Capsid Structure of the Fish Pathogen Syngnathus Scovelli Chapparvovirus Offers a New Perspective on Parvovirus Structural Biology
Abstract
1. Introduction
2. Materials and Methods
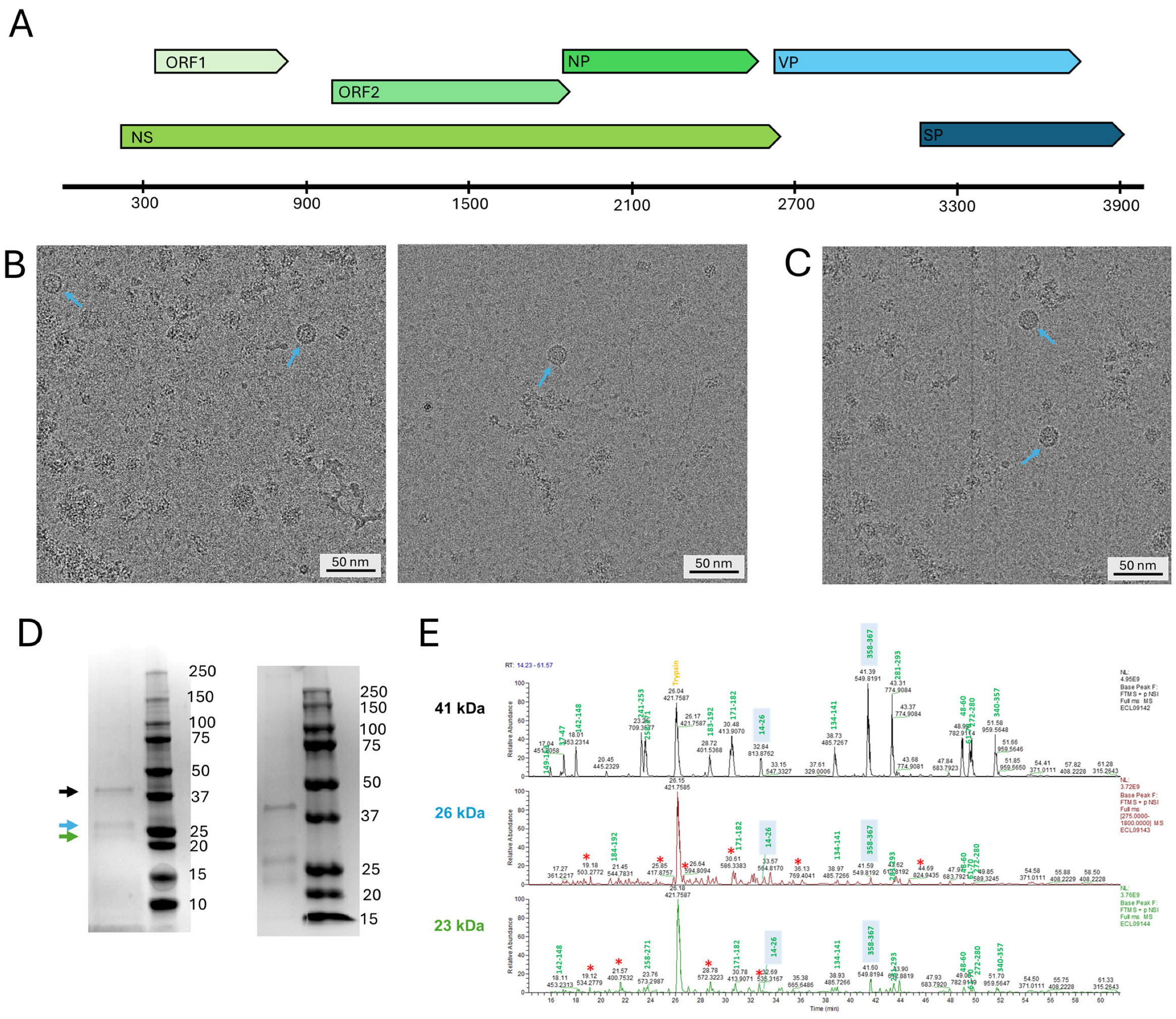
2.1. VLP Expression and Culturing Conditions
2.2. Purification of Virus-like Particles
2.3. Mass Spectrometry
2.4. Preparation of cryoEM Grids and Plunge Freezing
2.5. Collection of High-Resolution Data and 3D Reconstruction
3. Results
3.1. Protein Expression and Composition
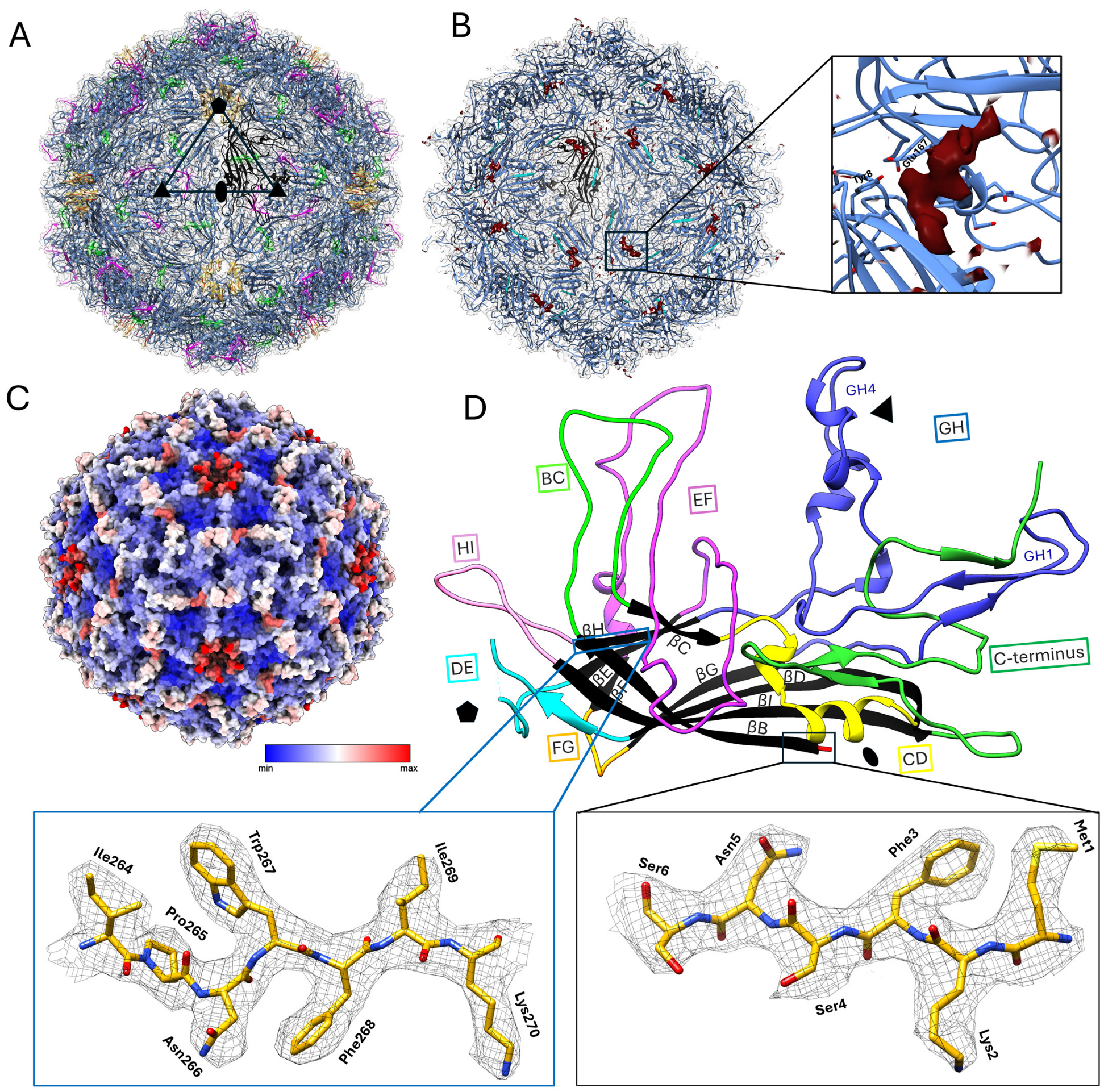
3.2. Structural Studies
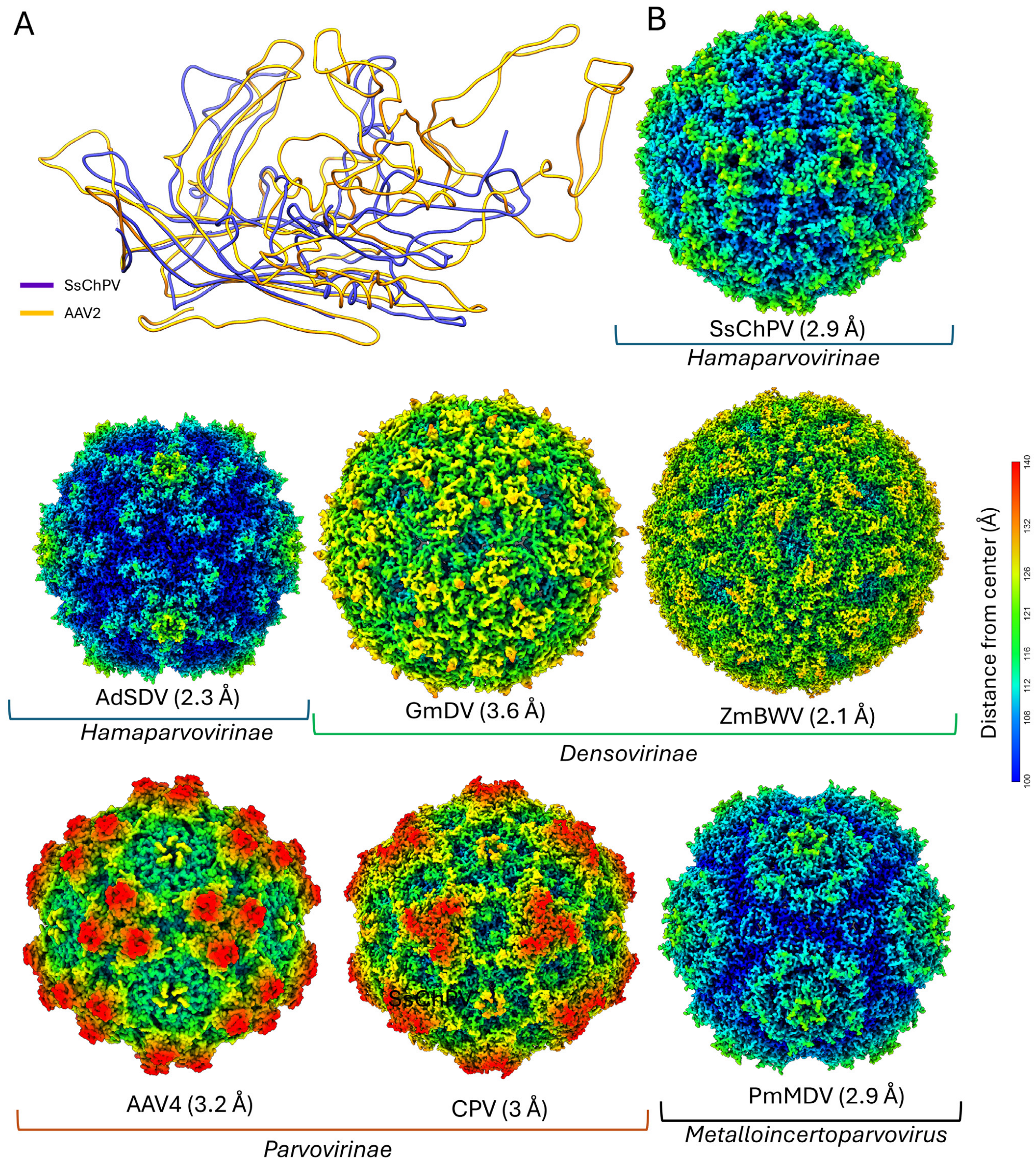
3.3. Structure Comparison and Multimer Interactions
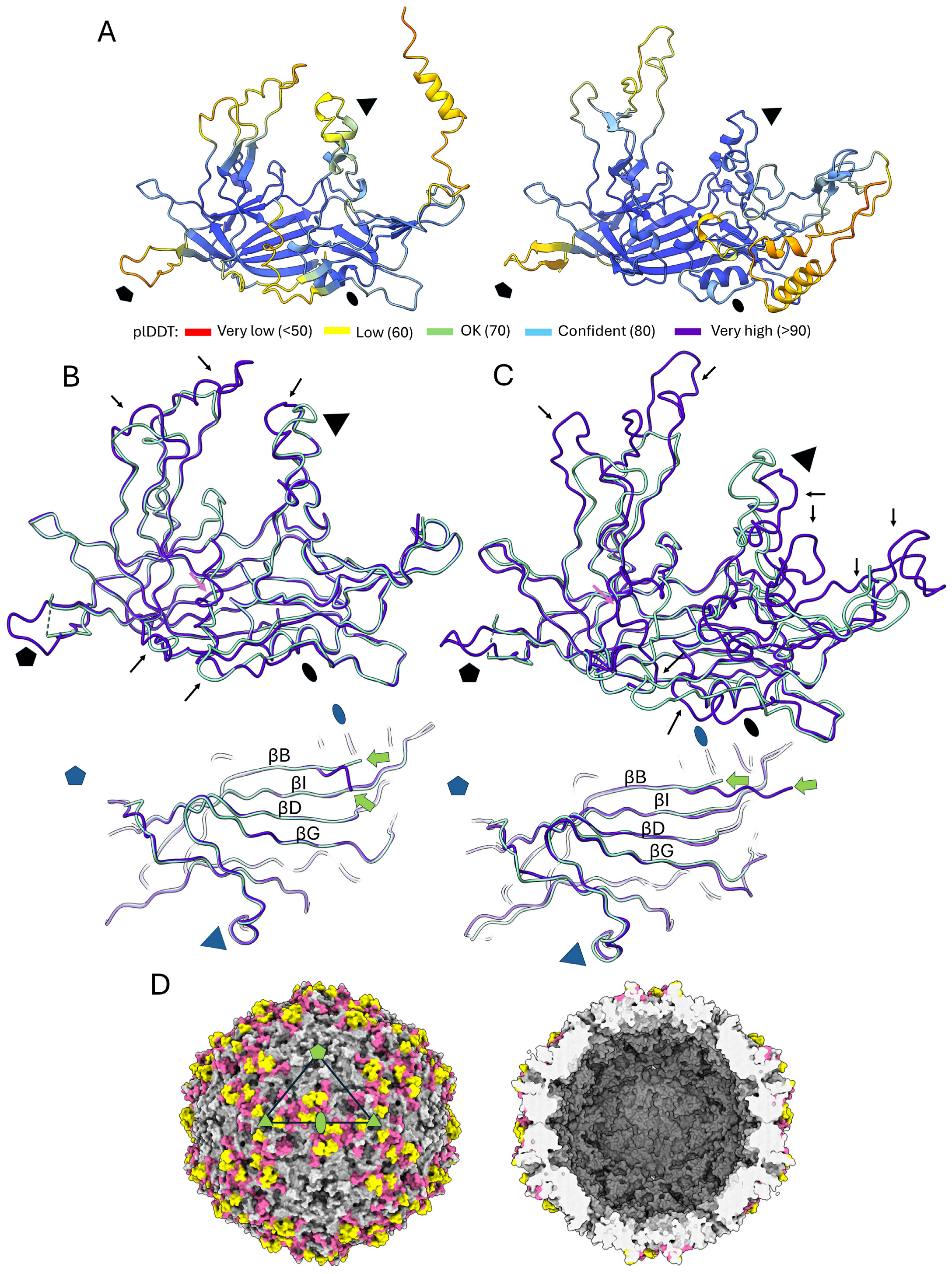
3.4. Structural Predictions of Further Chapparvoviral Structures Through the Syngnathus Scovelli Chapparvovirus Capsid
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cotmore, S.F.; Agbandje-McKenna, M.; Canuti, M.; Chiorini, J.A.; Eis-Hubinger, A.M.; Hughes, J.; Mietzsch, M.; Modha, S.; Ogliastro, M.; Penzes, J.J.; et al. ICTV Virus Taxonomy Profile: Parvoviridae. J. Gen. Virol. 2019, 100, 367–368. [Google Scholar] [CrossRef] [PubMed]
- Cotmore, S.F.; Tattersall, P. Parvoviruses: Small Does Not Mean Simple. Annu. Rev. Virol. 2014, 1, 517–537. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Cotmore, S.F.; Tattersall, P. Parvoviral left-end hairpin ears are essential during infection for establishing a functional intranuclear transcription template and for efficient progeny genome encapsidation. J. Virol. 2013, 87, 10501–10514. [Google Scholar] [CrossRef]
- Pénzes, J.J.; Söderlund-Venermo, M.; Canuti, M.; Eis-Hübinger, A.M.; Hughes, J.; Cotmore, S.F.; Harrach, B. Reorganizing the family Parvoviridae: A revised taxonomy independent of the canonical approach based on host association. Arch. Virol. 2020, 165, 2133–2146. [Google Scholar] [CrossRef]
- Mietzsch, M.; Penzes, J.J.; Agbandje-McKenna, M. Twenty-Five Years of Structural Parvovirology. Viruses 2019, 11, 362. [Google Scholar] [CrossRef]
- Rossmann, M.G.; Abad-Zapatero, C.; Murthy, M.R.; Liljas, L.; Jones, T.A.; Strandberg, B. Structural comparisons of some small spherical plant viruses. J. Mol. Biol. 1983, 165, 711–736. [Google Scholar] [CrossRef]
- Pénzes, J.J.; Pham, H.T.; Chipman, P.; Bhattacharya, N.; McKenna, R.; Agbandje-McKenna, M.; Tijssen, P. Molecular biology and structure of a novel penaeid shrimp densovirus elucidate convergent parvoviral host capsid evolution. Proc. Natl. Acad. Sci. USA 2020, 117, 20211–20222. [Google Scholar] [CrossRef]
- Sarker, S.; Terrón, M.C.; Khandokar, Y.; Aragão, D.; Hardy, J.M.; Radjainia, M.; Jiménez-Zaragoza, M.; de Pablo, P.J.; Coulibaly, F.; Luque, D.; et al. Structural insights into the assembly and regulation of distinct viral capsid complexes. Nat. Commun. 2016, 7, 13014. [Google Scholar] [CrossRef]
