Characterizations of Newly Isolated Erwinia amylovora Loessnervirus-like Bacteriophages from Hungary
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains
2.2. Isolation and Purification of Bacteriophages
2.3. Transmission Electron Microscopy
2.4. Plaque Morphology and Quantitative and Qualitative Evaluation of Bacteriophages
2.5. In Vitro Infection Assay
2.6. Stability of Bacteriophages
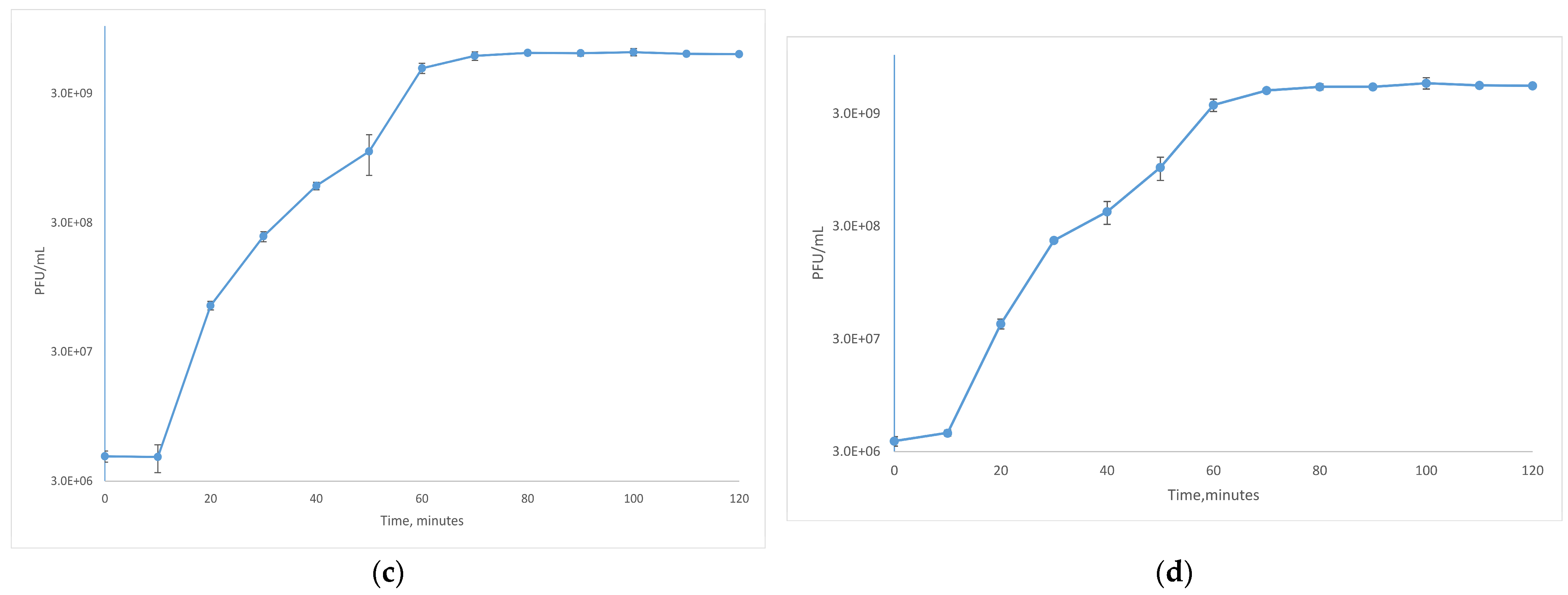
2.7. One-Step Growth Curve
2.8. DNA Isolation and Genome Sequencing
3. Results and Discussion
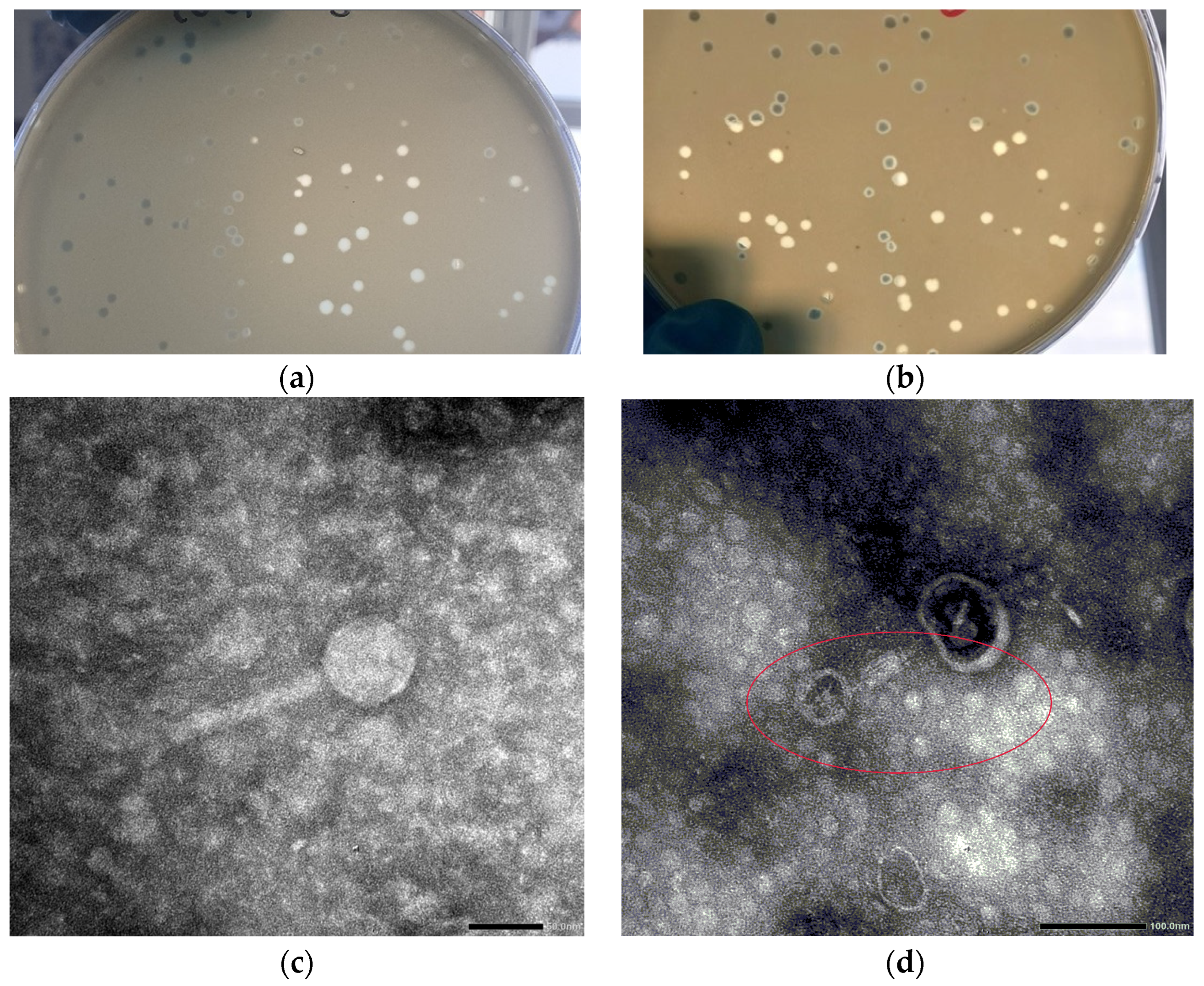
3.1. Viral and Plaque Morphology
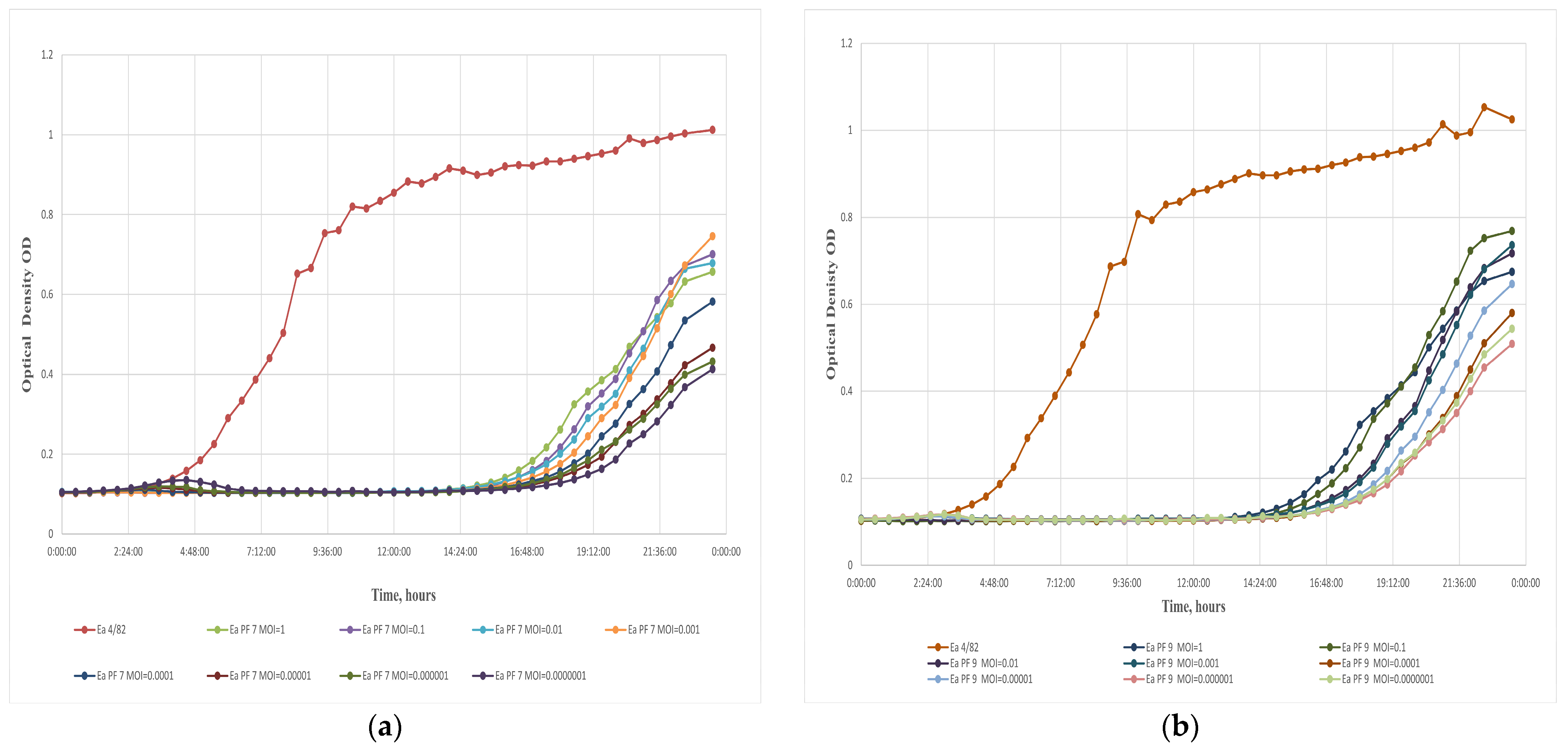
3.2. Quantitative and Qualitative Evaluation of Bacteriophages
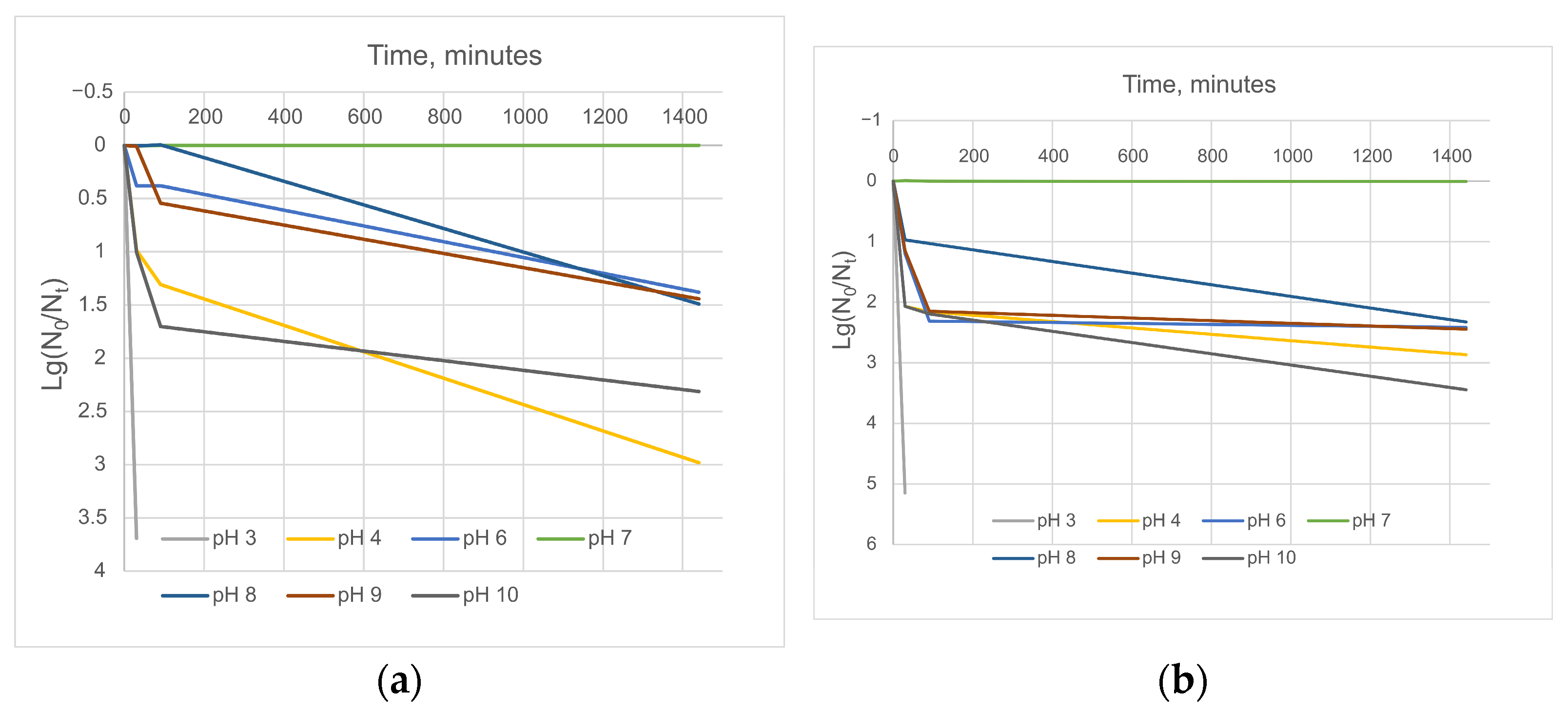
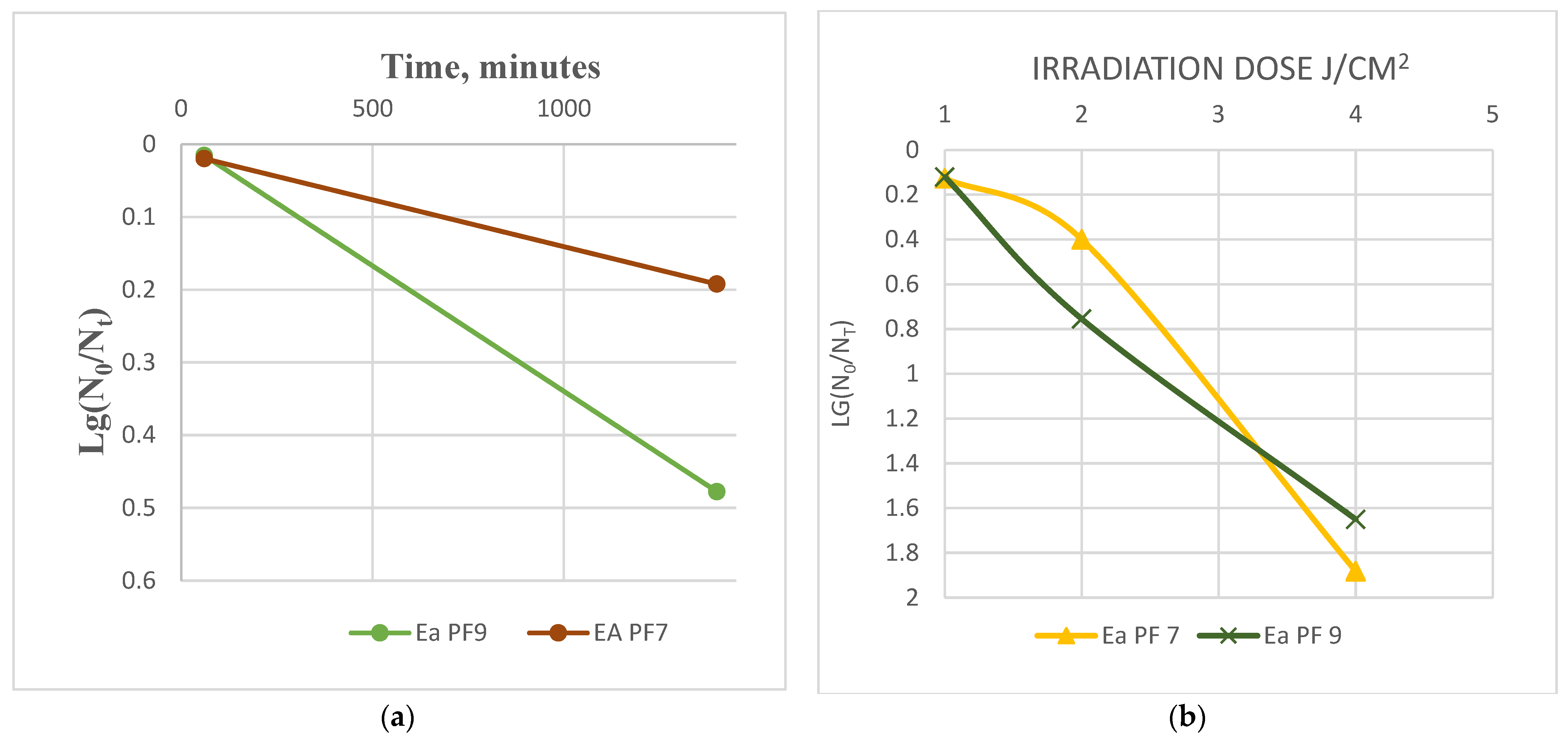
3.3. Stability to Biophysical Factors
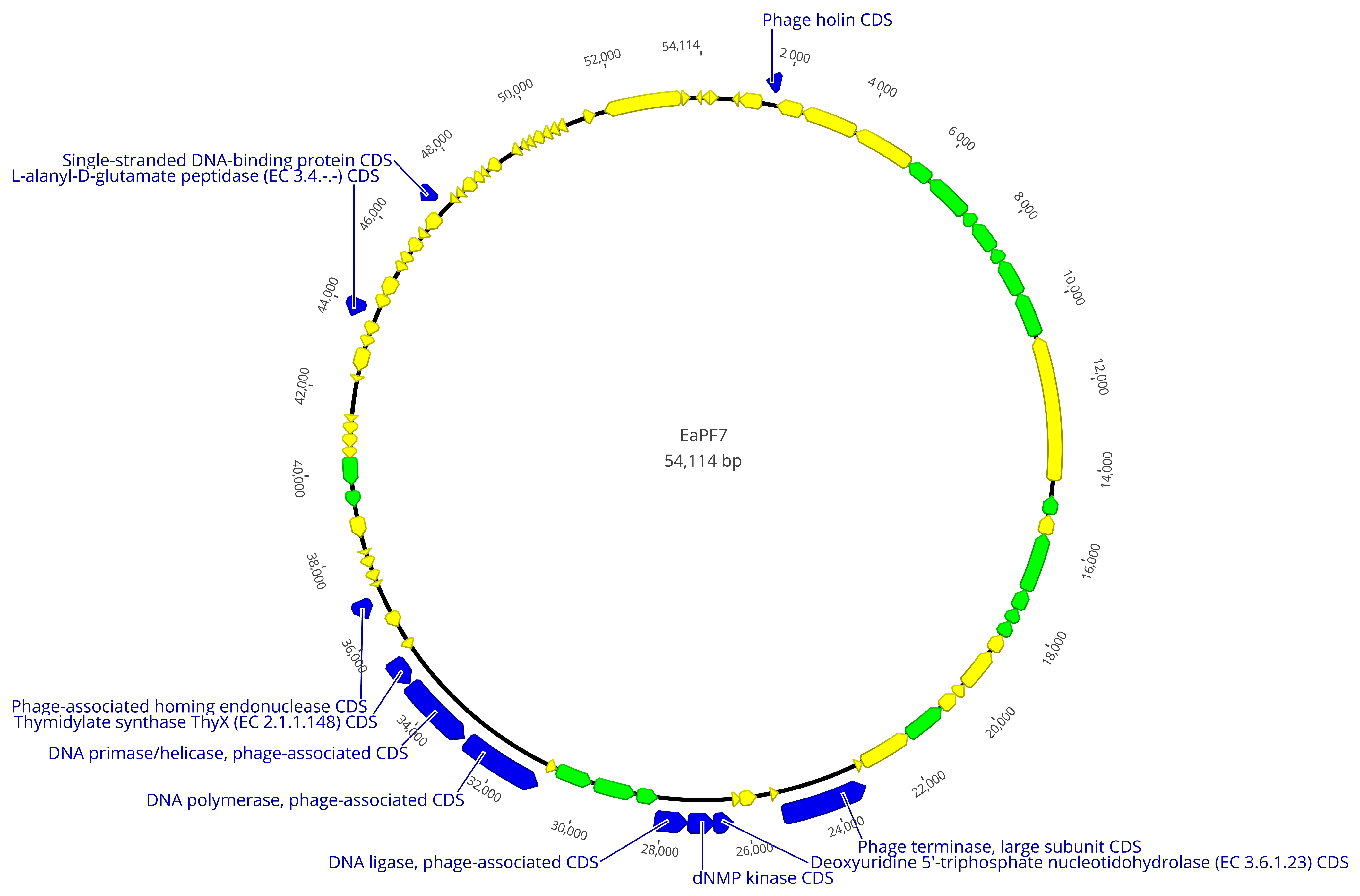
3.4. Genomic Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bonn, W.G.; van der Zwet, T. Distribution and economic importance of fire blight. In Fire Blight the Disease and Its Causative Agent, Erwinia amylovora; Vanneste, J.L., Ed.; CABI Publishing: Wallingford, UK, 2000; pp. 37–53. [Google Scholar]
