HIV–HPV Co-Infection and Identification of Novel High-Risk HPV Among Women at Two Hospital Centers in Cotonou, Republic of Benin
Abstract
1. Introduction
2. Methods
2.1. Study Design and Population
2.2. Study Procedure
2.3. DNA Extraction
2.4. ß-Globin Amplification
2.5. HPV L1 Gene Amplification
2.6. Gel Electrophoresis
2.7. Sanger Sequencing
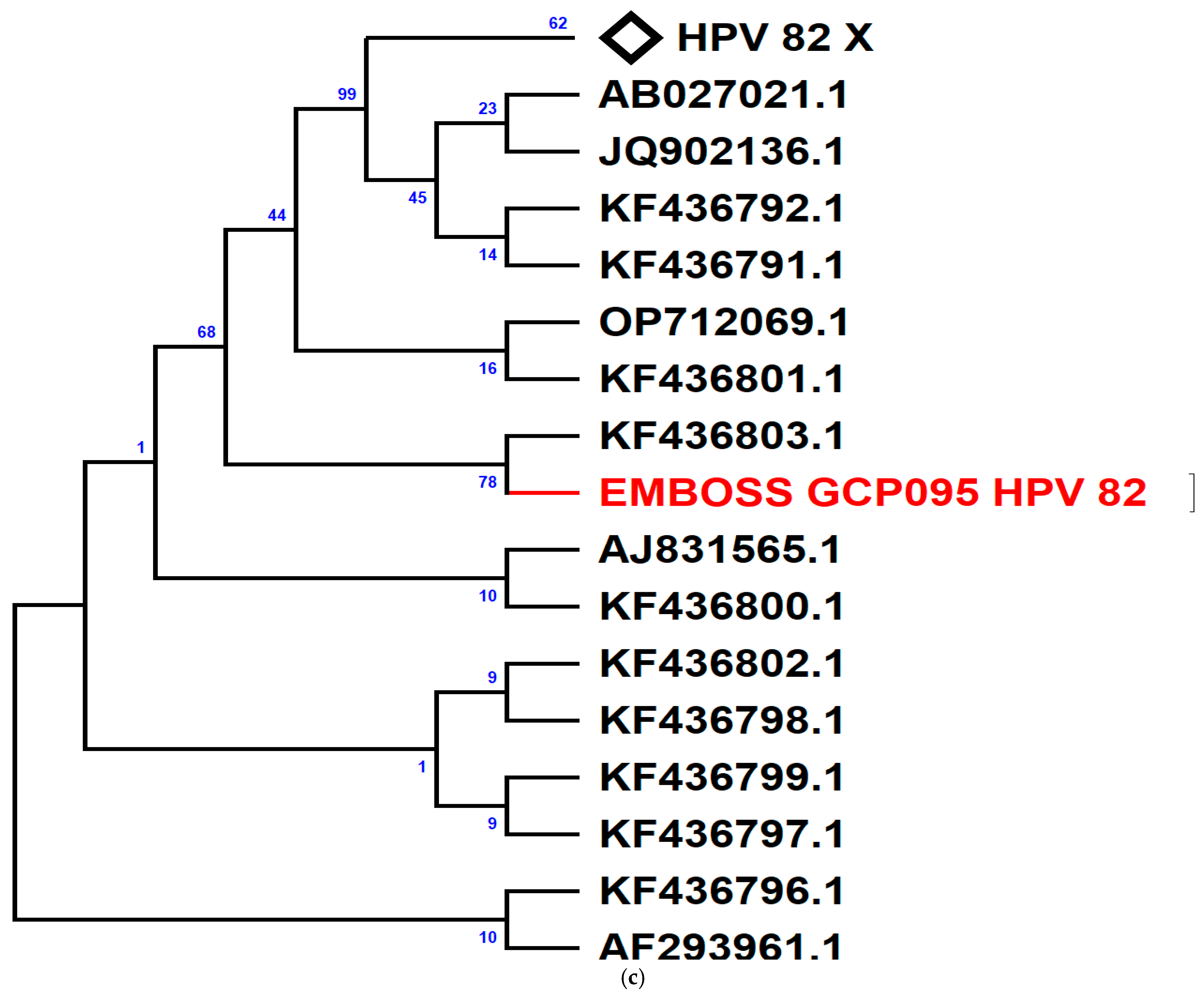
2.8. Bioinformatic Analyses
2.9. Statistical Analyses
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Milano, G.; Guarducci, G.; Nante, N.; Montomoli, E.; Manini, I. Human Papillomavirus Epidemiology and Prevention: Is There Still a Gender Gap? Vaccines 2023, 11, 1060. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Shao, H.; Zhang, T.; Pu, J.; Tang, C. Factors Influencing Men’s Attitudes toward HPV Vaccination in Males Included in the Chinese National Immunization Program. Vaccines 2022, 10, 1054. [Google Scholar] [CrossRef]
- De Sanjosé, S.; Diaz, M.; Castellsagué, X.; Clifford, G.; Bruni, L.; Muñoz, N.; Bosch, F.X. Worldwide prevalence and genotype distribution of cervical human papillomavirus DNA in women with normal cytology: A meta-analysis. Lancet Infect. Dis. 2007, 7, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Ranjeva, S.L.; Baskerville, E.B.; Dukic, V.; Villa, L.L.; Lazcano-Ponce, E.; Giuliano, A.R.; Dwyer, G.; Cobey, S. Recurring infection with ecologically distinct HPV types can explain high prevalence and diversity. Proc. Natl. Acad. Sci. USA 2017, 114, 13573–13578. [Google Scholar] [CrossRef] [PubMed]
- Faridi, R.; Zahra, A.; Khan, K.; Idrees, M. Oncogenic potential of Human Papillomavirus (HPV) and its relation with cervical cancer. Virol. J. 2011, 8, 269. [Google Scholar] [CrossRef]
- Mlynarczyk-Bonikowska, B.; Rudnicka, L. HPV Infections—Classification, Pathogenesis, and Potential New Therapies. Int. J. Mol. Sci. 2024, 25, 7616. [Google Scholar] [CrossRef]
- Choi, S.; Ismail, A.; Pappas-Gogos, G.; Boussios, S. HPV and Cervical Cancer: A Review of Epidemiology and Screening Uptake in the UK. Pathogens 2023, 12, 298. [Google Scholar] [CrossRef]
- Stanley, M.A.; Pett, M.R.; Coleman, N. HPV: From infection to cancer. Biochem. Soc. Trans. 2007, 35, 1456–1460. [Google Scholar] [CrossRef]
- Nor Rashid, N.; Yusof, R.; Watson, R.J. Disruption of repressive p130–DREAM complexes by human papillomavirus 16 E6/E7 oncoproteins is required for cell-cycle progression in cervical cancer cells. J. Gen. Virol. 2011, 92, 2620–2627. [Google Scholar] [CrossRef]
