Characterization of Papillomatous Lesions and Genetic Diversity of Bovine Papillomavirus from the Amazon Region
Abstract
1. Introduction
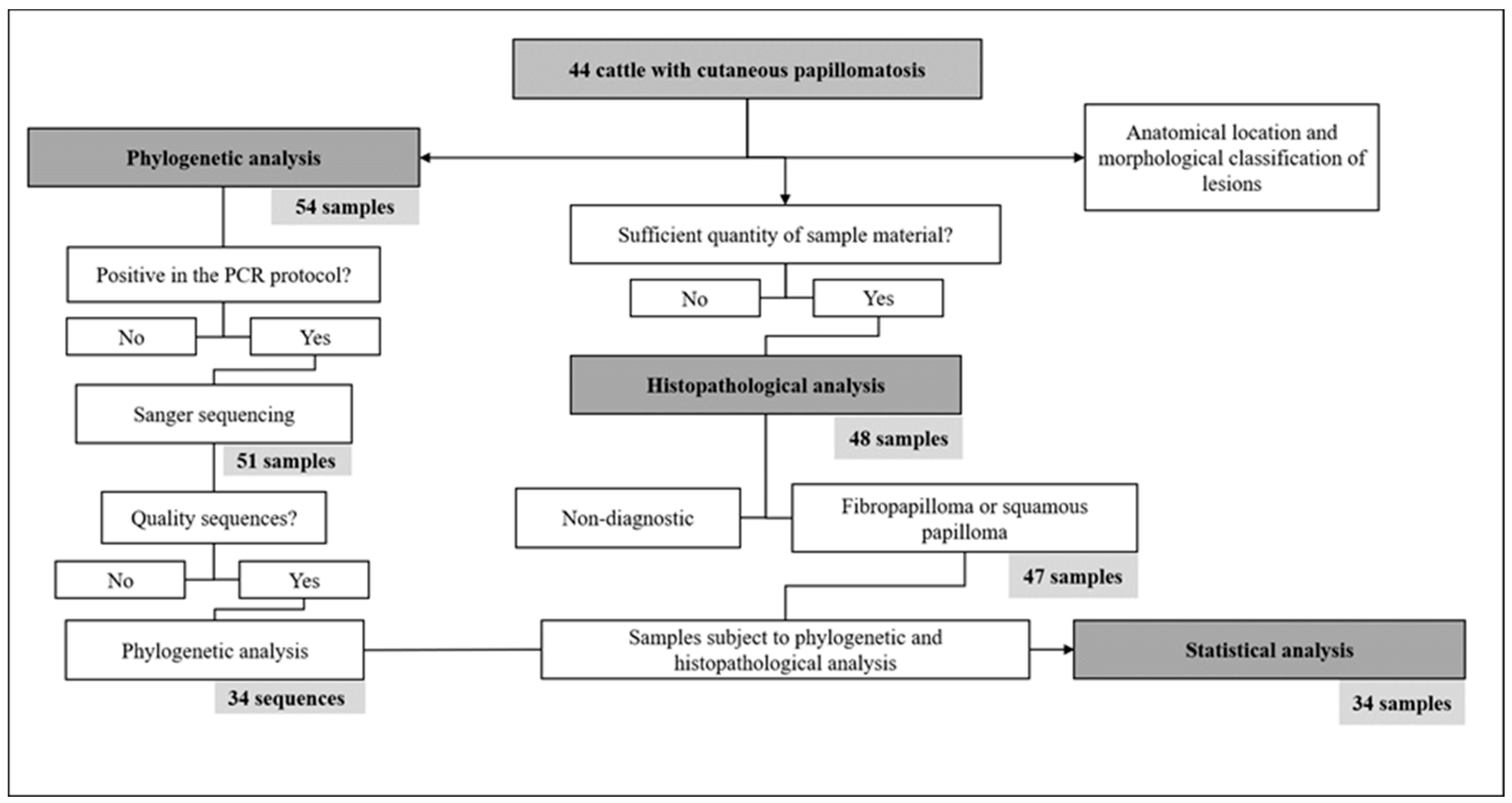
2. Materials and Methods
2.1. Sample Collection
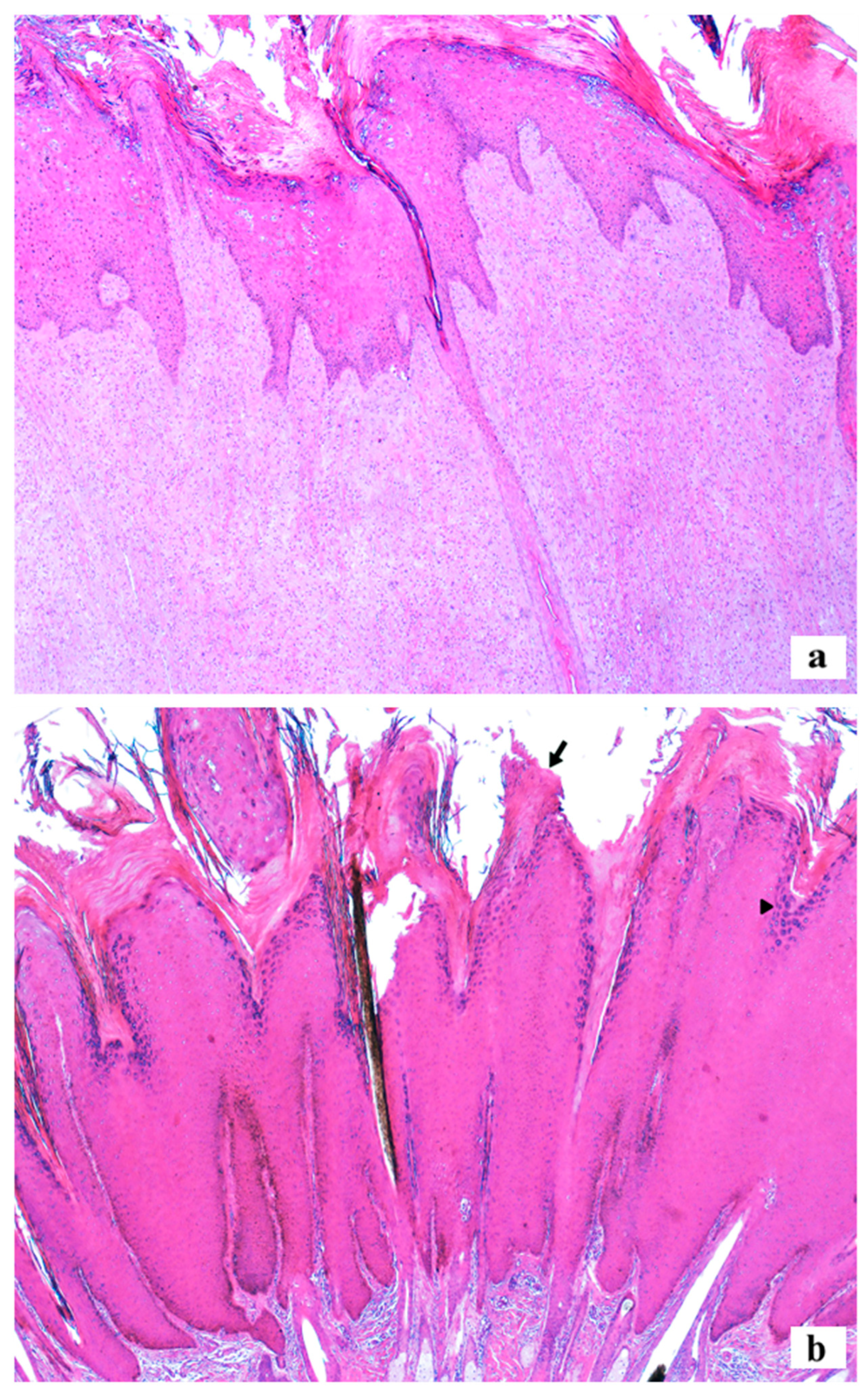
2.2. Macroscopic and Histopathological Analysis
2.3. DNA Extraction and PCR
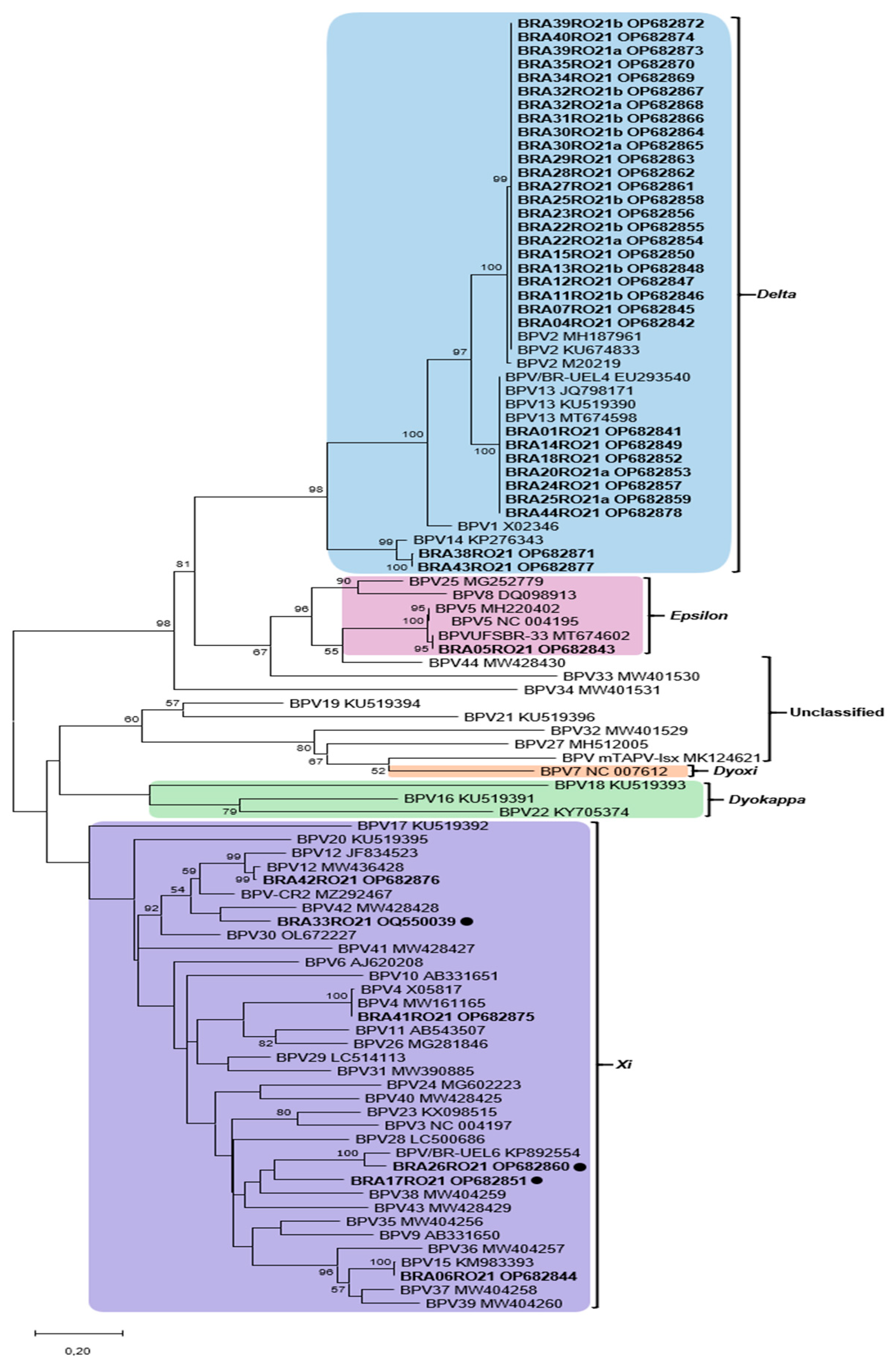
2.4. Sequencing and Phylogenetic Analysis
2.5. Statistical Analysis
3. Results
3.1. PCR, Sequencing, and Phylogenetic Analysis
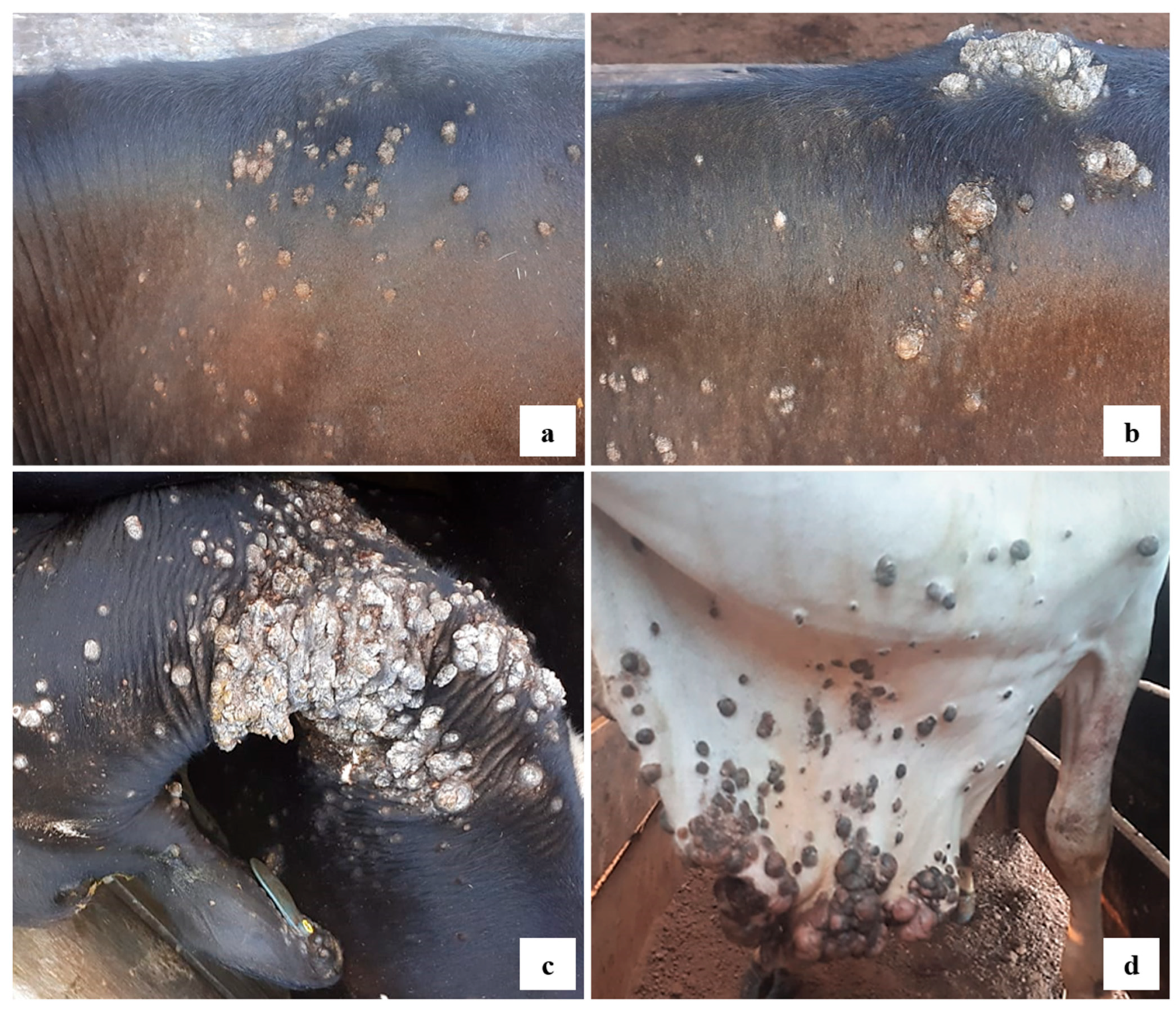