- Momol, M.; Aldwinckle, H.S. Genetic Diversity and Host Range of Erwinia amylovora; CABI Publishing: Wallingford, UK, 2000; pp. 55–72. [Google Scholar]
- Thomson, S.V. Epidemiology of fire blight. In Fire Blight: The Disease and Its Causative Agent Erwinia Amylovora; Vanneste, J.L., Ed.; CAB International: Wallingford, UK, 2000; pp. 9–36. [Google Scholar]
- Mansfield, J.; Genin, S.; Magori, S.; Citovsky, V.; Sriariyanum, M.; Ronald, P.; Dow, M.; Verdier, V.; Beer, S.V.; Machado, M.A.; et al. Top 10 plant pathogenic bacteria in molecular plant pathology. Mol. Plant Pathol. 2012, 13, 614–629. [Google Scholar] [CrossRef] [PubMed]
- Hevesi, M. Az Erwinia amylovora (Burill) Winslow et al. hazai megjelenése almán. Növényvédelem 1996, 32, 225–228. [Google Scholar]
- Végh, A.; Hevesi, M.; Pájtli, É.; Palkovics, L. Characterization of Erwinia amylovora strains from Hungary. Eur. J. Plant Pathol. 2017, 147, 455–461. [Google Scholar] [CrossRef]