- Li, Y.; Yu, P.; Bao, Y.; Ren, Y.; Zhao, S.; Zhang, X. Production of virus-like particles of porcine circovirus 2 in baculovirus expression system and its application for antibody detection. BMC Vet. Res. 2023, 19, 87. [Google Scholar] [CrossRef]
- Penzes, J.J.; Chipman, P.; Bhattacharya, N.; Zeher, A.; Huang, R.; McKenna, R.; Agbandje-McKenna, M. Adeno-associated Virus 9 Structural Rearrangements Induced by Endosomal Trafficking pH and Glycan Attachment. J. Virol. 2021, 95, e0084321. [Google Scholar] [CrossRef]
- Pénzes, J.J.; Pham, H.T.; Chipman, P.; Smith, E.W.; McKenna, R.; Tijssen, P. Bipartite genome and structural organization of the parvovirus Acheta domesticus segmented densovirus. Nat. Commun. 2023, 14, 3515. [Google Scholar] [CrossRef] [PubMed]
- Mietzsch, M.; Barnes, C.; Hull, J.A.; Chipman, P.; Xie, J.; Bhattacharya, N.; Sousa, D.; McKenna, R.; Gao, G.; Agbandje-McKenna, M. Comparative Analysis of the Capsid Structures of AAVrh.10, AAVrh.39, and AAV8. J. Virol. 2020, 94, 10-1128. [Google Scholar] [CrossRef] [PubMed]
- Plevka, P.; Hafenstein, S.; Li, L.; D’Abrgamo, A., Jr.; Cotmore, S.F.; Rossmann, M.G.; Tattersall, P. Structure of a packaging-defective mutant of minute virus of mice indicates that the genome is packaged via a pore at a 5-fold axis. J. Virol. 2011, 85, 4822–4827. [Google Scholar] [CrossRef] [PubMed]
- Venkatakrishnan, B.; Yarbrough, J.; Domsic, J.; Bennett, A.; Bothner, B.; Kozyreva, O.G.; Samulski, R.J.; Muzyczka, N.; McKenna, R.; Agbandje-McKenna, M. Structure and dynamics of adeno-associated virus serotype 1 VP1-unique N-terminal domain and its role in capsid trafficking. J. Virol. 2013, 87, 4974–4984. [Google Scholar] [CrossRef]
- Jager, M.C.; Tomlinson, J.E.; Lopez-Astacio, R.A.; Parrish, C.R.; Van de Walle, G.R. Small but mighty: Old and new parvoviruses of veterinary significance. Virol. J. 2021, 18, 210. [Google Scholar] [CrossRef]
- Pupo, A.; Fernández, A.; Low, S.H.; François, A.; Suárez-Amarán, L.; Samulski, R.J. AAV vectors: The Rubik's cube of human gene therapy. Mol. Ther. 2022, 30, 3515–3541. [Google Scholar] [CrossRef]
- Baker, K.S.; Leggett, R.M.; Bexfield, N.H.; Alston, M.; Daly, G.; Todd, S.; Tachedjian, M.; Holmes, C.E.; Crameri, S.; Wang, L.F.; et al. Metagenomic study of the viruses of African straw-coloured fruit bats: Detection of a chiropteran poxvirus and isolation of a novel adenovirus. Virology 2013, 441, 95–106. [Google Scholar] [CrossRef]
- Reuter, G.; Boros, Á.; Delwart, E.; Pankovics, P. Novel circular single-stranded DNA virus from turkey faeces. Arch. Virol. 2014, 159, 2161–2164. [Google Scholar] [CrossRef]
- Reuter, G.; Boros, Á.; Mátics, R.; Altan, E.; Delwart, E.; Pankovics, P. A novel parvovirus (family Parvoviridae) in a freshwater fish, zander (Sander lucioperca). Arch. Virol. 2022, 167, 1163–1167. [Google Scholar] [CrossRef]