- Niebler, M.; Qian, X.; Hofler, D.; Kogosov, V.; Kaewprag, J.; Kaufmann, A.M.; Ly, R.; Böhmer, G.; Zawatzky, R.; Rösl, F.; et al. Post-Translational Control of IL-1b via the Human Papillomavirus Type 16 E6 Oncoprotein: A Novel Mechanism of Innate Immune Escape Mediated by the E3-Ubiquitin Ligase E6-AP and p53. PLoS Pathog. 2013, 9, e1003536. [Google Scholar] [CrossRef]
- Veldhuijzen, N.J.; Snijders, P.J.; Reiss, P.; Meijer, C.J.L.M.; van de Wijgert, J.H.H.M. Factors affecting transmission of mucosal human papillomavirus. Lancet Infect. Dis. 2010, 10, 862–874. [Google Scholar] [CrossRef] [PubMed]
- Mane, A.; Sahasrabuddhe, V.V.; Nirmalkar, A.; Risbud, A.R.; Sahay, S.; Bhosale, R.A.; Vermund, S.H.; Mehendale, S.M. Rates and determinants of incidence and clearance of cervical HPV genotypes among HIV-seropositive women in Pune, India. J. Clin. Virol. 2017, 88, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Adebamowo, S.N.; Famooto, A.; Dareng, E.O.; Olawande, O.; Olaniyan, O.; Offiong, R.; Adebamowo, C.A. Clearance of Type-Specific, Low-Risk, and High-Risk Cervical Human Papillomavirus Infections in HIV-Negative and HIV-Positive Women. J. Glob. Oncol. 2018, 4, JGO.17.00129. [Google Scholar] [CrossRef]
- Seyoum, A.; Assefa, N.; Gure, T.; Seyoum, B.; Mulu, A.; Mihret, A. Prevalence and Genotype Distribution of High-Risk Human Papillomavirus Infection Among Sub-Saharan African Women: A Systematic Review and Meta-Analysis. Front. Public Health 2022, 10, 890880. [Google Scholar] [CrossRef]
- Capo-chichi, C.D.; Aguida, B.; Chabi, N.W.; Acapko-Ezin, J.; Sossah-Hiffo, J.; Agossou, V.K.; Anagbla, T.; Zannou, M.; Houngbé, F.; Sanni, A. Diversity of high risk human papilloma viruses in women treated with antiretroviral and in healthy controls and discordance with cervical dysplasia in the South of Benin. Infect. Agent. Cancer 2016, 11, 43. [Google Scholar] [CrossRef]
- Piras, F.; Piga, M.; De Montis, A.; Zannou, A.R.F.; Minerba, L.; Perra, M.T.; Murtas, D.; Atzori, M.; Pittau, M.; Maxia, C.; et al. Prevalence of human papillomavirus infection in women in Benin, West Africa. Virol. J. 2011, 8, 514. [Google Scholar] [CrossRef]
- Liu, G.; Sharma, M.; Tan, N.; Barnabas, R. HIV-positive women have higher risk of human papilloma virus infection, precancerous lesions, and cervical cancer. AIDS 2018, 32, 795–808. [Google Scholar] [CrossRef]
- Ntekim, A.; Campbell, O.; Rothenbacher, D. Optimal management of cervical cancer in HIV-positive patients: A systematic review. Cancer Med. 2015, 4, 1381–1393. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Human Papillomavirus Vaccines: WHO Position Paper, December 2022. Available online: https://www.who.int/publications/i/item/who-wer9750-645-672 (accessed on 30 April 2025).