3.2. Histopathology and Macroscopic Characteristics of the Lesions
3.3. Statistical Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Van Doorslaer, K.; Chen, Z.; Bernard, H.U.; Chan, P.K.S.; Desalle, R.; Dillner, J.; Forslund, O.; Haga, T.; McBride, A.A.; Villa, L.L.; et al. ICTV Report Consortium. ICTV Virus Taxonomy Profile: Papillomaviridae. J. Gen. Virol. 2018, 99, 989–990. [Google Scholar] [CrossRef]
- Daudt, C.; Da Silva, F.R.C.; Lunardi, M.; Alves, C.B.D.T.; Weber, M.N.; Cibulski, S.P.; Alfieri, A.F.; Alfieri, A.A.; Canal, C.W. Papillomaviruses in ruminants: An update. Transbound. Emerg. Dis. 2018, 65, 1381–1395. [Google Scholar] [CrossRef]
- Rector, A.; Van Ranst, M. Animal papillomaviruses. Virology 2013, 445, 213–223. [Google Scholar] [CrossRef]
- Borzacchiello, G.; Roperto, F. Bovine papillomaviruses, papillomas and cancer in cattle. Vet. Res. 2008, 39, 45. [Google Scholar] [CrossRef]
- Munday, J.S. Bovine and human papillomaviruses: A comparative review. Vet. Pathol. 2018, 51, 1063–1075. [Google Scholar] [CrossRef] [PubMed]
- Campo, M.S. Animal models of papillomavirus pathogenesis. Virus Res. 2002, 89, 249–261. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, R.M.; Alves, C.D.B.T.; Schwertz, C.I.; Panziera, W.; De Lorenzo, C.; Da Silva, F.S.; De Cecco, B.S.; Daudt, C.; Chaves, F.R.; Canal, C.W.; et al. Molecular and pathological characterization of teat papillomatosis in dairy cows in Southern Brazil. Braz. J. Microbiol. 2020, 51, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Bocaneti, F.; Altamura, G.; Corteggio, A.; Velescu, E.; Roperto, F.; Borzacchiello, G. Bovine Papillomavirus: New Insights into an Old Disease. Transbound. Emerg. Dis. 2016, 63, 14–23. [Google Scholar] [CrossRef]
- Bernard, H.U.; Burk, R.D.; Chen, Z.; Van Doorslaer, K.; Zur Hausen, H.; De Villiers, E.M. Classification of papillomaviruses (PVs) based on 189 PV types and proposal of taxonomic amendments. Virology 2019, 401, 70–79. [Google Scholar] [CrossRef]
- De Villiers, E.M.; Fauquet, C.; Broker, T.R.; Bernard, H.U.; Zur Hausen, H. Classification of papillomaviruses. Virology 2004, 324, 17–27. [Google Scholar] [CrossRef]
- PaVE. PaVE: The Papillomavirus Episteme. 2024. Available online: https://pave.niaid.nih.gov/ (accessed on 4 February 2025).