- Végh, A.; Némethy, Z.; Hajagos, L.; Palkovics, L. First report of Erwinia amylovora causing fire blight on plum (Prunus domestica L.) in Hungary. Plant Dis. 2012, 96, 759. [Google Scholar] [CrossRef]
- Végh, A.; Palkovics, L. First occurence of fire blight on apricot (Prunus armeniaca) in Hungary. Not. Bot. Horti Agrobot. Cluj-Napoca 2013, 41, 440–443. [Google Scholar] [CrossRef]
- Vanneste, J.L.; Eden-Green, S. Migration of Erwinia amylovora in host plant tissues. In Fire Blight: The Disease and Its Causative Agent, Erwinia amylovora; Vanneste, J.L., Ed.; CAB International: Wallingford, UK, 2000; pp. 73–83. [Google Scholar]
- Skoneczny, H.; Kubiak, K.; Spiralski, M.; Kotlarz, J.; Mikiciński, A.; Puławska, J. Fire Blight Disease Detection for Apple Trees: Hyperspectral Analysis of Healthy, Infected and Dry Leaves. Remote Sens. 2020, 12, 2101. [Google Scholar] [CrossRef]
- Zurn, J.D.; Norelli, J.L.; Montanari, S.; Bell, R.; Bassil, N.V. Dissecting Genetic Resistance to Fire Blight in Three Pear Populations. Phytopathology 2020, 110, 1305–1311. [Google Scholar] [CrossRef] [PubMed]
