Comparison of Three Commercial ELISA Kits for Detection of Antibodies Against SARS-CoV-2 in Serum Samples from Different Animal Species
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Antibody Detection Tests
2.2.1. ELISA-1: cPass SARS-CoV-2 Neutralization Antibody Detection Kit
2.2.2. ELISA-2: SARS-CoV-2 NeutraLISA Kit
2.2.3. ELISA-3: ID Screen® SARS-CoV-2 Double Antigen Multi-Species Assay
2.3. Pseudovirus Neutralization Assay (pVNT)
2.4. Statistical Analyses
3. Results
3.1. Detection of SARS-CoV-2 Humoral Response by pVNT
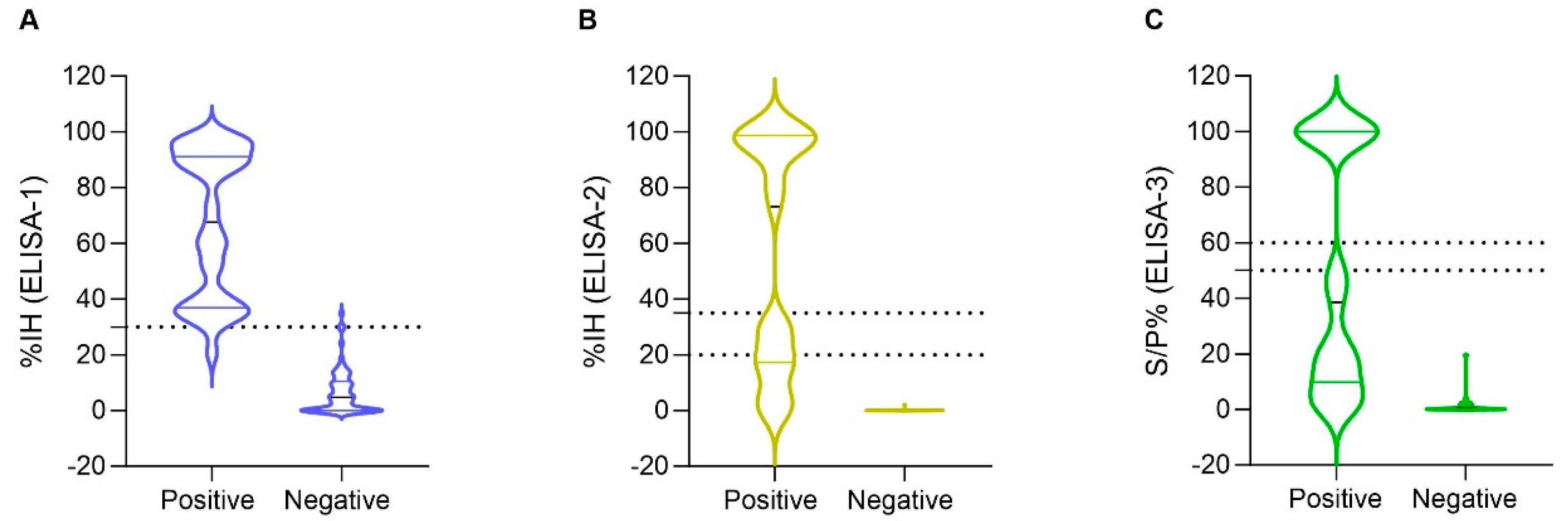
3.2. Detection of SARS-CoV-2 Humoral Response by ELISA-1, ELISA-2, and ELISA-3
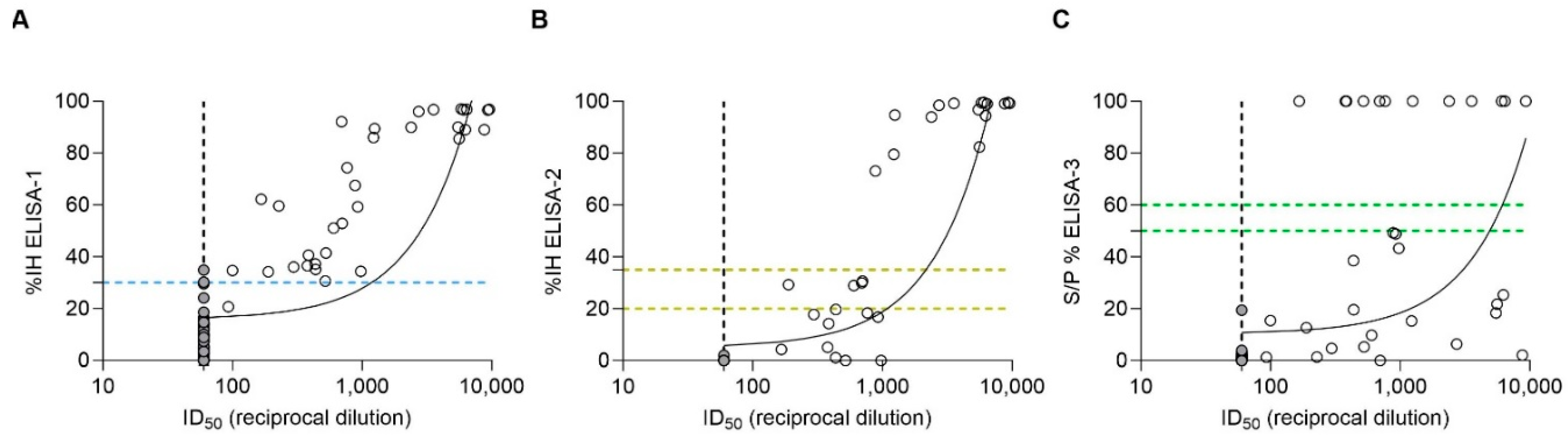
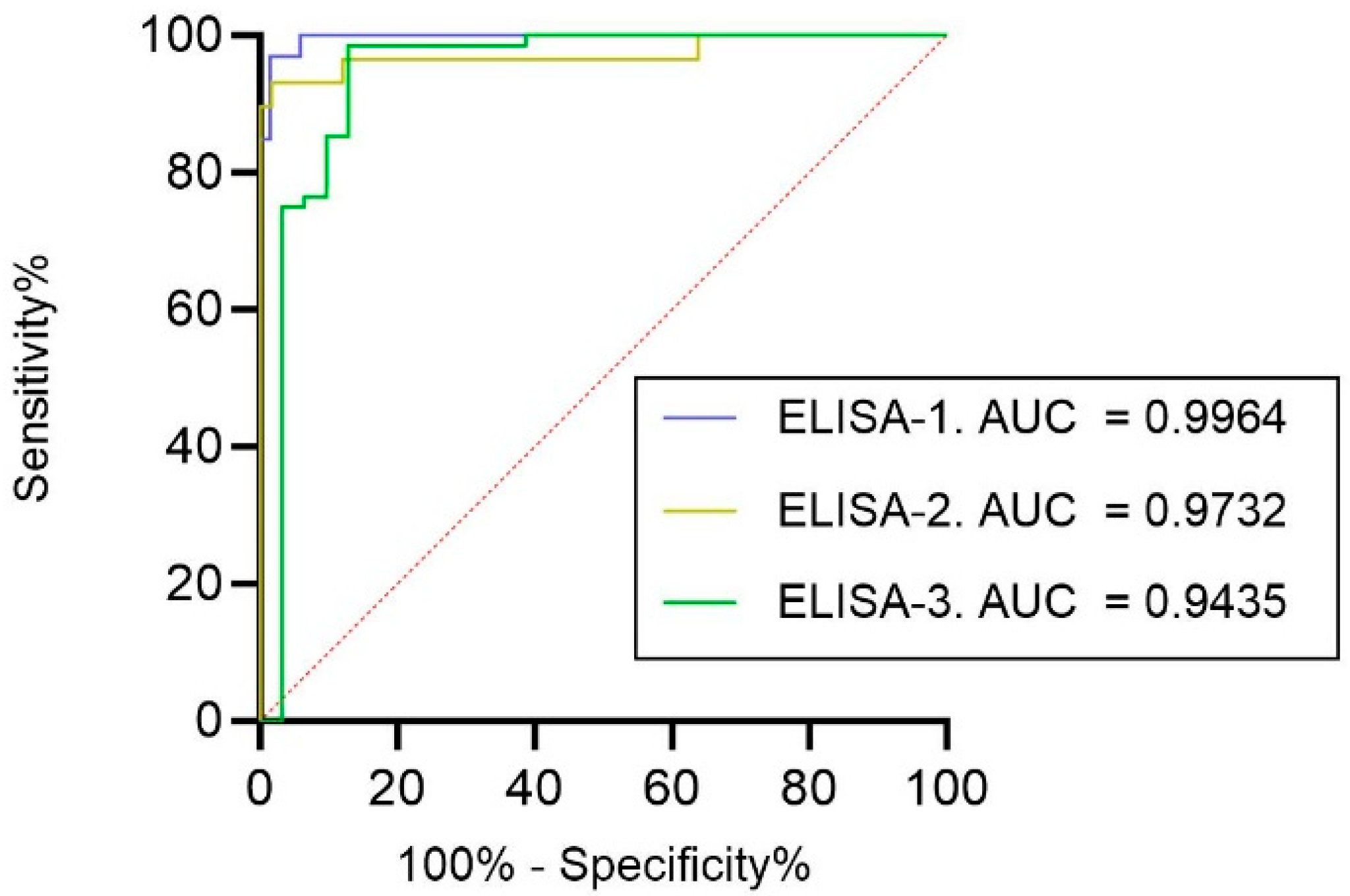
3.3. Qualitative Comparison of ELISAs with pVNT
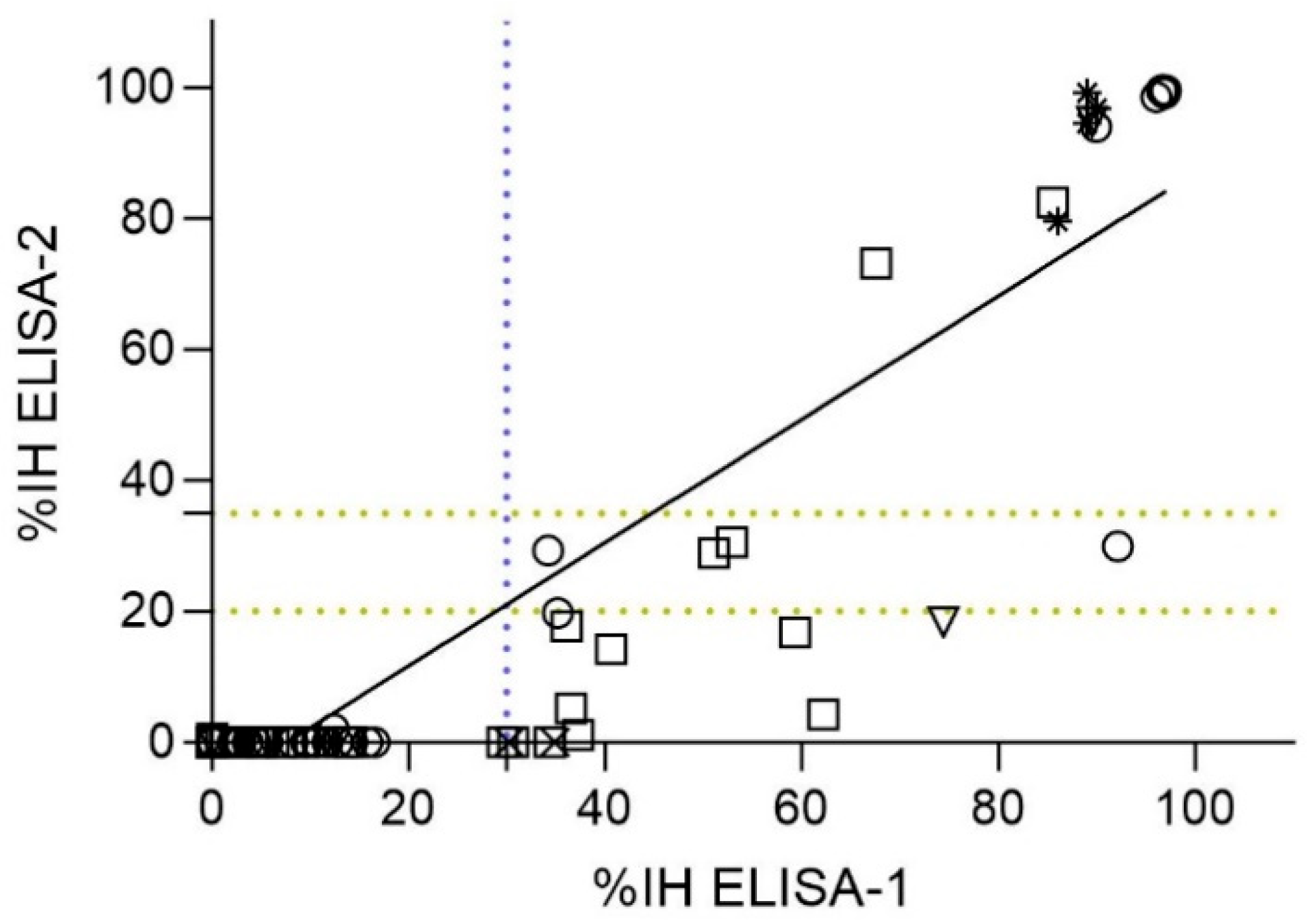
3.4. Correlation of ELISAs Detecting RBD nAbs (ELISA-1 vs. ELISA-2)
3.5. Qualitative and Quantitative Correlation of RBD nAbs (ELISA-1) and N Protein Antibodies (ELISA-3)
3.6. Serological Assays Associated with RT-qPCR Detection in Animal Samples
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Barroso, P.; López-Olvera, J.R.; Kiluba, T.K.W.; Gortázar, C. Overcoming the limitations of wildlife disease monitoring. Res. Dir. One Health 2024, 2, e3. [Google Scholar] [CrossRef]
- Lefrançois, T.; Malvy, D.; Atlani-Duault, L.; Benamouzig, D.; Druais, P.L.; Yazdanpanah, Y.; Delfraissy, J.F.; Linam, B. After 2 years of the COVID-19 pandemic, translating One Health into action is urgent. Lancet 2023, 401, 789–794. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.W.; Chia, W.N.; Qin, X.; Liu, P.; Chen, M.I.; Tiu, C.; Hu, Z.; Chen, V.C.; Young, B.E.; Sia, W.R.; et al. A SARS-CoV-2 surrogate virus neutralization test based on antibody-mediated blockage of ACE2–spike protein–protein interaction. Nat. Biotechnol. 2020, 38, 1073–1078. [Google Scholar] [CrossRef]
- Bewley, K.R.; Coombes, N.S.; Gagnon, L.; McInroy, L.; Baker, N.; Shaik, I.; St-Jean, J.R.; St-Amant, N.; Buttigieg, K.R.; Humphries, H.E.; et al. Quantification of SARS-CoV-2 neutralizing antibody by wild-type plaque reduction neutralization, microneutralization and pseudotyped virus neutralization assays. Nat. Protoc. 2021, 16, 3114–3140. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Q.; Li, L.; Wu, J.; Tian, M.; Fu, Y. Application of pseudovirus system in the development of vaccine, antiviral-drugs, and neutralizing antibodies. Microbiol. Res. 2022, 258, 126993. [Google Scholar] [CrossRef] [PubMed]