- Pils, S.; Joura, E.A. From the monovalent to the nine-valent HPV vaccine. Clin. Microbiol. Infect. 2015, 21, 827–833. [Google Scholar] [CrossRef]
- Illah, O.; Olaitan, A. Updates on HPV Vaccination. Diagnostics 2023, 13, 243. [Google Scholar] [CrossRef]
- Naing, L.; Winn, T.; Rusli, B.N. Practical Issues in Calculating the Sample Size for Prevalence Studies. Arch. Orofac. Sci. 2006, 1, 9–14. [Google Scholar]
- Perrons, C.; Kleter, B.; Jelley, R.; Jalal, H.; Quint, W.; Tedder, R. Detection and genotyping of human papillomavirus DNA by SPF10 and MY09/11 primers in cervical cells taken from women attending a colposcopy clinic. J. Med. Virol. 2002, 67, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Badial, R.M.; Dias, M.C.; Stuqui, B.; Melli, P.P.S.; Quintana, S.M.; Bonfim, C.M.; Cordeiro, J.A.; Rabachini, T.; Calmon, M.F.; Provazzi, P.J.S.; et al. Detection and genotyping of human papillomavirus (HPV) in HIV-infected women and its relationship with HPV/HIV co-infection. Medicine 2018, 97, e9545. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K.; Rozewicki, J.; Yamada, K.D. MAFFT online service: Multiple sequence alignment, interactive sequence choice and visualization. Brief. Bioinform. 2019, 20, 1160–1166. [Google Scholar] [CrossRef]
- Shahrestani, K.R.; Ideris, A.; Hair-bejo, M.; Tan, S.W.; Yeap, S.K.; Pirozyan, M.R.; Jazayeri, S.D.; Omar, A. Molecular characterisation and pathotyping of recently isolated newcastle disease virus isolates based on f protein’s cleavage site. In Proceeding of the World’s Poultry Science Association Scientific Conference 2013, Serdang, Malaysia, 30 November 2013. [Google Scholar]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Nei, M.; Kumar, S. Molecular Evolution and Phylogenetics; Oxford University Press: Oxford, UK, 2000. [Google Scholar]
- Felsenstein, J. Confidence Limits on Phylogenies: An Approach Using the Bootstrap. Evolution 1985, 39, 783. [Google Scholar] [CrossRef]
- Burk, R.D.; Harari, A.; Chen, Z. Human papillomavirus genome variants. Virology 2013, 445, 232–243. [Google Scholar] [CrossRef]
- Chakravarty, J.; Chourasia, A.; Thakur, M.; Singh, A.K.; Sundar, S.; Agrawal, N.R. Prevalence of human papillomavirus infection & cervical abnormalities in HIV-positive women in eastern India. Indian J. Med. Res. 2016, 143, 79. [Google Scholar]
- Tartaglia, E.; Falasca, K.; Vecchiet, J.; Sabusco, G.P.; Picciano, G.; Di Marco, R.; Ucciferri, C. Prevalence of HPV infection among HIV-positive and HIV-negative women in Central/Eastern Italy: Strategies of prevention. Oncol. Lett. 2017. Epub ahead of print. [Google Scholar] [CrossRef]
- Agyare Gyane, F.; Modey, E.; Maya, E.; Bonney, E.Y.; Abaidoo-Myles, A.; Paintsil, E.; Torpey, K. Prevalence and risk factors associated with high-risk human papillomavirus infection among women living with HIV (WLWH) at a tertiary health facility in Accra, Ghana. PLoS ONE 2024, 19, e0303535. [Google Scholar] [CrossRef]
- Abubakar, Y.; Ajang, A.Y.; Ella, E.E.; Oguntayo, A.O.; Aminu, M. Prevalence of Human Papillomavirus Infection and its Association with the Risk of Cervical Cancer among Hiv-Positive Women in Plateau State, North-Central Nigeria. UMYU J. Microbiol. Res. UJMR 2024, 9, 247–257. [Google Scholar] [CrossRef]
- Abdi, M.; Tamiru, A.; Tilahun, T.; Tiruneh, G.; Fite, M.B. Factors associated with human papillomavirus infections among women living with HIV in public health facilities in Western Oromia, Ethiopia. BMC Women’s Health 2024, 24, 423. [Google Scholar] [CrossRef] [PubMed]
- Mcharo, R.; Lennemann, T.; France, J.; Torres, L.; Garí, M.; Mbuya, W.; Mwalongo, W.; Mahenge, A.; Bauer, A.; Mnkai, J.; et al. HPV Type Distribution in HIV Positive and Negative Women With or Without Cervical Dysplasia or Cancer in East Africa. Front. Oncol. 2021, 11, 763717. [Google Scholar] [CrossRef]