- Palinski, R.M.; Mitra, N.; Hause, B.M. Discovery of a novel Parvovirinae virus, porcine parvovirus 7, by metagenomic sequencing of porcine rectal swabs. Virus Genes 2016, 52, 564–567. [Google Scholar] [CrossRef]
- Amargier, A.; Vago, C.; Meynadier, G. [Histopathological study of a new type of viral disease demonstrated in the lepidoptera Galleria mellonella]. Arch. Gesamte Virusforsch. 1965, 15, 659–667. [Google Scholar] [CrossRef] [PubMed]
- Atchison, R.W.; Casto, B.C.; Hammon, W.M. Adenovirus-associated defective virus particles. Science 1965, 149, 754–756. [Google Scholar] [CrossRef] [PubMed]
- Canuti, M.; Verhoeven, J.T.P.; Munro, H.J.; Roul, S.; Ojkic, D.; Robertson, G.J.; Whitney, H.G.; Dufour, S.C.; Lang, A.S. Investigating the Diversity and Host Range of Novel Parvoviruses from North American Ducks Using Epidemiology, Phylogenetics, Genome Structure, and Codon Usage Analysis. Viruses 2021, 13, 193. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.S.; Li, C.X.; Hall, J.; Eden, J.S.; Hyndman, T.H.; Holmes, E.C.; Rose, K. Meta-Transcriptomic Discovery of a Divergent Circovirus and a Chaphamaparvovirus in Captive Reptiles with Proliferative Respiratory Syndrome. Viruses 2020, 12, 1073. [Google Scholar] [CrossRef]
- Lee, Q.; Padula, M.P.; Pinello, N.; Williams, S.H.; O’Rourke, M.B.; Fumagalli, M.J.; Orkin, J.D.; Song, R.; Shaban, B.; Brenner, O.; et al. Murine and related chapparvoviruses are nephro-tropic and produce novel accessory proteins in infected kidneys. PLoS Pathog. 2020, 16, e1008262. [Google Scholar] [CrossRef]
- Li, Y.; Gordon, E.; Idle, A.; Altan, E.; Seguin, M.A.; Estrada, M.; Deng, X.; Delwart, E. Virome of a Feline Outbreak of Diarrhea and Vomiting Includes Bocaviruses and a Novel Chapparvovirus. Viruses 2020, 12, 506. [Google Scholar] [CrossRef]
- Liu, X.; Wang, H.; Li, Y.; Chen, J.; Zhang, J.; Wang, X.; Shen, S.; Deng, F.; Wang, M.; Guan, W.; et al. Genomic and transcriptional analyses of novel parvoviruses identified from dead peafowl. Virology 2020, 539, 80–91. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, Y.; Ma, J.; Jiang, N.; Fan, Y.; Zhou, Y.; Cain, K.; Yi, M.; Jia, K.; Wen, H.; et al. Determination of a novel parvovirus pathogen associated with massive mortality in adult tilapia. PLoS Pathog. 2020, 16, e1008765. [Google Scholar] [CrossRef]
- Roediger, B.; Lee, Q.; Tikoo, S.; Cobbin, J.C.A.; Henderson, J.M.; Jormakka, M.; O’Rourke, M.B.; Padula, M.P.; Pinello, N.; Henry, M.; et al. An Atypical Parvovirus Drives Chronic Tubulointerstitial Nephropathy and Kidney Fibrosis. Cell 2018, 175, 530–543.e524. [Google Scholar] [CrossRef]
- Penzes, J.J.; de Souza, W.M.; Agbandje-McKenna, M.; Gifford, R.J. An Ancient Lineage of Highly Divergent Parvoviruses Infects both Vertebrate and Invertebrate Hosts. Viruses 2019, 11, 525. [Google Scholar] [CrossRef]
- Mastronarde, D.N. Advanced data acquisition from electron microscopes with SerialEM. Microsc. Microanal. 2018, 24, 864–865. [Google Scholar] [CrossRef]
