Establishment and Implementation of the Point-of-Care RT-RAA-CRISPR/Cas13a Diagnostic Test for Foot-And-Mouth Disease Virus Serotype O in Pigs
Abstract
1. Introduction
2. Materials and Methods
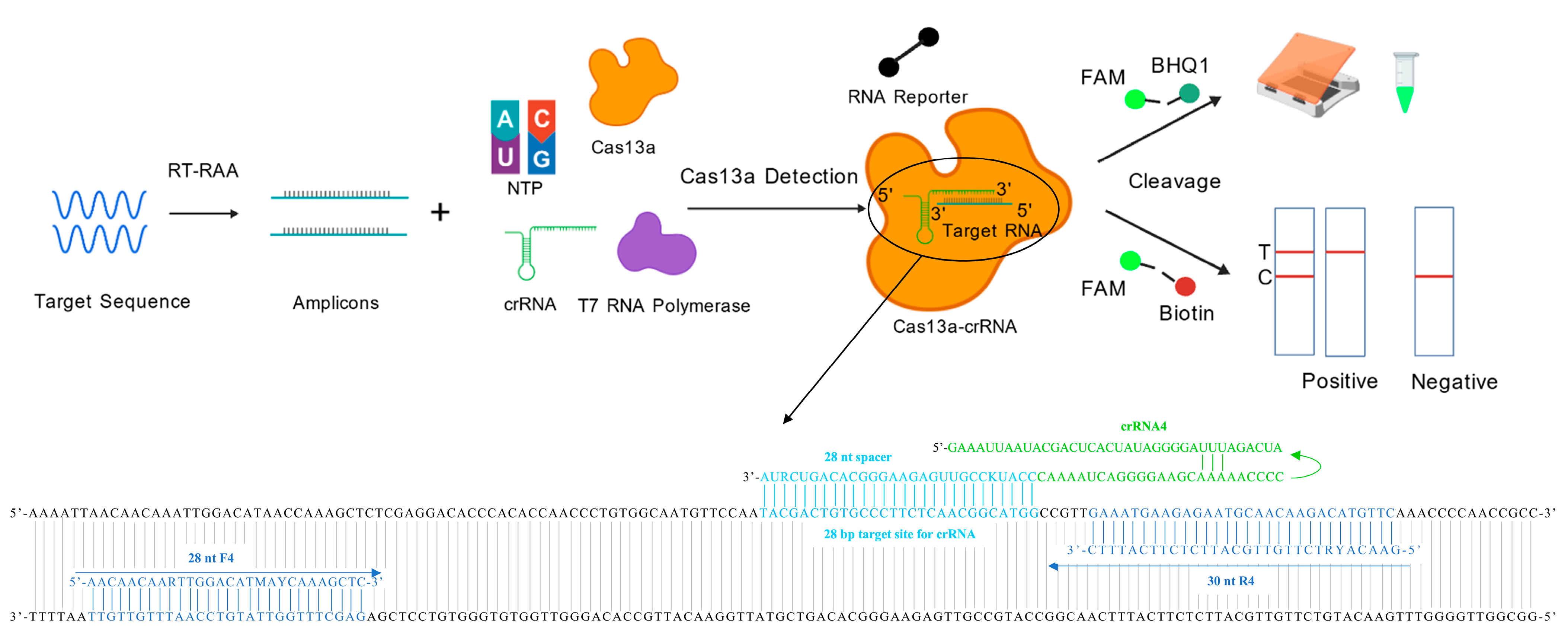
2.1. Design of RT-RAA Primers, CRISPR crRNAs, Digital Droplet PCR Primers, Probes, and RNA Reporters
2.2. Nucleic Acids and Samples
2.3. Screening of RT-RAA Primers and the Optimal Temperature for RT-RAA
2.4. Preparation of crRNAs
2.5. Screening of the crRNAs and the RT-RAA-CRISPR/Cas13a Reaction System
2.6. Lateral Flow Assay
2.7. RT-RAA-CRISPR/Cas13a Detection Method Specificity Test
2.8. Calibration of the Copy Number of FMDV Serotype O RNA by Digital PCR
2.9. Sensitivity of the RT-RAA-CRISPR/Cas13a Detection Method
2.10. RT-RAA-CRISPR/Cas13a Detection Method Repeatability Test
2.11. Simulated Clinical Samples Testing
2.12. Data Analysis
3. Results
3.1. Screening of RT-RAA Primers
3.2. Optimal Reaction Temperature Selection for RT-RAA
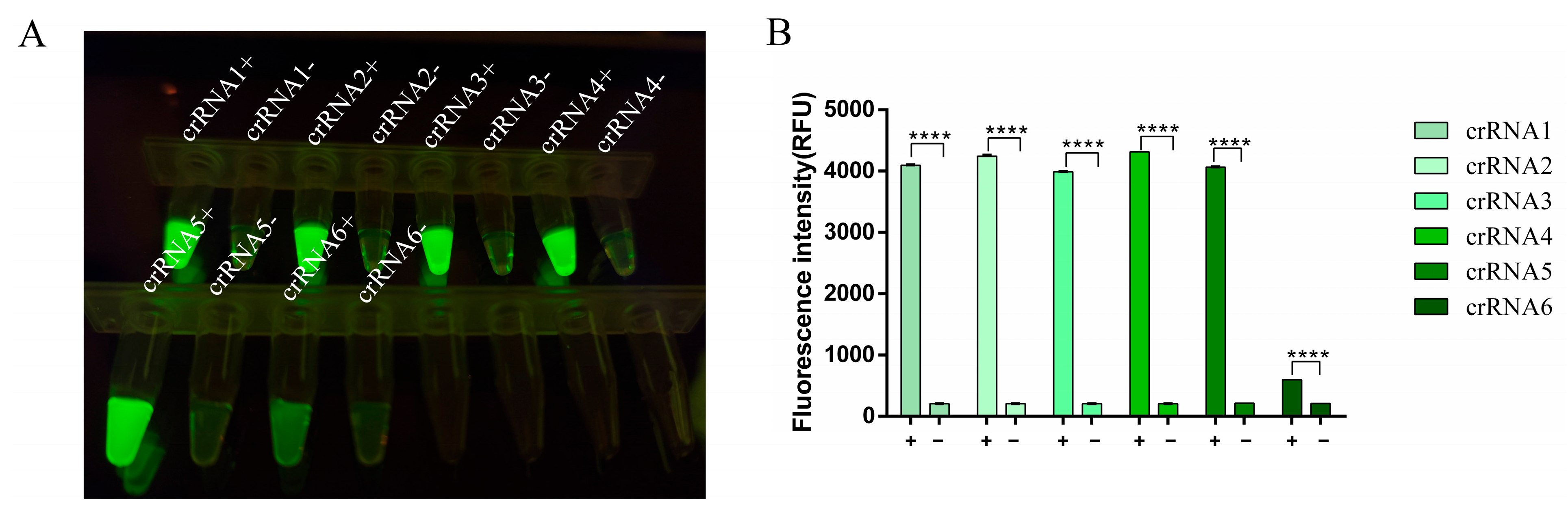
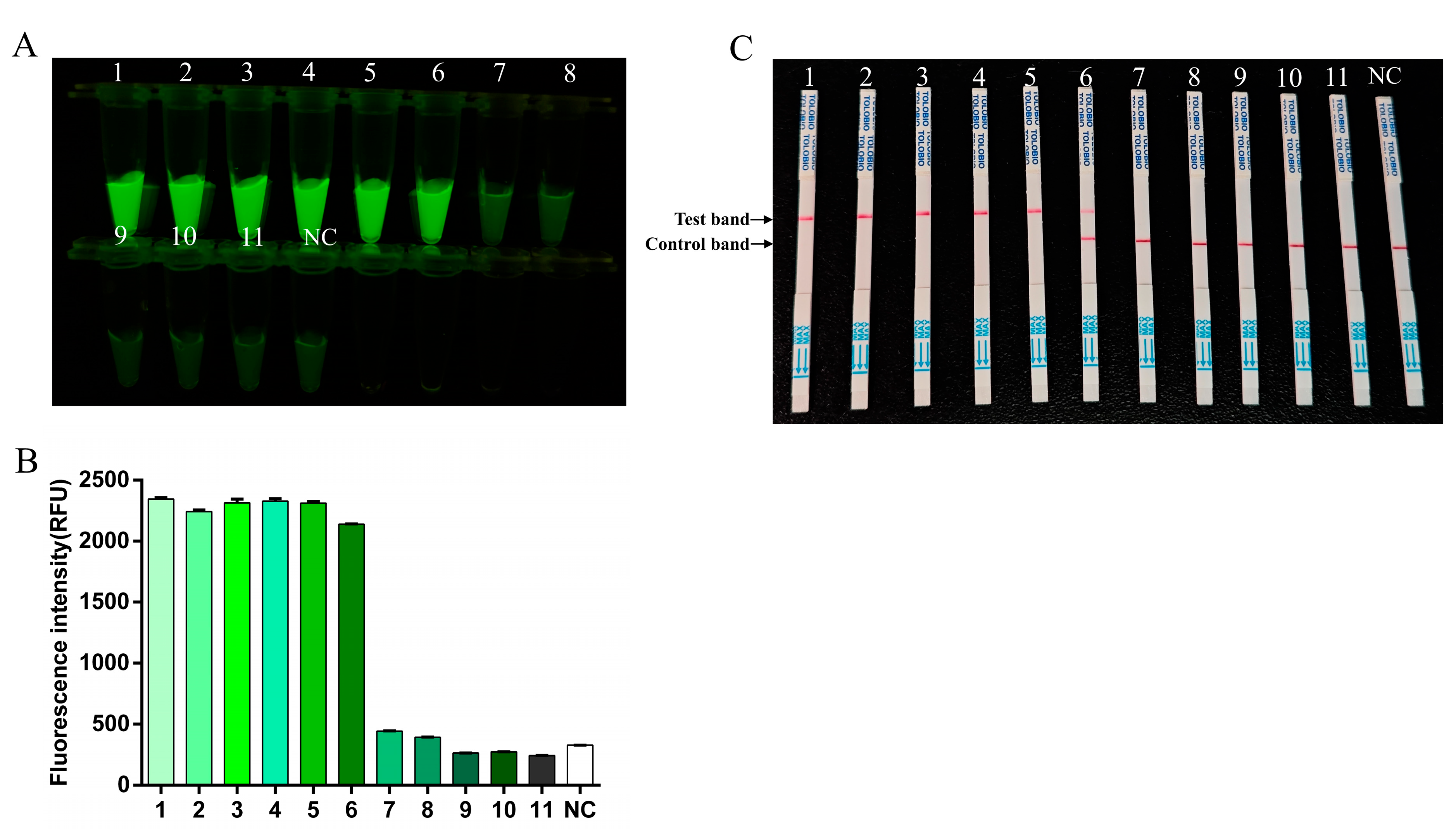
3.3. crRNA Selection for RT-RAA-CRISPR/Cas13a
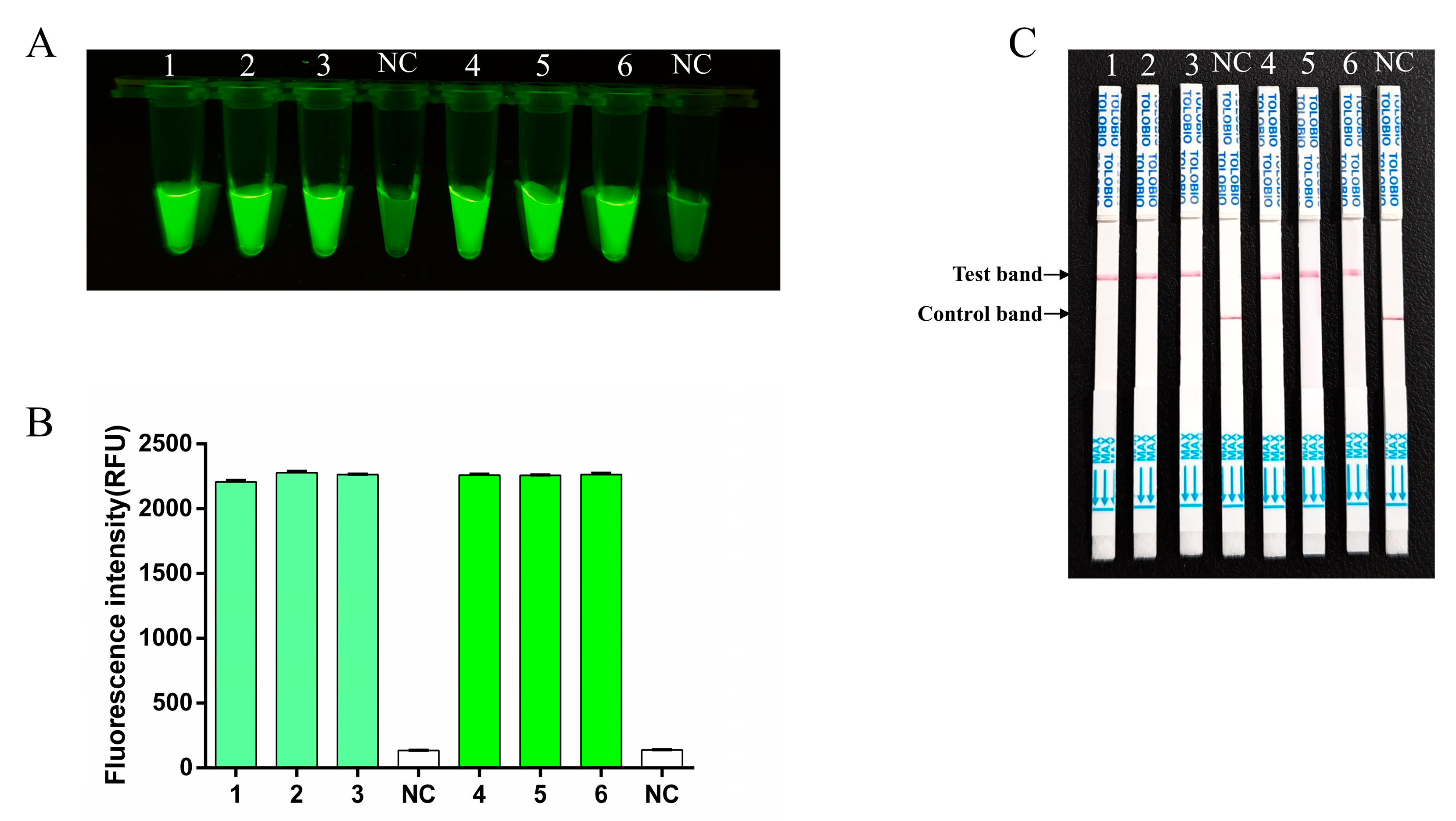
3.4. Specificity Testing for RT-RAA-CRISPR/Cas13a Assay
3.5. Sensitivity Testing for RT-RAA-CRISPR/Cas13a Assay
3.6. Repeatability Testing for RT-RAA-CRISPR/Cas13a Assay
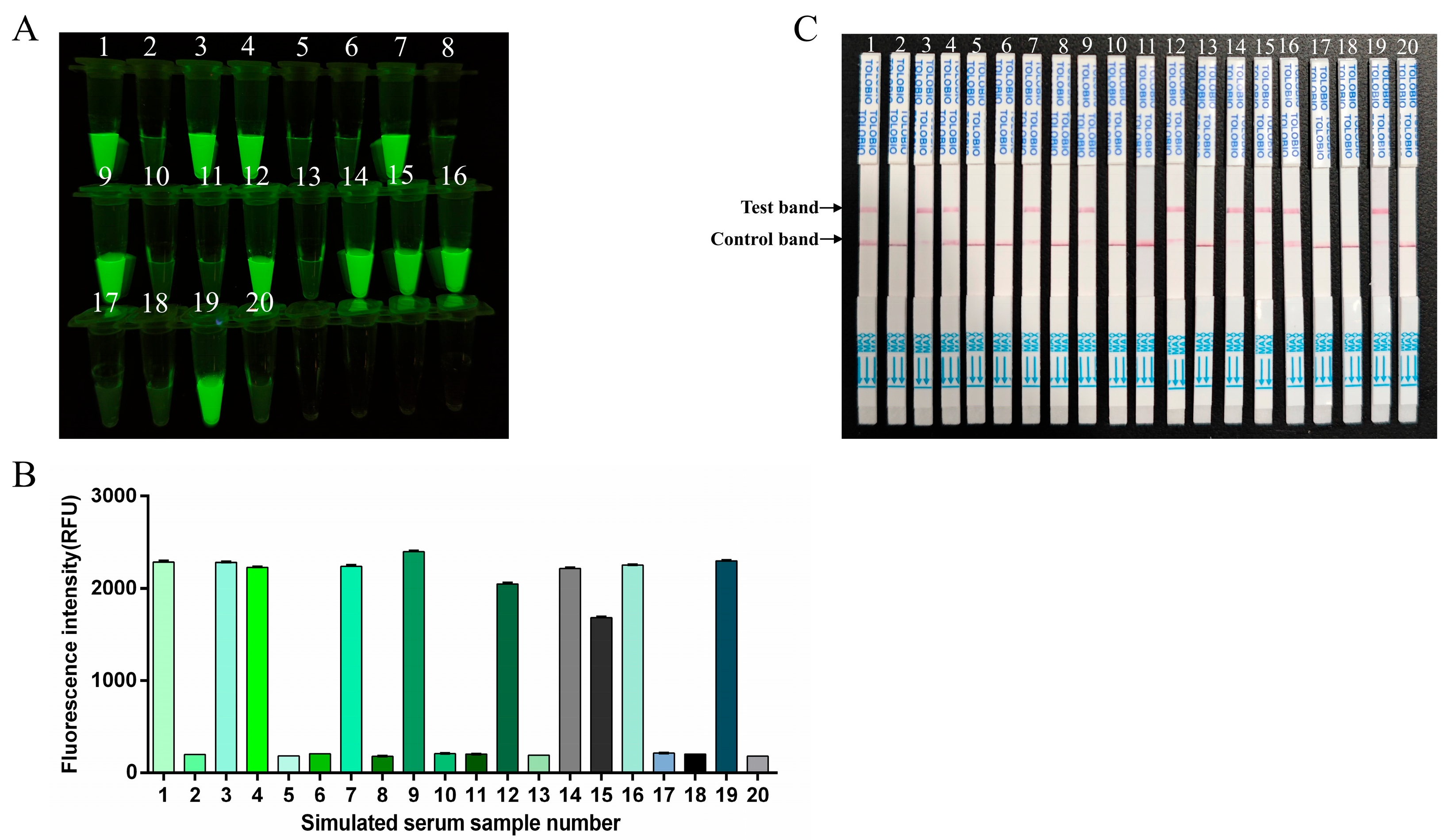
3.7. Simulated Clinical Sample Detection for RT-RAA-CRISPR/Cas13a Assay
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jamal, S.M.; Belsham, G.J. Foot-and-mouth disease: Past, present and future. Vet. Res. 2013, 44, 116. [Google Scholar] [CrossRef] [PubMed]
