Guinea Pigs Are Not a Suitable Model to Study Neurological Impacts of Ancestral SARS-CoV-2 Intranasal Infection
Abstract
1. Introduction
2. Materials and Methods
2.1. Viruses and Cells
2.2. Guinea Pig Infections, Assessment, Tissue Collection and Processing
2.3. RNA Extraction and SARS-CoV-2 RT-qPCR
2.4. Plaque Assays
2.5. Immunostaining
2.6. Quantification and Statistical Analysis
3. Results
3.1. Clinical Assessment Revealed No Overt Disease Following SARS-CoV-2 Inoculation
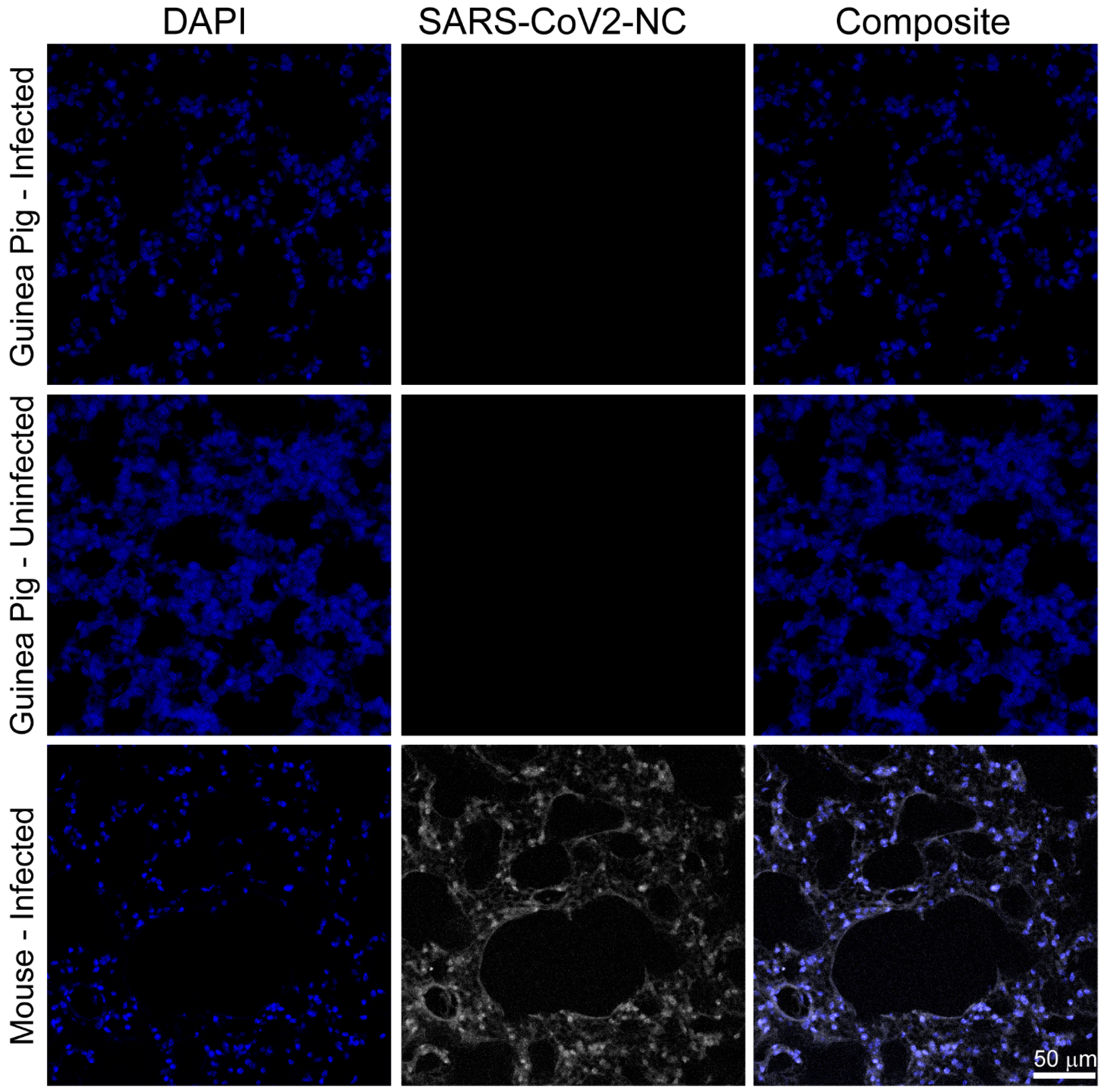
3.2. SARS-CoV-2 RNA and Nucleocapsid Are Undetectable in Tissues Three and Six Days Post Infection
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Padilla-Carlin, D.J.; McMurray, D.N.; Hickey, A.J. The guinea pig as a model of infectious diseases. Comp. Med. 2008, 58, 324–340. [Google Scholar] [PubMed]
- Canning, B.J.; Chou, Y. Using guinea pigs in studies relevant to asthma and COPD. Pulm. Pharmacol. Ther. 2008, 21, 702–720. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.K.; Lipatov, A.S.; Swayne, D.E. Bronchointerstitial pneumonia in guinea pigs following inoculation with H5N1 high pathogenicity avian influenza virus. Vet. Pathol. 2009, 46, 138–141. [Google Scholar] [CrossRef]
- Kumar, M.; Krause, K.K.; Azouz, F.; Nakano, E.; Nerurkar, V.R. A guinea pig model of Zika virus infection. Virol. J. 2017, 14, 75. [Google Scholar] [CrossRef]
- Hook, L.M.; Friedman, H.M.; Awasthi, S. Guinea Pig and Mouse Models for Genital Herpes Infection. Curr. Protoc. 2021, 1, e332. [Google Scholar] [CrossRef]
- Yadavalli, T.; Patil, C.; Sharma, P.; Volety, I.; Borase, H.; Kapoor, D.; Shukla, D. Unique Attributes of Guinea Pigs as New Models to Study Ocular Herpes Pathophysiology and Recurrence. Investig. Ophthalmol. Vis. Sci. 2023, 64, 41. [Google Scholar] [CrossRef]
- Laemmle, L.; Goldstein, R.S.; Kinchington, P.R. Modeling Varicella Zoster Virus Persistence and Reactivation—Closer to Resolving a Perplexing Persistent State. Front. Microbiol. 2019, 10, 1634. [Google Scholar] [CrossRef]
- Chepurnov, A.A.; Dadaeva, A.A.; Malkova, E.M.; Kolesnikov, S.I.; Sandakhchiev, L.S. Symptoms of infection caused by SARS coronavirus in laboratory mice and guinea pigs. Dokl. Biol. Sci. 2004, 397, 310–313. [Google Scholar] [CrossRef]
- Liang, L.; He, C.; Lei, M.; Li, S.; Hao, Y.; Zhu, H.; Duan, Q. Pathology of guinea pigs experimentally infected with a novel reovirus and coronavirus isolated from SARS patients. DNA Cell Biol. 2005, 24, 485–490. [Google Scholar] [CrossRef]
