al. Epidemiological survey of calf diarrhea related viruses in several areas of Guangdong Province. Front. Microbiol. 2024, 15, 1441419. [Google Scholar] [CrossRef] [PubMed]
- Simmonds, P.; Adriaenssens, E.M.; Lefkowitz, E.J.; Oksanen, H.M.; Siddell, S.G.; Zerbini, F.M.; Alfenas-Zerbini, P.; Aylward, F.O.; Dempsey, D.M.; Dutilh, B.E.; et al. Changes to virus taxonomy and the ICTV Statutes ratified by the International Committee on Taxonomy of Viruses (2024). Arch. Virol. 2024, 169, 236. [Google Scholar] [CrossRef]
- Moll, T.; Davis, A.D. Isolation and characterization of cytopathogenic enteroviruses from cattle with respiratory disease. Am. J. Vet. Res. 1959, 20, 27–32. [Google Scholar]
- Blas-Machado, U.; Saliki, J.T.; Boileau, M.J.; Goens, S.D.; Caseltine, S.L.; Duffy, J.C.; Welsh, R.D. Fatal ulcerative and hemorrhagic typhlocolitis in a pregnant heifer associated with natural bovine enterovirus type-1 infection. Vet. Pathol. 2007, 44, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Shaukat, S.; Angez, M.; Alam, M.M.; Sharif, S.; Khurshid, A.; Malik, F.; Rana, M.S.; Mahmood, T.; Zaidi, S.S. Molecular identification and characterization of a new type of bovine enterovirus. Appl. Environ. Microbiol. 2012, 78, 4497–4500. [Google Scholar] [CrossRef]
- Zhu, L.; Xing, Z.; Gai, X.; Li, S.; San, Z.; Wang, X. Identification of a novel enterovirus E isolates HY12 from cattle with severe respiratory and enteric diseases. PLoS ONE 2014, 9, e97730. [Google Scholar] [CrossRef]
- Sobhy, N.M.; Mor, S.K.; Mohammed, M.E.; Bastawecy, I.M.; Fakhry, H.M.; Youssef, C.R.; Abouzeid, N.Z.; Goyal, S.M. Isolation and molecular characterization of bovine enteroviruses in Egypt. Vet. J. 2015, 206, 317–321. [Google Scholar] [CrossRef] [PubMed]
- Kosoltanapiwat, N.; Yindee, M.; Chavez, I.F.; Leaungwutiwong, P.; Adisakwattana, P.; Singhasivanon, P.; Thawornkuno, C.; Thippornchai, N.; Rungruengkitkun, A.; Soontorn, J.; et al. Genetic variations in regions of bovine and bovine-like enteroviral 5′UTR from cattle, Indian bison and goat feces. Virol. J. 2016, 13, 13. [Google Scholar] [CrossRef]
- He, H.; Tang, C.; Chen, X.; Yue, H.; Ren, Y.; Liu, Y.; Zhang, B. Isolation and characterization of a new enterovirus F in yak feces in the Qinghai-Tibetan Plateau. Arch. Virol. 2017, 162, 523–527. [Google Scholar] [CrossRef]
- Karim, A. Unveiling the Potential of Probiotics in Osteoarthritis Management. Curr. Rheumatol. Rep. 2024, 27, 2. [Google Scholar] [CrossRef]
- Qin, M.; Huang, Z.; Huang, Y.; Huang, X.; Chen, C.; Wu, Y.; Wang, Z.; He, F.; Tang, B.; Long, C.; et al. Association analysis of gut microbiota with LDL-C metabolism and microbial pathogenicity in colorectal cancer patients. Lipids Health Dis. 2024, 23, 367. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Ding, X.; Tian, Y.; Chen, J.; Li, W. Effect of anthocyanins on metabolic syndrome through interacting with gut microbiota. Pharmacol. Res. 2024, 210, 107511. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, H.; Sang, Y.; Liu, M.; Wang, Q.; Yang, H.; Li, X. Gut microbiota in health and disease: Advances and future prospects. MedComm 2024, 5, e70012. [Google Scholar] [CrossRef]