- Punjani, A.; Rubinstein, J.L.; Fleet, D.J.; Brubaker, M.A. cryoSPARC: Algorithms for rapid unsupervised cryo-EM structure determination. Nat. Methods 2017, 14, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Jamali, K.; Käll, L.; Zhang, R.; Brown, A.; Kimanius, D.; Scheres, S.H.W. Automated model building and protein identification in cryo-EM maps. bioRxiv 2023. [Google Scholar] [CrossRef] [PubMed]
- Emsley, P.; Lohkamp, B.; Scott, W.G.; Cowtan, K. Features and development of Coot. Acta Crystallogr. D Biol. Crystallogr. 2010, 66, 486–501. [Google Scholar] [CrossRef]
- Croll, T.I. ISOLDE: A physically realistic environment for model building into low-resolution electron-density maps. Acta Crystallogr. D Struct. Biol. 2018, 74, 519–530. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef]
- Meng, E.C.; Goddard, T.D.; Pettersen, E.F.; Couch, G.S.; Pearson, Z.J.; Morris, J.H.; Ferrin, T.E. UCSF ChimeraX: Tools for structure building and analysis. Protein Sci. 2023, 32, e4792. [Google Scholar] [CrossRef]
- Adams, P.D.; Afonine, P.V.; Bunkoczi, G.; Chen, V.B.; Davis, I.W.; Echols, N.; Headd, J.J.; Hung, L.W.; Kapral, G.J.; Grosse-Kunstleve, R.W.; et al. PHENIX: A comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 2010, 66, 213–221. [Google Scholar] [CrossRef]
- Krissinel, E.; Henrick, K. Inference of macromolecular assemblies from crystalline state. J. Mol. Biol. 2007, 372, 774–797. [Google Scholar] [CrossRef]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef]
- Mirdita, M.; Schütze, K.; Moriwaki, Y.; Heo, L.; Ovchinnikov, S.; Steinegger, M. ColabFold: Making protein folding accessible to all. Nat. Methods 2022, 19, 679–682. [Google Scholar] [CrossRef] [PubMed]
- Speir, J.A.; Johnson, J.E. Nucleic acid packaging in viruses. Curr. Opin. Struct. Biol. 2012, 22, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Holm, L. DALI and the persistence of protein shape. Protein Sci. 2020, 29, 128–140. [Google Scholar] [CrossRef] [PubMed]
- Costa, V.A.; Ronco, F.; Mifsud, J.C.O.; Harvey, E.; Salzburger, W.; Holmes, E.C. Host adaptive radiation is associated with rapid virus diversification and cross-species transmission in African cichlid fishes. Curr. Biol. 2024, 34, 1247–1257.e1243. [Google Scholar] [CrossRef]
- Piewbang, C.; Jo, W.K.; Puff, C.; Ludlow, M.; van der Vries, E.; Banlunara, W.; Rungsipipat, A.; Kruppa, J.; Jung, K.; Techangamsuwan, S.; et al. Canine Bocavirus Type 2 Infection Associated With Intestinal Lesions. Vet. Pathol. 2018, 55, 434–441. [Google Scholar] [CrossRef]
- Robinson, W.F.; Wilcox, G.E.; Flower, R.L. Canine parvoviral disease: Experimental reproduction of the enteric form with a parvovirus isolated from a case of myocarditis. Vet. Pathol. 1980, 17, 589–599. [Google Scholar] [CrossRef]