- McCray, P.B., Jr.; Pewe, L.; Wohlford-Lenane, C.; Hickey, M.; Manzel, L.; Shi, L.; Netland, J.; Jia, H.P.; Halabi, C.; Sigmund, C.D.; et al. Lethal infection of K18-hACE2 mice infected with severe acute respiratory syndrome coronavirus. J. Virol. 2007, 81, 813–821. [Google Scholar] [CrossRef]
- Oladunni, F.S.; Park, J.G.; Pino, P.A.; Gonzalez, O.; Akhter, A.; Allue-Guardia, A.; Olmo-Fontanez, A.; Gautam, S.; Garcia-Vilanova, A.; Ye, C.; et al. Lethality of SARS-CoV-2 infection in K18 human angiotensin-converting enzyme 2 transgenic mice. Nat. Commun. 2020, 11, 6122. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.; Wu, Y.; Rui, X.; Yang, Y.; Ling, C.; Liu, S.; Liu, S.; Wang, Y. Animal models for COVID-19: Advances, gaps and perspectives. Signal Transduct. Target. Ther. 2022, 7, 220. [Google Scholar] [CrossRef] [PubMed]
- Imai, M.; Iwatsuki-Horimoto, K.; Hatta, M.; Loeber, S.; Halfmann, P.J.; Nakajima, N.; Watanabe, T.; Ujie, M.; Takahashi, K.; Ito, M.; et al. Syrian hamsters as a small animal model for SARS-CoV-2 infection and countermeasure development. Proc. Natl. Acad. Sci. USA 2020, 117, 16587–16595. [Google Scholar] [CrossRef]
- Niu, S.; Zhao, Z.; Liu, Z.; Rong, X.; Chai, Y.; Bai, B.; Han, P.; Shang, G.; Ren, J.; Wang, Y.; et al. Structural basis and analysis of hamster ACE2 binding to different SARS-CoV-2 spike RBDs. J. Virol. 2024, 98, e0115723. [Google Scholar] [CrossRef]
- Piplani, S.; Singh, P.K.; Winkler, D.A.; Petrovsky, N. In silico comparison of SARS-CoV-2 spike protein-ACE2 binding affinities across species and implications for virus origin. Sci. Rep. 2021, 11, 13063. [Google Scholar]
- Zheng, J.; Wong, L.R.; Li, K.; Verma, A.K.; Ortiz, M.E.; Wohlford-Lenane, C.; Leidinger, M.R.; Knudson, C.M.; Meyerholz, D.K.; McCray, P.B., Jr.; et al. COVID-19 treatments and pathogenesis including anosmia in K18-hACE2 mice. Nature 2021, 589, 603–607. [Google Scholar] [CrossRef]
- Rathnasinghe, R.; Strohmeier, S.; Amanat, F.; Gillespie, V.L.; Krammer, F.; Garcia-Sastre, A.; Coughlan, L.; Schotsaert, M.; Uccellini, M.B. Comparison of transgenic and adenovirus hACE2 mouse models for SARS-CoV-2 infection. Emerg. Microbes Infect. 2020, 9, 2433–2445. [Google Scholar] [CrossRef]