- Zhang, Z.; Huang, J.; Li, C.; Zhao, Z.; Cui, Y.; Yuan, X.; Wang, X.; Liu, Y.; Zhou, Y.; Zhu, Z. The gut microbiota contributes to the infection of bovine viral diarrhea virus in mice. J. Virol. 2024, 98, e0203523. [Google Scholar] [CrossRef]
- Cao, Z.; Ling, X.; Sun, P.; Zheng, X.; Zhang, H.; Zhong, J.; Yin, W.; Fan, K.; Sun, Y.; Li, H.; et al. Matrine Targets Intestinal Lactobacillus acidophilus to Inhibit Porcine Circovirus Type 2 Infection in Mice. Int. J. Mol. Sci. 2023, 24, 11878. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, C.; Huang, J.; Yuan, X.; Cui, Y.; Liu, Y.; Zhou, Y.; Zhu, Z.; Zhang, Z. Phlorizin Limits Bovine Viral Diarrhea Virus Infection in Mice via Regulating Gut Microbiota Composition. J. Agric. Food Chem. 2024, 72, 9906–9914. [Google Scholar] [CrossRef] [PubMed]
- Dou, B.; Wu, X.; He, Y.; Xu, G.; Zhang, H.; Huang, Q.; Chen, X.; Duan, N.; Zhou, L.; Zhang, W.; et al. Fei-Yan-Qing-Hua decoction attenuates influenza virus infection by enhancing host antiviral response through microbiota-derived acetate. Front. Pharmacol. 2024, 15, 1446749. [Google Scholar] [CrossRef]
- Pan, W.; Wu, R.; Zhang, Q.; Ma, Y.; Xiang, J.; Wang, J.; Chen, J. Ruhao Dashi granules exert therapeutic effects on H1N1 influenza virus infection by altering intestinal microflora composition. Front. Microbiol. 2024, 15, 1482785. [Google Scholar] [CrossRef]
- Jiang, J.; Hou, X.; Xu, K.; Ji, K.; Ji, Z.; Xi, J.; Wang, X. Bacteria-targeted magnolol-loaded multifunctional nanocomplexes for antibacterial and anti-inflammatory treatment. Biomed. Mater. 2024, 19, 025029. [Google Scholar] [CrossRef]
- Tseng, C.F.; Chen, H.M.; Liao, T.L.; Hsu, F.T.; Yeh, C.J.; Chen, W.T.; Kok, S.H. Magnolol’s Therapeutic Efficacy and Immunomodulatory Effects in Oral Squamous Cell Carcinoma. In Vivo 2024, 38, 2152–2164. [Google Scholar] [CrossRef]
- Peng, W.S.; Gao, M.; Yao, X.F.; Tong, Y.Y.; Zhang, H.H.; He, X. Magnolol supplementation alleviates diquat-induced oxidative stress via PI3K-Akt in broiler chickens. Anim. Sci. J. 2023, 94, e13891. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Guo, X.; Lin, B.; Huang, R.; Li, H.; Wang, Q.; Zeng, Y.; Shang, Y.; Wu, Y. Magnolol against enterovirus 71 by targeting Nrf2-SLC7A11-GSH pathway. Biomed. Pharmacother. 2024, 176, 116866. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, B.; Yu, R.; Si, F.; Xie, C.; Li, Z.; Dong, S.; Zhang, D. Magnolol, a Neolignan-like Drug, Inhibits Porcine Epidemic Diarrhea Virus Replication in Cultured Cells. Pathogens 2023, 12, 263. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Lim, C.Y.; Chung, M.S. Magnolia officinalis and Its Honokiol and Magnolol Constituents Inhibit Human Norovirus Surrogates. Foodborne Pathog. Dis. 2021, 18, 24–30. [Google Scholar] [CrossRef]