- Trinité, B.; Tarrés-Freixas, F.; Rodon, J.; Pradenas, E.; Urrea, V.; Marfil, S.; Rodríguez de la Concepción, M.L.; Ávila-Nieto, C.; Aguilar-Gurrieri, C.; Barajas, A.; et al. SARS-CoV-2 infection elicits a rapid neutralizing antibody response that correlates with disease severity. Sci. Rep. 2021, 11, 2608. [Google Scholar] [CrossRef]
- Khoury, D.S.; Cromer, D.; Reynaldi, A.; Schlub, T.E.; Wheatley, A.K.; Juno, J.A.; Subbarao, K.; Kent, S.J.; Triccas, J.A.; Davenport, M.P. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat. Med. 2021, 27, 1205–1211. [Google Scholar] [CrossRef]
- Bosco-Lauth, A.M.; Hartwig, A.E.; Porter, S.M.; Gordy, P.W.; Nehring, M.; Byas, A.D.; VandeWoude, S.; Ragan, I.K.; Maison, R.M.; Bowen, R.A. Experimental infection of domestic dogs and cats with SARS-CoV-2: Pathogenesis, transmission, and response to reexposure in cats. Proc. Natl. Acad. Sci. USA 2020, 117, 26382–26388. [Google Scholar] [CrossRef]
- Deng, W.; Bao, L.; Liu, J.; Xiao, C.; Liu, J.; Xue, J.; Lv, Q.; Qi, F.; Gao, H.; Yu, P.; et al. Primary exposure to SARS-CoV-2 protects against reinfection in rhesus macaques. Science 2020, 369, 818–823. [Google Scholar] [CrossRef]
- Rogers, T.F.; Zhao, F.; Huang, D.; Beutler, N.; Burns, A.; He, W.T.; Limbo, O.; Smith, C.; Song, G.; Woehl, J.; et al. Isolation of potent SARS-CoV-2 neutralizing antibodies and protection from disease in a small animal model. Science 2020, 369, 956–963. [Google Scholar] [CrossRef]
- Brustolin, M.; Rodon, J.; Rodríguez De La Concepción, M.L.; Ávila-Nieto, C.; Cantero, G.; Pérez, M.; Te, N.; Noguera-Julián, M.; Guallar, V.; Valencia, A.; et al. Protection against reinfection with D614- or G614-SARS-CoV-2 isolates in golden Syrian hamster. Emerg. Microbes Infect. 2021, 10, 797–809. [Google Scholar] [CrossRef]
- Qi, H.; Liu, B.; Wang, X.; Zhang, L. The humoral response and antibodies against SARS-CoV-2 infection. Nat. Immunol. 2022, 23, 1008–1020. [Google Scholar] [CrossRef]
- Dobaño, C.; Santano, R.; Jiménez, A.; Vidal, M.; Chi, J.; Rodrigo Melero, N.; Popovic, M.; López-Aladid, R.; Fernández-Barat, L.; Tortajada, M.; et al. Immunogenicity and crossreactivity of antibodies to the nucleocapsid protein of SARS-CoV-2: Utility and limitations in seroprevalence and immunity studies. Transl. Res. 2021, 232, 60–74. [Google Scholar] [CrossRef] [PubMed]