- Winkler, E.S.; Bailey, A.L.; Kafai, N.M.; Nair, S.; McCune, B.T.; Yu, J.; Fox, J.M.; Chen, R.E.; Earnest, J.T.; Keeler, S.P.; et al. SARS-CoV-2 infection of human ACE2-transgenic mice causes severe lung inflammation and impaired function. Nat. Immunol. 2020, 21, 1327–1335. [Google Scholar] [CrossRef]
- Golden, J.W.; Cline, C.R.; Zeng, X.; Garrison, A.R.; Carey, B.D.; Mucker, E.M.; White, L.E.; Shamblin, J.D.; Brocato, R.L.; Liu, J.; et al. Human angiotensin-converting enzyme 2 transgenic mice infected with SARS-CoV-2 develop severe and fatal respiratory disease. JCI Insight 2020, 5, e142032. [Google Scholar] [CrossRef]
- Liotta, E.M.; Batra, A.; Clark, J.R.; Shlobin, N.A.; Hoffman, S.C.; Orban, Z.S.; Koralnik, I.J. Frequent neurologic manifestations and encephalopathy-associated morbidity in Covid-19 patients. Ann. Clin. Transl. Neurol. 2020, 7, 2221–2230. [Google Scholar] [CrossRef]
- Chou, S.H.; Beghi, E.; Helbok, R.; Moro, E.; Sampson, J.; Altamirano, V.; Mainali, S.; Bassetti, C.; Suarez, J.I.; McNett, M.; et al. Global Incidence of Neurological Manifestations Among Patients Hospitalized With COVID-19-A Report for the GCS-NeuroCOVID Consortium and the ENERGY Consortium. JAMA Netw. Open 2021, 4, e2112131. [Google Scholar] [CrossRef] [PubMed]
- Misra, S.; Kolappa, K.; Prasad, M.; Radhakrishnan, D.; Thakur, K.T.; Solomon, T.; Michael, B.D.; Winkler, A.S.; Beghi, E.; Guekht, A.; et al. Frequency of Neurologic Manifestations in COVID-19: A Systematic Review and Meta-analysis. Neurology 2021, 97, e2269–e2281. [Google Scholar] [CrossRef] [PubMed]
- Pinzon, R.T.; Wijaya, V.O.; Jody, A.A.; Nunsio, P.N.; Buana, R.B. Persistent neurological manifestations in long COVID-19 syndrome: A systematic review and meta-analysis. J. Infect. Public Health 2022, 15, 856–869. [Google Scholar] [CrossRef]
- Siddiqui, S.; Alhamdi, H.W.S.; Alghamdi, H.A. Recent Chronology of COVID-19 Pandemic. Front. Public Health 2022, 10, 778037. [Google Scholar] [CrossRef]
- Chandrashekar, A.; Liu, J.; Yu, J.; McMahan, K.; Tostanoski, L.H.; Jacob-Dolan, C.; Mercado, N.B.; Anioke, T.; Chang, A.; Gardner, S.; et al. Prior infection with SARS-CoV-2 WA1/2020 partially protects rhesus macaques against reinfection with B.1.1.7 and B.1.351 variants. Sci. Transl. Med. 2021, 13, eabj2641. [Google Scholar] [CrossRef]
- World Health Organization. Weekly Epidemiological Update—29 December 2020. Emergency Situational Updates 2020. Available online: https://www.who.int/publications/m/item/weekly-epidemiological-update---29-december-2020 (accessed on 6 May 2025).