- Chen, X.; Hao, K.; Yu, X.; Huang, A.; Zhu, B.; Wang, G.X.; Ling, F. Magnolol protects Ctenopharyngodon idella kidney cells from apoptosis induced by grass carp reovirus. Fish Shellfish Immunol. 2018, 74, 426–435. [Google Scholar] [CrossRef]
- Li, J.; Huang, Y.; Guan, X.L.; Li, J.; Deng, S.P.; Wu, Q.; Zhang, Y.J.; Su, X.J.; Yang, R.Y. Anti-hepatitis B virus constituents from the stem bark of Streblus asper. Phytochemistry 2012, 82, 100–109. [Google Scholar] [CrossRef]
- Li, X. Effect of IRF3/7-Mediated Innate Immunity on HY12 Enterovirus Replication. Master’s Thesis, Jilin University, Changchun, China, 2019. (In Chinese). [Google Scholar]
- Wang, G. Isolation and Identification of Bovine Enterovirus and Development of Bivalent Inactivated Vaccine. Master’s Thesis, Inner Mongolia Agricultural University, Hohhot, China, 2021. (In Chinese). [Google Scholar]
- Tao, W.; Hu, Y.; Chen, Z.; Dai, Y.; Hu, Y.; Qi, M. Magnolol attenuates depressive-like behaviors by polarizing microglia towards the M2 phenotype through the regulation of Nrf2/HO-1/NLRP3 signaling pathway. Phytomedicine 2021, 91, 153692. [Google Scholar] [CrossRef]
- Bai, Y.; Song, L.; Dai, G.; Xu, M.; Zhu, L.; Zhang, W.; Jing, W.; Ju, W. Antidepressant effects of magnolol in a mouse model of depression induced by chronic corticosterone injection. Steroids 2018, 135, 73–78. [Google Scholar] [CrossRef]
- Matsui, N.; Akae, H.; Hirashima, N.; Kido, Y.; Tanabe, S.; Koseki, M.; Fukuyama, Y.; Akagi, M. Magnolol Enhances Hippocampal Neurogenesis and Exerts Antidepressant-like Effects in Olfactory Bulbectomized Mice. Phytother. Res. 2016, 30, 1856–1861. [Google Scholar] [CrossRef]
- Yang, J.; Wei, Y.; Zhao, T.; Li, X.; Zhao, X.; Ouyang, X.; Zhou, L.; Zhan, X.; Qian, M.; Wang, J.; et al. Magnolol effectively ameliorates diabetic peripheral neuropathy in mice. Phytomedicine 2022, 107, 154434. [Google Scholar] [CrossRef]
- Xu, C.; Ye, J.; Sun, Y.; Sun, X.; Liu, J.G. The Antidepressant Effect of Magnolol on Depression-like Behavior of CORT-Treated Mice. J. Mol. Neurosci. 2024, 74, 3. [Google Scholar] [CrossRef] [PubMed]
- Xia, T.; Zhang, J.; Han, L.; Jin, Z.; Wang, J.; Li, X.; Man, S.; Liu, C.; Gao, W. Protective effect of magnolol on oxaliplatin-induced intestinal injury in mice. Phytother. Res. 2019, 33, 1161–1172. [Google Scholar] [CrossRef]
- Chang, X.; Guo, Y.; Zhang, Q.; Zheng, X.; Cui, X.; Hu, J.; Zhang, Z.; Zhang, F.; Wang, X. GRP78 recognizes EV-F 3D protein and activates NF-kappaB to repress virus replication by interacting with CHUK/IKBKB. J. Virol. 2024, 98, e0026824. [Google Scholar] [CrossRef]
- Fluhr, L.; Mor, U.; Kolodziejczyk, A.A.; Dori-Bachash, M.; Leshem, A.; Itav, S.; Cohen, Y.; Suez, J.; Zmora, N.; Moresi, C.; et al. Gut microbiota modulates weight gain in mice after discontinued smoke exposure. Nature 2021, 600, 713–719. [Google Scholar] [CrossRef]