- Millá, F.; Feliu, E.; Ribera, J.M.; Juncà, J.; Flores, A.; Vidal, J.; Zarco, M.A.; Masat, T. Electron microscopic identification of parvovirus virions in erythroid and granulocytic-line cells in a patient with human parvovirus B19 induced pancytopenia. Leuk. Lymphoma 1993, 10, 483–487. [Google Scholar] [CrossRef]
- Chen, S.; Wang, S.; Cheng, X.; Xiao, S.; Zhu, X.; Lin, F.; Wu, N.; Wang, J.; Huang, M.; Zheng, M.; et al. Isolation and characterization of a distinct duck-origin goose parvovirus causing an outbreak of duckling short beak and dwarfism syndrome in China. Arch. Virol. 2016, 161, 2407–2416. [Google Scholar] [CrossRef]
- Brown, C.S.; Van Lent, J.W.; Vlak, J.M.; Spaan, W.J. Assembly of empty capsids by using baculovirus recombinants expressing human parvovirus B19 structural proteins. J. Virol. 1991, 65, 2702–2706. [Google Scholar] [CrossRef]
- Smith, R.H.; Levy, J.R.; Kotin, R.M. A simplified baculovirus-AAV expression vector system coupled with one-step affinity purification yields high-titer rAAV stocks from insect cells. Mol. Ther. 2009, 17, 1888–1896. [Google Scholar] [CrossRef]
- Kaufmann, B.; El-Far, M.; Plevka, P.; Bowman, V.D.; Li, Y.; Tijssen, P.; Rossmann, M.G. Structure of Bombyx mori densovirus 1, a silkworm pathogen. J. Virol. 2011, 85, 4691–4697. [Google Scholar] [CrossRef] [PubMed]
- Lecoq, L.; Wang, S.; Dujardin, M.; Zimmermann, P.; Schuster, L.; Fogeron, M.L.; Briday, M.; Schledorn, M.; Wiegand, T.; Cole, L.; et al. A pocket-factor-triggered conformational switch in the hepatitis B virus capsid. Proc. Natl. Acad. Sci. USA 2021, 118, e2022464118. [Google Scholar] [CrossRef] [PubMed]
- Smyth, M.; Pettitt, T.; Symonds, A.; Martin, J. Identification of the pocket factors in a picornavirus. Arch. Virol. 2003, 148, 1225–1233. [Google Scholar] [CrossRef]
- DiNunno, N.M.; Goetschius, D.J.; Narayanan, A.; Majowicz, S.A.; Moustafa, I.; Bator, C.M.; Hafenstein, S.L.; Jose, J. Identification of a pocket factor that is critical to Zika virus assembly. Nat. Commun. 2020, 11, 4953. [Google Scholar] [CrossRef]
- Kaufmann, B.; Bowman, V.D.; Li, Y.; Szelei, J.; Waddell, P.J.; Tijssen, P.; Rossmann, M.G. Structure of Penaeus stylirostris densovirus, a shrimp pathogen. J. Virol. 2010, 84, 11289–11296. [Google Scholar] [CrossRef]
- Pénzes, J.J.; Marsile-Medun, S.; Agbandje-McKenna, M.; Gifford, R.J. Endogenous amdoparvovirus-related elements reveal insights into the biology and evolution of vertebrate parvoviruses. Virus Evol. 2018, 4, vey026. [Google Scholar] [CrossRef]
- Hildebrandt, E.; Penzes, J.J.; Gifford, R.J.; Agbandje-Mckenna, M.; Kotin, R.M. Evolution of dependoparvoviruses across geological timescales-implications for design of AAV-based gene therapy vectors. Virus Evol. 2020, 6, veaa043. [Google Scholar] [CrossRef]
- Mietzsch, M.; Hull, J.A.; Makal, V.E.; Jimenez Ybargollin, A.; Yu, J.C.; McKissock, K.; Bennett, A.; Penzes, J.; Lins-Austin, B.; Yu, Q.; et al. Characterization of the Serpentine Adeno-Associated Virus (SAAV) Capsid Structure: Receptor Interactions and Antigenicity. J. Virol. 2022, 96, e0033522. [Google Scholar] [CrossRef]