- Xi, L.F.; Touré, P.; Critchlow, C.W.; Hawes, S.E.; Dembélé, B.; Sow, P.S.; Kiviat, N.B. Prevalence of specific types of human papillomavirus and cervical squamous intraepithelial lesions in consecutive, previously unscreened, West-African women over 35 years of age. Int. J. Cancer 2003, 103, 803–809. [Google Scholar] [CrossRef] [PubMed]
- Okoye, J.O.; Ofodile, C.A.; Adeleke, O.K.; Obioma, O. Prevalence of high-risk HPV genotypes in sub-Saharan Africa according to HIV status: A 20-year systematic review. Epidemiol. Health 2021, 43, e2021039. [Google Scholar] [CrossRef]
- Bruni, L.; Saura-Lázaro, A.; Montoliu, A.; Brotons, M.; Alemany, L.; Diallo, M.S.; Afsar, O.Z.; LaMontagne, D.S.; Mosina, L.; Contreras, M.; et al. HPV vaccination introduction worldwide and WHO and UNICEF estimates of national HPV immunization coverage 2010–2019. Prev. Med. 2021, 144, 106399. [Google Scholar] [CrossRef]
- Okolie, E.A.; Nwadike, B.I. Spotlight on Human Papillomavirus Vaccination Coverage: Is Nigeria Making Any Progress? JCO Glob. Oncol. 2023, 9, e2300088. [Google Scholar] [CrossRef]
- Massad, L.S.; Einstein, M.H.; Huh, W.K.; Katki, H.A.; Kinney, W.K.; Schiffman, M.; Solomon, D.; Wentzensen, N.; Lawson, H.W. 2012 Updated Consensus Guidelines for the Management of Abnormal Cervical Cancer Screening Tests and Cancer Precursors. J. Low. Genit. Tract Dis. 2013, 17, S1–S27. [Google Scholar] [CrossRef]
- Wentzensen, N.; Schiffman, M.; Palmer, T.; Arbyn, M. Triage of HPV positive women in cervical cancer screening. J. Clin. Virol. 2016, 76, S49–S55. [Google Scholar] [CrossRef]
- Ping, Q.Y.; Ling, W.A.; Wen, F.L.; Hann, K.; Lin Hong, W. High-risk Human Papillomavirus Infection and Associated Factors among HIV-positive Women in High HIV-burden Areas of China. Biomed. Environ. Sci. 2020, 33, 206–212. [Google Scholar]
- Zeier, M.D.; Botha, M.H.; Engelbrecht, S.; Machekano, R.N.; Jacobs, G.B.; Isaacs, S.; van Schalkwyk, M.; van der Merwe, H.; Mason, D.; Nachega, J.B. Combination antiretroviral therapy reduces the detection risk of cervical human papilloma virus infection in women living with HIV. AIDS 2015, 29, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Cercato, M.C.; Mariani, L.; Vocaturo, A.; Carrone, A.; Terrenato, I.; Morano, G.; Benevolo, M.; Rollo, F.; Germelli, C.; Paolini, F.; et al. Predictors of human papilloma virus (HPV) infection in Italian women. J. Med. Virol. 2010, 82, 1921–1927. [Google Scholar] [CrossRef]
- Aziz, H.; Sattar, A.A.; Mahmood, H.; Fatima, S.; Khurshid, M.; Faheem, M. Prevalence of HPV types in HIV-positive and negative females with normal cervical cytology or dysplasia. J. Clin. Lab. Anal. 2023, 37, e24851. [Google Scholar] [CrossRef]
- Luque, A.E.; Demeter, L.M.; Reichman, R.C. Association of Human Papillomavirus Infection and Disease with Magnitude of Human Immunodeficiency Virus Type 1 (HIV-1) RNA Plasma Level among Women with HIV-1 Infection. J. Infect. Dis. 1999, 179, 1405–1409. [Google Scholar] [CrossRef] [PubMed]
- Strickler, H.D.; Burk, R.D.; Fazzari, M.; Anastos, K.; Minkoff, H.; Massad, L.S.; Hall, C.; Bacon, M.; Levine, A.M.; Watts, D.H.; et al. Natural History and Possible Reactivation of Human Papillomavirus in Human Immunodeficiency Virus–Positive Women. JNCI J. Natl. Cancer Inst. 2005, 97, 577–586. [Google Scholar] [CrossRef]
- Konopnicki, D.; Manigart, Y.; Gilles, C.; Barlow, P.; de Marchin, J.; Feoli, F.; Larsimont, D.; Delforge, M.; De Wit, S.; Clumeck, N. Sustained Viral Suppression and Higher CD4+ T-Cell Count Reduces the Risk of Persistent Cervical High-Risk Human Papillomavirus Infection in HIV-Positive Women. J. Infect. Dis. 2013, 207, 1723–1729. [Google Scholar] [CrossRef] [PubMed]
- Dom-Chima, N.; Ajang, Y.A.; Dom-Chima, C.I.; Biswas-Fiss, E.; Aminu, M.; Biswas, S.B. Human papillomavirus spectrum of HPV-infected women in Nigeria: An analysis by next-generation sequencing and type-specific PCR. Virol. J. 2023, 20, 144. [Google Scholar] [CrossRef]
- Menzo, S.; Trozzi, C.; Clementi, M.M. Human Papillomavirus Type 67 L1 Gene, Partial, Isolate han 1464. 2016. Available online: http://www.ncbi.nlm.nih.gov/nuccore/Y12207.1 (accessed on 30 April 2025).