- Huang, A.; Cai, R.; Wang, Q.; Shi, L.; Li, C.; Yan, H. Dynamic Change of Gut Microbiota During Porcine Epidemic Diarrhea Virus Infection in Suckling Piglets. Front. Microbiol. 2019, 10, 322. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Fan, X.; Mao, X.; Yu, B.; He, J.; Yan, H.; Wang, J. The protective role of prebiotics and probiotics on diarrhea and gut damage in the rotavirus-infected piglets. J. Anim. Sci. Biotechnol. 2024, 15, 61. [Google Scholar] [CrossRef]
- Lee, H.; Ko, G. New perspectives regarding the antiviral effect of vitamin A on norovirus using modulation of gut microbiota. Gut Microbes 2017, 8, 616–620. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Yu, M.L.; Tao, X.; Cheng, M.H.; Liu, C.C.; Liu, Y.; Li, Y.G. Analysis of the intestinal microbial community altered during rotavirus infection in suckling mice. Virol. J. 2021, 18, 254. [Google Scholar] [CrossRef]
- Parker, B.J.; Wearsch, P.A.; Veloo, A.C.M.; Rodriguez-Palacios, A. The Genus Alistipes: Gut Bacteria With Emerging Implications to Inflammation, Cancer, and Mental Health. Front. Immunol. 2020, 11, 906. [Google Scholar] [CrossRef]
- Rodriguez-Palacios, A.; Harding, A.; Menghini, P.; Himmelman, C.; Retuerto, M.; Nickerson, K.P.; Lam, M.; Croniger, C.M.; McLean, M.H.; Durum, S.K.; et al. The Artificial Sweetener Splenda Promotes Gut Proteobacteria, Dysbiosis, and Myeloperoxidase Reactivity in Crohn’s Disease-like Ileitis. Inflamm. Bowel Dis. 2018, 24, 1005–1020. [Google Scholar] [CrossRef]
- Lv, Y.; Ge, C.; Wu, L.; Hu, Z.; Luo, X.; Huang, W.; Zhan, S.; Shen, X.; Yu, D.; Liu, B. Hepatoprotective effects of magnolol in fatty liver hemorrhagic syndrome hens through shaping gut microbiota and tryptophan metabolic profile. J. Anim. Sci. Biotechnol. 2024, 15, 120. [Google Scholar] [CrossRef] [PubMed]
- Mo, J.; Xiang, J.; Li, J.; Yang, M.; Zhang, Z.; Zhang, L.; Zhang, G.; Yang, Y.; Liu, G.; Lu, Y.; et al. Natural Magnolol ameliorates coccidiosis infected with Eimeria tenella by affecting antioxidant, anti-inflammatory, and gut microbiota of chicks. Poult. Sci. 2023, 102, 102975. [Google Scholar] [CrossRef] [PubMed]
- Toomer, O.T.; Redhead, A.K.; Vu, T.C.; Santos, F.; Malheiros, R.; Proszkowiec-Weglarz, M. The effect of peanut skins as a natural antimicrobial feed additive on ileal and cecal microbiota in broiler chickens inoculated with Salmonella enterica Enteritidis. Poult. Sci. 2024, 103, 104159. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Zhang, H.; Du, E.; Fan, Q.; Zhao, N.; Jin, F.; Zhang, W.; Guo, W.; Huang, S.; Wei, J. Supplemental magnolol or honokiol attenuates adverse effects in broilers infected with Salmonella pullorum by modulating mucosal gene expression and the gut microbiota. J. Anim. Sci. Biotechnol. 2021, 12, 87. [Google Scholar] [CrossRef]