- Batista, M.V.A.; Silva, M.A.R.; Pontes, N.E.; Reis, M.C.; Corteggio, A.; Castro, R.S.; Borzacchiello, G.; Balbino, V.Q.; Freitas, A.C. Molecular epidemiology of bovine papillomatosis and the identification of a putative new virus type in Brazilian cattle. Vet. J. 2013, 197, 368–373. [Google Scholar] [CrossRef] [PubMed]
- Daudt, C.; Da Silva, F.R.C.; Streck, A.F.; Weber, M.N.; Mayer, F.Q.; Cibulski, S.P.; Canal, C.W. How many papillomavirus species can go undetected in papilloma lesions? Sci. Rep. 2016, 6, 36480. [Google Scholar] [CrossRef][Green Version]
- Da Silva, F.R.C.; Daudt, C.; Streck, A.F.; Weber, M.N.; Leite Filho, R.V.; Driemeier, D.; Canal, C.W. Genetic characterization of Amazonian bovine papillomavirus reveals the existence of four new putative types. Virus Genes 2015, 51, 77–84. [Google Scholar] [CrossRef]
- Lunardi, M.; Tozato, C.C.; Alfieri, A.F.; De Alcântara, B.K.; Vilas-Boas, L.A.; Otonel, R.A.A.; Headley, S.A.; Alfieri, A.A. Genetic diversity of bovine papillomavirus types, including two putative new types, in teat warts from dairy cattle herds. Arch. Virol. 2016, 161, 1569–1577. [Google Scholar] [CrossRef] [PubMed]
- Araldi, R.P.; Assaf, S.M.R.; De Carvalho, R.F.; De Carvalho, M.A.C.R.; Mazzuchelli-de-Souza, J.; Magnelli, R.F.; Módolo, D.G.; Roperto, F.P.; Stocco, R.C.; Beçak, W. Papillomaviruses: A systematic review. Genet. Mol. Biol. 2017, 40, 1–21. [Google Scholar] [CrossRef]
- Goldschmidt, M.H.; Goldschmidt, K.H. Epithelial and Melanocytic Tumors of the Skin. In Tumors in Domestic Animals; Meuten, D.J., Ed.; Wiley: Hoboken, NJ, USA, 2016; pp. 88–141. [Google Scholar] [CrossRef]
- Mauldin, E.A.; Peters-Kennedy, J. Integumentary System. In Jubb, Kennedy & Palmer’s Pathology of Domestic Animals; Maxie, M.G., Ed.; W.B. Saunders: Philadelphia, PA, USA, 2016; pp. 509–736.e1. [Google Scholar] [CrossRef]
- Forslund, O.; Antonsson, A.; Nordin, P.; Stenquist, B.; Hansson, B.G. A broad range of human papillomavirus types detected with a general PCR method suitable for analysis of cutaneous tumours and normal skin. J. Gen. Virol. 1999, 80, 2437–2443. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Larkin, M.A.; Blackshields, G.; Brown, N.P.; Chenna, R.; Mcgettigan, P.A.; McWilliam, H.; Valentin, F.; Wallace, I.M.; Wilm, A.; Lopez, R.; et al. Clustal W and Clustal X version 2.0. Bioinformatics 2007, 23, 2947–2948. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K. Estimation of the number of nucleotide substitutions when there are strong transition-transversion and G+C-content biases. Mol. Biol. Evol. 1992, 9, 678–687. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Upton, G.J.G. Fisher’s Exact Test. J. R. Statist. Soc. Ser. A 1992, 155, 395–402. [Google Scholar] [CrossRef]
- Turner, J.E.; Wright, H.A.; Hamm, R.N. A Monte Carlo primer for health physicists. Health Phys. 1985, 48, 717–733. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023; Available online: https://www.R-project.org/ (accessed on 4 February 2025).
- Araldi, R.P.; Carvalho, R.F.; Melo, T.C.; Diniz, N.S.P.; Sant’Ana, T.A.; Mazzuchelli-de-Souza, J.; Spadacci-Morena, D.D.; Beçak, W.; Stocco, R.C. Bovine papillomavirus in beef cattle: First description of BPV-12 and putative type BAPV8 in Brazil. Genet. Mol. Res. 2014, 13, 5644–5653. [Google Scholar] [CrossRef]