- Gandekon, C.; Imorou, R.S.; Zohoncon, T.; Gomina, M.; Ouedraogo, A.; Traore, I.; Dolou, E.; Bado, P.; Traore, E.; Ouedraogo, C.; et al. Prevalence of Oncogenic Human Papillomavirus Genotypes Among Sexually Active Women in Parakou (Benin, West Africa). J. Gynecol. Obstet. 2020, 8, 102. [Google Scholar] [CrossRef]
- Denny, L.; Adewole, I.; Anorlu, R.; Dreyer, G.; Moodley, M.; Smith, T.; Snyman, L.; Wiredu, E.; Molijn, A.; Quint, W.; et al. Human papillomavirus prevalence and type distribution in invasive cervical cancer in sub-Saharan Africa. Int. J. Cancer 2014, 134, 1389–1398. [Google Scholar] [CrossRef]
- Paavonen, J.; Naud, P.; Salmerón, J.; Wheeler, C.M.; Chow, S.-N.; Apter, D.; Kitchener, H.; Castellsagué, X.; Teixeira, J.C.; Skinner, S.R.; et al. Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): Final analysis of a double-blind, randomised study in young women. Lancet 2009, 374, 301–314. [Google Scholar] [CrossRef]
- Brown, D.R.; Kjaer, S.K.; Sigurdsson, K.; Iversen, O.-E.; Hernandez-Avila, M.; Wheeler, C.M.; Perez, G.; Koutsky, L.A.; Tay, E.H.; Garcia, P.; et al. The Impact of Quadrivalent Human Papillomavirus (HPV; Types 6, 11, 16, and 18) L1 Virus-Like Particle Vaccine on Infection and Disease Due to Oncogenic Nonvaccine HPV Types in Generally HPV-Naive Women Aged 16–26 Years. J. Infect. Dis. 2009, 199, 926–935. [Google Scholar] [CrossRef] [PubMed]
- Michaeli, D.T.; Stoycheva, S.; Marcus, S.M.; Zhang, W.; Michaeli, J.C.; Michaeli, T. Cost-Effectiveness of Bivalent, Quadrivalent, and Nonavalent HPV Vaccination in South Africa. Clin. Drug Investig. 2022, 42, 333–343. [Google Scholar] [CrossRef] [PubMed]


| Baseline Characteristics | WLWHIV (n = 100) (%) | WWHIV (n = 51) (%) | p-Value |