- Escursell, M.M.; Roschi, A.; Smits, T.H.M.; Rezzonico, F. Characterization and direct molecular discrimination of rpsL mutations leading to high streptomycin resistance in Erwinia amylovora. J. Plant Pathol. 2021, 103, 99–108. [Google Scholar] [CrossRef]
- Evrenosoğlu, Y.; Mertoglu, K.; Bilgin, N.; Misirli, A.; Altay, Y. An Analysis on Some Reciprocal Pear Hybridization Combinations in Terms of Transferring Resistance to Fire BlightUntersuchung einiger reziproker Birnen-Hybridisierungskombinationen im Hinblick auf die Übertragung von Feuerbrandresistenz. Erwerbs-Obstbau 2020, 62, 189–194. [Google Scholar] [CrossRef]
- Seed, K.D. Battling Phages: How Bacteria Defend against Viral Attack. PLoS Pathog. 2015, 11, e1004847. [Google Scholar] [CrossRef]
- Harvey, H.; Bondy-Denomy, J.; Marquis, H.; Sztanko, K.M.; Davidson, A.R.; Burrows, L.L. Pseudomonas aeruginosa defends against phages through type IV pilus glycosylation. Nat. Microbiol. 2018, 3, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Destoumieux-Garzon, D.; Duquesne, S.; Peduzzi, J.; Goulard, C.; Desmadril, M.; Letellier, L.; Rebuffat, S.; Boulanger, P. The iron-siderophore transporter FhuA is the receptor for the antimicrobial peptide microcin J25: Role of the microcin Val11-Pro16 beta-hairpin region in the recognition mechanism. Biochem. J. 2005, 389 Pt 3, 869–876. [Google Scholar] [CrossRef]
- Bebeacua, C.; Lorenzo Fajardo, J.C.; Blangy, S.; Spinelli, S.; Bollmann, S.; Neve, H.; Cambillau, C.; Heller, K.J. X-ray structure of a superinfection exclusion lipoprotein from phage TP-J34 and identification of the tape measure protein as its target. Mol. Microbiol. 2013, 89, 152–165. [Google Scholar] [CrossRef] [PubMed]
- Dupuis, M.E.; Villion, M.; Magadan, A.H.; Moineau, S. CRISPR-Cas and restriction-modification systems are compatible and increase phage resistance. Nat. Commun. 2013, 4, 2087. [Google Scholar] [CrossRef]
- Aziz, R.K.; Bartels, D.; Best, A.A.; DeJongh, M.; Disz, T.; Edwards, R.A.; Formsma, K.; Gerdes, S.; Glass, E.M.; Kubal, M.; et al. The RAST Server: Rapid annotations using subsystems technology. BMC Genom. 2008, 9, 75. [Google Scholar] [CrossRef]