- Grubman, M.J.; Baxt, B. Foot-and-mouth disease. Clin. Microbiol. Rev. 2004, 17, 465–493. [Google Scholar] [CrossRef]
- Wang, J.; Teng, Z.; Cui, X.; Li, C.; Pan, H.; Zheng, Y.; Mao, S.; Yang, Y.; Wu, L.; Guo, X.; et al. Epidemiological and serological surveillance of hand-foot-and-mouth disease in Shanghai, China, 2012–2016. Emerg. Microbes Infect. 2018, 7, 1–12. [Google Scholar] [CrossRef]
- Gaboiphiwe, K.; Kabelo, T.I.; Mosholombe, P.T.; Hyera, J.; Fana, E.M.; Masisi, K.; Lebani, K. A Review of the Utility of Established Cell Lines for Isolation and Propagation of the Southern African Territories Serotypes of Foot-and-Mouth Disease Virus. Viruses 2024, 17, 39. [Google Scholar] [CrossRef]
- Carrillo, C.; Tulman, E.R.; Delhon, G.; Lu, Z.; Carreno, A.; Vagnozzi, A.; Kutish, G.F.; Rock, D.L. Comparative genomics of foot-and-mouth disease virus. J. Virol. 2005, 79, 6487–6504. [Google Scholar] [CrossRef] [PubMed]
- Freimanis, G.L.; Di Nardo, A.; Bankowska, K.; King, D.J.; Wadsworth, J.; Knowles, N.J.; King, D.P. Genomics and outbreaks: Foot and mouth disease. Rev. Sci. Tech. Int. Off. Epizoot. 2016, 35, 175–189. [Google Scholar] [CrossRef]
- Li, P.; Huang, S.; Zha, J.; Sun, P.; Li, D.; Bao, H.; Cao, Y.; Bai, X.; Fu, Y.; Ma, X.; et al. Evaluation of immunogenicity and cross-reactive responses of vaccines prepared from two chimeric serotype O foot-and-mouth disease viruses in pigs and cattle. Vet. Res. 2022, 53, 56. [Google Scholar] [CrossRef]
- Ren, X.; Li, P.; Li, X.; Qian, P. Epidemiological and genetic characteristics of foot-and-mouth disease virus in China from 2010 to 2022. Virology 2024, 589, 109940. [Google Scholar] [CrossRef] [PubMed]