|---|---|---|---|
| Age (year) 20–29 30–39 40–49 50–59 60–69 No information | 11 (11) 21 (21) 38 (38) 24 (24) 6 (6) 0 (0) | 11 (21.56) 14 (27.45) 12 (23.53) 10 (19.61) 2 (3.92) 2 (3.92) | p = 0.033 |
| Marital status Married Single Widow Divorced | 35 (35) 45 (45) 17 (17) 3 (3) | 42 (82.3) 8 (15.7) 0 (0) 1 (1.9) | p < 0.001 |
| Education level No education Primary Secondary High | 26 (26) 29 (29) 40 (40) 5 (5) | 1 (1.9) 6 (11.8) 18 (35.3) 26 (51) | p < 0.001 |
| Number of sexual partners ≤5 6–10 >10 Not disclosed | 77 (77) 6 (6) 3 (3) 14 (14) | 23 (45.1) 0 (0) 0 (0) 28 (54.9) | p = 0.001 |
| Age at first sexual intercourse <15 15–17 18–20 21–24 >24 No information | 3 (3) 30 (30) 52 (52) 7 (7) 3 (3) 5 (5) | 3 (5.88) 9 (17.64) 21 (41.17) 6 (11.76) 5 (9.80) 7 (13.72) | p = 0.003 |
| Use of Oral contraceptives Yes No No information | 38 (38) 54 (54) 8 (8) | 17 (33.33) 32 (62.74) 2 (3.92) | p = 0.034 |
| Alcohol consumption Yes No No information | 81 (81) 19 (19) 0 (0) | 12 (23.52) 34 (66.66) 5 | p < 0.001 |
| Use of condom Always Sometimes Never No information | 11 (11) 52 (52) 35 (35) 2 (2) | 1 (1.9) 32 (62.74) 16 (31.37) 2 (3.92) | p = 0.055 |
| HIV viral load Undetectable <1000 1000–10,000 >10,000 No information | 42 (42%) 8 (8%) 14 (14%) 12 (12%) 24 (24%) | ||
| Recent CD4+ counts <200 200–500 >500 No information | 6 (6%) 29 (29%) 52 (52%) 13 (13%) |
| Variables | Total | HPV | OR (CI) | p-Value | AOR | p-Value |
|---|---|---|---|---|---|---|
| Age (years old) ≥35 <35 | 83 17 | 69 16 | 1 3.2 (0.4–26.5) | 0.5 | - | - |
| Marital status Married Single | 35 65 | 25 60 | 1 4.8 (1.5–15.5) | 0.01 | 1 4.6 (1.3–15.9) | 0.02 |
| Use of condom Yes No | 63 35 | 53 30 | 1 1.1 (0.3–3.6) | 1.0 | - | - |