- Li, N.; Ma, W.T.; Pang, M.; Fan, Q.L.; Hua, J.L. The Commensal Microbiota and Viral Infection: A Comprehensive Review. Front. Immunol. 2019, 10, 1551. [Google Scholar] [CrossRef]
- Zhang, Y.; Si, L.; Shu, X.; Qiu, C.; Wan, X.; Li, H.; Ma, S.; Jin, X.; Wei, Z.; Hu, H. Gut microbiota contributes to protection against porcine deltacoronavirus infection in piglets by modulating intestinal barrier and microbiome. Microbiome 2025, 13, 93. [Google Scholar] [CrossRef]
- Zang, R.; Zhou, R.; Li, Y.; Wu, H.; Lu, L.; Xu, H. The probiotic Lactobacillus plantarum alleviates colitis by modulating gut microflora to activate PPARgamma and inhibit MAPKs/NF-kappaB. Eur. J. Nutr. 2024, 64, 32. [Google Scholar] [CrossRef]
- Liu, W.; Cheng, H.; Zhang, H.; Liu, G.; Yin, X.; Zhang, C.; Jiang, R.; Wang, Z.; Ding, X. Effect of Lactobacillus paracasei LK01 on Growth Performance, Antioxidant Capacity, Immunity, Intestinal Health, and Serum Biochemical Indices in Broilers. Animals 2024, 14, 3474. [Google Scholar] [CrossRef]
- Ang, L.Y.; Too, H.K.; Tan, E.L.; Chow, T.K.; Shek, L.P.; Tham, E.H.; Alonso, S. Antiviral activity of Lactobacillus reuteri Protectis against Coxsackievirus A and Enterovirus 71 infection in human skeletal muscle and colon cell lines. Virol. J. 2016, 13, 111. [Google Scholar] [CrossRef]
- Mao, X.; Gu, C.; Hu, H.; Tang, J.; Chen, D.; Yu, B.; He, J.; Yu, J.; Luo, J.; Tian, G. Dietary Lactobacillus rhamnosus GG Supplementation Improves the Mucosal Barrier Function in the Intestine of Weaned Piglets Challenged by Porcine Rotavirus. PLoS ONE 2016, 11, e0146312. [Google Scholar] [CrossRef]
- Wang, J.; Huang, M.; Du, Y.; Chen, H.; Li, Z.; Zhai, T.; Ou, Z.; Huang, Y.; Bu, F.; Zhen, H.; et al. Lactobacillus rhamnosus GG Regulates Host IFN-I Through the RIG-I Signalling Pathway to Inhibit Herpes Simplex Virus Type 2 Infection. Probiotics Antimicrob. Proteins 2024, 16, 1966–1978. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Zhang, Q.; Wu, M.; Zhang, Y.; Li, Z.; Li, H.; Yu, C.; Zhang, X.; Zhao, D.; Wang, L.; et al. Lactobacillus rhamnosus GG powder supplementation alleviates intestinal injury in piglets challenged by porcine epidemic diarrhea virus. Front. Cell. Infect. Microbiol. 2024, 14, 1371916. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.J.; Xing, J.H.; Yan, Q.S.; Zou, B.S.; Wang, Y.J.; Niu, T.M.; Yu, T.; Huang, H.B.; Zhang, D.; Zhang, S.M.; et al. The Acetic Acid Produced by Lactobacillus Species Regulates Immune Function to Alleviate PEDV Infection in Piglets. Probiotics Antimicrob. Proteins 2024, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Nelson, C.A.; Wilen, C.B.; Dai, Y.N.; Orchard, R.C.; Kim, A.S.; Stegeman, R.A.; Hsieh, L.L.; Smith, T.J.; Virgin, H.W.; Fremont, D.H. Structural basis for murine norovirus engagement of bile acids and the CD300lf receptor. Proc. Natl. Acad. Sci. USA 2018, 115, E9201–E9210. [Google Scholar] [CrossRef]