- Alexandersen, S.; Brotherhood, I.; Donaldson, A.I. Natural aerosol transmission of foot-and-mouth disease virus to pigs: Minimal infectious dose for strain O1 Lausanne. Epidemiol. Infect. 2002, 128, 301–312. [Google Scholar] [CrossRef]
- Brehm, K.E.; Kumar, N.; Thulke, H.H.; Haas, B. High potency vaccines induce protection against heterologous challenge with foot-and-mouth disease virus. Vaccine 2008, 26, 1681–1687. [Google Scholar] [CrossRef]
- Kabelo, T.I.; Fana, E.M.; Hyera, J.M.; Lebani, K. A review of foot-and-mouth disease status and control measures in Botswana. Trop. Anim. Health Prod. 2023, 55, 278. [Google Scholar] [CrossRef] [PubMed]
- Gibbens, J.C.; Sharpe, C.E.; Wilesmith, J.W.; Mansley, L.M.; Michalopoulou, E.; Ryan, J.B.; Hudson, M. Descriptive epidemiology of the 2001 foot-and-mouth disease epidemic in Great Britain: The first five months. Vet. Rec. 2001, 149, 729–743. [Google Scholar] [CrossRef] [PubMed]
- Fernández, J.; Agüero, M.; Romero, L.; Sánchez, C.; Belák, S.; Arias, M.; Sánchez-Vizcaíno, J.M. Rapid and differential diagnosis of foot-and-mouth disease, swine vesicular disease, and vesicular stomatitis by a new multiplex RT-PCR assay. J. Virol. Methods 2008, 147, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Ferris, N.P.; Grazioli, S.; Hutchings, G.H.; Brocchi, E. Validation of a recombinant integrin αvβ6/monoclonal antibody based antigen ELISA for the diagnosis of foot-and-mouth disease. J. Virol. Methods 2011, 175, 253–260. [Google Scholar] [CrossRef]