- Bahnan, W.; Wrighton, S.; Sundwall, M.; Bläckberg, A.; Larsson, O.; Höglund, U.; Khakzad, H.; Godzwon, M.; Walle, M.; Elder, E.; et al. Spike-Dependent Opsonization Indicates Both Dose-Dependent Inhibition of Phagocytosis and That Non-Neutralizing Antibodies Can Confer Protection to SARS-CoV-2. Front. Immunol. 2022, 12, 808932. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Gonzalez, J.C.; Sievers, B.L.; Mallajosyula, V.; Chakraborty, S.; Dubey, M.; Ashraf, U.; Cheng, B.Y.; Kathale, N.; Tran, K.Q.T.; et al. Early non-neutralizing, afucosylated antibody responses are associated with COVID-19 severity. Sci. Transl. Med. 2022, 14, eabm7853. [Google Scholar] [CrossRef]
- Meyer, B.; Drosten, C.; Müller, M.A. Serological assays for emerging coronaviruses: Challenges and pitfalls. Virus Res. 2014, 194, 175–183. [Google Scholar] [CrossRef]
- Diezma-Díaz, C.; Álvarez-García, G.; Regidor-Cerrillo, J.; Miró, G.; Villanueva-Saz, S.; Dolores Pérez, M.; Verde, M.T.; Galán-Malo, P.; Brun, A.; Moreno, S.; et al. A comparative study of eight serological methods shows that spike protein-based ELISAs are the most accurate tests for serodiagnosing SARS-CoV-2 infections in cats and dogs. Front. Vet. Sci. 2023, 10, 1121935. [Google Scholar] [CrossRef]
- Zhou, Z.; Qiu, Y.; Ge, X. The taxonomy, host range and pathogenicity of coronaviruses and other viruses in the Nidovirales order. Anim. Dis. 2021, 1, 5. [Google Scholar] [CrossRef]
- Zhao, S.; Schuurman, N.; Li, W.; Wang, C.; Smit, L.A.M.; Broens, E.M.; Wagenaar, J.A.; van Kuppeveld, F.J.M.; Bosch, B.J.; Egberink, H.; et al. Serologic screening of severe acute respiratory syndrome coronavirus 2 infection in cats and dogs during first coronavirus disease wave, the Netherlands. Emerg. Infect. Dis. 2021, 27, 5. [Google Scholar] [CrossRef]
- Barua, S.; Hoque, M.; Adekanmbi, F.; Kelly, P.; Jenkins-Moore, M.; Torchetti, M.K.; Chenoweth, K.; Wood, T.; Wang, C. Antibodies to SARS-CoV-2 in dogs and cats, USA. Emerg. Microbes Infect. 2021, 10, 1669–1674. [Google Scholar] [CrossRef]
- Wernike, K.; Böttcher, J.; Amelung, S.; Albrecht, K.; Gärtner, T.; Donat, K.; Beer, M. Antibodies against SARS-CoV-2 suggestive of single events of spillover to cattle, Germany. Emerg. Infect. Dis. 2022, 28, 1916–1918. [Google Scholar] [CrossRef]