- Li, W.; Chen, H.; Tang, J. Interplay between Bile Acids and Intestinal Microbiota: Regulatory Mechanisms and Therapeutic Potential for Infections. Pathogens 2024, 13, 702. [Google Scholar] [CrossRef]
- Lin, Q.; Zhao, J.; Xie, K.; Wang, Y.; Hu, G.; Jiang, G.; Dai, Q.; Fan, Z.; He, J.; He, X.; et al. Magnolol additive as a replacer of antibiotic enhances the growth performance of Linwu ducks. Anim. Nutr. 2017, 3, 132–138. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Q.; Shi, X.; Guo, Z.; He, X.; Zhang, T.; Zhao, X. Effects of magnolol solid dispersion on growth performance, serum antioxidant capacity and intestinal microbiome of calves. Acta Vet. et Zootech. Sin. 2025, 56, 943–952. (In Chinese) [Google Scholar] [CrossRef]
- Murtaza, N.; Nawaz, M.; Yaqub, T.; Mehmood, A.K. Impact of Limosilactobacillus fermentum probiotic treatment on gut microbiota composition in sahiwal calves with rotavirus diarrhea: A 16S metagenomic analysis study. BMC Microbiol. 2024, 24, 114. [Google Scholar] [CrossRef]
- Gandhar, J.S.; De, U.K.; Kala, A.; Malik, Y.S.; Yadav, S.; Paul, B.R.; Dixit, S.K.; Sircar, S.; Chaudhary, P.; Patra, M.K.; et al. Efficacy of Microencapsulated Probiotic as Adjunct Therapy on Resolution of Diarrhea, Copper-Zinc Homeostasis, Immunoglobulins, and Inflammatory Markers in Serum of Spontaneous Rotavirus-Infected Diarrhoetic Calves. Probiotics Antimicrob. Proteins 2022, 14, 1054–1066. [Google Scholar] [CrossRef]
- Kim, H.S.; Whon, T.W.; Sung, H.; Jeong, Y.S.; Jung, E.S.; Shin, N.R.; Hyun, D.W.; Kim, P.S.; Lee, J.Y.; Lee, C.H.; et al. Longitudinal evaluation of fecal microbiota transplantation for ameliorating calf diarrhea and improving growth performance. Nat. Commun. 2021, 12, 161. [Google Scholar] [CrossRef]

| Gene Name | Forward Primer Sequence (5′-3′) | Reverse Primer Sequence (5′-3′) |
|---|---|---|
| HY12-VP1 | CCACTGATGCAACACCCGCTCTA | CGCTTGTTTCATGTATGCCGTGTG |
| m-GAPDH | AGGTCGGTGTGAACGGATTTG | GGGGTCGTTGATGGCAACA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, J.; Zhang, Q.; Liu, D.; Cui, X.; Wang, Q.; Gong, W.; Wang, X. Inhibition of Bovine Enterovirus Infection by Magnolol via Modulating the Gut Microbiota in Mice. Viruses 2025, 17, 750. https://doi.org/10.3390/v17060750
Hu J, Zhang Q, Liu D, Cui X, Wang Q, Gong W, Wang X. Inhibition of Bovine Enterovirus Infection by Magnolol via Modulating the Gut Microbiota in Mice. Viruses. 2025; 17(6):750. https://doi.org/10.3390/v17060750
Chicago/Turabian StyleHu, Junying, Qun Zhang, Dan Liu, Xuyuan Cui, Qianying Wang, Wenjie Gong, and Xinping Wang. 2025. "Inhibition of Bovine Enterovirus Infection by Magnolol via Modulating the Gut Microbiota in Mice" Viruses 17, no. 6: 750. https://doi.org/10.3390/v17060750
APA StyleHu, J., Zhang, Q., Liu, D., Cui, X., Wang, Q., Gong, W., & Wang, X. (2025). Inhibition of Bovine Enterovirus Infection by Magnolol via Modulating the Gut Microbiota in Mice. Viruses, 17(6), 750. https://doi.org/10.3390/v17060750