- Araldi, R.P.; Giovanni, D.N.S.; Melo, T.C.; Diniz, N.; Mazzuchelli-de-Souza, J.; Sant’Ana, T.A.; Carvalho, R.F.; Beçak, W.; Stocco, R.C. Bovine papillomavirus isolation by ultracentrifugation. J. Virol. Methods 2014, 208, 119–124. [Google Scholar] [CrossRef]
- Figueirêdo, R.P.; Santos, G.F.; Oliveira, L.B.; Santos, L.A.B.O.; Barreto, D.M.; Cândido, A.L.; Campos, A.C.; Azevedo, E.O.; Batista, M.V.A. High Genotypic Diversity, Putative New Types and Intra-Genotype Variants of Bovine Papillomavirus in Northeast Brazil. Pathogens 2020, 9, 748. [Google Scholar] [CrossRef] [PubMed]
- Daudt, C.; Da Silva, F.R.C.; Cibulski, S.P.; Streck, A.F.; Laurie, R.E.; Munday, J.S.; Canal, C.W. Bovine Papillomavirus 24: A novel member of the genus Xipapillomavirus detected in the Amazon region. Arch. Virol. 2019, 164, 637–641. [Google Scholar] [CrossRef]
- Da Silva, F.R.C.; Daudt, C.; Cibulski, S.P.; Weber, M.N.; Varela, A.P.M.; Mayer, F.Q.; Roehe, P.M.; Canal, C.W. Genome characterization of a bovine papillomavirus type 5 from cattle in the Amazon region, Brazil. Virus Genes 2017, 53, 130–133. [Google Scholar] [CrossRef] [PubMed]
- Gasparotto, P.H.G.; Dos Santos, I.R.; Dantas Filho, J.V.; Da Silva, M.S.; Souza, F.A.; Sousa, J.C.M.; Driemeier, D.; Canal, C.W.; Da Silva, F.R.C.; Daudt, C. Characterization of Bovine Papillomavirus Types Detected in Cattle Rumen Tissues from Amazon Region, Brazil. Animals 2024, 14, 2262. [Google Scholar] [CrossRef]
- Roperto, S.; Munday, J.S.; Corrado, F.; Goria, M.; Roperto, F. Detection of bovine papillomavirus type 14 DNA sequences in urinary bladder tumors in cattle. Vet. Microbiol. 2016, 190, 1–4. [Google Scholar] [CrossRef]
- Medeiros-Fonseca, B.; Abreu-Silva, A.L.; Medeiros, R.; Oliveira, P.A.; Da Costa, R.M.G. Pteridium spp. and Bovine Papillomavirus: Partners in Cancer. Front. Vet. Sci. 2021, 8, 758720. [Google Scholar] [CrossRef]
- Sauthier, J.T.; Daudt, C.; Da Silva, F.R.C.; Alves, C.D.B.T.; Mayer, F.Q.; Bianchi, R.M.; Driemeier, D.; Streit, R.S.A.; Staats, C.C.; Canal, C.W.; et al. The genetic diversity of “papillomavirome” in bovine teat papilloma lesions. Anim. Microbiome 2021, 3, 51. [Google Scholar] [CrossRef]
- Zhu, W.; Dong, J.; Shimizu, E.; Hatama, S.; Kadota, K.; Goto, Y.; Haga, T. Characterization of novel bovine papillomavirus type 12 (BPV-12) causing epithelial papilloma. Arch. Virol. 2012, 157, 85–91. [Google Scholar] [CrossRef]
- Merchioratto, I.; De Oliveira, P.S.B.; Silva Júnior, J.V.J.; Brum, M.C.S.; Weiblen, R.; Flores, E.F. Phylogeny and amino acid analysis in single and mixed bovine papillomavirus infections in Southern Brazil, 2016–2020. Arch. Virol. 2023, 168, 52. [Google Scholar] [CrossRef]
- Bertagnolli, A.C.; Bezerra, A.V.A.; Santos, R.N.; Cavalli, L.S.; Varela, A.P.M.; Reis, E.M.; Cibulsky, S.P.; Roehe, P.M.; Mayer, F.Q. Clinicopathological characteristics and papillomavirus types in cutaneous warts in bovine. Braz. J. Microbiol. 2020, 51, 395–401. [Google Scholar] [CrossRef]
- Tsirimonaki, E.; O’Neil, B.; Williams, R.; Campo, M. Extensive papillomatosis of the bovine upper gastrointestinal tract. J. Comp. Pathol. 2003, 129, 93–99. [Google Scholar] [CrossRef]