- Fernández-Bastit, L.; Rodon, J.; Pradenas, E.; Marfil, S.; Trinité, B.; Parera, M.; Roca, N.; Pou, A.; Cantero, G.; Lorca-Oró, C.; et al. First detection of SARS-CoV-2 Delta (B.1.617.2) variant of concern in a dog with clinical signs in Spain. Viruses 2021, 13, 2526. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Bellon, H.; Rodon, J.; Fernández-Bastit, L.; Almagro, V.; Padilla-Solé, P.; Lorca-Oró, C.; Valle, R.; Roca, N.; Grazioli, S.; Trogu, T.; et al. Monitoring natural SARS-CoV-2 infection in lions (Panthera leo) at the Barcelona Zoo: Viral dynamics and host responses. Viruses 2021, 13, 1683. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Bastit, L.; Marfil, S.; Pradenas, E.; Valle, R.; Roca, N.; Rodon, J.; Pailler-García, L.; Trinité, B.; Parera, M.; Noguera-Julian, M.; et al. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection and humoral responses against different variants of concern in domestic pet animals and stray cats from north-eastern Spain. Transbound. Emerg. Dis. 2022, 69, 3518–3529. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Bastit, L.; Roca, N.; Romero-Durana, M.; Rodon, J.; Cantero, G.; García, Ó.; López, C.; Pérez, M.; López, R.; Carrillo, J.; et al. Susceptibility of domestic goat (Capra aegagrus hircus) to experimental infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) B.1.351/Beta variant. Viruses 2022, 14, 2002. [Google Scholar] [CrossRef]
- Vergara-Alert, J.; Rodon, J.; Carrillo, J.; Te, N.; Izquierdo-Useros, N.; Rodríguez de la Concepción, M.L.; Ávila-Nieto, C.; Guallar, V.; Valencia, A.; Cantero, G.; et al. Pigs are not susceptible to SARS-CoV-2 infection but are a model for viral immunogenicity studies. Transbound. Emerg. Dis. 2021, 68, 1721–1725. [Google Scholar] [CrossRef]
- Premkumar, L.; Segovia-Chumbez, B.; Jadi, R.; Martinez, D.R.; Raut, R.; Markmann, A.; Cornaby, C.; Bartelt, L.; Weiss, S.; Park, Y.; et al. The receptor-binding domain of the viral spike protein is an immunodominant and highly specific target of antibodies in SARS-CoV-2 patients. Sci. Immunol. 2020, 5, eabc8413. [Google Scholar] [CrossRef]
- Lauring, A.S.; Hodcroft, E.B. Genetic variants of SARS-CoV-2—What do they mean? JAMA 2021, 325, 529–531. [Google Scholar] [CrossRef]
- Fernández-Bastit, L.; Cano-Terriza, D.; Caballero-Gómez, J.; Beato-Benítez, A.; Fernández, A.; García-Párraga, D.; Domingo, M.; Sierra, C.; Canales, R.; Borragan, S.; et al. Survey of severe acute respiratory syndrome coronavirus 2 in captive and free-ranging wildlife from Spain. Vet. Res. 2024, 55, 90. [Google Scholar] [CrossRef]