- Aleem, A.; Akbar Samad, A.B.; Vaqar, S. Emerging Variants of SARS-CoV-2 and Novel Therapeutics Against Coronavirus (COVID-19). In StatPearls; Treasure Island (FL) Ineligible Companies: Treasure Island, FL, USA, 2025. [Google Scholar]
- Joyce, J.D.; Moore, G.A.; Goswami, P.; Harrell, T.L.; Taylor, T.M.; Hawks, S.A.; Green, J.C.; Jia, M.; Irwin, M.D.; Leslie, E.; et al. SARS-CoV-2 Rapidly Infects Peripheral Sensory and Autonomic Neurons, Contributing to Central Nervous System Neuroinvasion before Viremia. Int. J. Mol. Sci. 2024, 25, 8245. [Google Scholar] [CrossRef]
- Kumari, P.; Rothan, H.A.; Natekar, J.P.; Stone, S.; Pathak, H.; Strate, P.G.; Arora, K.; Brinton, M.A.; Kumar, M. Neuroinvasion and Encephalitis Following Intranasal Inoculation of SARS-CoV-2 in K18-hACE2 Mice. Viruses 2021, 13, 132. [Google Scholar] [CrossRef]
- Yinda, C.K.; Port, J.R.; Bushmaker, T.; Offei Owusu, I.; Purushotham, J.N.; Avanzato, V.A.; Fischer, R.J.; Schulz, J.E.; Holbrook, M.G.; Hebner, M.J.; et al. K18-hACE2 mice develop respiratory disease resembling severe COVID-19. PLoS Pathog. 2021, 17, e1009195. [Google Scholar] [CrossRef]
- Li, C.; Song, W.; Chan, J.F.; Chen, Y.; Liu, F.; Ye, Z.; Lam, A.H.; Cai, J.; Lee, A.C.; Wong, B.H.; et al. Intranasal infection by SARS-CoV-2 Omicron variants can induce inflammatory brain damage in newly weaned hamsters. Emerg. Microbes Infect. 2023, 12, 2207678. [Google Scholar] [CrossRef]
- Carossino, M.; Kenney, D.; O’Connell, A.K.; Montanaro, P.; Tseng, A.E.; Gertje, H.P.; Grosz, K.A.; Ericsson, M.; Huber, B.R.; Kurnick, S.A.; et al. Fatal Neurodissemination and SARS-CoV-2 Tropism in K18-hACE2 Mice Is Only Partially Dependent on hACE2 Expression. Viruses 2022, 14, 535. [Google Scholar] [CrossRef]
- Lau, S.K.; Woo, P.C.; Wong, B.H.; Tsoi, H.W.; Woo, G.K.; Poon, R.W.; Chan, K.H.; Wei, W.I.; Peiris, J.S.; Yuen, K.Y. Detection of severe acute respiratory syndrome (SARS) coronavirus nucleocapsid protein in sars patients by enzyme-linked immunosorbent assay. J. Clin. Microbiol. 2004, 42, 2884–2889. [Google Scholar] [CrossRef] [PubMed]
- Lien, S.P.; Shih, Y.P.; Chen, H.W.; Tsai, J.P.; Leng, C.H.; Lin, M.H.; Lin, L.H.; Liu, H.Y.; Chou, A.H.; Chang, Y.W.; et al. Identification of synthetic vaccine candidates against SARS CoV infection. Biochem. Biophys. Res. Commun. 2007, 358, 716–721. [Google Scholar] [CrossRef] [PubMed]
- Vlasova, A.N.; Zhang, X.; Hasoksuz, M.; Nagesha, H.S.; Haynes, L.M.; Fang, Y.; Lu, S.; Saif, L.J. Two-way antigenic cross-reactivity between severe acute respiratory syndrome coronavirus (SARS-CoV) and group 1 animal CoVs is mediated through an antigenic site in the N-terminal region of the SARS-CoV nucleoprotein. J. Virol. 2007, 81, 13365–13377. [Google Scholar] [CrossRef]
- Totura, A.L.; Bavari, S. Broad-spectrum coronavirus antiviral drug discovery. Expert. Opin. Drug Discov. 2019, 14, 397–412. [Google Scholar] [CrossRef]
- Zhou, P.; Yang, X.L.; Wang, X.G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.R.; Zhu, Y.; Li, B.; Huang, C.L.; et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 579, 270–273. [Google Scholar] [CrossRef]