- Munday, J.S.; Thomson, N.; Dunowska, M.; Knight, C.G.; Laurie, R.E.; Hills, S. Genomic characterisation of the feline sarcoid-associated papillomavirus and proposed classification as Bos taurus papillomavirus type 14. Vet. Microbiol. 2015, 177, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, C.C.R.; Batista, M.V.A.; Silva, M.A.R.; Balbino, V.Q.; Freitas, A.C. Detection of bovine papillomavirus types, co-infection and a putative new BPV11 subtype in cattle. Transbound. Emerg. Dis. 2012, 59, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Dagalp, S.B.; Dogan, F.; Farzanı, T.A.; Salar, S.; Bastan, A. The genetic diversity of bovine papillomaviruses (BPV) from different papillomatosis cases in dairy cows in Turkey. Arch. Virol. 2017, 162, 1507–1518. [Google Scholar] [CrossRef] [PubMed]
- Kale, M.; Yıldırım, Y.; Özmen, Ö.; Çağırgan, A.A.; Sökel, S.; Usta, A.; Küçük, A.; Orta, Y.S. Diagnosis and phylogenetic analysis of bovine papillomaviruses in cattle papillomatosis cases by different methods. Trop. Anim. Health Prod. 2023, 55, 191. [Google Scholar] [CrossRef]
- Silva, M.A.R.; Batista, M.V.A.; Pontes, N.E.; Santos, E.U.D.; Coutinho, L.C.A.; Castro, R.S.; Balbino, V.Q.; Freitas, A.C. Comparison of two PCR strategies for the detection of bovine papillomavirus. J. Virol. Methods 2013, 192, 55–58. [Google Scholar] [CrossRef]
- Diaz, J.H. The Disease Ecology, Epidemiology, Clinical Manifestations, Management, Prevention, and Control of Increasing Human Infections with Animal Orthopoxviruses. Wilderness Environ. Med. 2021, 32, 528–536. [Google Scholar] [CrossRef] [PubMed]
- Daudt, C.; Da Silva, F.R.C.; Cibulski, S.P.; Weber, M.N.; Mayer, F.Q.; Varela, A.P.M.; Roehe, P.M.; Canal, C.W. Complete genome sequence of Deltapapillomavirus 4 (bovine papillomavirus 2) from a bovine papillomavirus lesion in Amazon Region, Brazil. Mem. Inst. Oswaldo Cruz 2016, 111, 277–279. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Souza, F.d.A.; Daudt, C.; Lins, A.d.M.C.; dos Santos, I.R.; Tomaya, L.Y.C.; Lima, A.d.S.; Reis, E.M.B.; Satrapa, R.A.; Driemeier, D.; Bagon, A.; et al. Characterization of Papillomatous Lesions and Genetic Diversity of Bovine Papillomavirus from the Amazon Region. Viruses 2025, 17, 719. https://doi.org/10.3390/v17050719
Souza FdA, Daudt C, Lins AdMC, dos Santos IR, Tomaya LYC, Lima AdS, Reis EMB, Satrapa RA, Driemeier D, Bagon A, et al. Characterization of Papillomatous Lesions and Genetic Diversity of Bovine Papillomavirus from the Amazon Region. Viruses. 2025; 17(5):719. https://doi.org/10.3390/v17050719
Chicago/Turabian StyleSouza, Fernanda dos Anjos, Cíntia Daudt, André de Medeiros Costa Lins, Igor Ribeiro dos Santos, Lorena Yanet Cáceres Tomaya, Agnes de Souza Lima, Eduardo Mitke Brandão Reis, Rafael Augusto Satrapa, David Driemeier, Audrey Bagon, and et al. 2025. "Characterization of Papillomatous Lesions and Genetic Diversity of Bovine Papillomavirus from the Amazon Region" Viruses 17, no. 5: 719. https://doi.org/10.3390/v17050719
APA StyleSouza, F. d. A., Daudt, C., Lins, A. d. M. C., dos Santos, I. R., Tomaya, L. Y. C., Lima, A. d. S., Reis, E. M. B., Satrapa, R. A., Driemeier, D., Bagon, A., Canal, C. W., Salvarani, F. M., & da Silva, F. R. C. (2025). Characterization of Papillomatous Lesions and Genetic Diversity of Bovine Papillomavirus from the Amazon Region. Viruses, 17(5), 719. https://doi.org/10.3390/v17050719