- Armson, B.; Walsh, C.; Morant, N.; Fowler, V.L.; Knowles, N.J.; Clark, D. The development of two field-ready reverse transcription loop-mediated isothermal amplification assays for the rapid detection of Seneca Valley virus 1. Transbound. Emerg. Dis. 2019, 66, 497–504. [Google Scholar] [CrossRef]
- Shimmon, G.; Kotecha, A.; Ren, J.; Asfor, A.S.; Newman, J.; Berryman, S.; Cottam, E.M.; Gold, S.; Tuthill, T.J.; King, D.P.; et al. Generation and characterisation of recombinant FMDV antibodies: Applications for advancing diagnostic and laboratory assays. PLoS ONE 2018, 13, e0201853. [Google Scholar] [CrossRef]
- Wang, H.; Ding, X.; Sun, W.; Chen, Z.; Bai, L.; Liang, H.; Liu, Y.; Zhang, W.; Wang, G.; Yang, G.; et al. Recombinase polymerase amplification assay for rapid detection of Seneca Valley Virus. Anal. Biochem. 2022, 642, 114564. [Google Scholar] [CrossRef]
- Chen, J.S.; Ma, E.; Harrington, L.B.; Da Costa, M.; Tian, X.; Palefsky, J.M.; Doudna, J.A. CRISPR-Cas12a target binding unleashes indiscriminate single-stranded DNase activity. Science 2018, 360, 436–439. [Google Scholar] [CrossRef]
- Gootenberg, J.S.; Abudayyeh, O.O.; Kellner, M.J.; Joung, J.; Collins, J.J.; Zhang, F. Multiplexed and portable nucleic acid detection platform with Cas13, Cas12a, and Csm6. Science 2018, 360, 439–444. [Google Scholar] [CrossRef]
- Knott, G.J.; East-Seletsky, A.; Cofsky, J.C.; Holton, J.M.; Charles, E.; O’Connell, M.R.; Doudna, J.A. Guide-bound structures of an RNA-targeting A-cleaving CRISPR-Cas13a enzyme. Nat. Struct. Mol. Biol. 2017, 24, 825–833. [Google Scholar] [CrossRef]
- Zhao, L.; Qiu, M.; Li, X.; Yang, J.; Li, J. CRISPR-Cas13a system: A novel tool for molecular diagnostics. Front. Microbiol. 2022, 13, 1060947. [Google Scholar] [CrossRef]