| Use of contraception No Yes | 54 38 | 44 34 | 1 2 (0.6–6.7) | 0.4 | - | - |
| Alcohol consumption No Yes | 19 81 | 17 68 | 1 0.6 (0.13–3.0) | 0.7 | - | - |
| Age of first sexual intercourse ≥18 <18 | 62 33 | 51 29 | 1 1.6 (0.5–5.4) | 0.6 | - | - |
| Number of sexual partners <4 ≥4 | 55 31 | 46 28 | 1 1.8 (0.4–7.3) | 0.5 | - | - |
| Enrollment’s year of ART ≥2014 <2014 | 48 46 | 44 36 | 1 0.3 (0.1–1.1) | 0.09 | - | - |
| Initial CD4+ ≥200 <200 | 41 38 | 35 33 | 1 1.1 (0.3–4.1) | 1.0 | - | - |
| Frequency of ART use Regular Irregular | 29 20 | 25 16 | 1 0.6 (0.1–2.9) | 0.7 | - | - |
| HIV viral load Undetectable Detectable | 53 36 | 41 34 | 1 5.0 (1.0–23.7) | 0.04 | 1 5.3 (1.1–26.4) | 0.04 |
| Recent CD4+ count ≥500 <500 | 52 35 | 42 31 | 1 1.4 (0.5–6.4) | 0.4 | - | - |
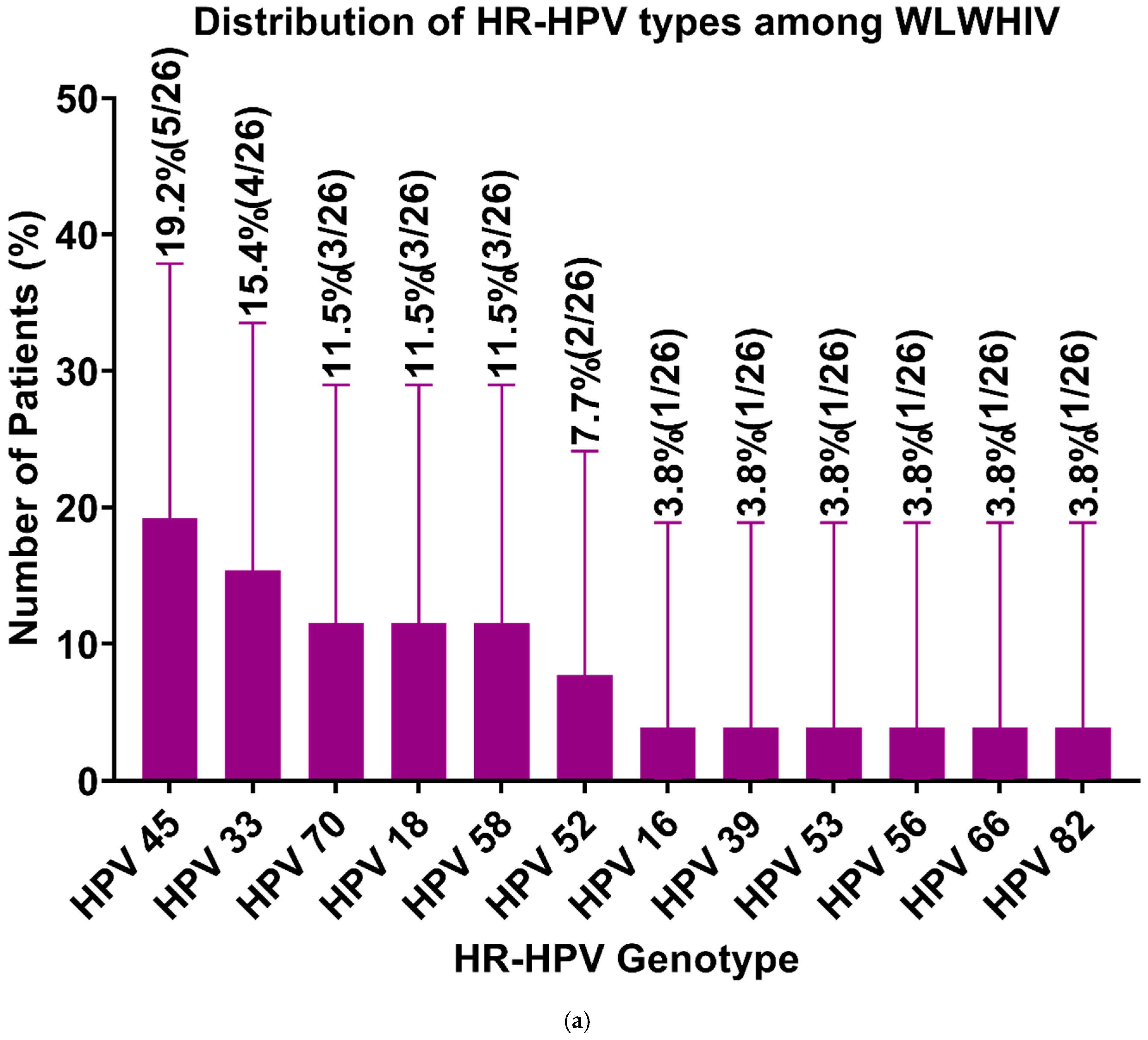
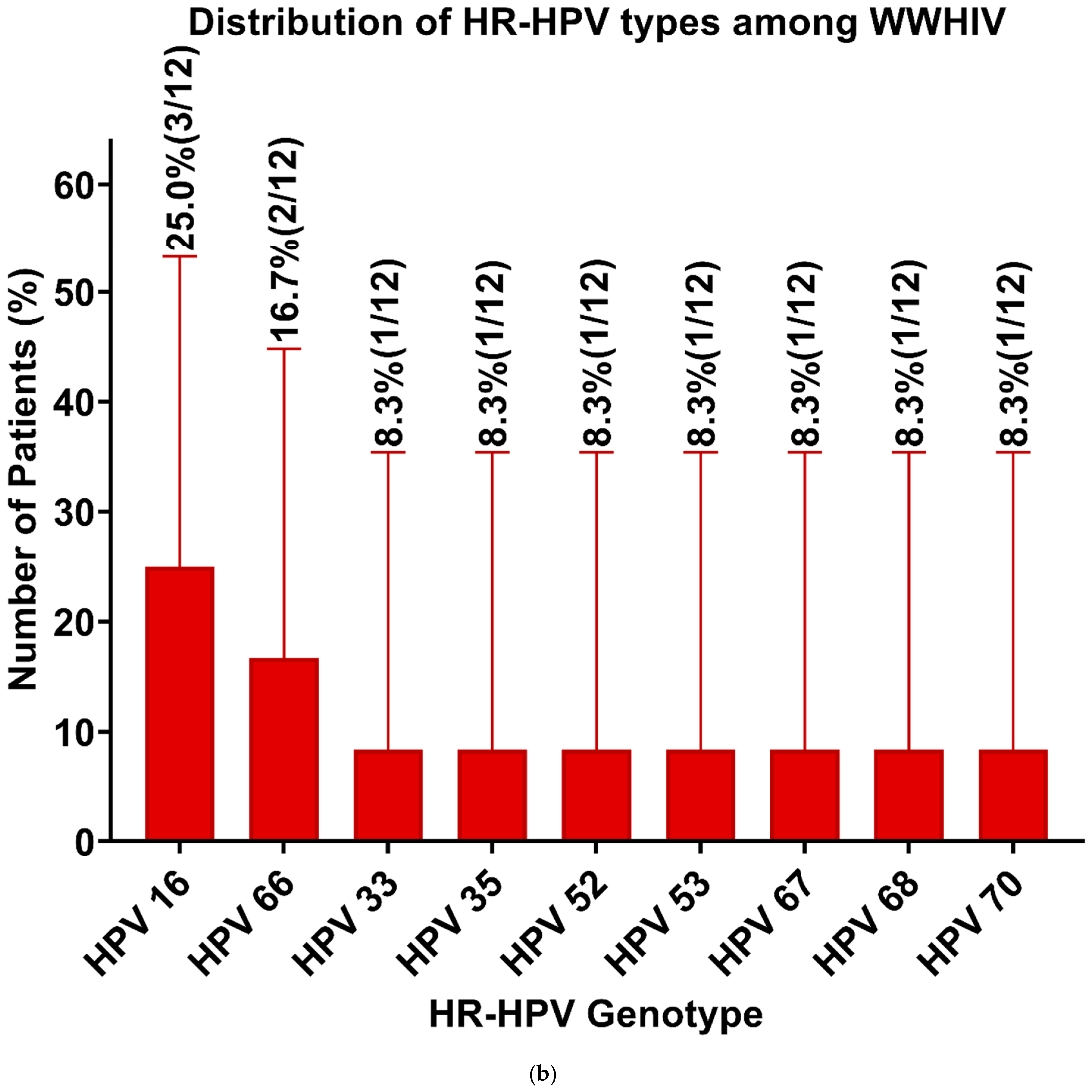
| HPV Genotypes | Generus + Species | WLWHIV (n = 46) (%) | WWHIV (n = 21) (%) |
|---|---|---|---|
| High-risk HPV | |||
| HPV 16 | α- 9 | 1 (2.2) | 3 (14.3) |
| HPV 18 | α- 7 | 3 (6.5) | 0 (0) |
| HPV 33 | α- 9 | 4 (8.7) | 1 (4.8) |
| HPV 35 | α- 9 | 0 (0) | 1 (4.8) |
| HPV 39 | α- 7 | 1 (2.2) | 0 (0) |
| HPV 45 | α- 7 | 5 (10.9) | 0 (0) |
| HPV 52 | α- 9 | 2 (4.3) | 1 (4.8) |
| HPV 53 | α- 6 | 1 (2.2) | 1 (4.8) |
| HPV 56 | α- 6 | 1 (2.2) | 0 (0) |
| HPV 58 | α- 9 | 3 (6.5) | 0 (0) |
| HPV 66 | α- 6 | 1 (2.2) | 2 (9.5) |
| HPV 67 | α- 9 | 0 (0) | 1 (4.8) |
| HPV 68 | α- 7 | 0 (0) | 1 (4.8) |
| HPV 70 | α- 7 | 3 (6.5) | 1 (4.8) |
| HPV 82 | α- 5 | 1 (2.2) | 0 (0) |
| Low-risk HPV | |||
| HPV 6 | α- 10 | 2 (4.3) | 0 (0) |
| HPV 32 | α- 1 | 0 (0) | 1 (4.8) |
| HPV 42 | α- 1 | 1 (2.2) | 0 (0) |
| HPV 54 | α- 13 | 3 (6.5) | 3 (14.3) |
| HPV 61 | α- 3 | 0 (0) | 1 (4.8) |