- Graninger, M.; Jani, C.M.; Reuberger, E.; Prüger, K.; Gaspar, P.; Springer, D.N.; Borsodi, C.; Weidner, L.; Rabady, S.; Puchhammer-Stöckl, E.; et al. Comprehensive comparison of seven SARS-CoV-2-specific surrogate virus neutralization and anti-spike IgG antibody assays using a live-virus neutralization assay as a reference. Microbiol. Spectr. 2023, 11, e02314-22. [Google Scholar] [CrossRef]
- Müller, K.; Girl, P.; von Buttlar, H.; Dobler, G.; Wölfel, R. Comparison of two commercial surrogate ELISAs to detect a neutralising antibody response to SARS-CoV-2. J. Virol. Methods 2021, 292, 114122. [Google Scholar] [CrossRef] [PubMed]
- Perera, R.A.P.M.; Ko, R.; Tsang, O.T.Y.; Hui, D.S.C.; Kwan, M.Y.M.; Brackman, C.J.; To, E.M.W.; Yen, H.L.; Leung, K.; Cheng, S.M.S.; et al. Evaluation of a SARS-CoV-2 Surrogate Virus Neutralization Test for Detection of Antibody in Human, Canine, Cat, and Hamster Sera. J. Clin. Microbiol. 2021, 59, e02504-20. [Google Scholar] [CrossRef] [PubMed]
- Kakavandi, S.; Zare, I.; VaezJalali, M.; Dadashi, M.; Azarian, M.; Akbari, A.; Ramezani Farani, M.; Zalpoor, H.; Hajikhani, B. Structural and Non-Structural Proteins in SARS-CoV-2: Potential Aspects to COVID-19 Treatment or Prevention of Progression of Related Diseases. Cell Commun. Signal. 2023, 21, 110. [Google Scholar] [CrossRef]
- Taylor, S.C.; Hurst, B.; Charlton, C.L.; Bailey, A.; Kanji, J.N.; McCarthy, M.K.; Morrison, T.E.; Huey, L.; Annen, K.; DomBourian, M.G.; et al. A New SARS-CoV-2 Dual-Purpose Serology Test: Highly Accurate Infection Tracing and Neutralizing Antibody Response Detection. J. Clin. Microbiol. 2021, 59, e02438-20. [Google Scholar] [CrossRef]
- Chaintoutis, S.C.; Thomou, Z.; Mouchtaropoulou, E.; Tsiolas, G.; Chassalevris, T.; Stylianaki, I.; Lagou, M.; Michailidou, S.; Moutou, E.; Koenen, J.J.H.; et al. Outbreaks of SARS-CoV-2 in Naturally Infected Mink Farms: Impact, Transmission Dynamics, Genetic Patterns, and Environmental Contamination. PLoS Pathog. 2021, 17, e1009883. [Google Scholar] [CrossRef]
- Embregts, C.W.E.; Verstrepen, B.; Langermans, J.A.M.; Böszörményi, K.P.; Sikkema, R.S.; de Vries, R.D.; Hoffmann, D.; Wernike, K.; Smit, L.A.M.; Zhao, S.; et al. Evaluation of a Multi-Species SARS-CoV-2 Surrogate Virus Neutralization Test. One Health 2021, 13, 100313. [Google Scholar] [CrossRef]
- Neira, V.; Brito, B.; Agüero, B.; Berrios, F.; Valdés, V.; Gutierrez, A.; Ariyama, N.; Espinoza, P.; Retamal, P.; Holmes, E.C.; et al. A Household Case Evidences Shorter Shedding of SARS-CoV-2 in Naturally Infected Cats Compared to Their Human Owners. Emerg. Microbes Infect. 2021, 10, 376–383. [Google Scholar] [CrossRef]