- Shang, J.; Ye, G.; Shi, K.; Wan, Y.; Luo, C.; Aihara, H.; Geng, Q.; Auerbach, A.; Li, F. Structural basis of receptor recognition by SARS-CoV-2. Nature 2020, 581, 221–224. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhang, Y.; Wu, L.; Niu, S.; Song, C.; Zhang, Z.; Lu, G.; Qiao, C.; Hu, Y.; Yuen, K.Y.; et al. Structural and Functional Basis of SARS-CoV-2 Entry by Using Human ACE2. Cell 2020, 181, 894–904 e9. [Google Scholar] [CrossRef]
- Mao, L.; Jin, H.; Wang, M.; Hu, Y.; Chen, S.; He, Q.; Chang, J.; Hong, C.; Zhou, Y.; Wang, D.; et al. Neurologic Manifestations of Hospitalized Patients With Coronavirus Disease 2019 in Wuhan, China. JAMA Neurol. 2020, 77, 683–690. [Google Scholar] [CrossRef]
- Conceicao, C.; Thakur, N.; Human, S.; Kelly, J.T.; Logan, L.; Bialy, D.; Bhat, S.; Stevenson-Leggett, P.; Zagrajek, A.K.; Hollinghurst, P.; et al. The SARS-CoV-2 Spike protein has a broad tropism for mammalian ACE2 proteins. PLoS Biol. 2020, 18, e3001016. [Google Scholar] [CrossRef]
- Li, K.K.; Yip, C.W.; Hon, C.C.; Lam, C.Y.; Zeng, F.; Leung, F.C. Characterisation of animal angiotensin-converting enzyme 2 receptors and use of pseudotyped virus to correlate receptor binding with susceptibility of SARS-CoV infection. Hong. Kong Med. J. 2012, 18 (Suppl. S3), 35–38. [Google Scholar]
- Brooke, G.N.; Prischi, F. Structural and functional modelling of SARS-CoV-2 entry in animal models. Sci. Rep. 2020, 10, 15917. [Google Scholar] [CrossRef]
- Iwatsuki-Horimoto, K.; Kiso, M.; Ito, M.; Yamayoshi, S.; Kawaoka, Y. Sensitivity of rodents to SARS-CoV-2: Gerbils are susceptible to SARS-CoV-2, but guinea pigs are not. NPJ Viruses 2024, 2, 59. [Google Scholar] [CrossRef]
- Smith, T.R.F.; Patel, A.; Ramos, S.; Elwood, D.; Zhu, X.; Yan, J.; Gary, E.N.; Walker, S.N.; Schultheis, K.; Purwar, M.; et al. Immunogenicity of a DNA vaccine candidate for COVID-19. Nat. Commun. 2020, 11, 2601. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, Y.; Huang, B.; Deng, W.; Quan, Y.; Wang, W.; Xu, W.; Zhao, Y.; Li, N.; Zhang, J.; et al. Development of an Inactivated Vaccine Candidate, BBIBP-CorV, with Potent Protection against SARS-CoV-2. Cell 2020, 182, 713–721 e9. [Google Scholar] [CrossRef]
- Malladi, S.K.; Singh, R.; Pandey, S.; Gayathri, S.; Kanjo, K.; Ahmed, S.; Khan, M.S.; Kalita, P.; Girish, N.; Upadhyaya, A.; et al. Design of a highly thermotolerant, immunogenic SARS-CoV-2 spike fragment. J. Biol. Chem. 2021, 296, 100025. [Google Scholar] [CrossRef]
- Kandeil, A.; Mostafa, A.; Hegazy, R.R.; El-Shesheny, R.; El Taweel, A.; Gomaa, M.R.; Shehata, M.; Elbaset, M.A.; Kayed, A.E.; Mahmoud, S.H.; et al. Immunogenicity and Safety of an Inactivated SARS-CoV-2 Vaccine: Preclinical Studies. Vaccines 2021, 9, 214. [Google Scholar] [CrossRef]
- Dey, A.; Chozhavel Rajanathan, T.M.; Chandra, H.; Pericherla, H.P.R.; Kumar, S.; Choonia, H.S.; Bajpai, M.; Singh, A.K.; Sinha, A.; Saini, G.; et al. Immunogenic potential of DNA vaccine candidate, ZyCoV-D against SARS-CoV-2 in animal models. Vaccine 2021, 39, 4108–4116. [Google Scholar] [CrossRef]