- Gootenberg, J.S.; Abudayyeh, O.O.; Lee, J.W.; Essletzbichler, P.; Dy, A.J.; Joung, J.; Verdine, V.; Donghia, N.; Daringer, N.M.; Freije, C.A.; et al. Nucleic acid detection with CRISPR-Cas13a/C2c2. Science 2017, 356, 438–442. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Wu, Y.; Liu, G.; Gooding, J.J. CRISPR Mediated Biosensing Toward Understanding Cellular Biology and Point-of-Care Diagnosis. Angew. Chem. (Int. Ed. Engl.) 2020, 59, 20754–20766. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, R.; Li, J. CRISPR/cas systems redefine nucleic acid detection: Principles and methods. Biosens. Bioelectron. 2020, 165, 112430. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Man, S.; Ye, S.; Liu, G.; Ma, L. CRISPR-Cas based virus detection: Recent advances and perspectives. Biosens. Bioelectron. 2021, 193, 113541. [Google Scholar] [CrossRef] [PubMed]
- Miao, J.; Zuo, L.; He, D.; Fang, Z.; Berthet, N.; Yu, C.; Wong, G. Rapid detection of Nipah virus using the one-pot RPA-CRISPR/Cas13a assay. Virus Res. 2023, 332, 199130. [Google Scholar] [CrossRef]
- Stenfeldt, C.; Diaz-San Segundo, F.; de Los Santos, T.; Rodriguez, L.L.; Arzt, J. The Pathogenesis of Foot-and-Mouth Disease in Pigs. Front. Vet. Sci. 2016, 3, 41. [Google Scholar] [CrossRef]
- Fernandez-Sainz, I.; Medina, G.N.; Ramirez-Medina, E.; Koster, M.J.; Grubman, M.J.; de Los Santos, T. Adenovirus-vectored foot-and-mouth disease vaccine confers early and full protection against FMDV O1 Manisa in swine. Virology 2017, 502, 123–132. [Google Scholar] [CrossRef]