- Udom, K.; Jairak, W.; Chamsai, E.; Charoenkul, K.; Boonyapisitsopa, S.; Bunpapong, N.; Techakriengkrai, N.; Amonsin, A. Serological Survey of Antibodies Against SARS-CoV-2 in Dogs and Cats, Thailand. Transbound. Emerg. Dis. 2022, 69, 2140–2147. [Google Scholar] [CrossRef]
- Miller, M.R.; Braun, E.; Ip, H.S.; Tyson, G.H. Domestic and Wild Animal Samples and Diagnostic Testing for SARS-CoV-2. Vet. Q. 2023, 43, 1–11. [Google Scholar] [CrossRef]
- Fernández-Bastit, L.; Montalvo, T.; Franco, S.; Barahona, L.; López-Bejar, M.; Carbajal, A.; Casas-Díaz, E.; Closa-Sebastià, F.; Segalés, J.; Vergara-Alert, J. Monitoring SARS-CoV-2 Infection in Urban and Peri-Urban Wildlife Species from Catalonia (Spain). One Health Outlook 2024, 6, 15. [Google Scholar] [CrossRef]
- Hulst, M.; Kant, A.; Harders-Westerveen, J.; Hoffmann, M.; Xie, Y.; Laheij, C.; Murk, J.L.; Van der Poel, W.H.M. Cross-Reactivity of Human, Wild Boar, and Farm Animal Sera from Pre- and Post-Pandemic Periods with Alpha- and Beta-Coronaviruses (CoV), Including SARS-CoV-2. Viruses 2023, 16, 34. [Google Scholar] [CrossRef] [PubMed]
- Ly, H. Assessing the Prevalence of SARS-CoV-2 in Free-Living and Captive Animals. Pathogens 2022, 11, 1405. [Google Scholar] [CrossRef]
- Vlasova, A.N.; Zhang, X.; Hasoksuz, M.; Nagesha, H.S.; Haynes, L.M.; Fang, Y.; Lu, S.; Saif, L.J. Two-Way Antigenic Cross-Reactivity Between Severe Acute Respiratory Syndrome Coronavirus (SARS-CoV) and Group 1 Animal CoVs Is Mediated Through an Antigenic Site in the N-Terminal Region of the SARS-CoV Nucleoprotein. J. Virol. 2007, 81, 13365–13377. [Google Scholar] [CrossRef] [PubMed]
- Klompus, S.; Leviatan, S.; Vogl, T.; Mazor, R.D.; Kalka, I.N.; Stoler-Barak, L.; Nathan, N.; Peres, A.; Moss, L.; Godneva, A.; et al. Cross-Reactive Antibodies Against Human Coronaviruses and the Animal Coronavirome Suggest Diagnostics for Future Zoonotic Spillovers. Sci. Immunol. 2021, 6, eabe9950. [Google Scholar] [CrossRef] [PubMed]
- Laidoudi, Y.; Sereme, Y.; Medkour, H.; Watier-Grillot, S.; Scandola, P.; Ginesta, J.; Andréo, V.; Labarde, C.; Comtet, L.; Pourquier, P.; et al. SARS-CoV-2 Antibodies Seroprevalence in Dogs from France Using ELISA and an Automated Western Blotting Assay. One Health 2021, 13, 100293. [Google Scholar] [CrossRef]
- Hancock, T.J.; Hickman, P.; Kazerooni, N.; Kennedy, M.; Kania, S.A.; Dennis, M.; Szafranski, N.; Gerhold, R.; Su, C.; Masi, T.; et al. Possible Cross-Reactivity of Feline and White-Tailed Deer Antibodies Against the SARS-CoV-2 Receptor Binding Domain. J. Virol. 2022, 96, e0025022. [Google Scholar] [CrossRef]