- Goodridge, L.D.; Abedon, S.T. Bacteriophage biocontrol: The technology matures. Microbiol. Aust. 2008, 29, 48–49. [Google Scholar] [CrossRef]
- Polaska, M.; Sokolowska, B. Bacteriophages—A new hope or a huge problem in the food industry. AIMS Microbiol. 2019, 5, 324–346. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Dai, D.; Lv, L.; Ahmed, T.; Chen, L.; Wang, Y.; An, Q.; Sun, G.; Li, B. Advancements in the Use of Bacteriophages to Combat the Kiwifruit Canker Phytopathogen Pseudomonas syringae pv. actinidiae. Viruses 2022, 14, 2704. [Google Scholar] [CrossRef]
- Yin, Y.; Pei’en, N.; Bohan, D.; Shiping, W.; Wenping, X.; Wang, D. Isolation and characterisation of phages against Pseudomonas syringae pv. actinidiae. Acta Agric. Scand. Sect. B—Soil Plant Sci. 2019, 69, 199–208. [Google Scholar]
- Sieiro, C.; Areal-Hermida, L.; Pichardo-Gallardo, Á.; Almuiña-González, R.; de Miguel, T.; Sánchez, S.; Sánchez-Pérez, Á.; Villa, T.G. A Hundred Years of Bacteriophages: Can Phages Replace Antibiotics in Agriculture and Aquaculture? Antibiotics 2020, 9, 493. [Google Scholar] [CrossRef]
- Svircev, A.; Roach, D.; Castle, A. Framing the Future with Bacteriophages in Agriculture. Viruses 2018, 10, 218. [Google Scholar] [CrossRef]
- Jamal, M.; Bukhari, S.; Andleeb, S.; Ali, M.; Raza, S.; Nawaz, M.A.; Hussain, T.; Rahman, S.U.; Shah, S.S.A. Bacteriophages: An overview of the control strategies against multiple bacterial infections in different fields. J. Basic Microbiol. 2019, 59, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Domotor, D.; Becsagh, P.; Rakhely, G.; Schneider, G.; Kovacs, T. Complete genomic sequence of Erwinia amylovora phage PhiEaH2. J. Virol. 2012, 86, 10899. [Google Scholar] [CrossRef] [PubMed]
- Meczker, K.; Domotor, D.; Vass, J.; Rakhely, G.; Schneider, G.; Kovacs, T. The genome of the Erwinia amylovora phage PhiEaH1 reveals greater diversity and broadens the applicability of phages for the treatment of fire blight. FEMS Microbiol. Lett. 2014, 350, 25–27. [Google Scholar] [CrossRef]