- Ren, H.R.; Li, M.T.; Wang, Y.M.; Jin, Z.; Zhang, J. The risk factor assessment of the spread of foot-and-mouth disease in mainland China. J. Theor. Biol. 2021, 512, 110558. [Google Scholar] [CrossRef]
- Chen, W.; Wang, W.; Wang, X.; Li, Z.; Wu, K.; Li, X.; Li, Y.; Yi, L.; Zhao, M.; Ding, H.; et al. Advances in the differential molecular diagnosis of vesicular disease pathogens in swine. Front. Microbiol. 2022, 13, 1019876. [Google Scholar] [CrossRef]
- Yeo, S.; Yang, M.; Nyachoti, M.; Rauh, R.; Callahan, J.D.; Nfon, C. Detection of Foot-and-Mouth Disease Virus in Swine Meat Juice. Pathogens 2020, 9, 424. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wang, F.; Xue, B.; Zhou, X. Field-deployable assay based on CRISPR-Cas13a coupled with RT-RPA in one tube for the detection of SARS-CoV-2 in wastewater. J. Hazard. Mater. 2023, 459, 132077. [Google Scholar] [CrossRef] [PubMed]
- East-Seletsky, A.; O’Connell, M.R.; Knight, S.C.; Burstein, D.; Cate, J.H.; Tjian, R.; Doudna, J.A. Two distinct RNase activities of CRISPR-C2c2 enable guide-RNA processing and RNA detection. Nature 2016, 538, 270–273. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xu, H.; Liu, C.; Peng, L.; Khan, H.; Cui, L.; Huang, R.; Wu, C.; Shen, S.; Wang, S.; et al. CRISPR-Cas13a Nanomachine Based Simple Technology for Avian Influenza A (H7N9) Virus On-Site Detection. J. Biomed. Nanotechnol. 2019, 15, 790–798. [Google Scholar] [CrossRef]
- Kellner, M.J.; Koob, J.G.; Gootenberg, J.S.; Abudayyeh, O.O.; Zhang, F. SHERLOCK: Nucleic acid detection with CRISPR nucleases. Nat. Protoc. 2019, 14, 2986–3012. [Google Scholar] [CrossRef]