- Yamamoto, J.K.; Edison, L.K.; Rowe-Haas, D.K.; Takano, T.; Gilor, C.; Crews, C.D.; Tuanyok, A.; Arukha, A.P.; Shiomitsu, S.; Walden, H.D.S.; et al. Both Feline Coronavirus Serotypes 1 and 2 Infected Domestic Cats Develop Cross-Reactive Antibodies to SARS-CoV-2 Receptor Binding Domain: Its Implication to Pan-CoV Vaccine Development. Viruses 2023, 15, 914. [Google Scholar] [CrossRef]
- Zhang, L.; Jackson, C.B.; Mou, H.; Ojha, A.; Peng, H.; Quinlan, B.D.; Rangarajan, E.S.; Pan, A.; Vanderheiden, A.; Suthar, M.S.; et al. SARS-CoV-2 Spike-Protein D614G Mutation Increases Virion Spike Density and Infectivity. Nat. Commun. 2020, 11, 6013. [Google Scholar] [CrossRef]
- Shi, J.; Wen, Z.; Zhong, G.; Yang, H.; Wang, C.; Huang, B.; Liu, R.; He, X.; Shuai, L.; Sun, Z.; et al. Susceptibility of Ferrets, Cats, Dogs, and Other Domesticated Animals to SARS–Coronavirus 2. Science 2020, 368, 1016–1020. [Google Scholar] [CrossRef]
- Fenwick, C.; Croxatto, A.; Coste, A.T.; Pojer, F.; André, C.; Pellaton, C.; Farina, A.; Campos, J.; Hacker, D.; Lau, K.; et al. Changes in SARS-CoV-2 Spike Versus Nucleoprotein Antibody Responses Impact the Estimates of Infections in Population-Based Seroprevalence Studies. J. Virol. 2021, 95, e01828-20. [Google Scholar] [CrossRef]
- Van Elslande, J.; Oyaert, M.; Lorent, N.; Vande Weygaerde, Y.; Van Pottelbergh, G.; Godderis, L.; Van Ranst, M.; André, E.; Padalko, E.; Lagrou, K.; et al. Lower Persistence of Anti-Nucleocapsid Compared to Anti-Spike Antibodies up to One Year After SARS-CoV-2 Infection. Diagn. Microbiol. Infect. Dis. 2022, 103, 115659. [Google Scholar] [CrossRef] [PubMed]

| pVNT | ||||||||
|---|---|---|---|---|---|---|---|---|
| P | N | Sensitivity (%95 CI) | Specificity (%95 CI) | PPV (%95 CI) | NPV (%95 CI) | |||
| ELISA 1 | P | 32 | 2 | 34 | 96.90% (91.1–102.8%) | 97.05% (93.0–101.1%) | 94.10% (86.2–102.0%) | 98.50% (50.6–101.4%) |
| N | 1 | 66 | 67 | |||||
| 33 | 68 | 101 | ||||||
| ELISA 2 | P | 15 | 0 | 15 | 51.72% a (33.5–69.9%) 65.50% (48.2–82.8%) | 100.00% (100.0–100.0%) | 100.0% (100.0–100.0%) | 80.60% b (71.4–89.7%) 85.30% (76.9–93.7%) |
| N | 14 | 58 | 72 | |||||
| 29 | 58 | 87 | ||||||
| ELISA 3 | P | 12 | 0 | 12 | 38.70% (21.6–55.9%) | 100.00% (100.0–100.0%) | 100.00% (100.0–100.0%) | 78.20% (69.5–6.8%) |
| N | 19 | 68 | 87 | |||||
| 31 | 68 | 99 | ||||||