- Malladi, S.K.; Patel, U.R.; Rajmani, R.S.; Singh, R.; Pandey, S.; Kumar, S.; Khaleeq, S.; van Vuren, P.J.; Riddell, S.; Goldie, S.; et al. Immunogenicity and Protective Efficacy of a Highly Thermotolerant, Trimeric SARS-CoV-2 Receptor Binding Domain Derivative. ACS Infect. Dis. 2021, 7, 2546–2564. [Google Scholar] [CrossRef]
- Langereis, M.A.; Albulescu, I.C.; Stammen-Vogelzangs, J.; Lambregts, M.; Stachura, K.; Miller, S.; Bosco-Lauth, A.M.; Hartwig, A.E.; Porter, S.M.; Allen, M.; et al. An alphavirus replicon-based vaccine expressing a stabilized Spike antigen induces protective immunity and prevents transmission of SARS-CoV-2 between cats. NPJ Vaccines 2021, 6, 122. [Google Scholar] [CrossRef]
- Ghasemi, S.; Naderi Saffar, K.; Ebrahimi, F.; Khatami, P.; Monazah, A.; Alizadeh, G.A.; Ettehadi, H.A.; Rad, I.; Nojehdehi, S.; Kehtari, M.; et al. Development of Inactivated FAKHRAVAC((R)) Vaccine against SARS-CoV-2 Virus: Preclinical Study in Animal Models. Vaccines 2021, 9, 1271. [Google Scholar] [CrossRef]
- Kozlovskaya, L.I.; Piniaeva, A.N.; Ignatyev, G.M.; Gordeychuk, I.V.; Volok, V.P.; Rogova, Y.V.; Shishova, A.A.; Kovpak, A.A.; Ivin, Y.Y.; Antonova, L.P.; et al. Long-term humoral immunogenicity, safety and protective efficacy of inactivated vaccine against COVID-19 (CoviVac) in preclinical studies. Emerg. Microbes Infect. 2021, 10, 1790–1806. [Google Scholar] [CrossRef] [PubMed]
- Konrath, K.M.; Liaw, K.; Wu, Y.; Zhu, X.; Walker, S.N.; Xu, Z.; Schultheis, K.; Chokkalingam, N.; Chawla, H.; Du, J.; et al. Nucleic acid delivery of immune-focused SARS-CoV-2 nanoparticles drives rapid and potent immunogenicity capable of single-dose protection. Cell Rep. 2022, 38, 110318. [Google Scholar] [CrossRef]
- Abdoli, A.; Aalizadeh, R.; Aminianfar, H.; Kianmehr, Z.; Teimoori, A.; Azimi, E.; Emamipour, N.; Eghtedardoost, M.; Siavashi, V.; Jamshidi, H.; et al. Safety and potency of BIV1-CovIran inactivated vaccine candidate for SARS-CoV-2: A preclinical study. Rev. Med. Virol. 2022, 32, e2305. [Google Scholar] [CrossRef]
- Banihashemi, S.R.; Es-Haghi, A.; Fallah Mehrabadi, M.H.; Nofeli, M.; Mokarram, A.R.; Ranjbar, A.; Salman, M.; Hajimoradi, M.; Razaz, S.H.; Taghdiri, M.; et al. Safety and Efficacy of Combined Intramuscular/Intranasal RAZI-COV PARS Vaccine Candidate Against SARS-CoV-2: A Preclinical Study in Several Animal Models. Front. Immunol. 2022, 13, 836745. [Google Scholar] [CrossRef]
- Huang, Z.; Li, Y.; Yi, H.; Wu, Z.; Li, C.; Du, T.; Yang, J.; Wang, Y.; Jiang, Q.; Fan, S.; et al. Absence of active systemic anaphylaxis in guinea pigs upon intramuscular injection of inactivated SARS-CoV-2 vaccine (Vero cells). Immunopharmacol. Immunotoxicol. 2022, 44, 633–640. [Google Scholar] [CrossRef]
- Vishwanath, S.; Carnell, G.W.; Ferrari, M.; Asbach, B.; Billmeier, M.; George, C.; Sans, M.S.; Nadesalingam, A.; Huang, C.Q.; Paloniemi, M.; et al. A computationally designed antigen eliciting broad humoral responses against SARS-CoV-2 and related sarbecoviruses. Nat. Biomed. Eng. 2025, 9, 153–166. [Google Scholar] [CrossRef]