- Liu, H.; Chang, S.; Chen, S.; Du, Y.; Wang, H.; Wang, C.; Xiang, Y.; Wang, Q.; Li, Z.; Wang, S.; et al. Highly sensitive and rapid detection of SARS-CoV-2 via a portable CRISPR-Cas13a-based lateral flow assay. J. Med. Virol. 2022, 94, 5858–5866. [Google Scholar] [CrossRef]

| Primer Name | Primer Sequence (5′ → 3′) |
|---|---|
| FMDV-O-F1 | GAAATTAATACGACTCACTATAGGGGACCCAMCATGTGTGCAACCCC |
| FMDV-O-R1 | CCGGTCRCCTATTCAGGCATAGAAGC |
| FMDV-O-F2 | GAAATTAATACGACTCACTATAGGGCTATGAGAACAAACGCATYACMGTTGARG |
| FMDV-O-R2 | GTAGATGTTGTTCARAATTGTGTTGATGAT |
| FMDV-O-F3 | GAAATTAATACGACTCACTATAGGGCTTYAAATCYCTTGGYCAAACCATYACTCC |
| FMDV-O-R3 | TTTGTAAAACCCAGTTCCATARTCCATGTG |
| FMDV-O-F4 | GAAATTAATACGACTCACTATAGGGAACAACAARTTGGACATMAYCAAAGCTC |
| FMDV-O-R4 | GAACAYRTCTTGTTGCATTCTCTTCATTTC |
| FMDV-O-F5 | GAAATTAATACGACTCACTATAGGGTCGAAGACCCTCGARGCYATCCTCTC |
| FMDV-O-R5 | CGTTCACCCADCGCAGGTAAAGTGA |
| FMDV-O-F6 | GAAATTAATACGACTCACTATAGGGCAAACCATYACTCCAGCTGACAAAAGC |
| FMDV-O-R6 | ATCACAGGTTTRTAAAACCCAGTTCCRTA |
| crRNA Template 1 | CARTAYGACTGTGCCCTTCTCAACGGCAGTTTTAGTCCCCTTCGTTTTTGGGGTAGTCTAAATCCCCTATAGTGAGTCGTATTAATTTC |
| crRNA Template 2 | AATAYGACTGTGCCCTTCTCAACGGMATGTTTTAGTCCCCTTCGTTTTTGGGGTAGTCTAAATCCCCTATAGTGAGTCGTATTAATTTC |
| crRNA Template 3 | ATAYGACTGTGCCCTTCTCAACGGMATGGTTTTAGTCCCCTTCGTTTTTGGGGTAGTCTAAATCCCCTATAGTGAGTCGTATTAATTTC |
| crRNA Template 4 | TAYGACTGTGCCCTTCTCAACGGMATGGGTTTTAGTCCCCTTCGTTTTTGGGGTAGTCTAAATCCCCTATAGTGAGTCGTATTAATTTC |
| crRNA Template 5 | AYGACTGTGCCCTTCTCAACGGMATGGCGTTTTAGTCCCCTTCGTTTTTGGGGTAGTCTAAATCCCCTATAGTGAGTCGTATTAATTTC |