| HPV 62 | α- 3 | 4 (8.7) | 2 (9.5) |
| HPV 72 | α- 3 | 2 (4.3) | 0 (0) |
| HPV 81 | α- 3 | 4 (8.7) | 0 (0) |
| HPV 83 | α- 3 | 3 (6.5) | 1 (4.8) |
| HPV 90 | 0 (0) | 1 (4.8) | |
| HPV 114 | α- 3 | 1 (2.2) | 0 (0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gouton, C.D.; Bejide, I.O.; Ope-ewe, O.O.; Adjagba, M.; Azonbakin, S.; Muzanywa, G.; Akinyi, F.T.; Agbanlinsou, A.; Goussanou, Y.; Folarin, O.; et al. HIV–HPV Co-Infection and Identification of Novel High-Risk HPV Among Women at Two Hospital Centers in Cotonou, Republic of Benin. Viruses 2025, 17, 714. https://doi.org/10.3390/v17050714
Gouton CD, Bejide IO, Ope-ewe OO, Adjagba M, Azonbakin S, Muzanywa G, Akinyi FT, Agbanlinsou A, Goussanou Y, Folarin O, et al. HIV–HPV Co-Infection and Identification of Novel High-Risk HPV Among Women at Two Hospital Centers in Cotonou, Republic of Benin. Viruses. 2025; 17(5):714. https://doi.org/10.3390/v17050714
Chicago/Turabian StyleGouton, Clémence D., Ifeoluwa O. Bejide, Oludayo O. Ope-ewe, Marius Adjagba, Simon Azonbakin, Gaonyadiwe Muzanywa, Florence T. Akinyi, Arnaud Agbanlinsou, Yanique Goussanou, Onikepe Folarin, and et al. 2025. "HIV–HPV Co-Infection and Identification of Novel High-Risk HPV Among Women at Two Hospital Centers in Cotonou, Republic of Benin" Viruses 17, no. 5: 714. https://doi.org/10.3390/v17050714
APA StyleGouton, C. D., Bejide, I. O., Ope-ewe, O. O., Adjagba, M., Azonbakin, S., Muzanywa, G., Akinyi, F. T., Agbanlinsou, A., Goussanou, Y., Folarin, O., Laleye, A., Happi, C. T., & Ugwu, C. A. (2025). HIV–HPV Co-Infection and Identification of Novel High-Risk HPV Among Women at Two Hospital Centers in Cotonou, Republic of Benin. Viruses, 17(5), 714. https://doi.org/10.3390/v17050714