- Andrade, V.M.; Maricic, I.; Kalia, R.; Jachimowicz, L.; Bedoya, O.; Kulp, D.W.; Humeau, L.; Smith, T.R.F. Delineation of DNA and mRNA COVID-19 vaccine-induced immune responses in preclinical animal models. Hum. Vaccin. Immunother. 2023, 19, 2281733. [Google Scholar] [CrossRef]
- Li, T.; Cui, Z.; Jia, Y.; Liang, Z.; Nie, J.; Zhang, L.; Wang, M.; Li, Q.; Wu, J.; Xu, N.; et al. Aggregation of high-frequency RBD mutations of SARS-CoV-2 with three VOCs did not cause significant antigenic drift. J. Med. Virol. 2022, 94, 2108–2125. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, M.; Liang, Z.; Zhang, L.; Wu, X.; Yang, C.; An, Y.; Tong, J.; Liu, S.; Li, T.; et al. Antigenicity comparison of SARS-CoV-2 Omicron sublineages with other variants contained multiple mutations in RBD. MedComm (2020) 2022, 3, e130. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, L.; Liang, Z.; Wang, N.; Liu, S.; Li, T.; Yu, Y.; Cui, Q.; Wu, X.; Nie, J.; et al. Cross-reactivity of eight SARS-CoV-2 variants rationally predicts immunogenicity clustering in sarbecoviruses. Signal Transduct. Target. Ther. 2022, 7, 256. [Google Scholar] [CrossRef]
- Kolehmainen, P.; Huttunen, M.; Iakubovskaia, A.; Maljanen, S.; Tauriainen, S.; Yatkin, E.; Pasternack, A.; Naves, R.; Toivonen, L.; Tahtinen, P.A.; et al. Coronavirus spike protein-specific antibodies indicate frequent infections and reinfections in infancy and among BNT162b2-vaccinated healthcare workers. Sci. Rep. 2023, 13, 8416. [Google Scholar] [CrossRef]
- Liang, Z.; Tong, J.; Sun, Z.; Liu, S.; Wu, J.; Wu, X.; Li, T.; Yu, Y.; Zhang, L.; Zhao, C.; et al. Rational prediction of immunogenicity clustering through cross-reactivity analysis of thirteen SARS-CoV-2 variants. J. Med. Virol. 2024, 96, e29314. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, K.; Kitazawa, H.; Kawahata, T.; Yuhara, K.; Masuya, T.; Kuroita, T.; Waki, K.; Koike, S.; Isobe, M.; Kurosawa, N. The development of a highly sensitive and quantitative SARS-CoV-2 rapid antigen test applying newly developed monoclonal antibodies to an automated chemiluminescent flow-through membrane immunoassay device. BMC Immunol. 2023, 24, 34. [Google Scholar] [CrossRef]
- Walker, S.N.; Chokkalingam, N.; Reuschel, E.L.; Purwar, M.; Xu, Z.; Gary, E.N.; Kim, K.Y.; Helble, M.; Schultheis, K.; Walters, J.; et al. SARS-CoV-2 Assays To Detect Functional Antibody Responses That Block ACE2 Recognition in Vaccinated Animals and Infected Patients. J. Clin. Microbiol. 2020, 58, 10–1128. [Google Scholar] [CrossRef]
- Cordero-Ortiz, M.; Resendiz-Sandoval, M.; Dehesa-Canseco, F.; Solis-Hernandez, M.; Perez-Sanchez, J.; Martinez-Borges, C.; Mata-Haro, V.; Hernandez, J. Development of a Multispecies Double-Antigen Sandwich ELISA Using N and RBD Proteins to Detect Antibodies against SARS-CoV-2. Animals 2023, 13, 3487. [Google Scholar] [CrossRef]