| crRNA Template 6 | YGACTGTGCCCTTCTCAACGGMATGGCCGTTTTAGTCCCCTTCGTTTTTGGGGTAGTCTAAATCCCCTATAGTGAGTCGTATTAATTTC |
| T7-oligo | GAAATTAATACGACTCACTATAGGG |
| Fluorescence RNA reporter | 5′-FAM/UUUUUUUUUUU/BHQ1-3′ |
| LFS RNA reporter | 5′-FAM/UUUUUUUUUUU/Biotin-3′ |
| FMDV 3D F | GCGAGTCCTGCCACGGA |
| FMDV 3D R | ACTGGGTTTTACAAACCTGTGA |
| FMDV 3D probe | 5′-FAM/TCCTTTGCACGCCGTGGGAC/BHQ1-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, P.; Ni, B.; Li, C.; Sha, Z.; Liu, C.; Ren, W.; Wei, R.; Liu, F.; Li, J.; Wang, Z. Establishment and Implementation of the Point-of-Care RT-RAA-CRISPR/Cas13a Diagnostic Test for Foot-And-Mouth Disease Virus Serotype O in Pigs. Viruses 2025, 17, 721. https://doi.org/10.3390/v17050721
Meng P, Ni B, Li C, Sha Z, Liu C, Ren W, Wei R, Liu F, Li J, Wang Z. Establishment and Implementation of the Point-of-Care RT-RAA-CRISPR/Cas13a Diagnostic Test for Foot-And-Mouth Disease Virus Serotype O in Pigs. Viruses. 2025; 17(5):721. https://doi.org/10.3390/v17050721
Chicago/Turabian StyleMeng, Ping, Bo Ni, Chenyu Li, Zhou Sha, Chunju Liu, Weijie Ren, Rong Wei, Fuxiao Liu, Jinming Li, and Zhiliang Wang. 2025. "Establishment and Implementation of the Point-of-Care RT-RAA-CRISPR/Cas13a Diagnostic Test for Foot-And-Mouth Disease Virus Serotype O in Pigs" Viruses 17, no. 5: 721. https://doi.org/10.3390/v17050721
APA StyleMeng, P., Ni, B., Li, C., Sha, Z., Liu, C., Ren, W., Wei, R., Liu, F., Li, J., & Wang, Z. (2025). Establishment and Implementation of the Point-of-Care RT-RAA-CRISPR/Cas13a Diagnostic Test for Foot-And-Mouth Disease Virus Serotype O in Pigs. Viruses, 17(5), 721. https://doi.org/10.3390/v17050721