- Girod, A.; Wobus, C.E.; Zádori, Z.; Ried, M.; Leike, K.; Tijssen, P.; Kleinschmidt, J.A.; Hallek, M. The VP1 capsid protein of adeno-associated virus type 2 is carrying a phospholipase A2 domain required for virus infectivity. J. Gen. Virol. 2002, 83, 973–978. [Google Scholar] [CrossRef]
- Vihinen-Ranta, M.; Wang, D.; Weichert, W.S.; Parrish, C.R. The VP1 N-terminal sequence of canine parvovirus affects nuclear transport of capsids and efficient cell infection. J. Virol. 2002, 76, 1884–1891. [Google Scholar] [CrossRef]
- Zadori, Z.; Szelei, J.; Lacoste, M.C.; Li, Y.; Gariepy, S.; Raymond, P.; Allaire, M.; Nabi, I.R.; Tijssen, P. A viral phospholipase A2 is required for parvovirus infectivity. Dev. Cell 2001, 1, 291–302. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Li, Y.; Jousset, F.X.; Zadori, Z.; Szelei, J.; Yu, Q.; Pham, H.T.; Lépine, F.; Bergoin, M.; Tijssen, P. The Acheta domesticus densovirus, isolated from the European house cricket, has evolved an expression strategy unique among parvoviruses. J. Virol. 2011, 85, 10069–10078. [Google Scholar] [CrossRef] [PubMed]
- Penzes, J.J.; Holm, M.; Yost, S.A.; Kaelber, J.T. Cryo-EM-based discovery of a pathogenic parvovirus causing epidemic mortality by black wasting disease in farmed beetles. Cell 2024, 187, 5604–5619.e5614. [Google Scholar] [CrossRef] [PubMed]
- Meng, G.; Zhang, X.; Plevka, P.; Yu, Q.; Tijssen, P.; Rossmann, M.G. The structure and host entry of an invertebrate parvovirus. J. Virol. 2013, 87, 12523–12530. [Google Scholar] [CrossRef]
- Simpson, A.A.; Chipman, P.R.; Baker, T.S.; Tijssen, P.; Rossmann, M.G. The structure of an insect parvovirus (Galleria mellonella densovirus) at 3.7 A resolution. Structure 1998, 6, 1355–1367. [Google Scholar] [CrossRef]
- Kaufmann, B.; Chipman, P.R.; Kostyuchenko, V.A.; Modrow, S.; Rossmann, M.G. Visualization of the externalized VP2 N termini of infectious human parvovirus B19. J. Virol. 2008, 82, 7306–7312. [Google Scholar] [CrossRef]
- Sherman, M.B.; Guenther, R.; Reade, R.; Rochon, D.; Sit, T.; Smith, T.J. Near-Atomic-Resolution Cryo-Electron Microscopy Structures of Cucumber Leaf Spot Virus and Red Clover Necrotic Mosaic Virus: Evolutionary Divergence at the Icosahedral Three-Fold Axes. J. Virol. 2020, 94, 10-1128. [Google Scholar] [CrossRef]
- Gellman, S.H. On the role of methionine residues in the sequence-independent recognition of nonpolar protein surfaces. Biochemistry 1991, 30, 6633–6636. [Google Scholar] [CrossRef]
- Heiby, J.C.; Goretzki, B.; Johnson, C.M.; Hellmich, U.A.; Neuweiler, H. Methionine in a protein hydrophobic core drives tight interactions required for assembly of spider silk. Nat. Commun. 2019, 10, 4378. [Google Scholar] [CrossRef]