| pVNT | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| P | N | Sensitivity (%95 CI) | Specificity (%95 CI) | PPV (%95 CI) | NPV (%95 CI) | ||||
| CATS | ELISA 1 | P | 11 | 0 | 11 | 100% (100.0–100.0%) | 100% (100.0–100.0%) | 100% (100.0–100.0%) | 100% (100.0–100.0%) |
| N | 0 | 25 | 25 | ||||||
| 11 | 2 | 36 | |||||||
| ELISA 2 | P | 8 | 0 | 8 | 72.70% a (46.4–99.9%) 90.90% (73.9–107.9%) | 100% (100.0–100.0%) | 100% (100.0–100.0%) | 89.3% b (77.8–100.7%) 96.2% (88.8–103.5%) | |
| N | 3 | 25 | 28 | ||||||
| 11 | 25 | 36 | |||||||
| ELISA 3 | P | 6 | 0 | 6 | 66.70% (35.9–97.5%) | 100% (100.0–100.0%) | 100% (100.0–100.0%) | 89.3% (77.8–100.7%) | |
| N | 3 | 25 | 28 | ||||||
| 9 | 25 | 34 | |||||||
| DOGS | ELISA 1 | P | 12 | 0 | 12 | 100% (100.0–100.0%) | 100% (100.0–100.0%) | 100% (100.0–100.0%) | 100% (100.0–100.0%) |
| N | 0 | 29 | 29 | ||||||
| 12 | 29 | 41 | |||||||
| ELISA 2 | P | 2 | 0 | 2 | 16.70% c (−4.4–37.8%) 33.30% (6.70–60.0%) | 100% (100.0–100.0%) | 100% (100.0–100.0%) | 74.40% d (60.7–88.1%) 78.4% (65.1–91.6%) | |
| N | 10 | 29 | 39 | ||||||
| 12 | 29 | 41 | |||||||
| ELISA 3 | P | 4 | 0 | 4 | 33.30% (6.70–60.0%) | 100% (100.0–100.0%) | 100% (100.0–100.0%) | 78.40% (65.10–91.6%) | |
| N | 8 | 29 | 37 | ||||||
| 12 | 41 | 41 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-Bastit, L.; Marfil, S.; Pradenas, E.; Blanco, J.; Vergara-Alert, J.; Segalés, J. Comparison of Three Commercial ELISA Kits for Detection of Antibodies Against SARS-CoV-2 in Serum Samples from Different Animal Species. Viruses 2025, 17, 716. https://doi.org/10.3390/v17050716
Fernández-Bastit L, Marfil S, Pradenas E, Blanco J, Vergara-Alert J, Segalés J. Comparison of Three Commercial ELISA Kits for Detection of Antibodies Against SARS-CoV-2 in Serum Samples from Different Animal Species. Viruses. 2025; 17(5):716. https://doi.org/10.3390/v17050716
Chicago/Turabian StyleFernández-Bastit, Leira, Sílvia Marfil, Edwards Pradenas, Julià Blanco, Júlia Vergara-Alert, and Joaquim Segalés. 2025. "Comparison of Three Commercial ELISA Kits for Detection of Antibodies Against SARS-CoV-2 in Serum Samples from Different Animal Species" Viruses 17, no. 5: 716. https://doi.org/10.3390/v17050716
APA StyleFernández-Bastit, L., Marfil, S., Pradenas, E., Blanco, J., Vergara-Alert, J., & Segalés, J. (2025). Comparison of Three Commercial ELISA Kits for Detection of Antibodies Against SARS-CoV-2 in Serum Samples from Different Animal Species. Viruses, 17(5), 716. https://doi.org/10.3390/v17050716