- Yen, H.L.; Sit, T.H.C.; Brackman, C.J.; Chuk, S.S.Y.; Gu, H.; Tam, K.W.S.; Law, P.Y.T.; Leung, G.M.; Peiris, M.; Poon, L.L.M.; et al. Transmission of SARS-CoV-2 delta variant (AY.127) from pet hamsters to humans, leading to onward human-to-human transmission: A case study. Lancet 2022, 399, 1070–1078. [Google Scholar] [CrossRef]
- Borel, N.; Ciuria, S.; Flury, T.; Basso, W.U.; Ruetten, M. Zoonotic potential of guinea pigs: Outbreak of cryptosporidiosis combined with chlamydiosis in a breeding guinea pig herd. Schweiz. Arch. Tierheilkd. 2023, 165, 59–63. [Google Scholar] [CrossRef]
- Osterrieder, N.; Bertzbach, L.D.; Dietert, K.; Abdelgawad, A.; Vladimirova, D.; Kunec, D.; Hoffmann, D.; Beer, M.; Gruber, A.D.; Trimpert, J. Age-Dependent Progression of SARS-CoV-2 Infection in Syrian Hamsters. Viruses 2020, 12, 779. [Google Scholar] [CrossRef]
- Yuan, L.; Zhu, H.; Zhou, M.; Ma, J.; Chen, R.; Chen, Y.; Chen, L.; Wu, K.; Cai, M.; Hong, J.; et al. Gender associates with both susceptibility to infection and pathogenesis of SARS-CoV-2 in Syrian hamster. Signal Transduct. Target. Ther. 2021, 6, 136. [Google Scholar] [CrossRef]
- Dhakal, S.; Ruiz-Bedoya, C.A.; Zhou, R.; Creisher, P.S.; Villano, J.S.; Littlefield, K.; Ruelas Castillo, J.; Marinho, P.; Jedlicka, A.E.; Ordonez, A.A.; et al. Sex Differences in Lung Imaging and SARS-CoV-2 Antibody Responses in a COVID-19 Golden Syrian Hamster Model. mBio 2021, 12, e0097421. [Google Scholar] [CrossRef]
- Sia, S.F.; Yan, L.M.; Chin, A.W.H.; Fung, K.; Choy, K.T.; Wong, A.Y.L.; Kaewpreedee, P.; Perera, R.; Poon, L.L.M.; Nicholls, J.M.; et al. Pathogenesis and transmission of SARS-CoV-2 in golden hamsters. Nature 2020, 583, 834–838. [Google Scholar] [CrossRef]
- Francis, M.E.; Goncin, U.; Kroeker, A.; Swan, C.; Ralph, R.; Lu, Y.; Etzioni, A.L.; Falzarano, D.; Gerdts, V.; Machtaler, S.; et al. SARS-CoV-2 infection in the Syrian hamster model causes inflammation as well as type I interferon dysregulation in both respiratory and non-respiratory tissues including the heart and kidney. PLoS Pathog. 2021, 17, e1009705. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Joyce, J.D.; Moore, G.A.; Thompson, C.K.; Bertke, A.S. Guinea Pigs Are Not a Suitable Model to Study Neurological Impacts of Ancestral SARS-CoV-2 Intranasal Infection. Viruses 2025, 17, 706. https://doi.org/10.3390/v17050706
Joyce JD, Moore GA, Thompson CK, Bertke AS. Guinea Pigs Are Not a Suitable Model to Study Neurological Impacts of Ancestral SARS-CoV-2 Intranasal Infection. Viruses. 2025; 17(5):706. https://doi.org/10.3390/v17050706
Chicago/Turabian StyleJoyce, Jonathan D., Greyson A. Moore, Christopher K. Thompson, and Andrea S. Bertke. 2025. "Guinea Pigs Are Not a Suitable Model to Study Neurological Impacts of Ancestral SARS-CoV-2 Intranasal Infection" Viruses 17, no. 5: 706. https://doi.org/10.3390/v17050706
APA StyleJoyce, J. D., Moore, G. A., Thompson, C. K., & Bertke, A. S. (2025). Guinea Pigs Are Not a Suitable Model to Study Neurological Impacts of Ancestral SARS-CoV-2 Intranasal Infection. Viruses, 17(5), 706. https://doi.org/10.3390/v17050706