- Reguera, J.; Carreira, A.; Riolobos, L.; Almendral, J.M.; Mateu, M.G. Role of interfacial amino acid residues in assembly, stability, and conformation of a spherical virus capsid. Proc. Natl. Acad. Sci. USA 2004, 101, 2724–2729. [Google Scholar] [CrossRef]
- Riolobos, L.; Reguera, J.; Mateu, M.G.; Almendral, J.M. Nuclear transport of trimeric assembly intermediates exerts a morphogenetic control on the icosahedral parvovirus capsid. J. Mol. Biol. 2006, 357, 1026–1038. [Google Scholar] [CrossRef] [PubMed]
- Bennett, A.; Gargas, J.; Kansol, A.; Lewis, J.; Hsi, J.; Hull, J.; Mietzsch, M.; Tartaglia, L.; Muzyczka, N.; Bhattacharya, N.; et al. Structural and Biophysical Analysis of Adeno-Associated Virus Serotype 2 Capsid Assembly Variants. J. Virol. 2023, 97, e0177222. [Google Scholar] [CrossRef] [PubMed]
- Inaba, H.; Matsuura, K. Functional Peptide Nanocapsules Self-Assembled from β-Annulus Peptides. Methods Mol. Biol. 2021, 2208, 101–121. [Google Scholar] [CrossRef]
- Matsuura, K.; Nakamura, T.; Watanabe, K.; Noguchi, T.; Minamihata, K.; Kamiya, N.; Kimizuka, N. Self-assembly of Ni-NTA-modified β-annulus peptides into artificial viral capsids and encapsulation of His-tagged proteins. Org. Biomol. Chem. 2016, 14, 7869–7874. [Google Scholar] [CrossRef] [PubMed]


| Processing and Refinement Parameters | |
|---|---|
| Total number of micrographs | 11,904 |
| Number of micrographs involved in reconstruction | 7182 |
| Reconstruction software | CryoSparc v4.5.3 |
| Defocus range (µm) | 0.6–2.4 |
| Electron dose (e−/Å2) | 31 |
| Frames/micrograph | 30 |
| Pixel size at acquisition (Å/pixel) | 1.038 |
| Calibrated pixel size (Å/pixel) | 1.011 |
| Starting number of particles | 14,213 |
| Particles used for final map | 2202 |
| Resolution of final map (Å) | 2.93 |
| PDB ID | 9NEK |
| Residue range (VP2) | 1–356 |
| Map correlation coefficient | 0.8339 |
| RMSD (root-mean-square deviation) [bonds] (Å) | 0.004 |
| RMSD [angles] (Å) | 0.554 |
| All-atom clash score | 6.74 |
| Favored (%) | 95.46 |
| Allowed (%) | 4.54 |
| Outliers (%) | 0.00 |
| Rotamer outliers (%) | 0 |
| C-β deviations | 0 |
| Inner volume (Å3) | Genome Size (nt) | Genus | Subfamily | Vm (Å3/Da) | |
|---|---|---|---|---|---|
| SsChPV | 2.179 × 106 | 4002 * | Ichthamaparvovirus | Hamaparvovirinae | 1.67 |
| AdSDV | 1.863 × 106 | 3332/3316 | Brevihamaparvovirus | Hamaparvovirinae | 1.71 |
| PstDV | 2.143 × 106 | 3914 | Penstylhamaparvovirus | Hamaparvovirinae | 1.67 |
| PmMDV | 2.059 × 106 | 4371 | Incertometalloparvovirus | Not assigned | 1.44 |
| AAV2 | 2.518 × 106 | 4679 | Dependoparvovirus | Parvovirinae | 1.65 |
| AAV6 | 2.7 × 106 † | 4683 | Dependoparvovirus | Parvovirinae | 1.76 † |
| Z-Scores | PDB ID | Identity % | Genus | Subfamily | Family |
|---|---|---|---|---|---|
| 18.4 | 2g8g | 13 | Dependoparvovirus | Parvovirinae | Parvoviridae |
| 18.2 | 3ntt | 14 | Dependoparvovirus | Parvovirinae | Parvoviridae |
| 18.1 | 4rso | 14 | Dependoparvovirus | Parvovirinae | Parvoviridae |
| 18.1 | 4qc8 | 10 | Dependoparvovirus | Parvovirinae | Parvoviridae |
| 18 | 4g0r | 11 | Dependoparvovirus | Parvovirinae | Parvoviridae |
| 17.8 | 1mvm | 11 | Protoparvovirus | Parvovirinae | Parvoviridae |
| 17.8 | 9c27 | 12 | Erythroparvovirus | Parvovirinae | Parvoviridae |
| 17.7 | 4dpv | 10 | Protoparvovirus | Parvovirinae | Parvoviridae |
| 17.6 | 7kfr | 14 | Dependoparvovirus | Parvovirinae | Parvoviridae |
| 17.6 | 6nf9 | 13 | Protoparvovirus | Parvovirinae | Parvoviridae |
| 17.5 | 7jot | 14 | Dependoparvovirus | Parvovirinae | Parvoviridae |
| 17.5 | 4iov | 13 | Dependoparvovirus | Parvovirinae | Parvoviridae |
| 17 | 7wq | 14 | Dependoparvovirus | Parvovirinae | Parvoviridae |
| 17.4 | 2qa0 | 14 | Dependoparvovirus | Parvovirinae | Parvoviridae |
| 17.3 | 3ng9 | 14 | Dependoparvovirus | Parvovirinae | Parvoviridae |
| 17.1 | 1k3v | 13 | Protoparvovirus | Parvovirinae | Parvoviridae |
| 17.1 | 3kic | 14 | Dependoparvovirus | Parvovirinae | Parvoviridae |
| 16.8 | 1s58 | 12 | Erythroparvovirus | Parvovirinae | Parvoviridae |
| 15.2 | 3p0s | 10 | Iteradensovirus | Densovirinae | Parvoviridae |
| 13.1 | 8t9c | 9 | Blattambidensovirus | Densovirinae | Parvoviridae |
| 13 | 1dnv | 9 | Protoambidensovirus | Densovirinae | Parvoviridae |
| 12 | 4mgu | 9 | Scindoambidensovirus | Densovirinae | Parvoviridae |
| 11.8 | 3n7x | 11 | Penstylhamaparvovirus | Hamaparvovirinae | Parvoviridae |
| 11.3 | 8er8 | 12 | Brevihamaparvovirus | Hamaparvovirinae | Parvoviridae |
| 5.6 | 7bgk | 12 | Aparavirus | Dicistoviridae | |
| 5.3 | 6shl | 11 | Bacillarnavirus | Marnaviridae | |
| 5.3 | 1b35 | 7 | Cripavirus | Dicistoviridae | |
| 5.2 | 5lwg | 11 | Aparavirus | Dicistoviridae | |
| Interface | Buried Interface Area (Å2) | ΔiG (kcal/mol) |
|---|---|---|
| Dimer | 751.9 | −8 |
| Trimer | 2521.8 | −27.1 |
| Pentamer | 1508.4 | −19.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Penzes, J.J.; Kaelber, J.T. Capsid Structure of the Fish Pathogen Syngnathus Scovelli Chapparvovirus Offers a New Perspective on Parvovirus Structural Biology. Viruses 2025, 17, 679. https://doi.org/10.3390/v17050679
Penzes JJ, Kaelber JT. Capsid Structure of the Fish Pathogen Syngnathus Scovelli Chapparvovirus Offers a New Perspective on Parvovirus Structural Biology. Viruses. 2025; 17(5):679. https://doi.org/10.3390/v17050679
Chicago/Turabian StylePenzes, Judit J., and Jason T. Kaelber. 2025. "Capsid Structure of the Fish Pathogen Syngnathus Scovelli Chapparvovirus Offers a New Perspective on Parvovirus Structural Biology" Viruses 17, no. 5: 679. https://doi.org/10.3390/v17050679
APA StylePenzes, J. J., & Kaelber, J. T. (2025). Capsid Structure of the Fish Pathogen Syngnathus Scovelli Chapparvovirus Offers a New Perspective on Parvovirus Structural Biology. Viruses, 17(5), 679. https://doi.org/10.3390/v17050679