- Schwarczinger, I.; Kolozsváriné Nagy, J.; Künstler, A.; Szabó, L.; Geider, K.; Király, L.; Pogány, M. Characterization of Myoviridae and Podoviridae family bacteriophages of Erwinia amylovora from Hungary—Potential of application in biological control of fire blight. Eur. J. Plant Pathol. 2017, 149, 639–652. [Google Scholar] [CrossRef]
- Kim, H.J.; Jun, J.W.; Giri, S.S.; Kim, S.G.; Kim, S.W.; Kwon, J.; Lee, S.B.; Chi, C.; Park, S.C. Bacteriophage Cocktail for the Prevention of Multiple-Antibiotic-Resistant and Mono-Phage-Resistant Vibrio coralliilyticus Infection in Pacific Oyster (Crassostrea gigas) Larvae. Pathogens 2020, 9, 831. [Google Scholar] [CrossRef]
- Kim, S.G.; Lee, S.B.; Jo, S.J.; Cho, K.; Park, J.K.; Kwon, J.; Giri, S.S.; Kim, S.W.; Kang, J.W.; Jung, W.J.; et al. Phage Cocktail in Combination with Kasugamycin as a Potential Treatment for Fire Blight Caused by Erwinia amylovora. Antibiotics 2022, 11, 1566. [Google Scholar] [CrossRef]
- Knecht, L.E.; Born, Y.; Pelludat, C.; Pothier, J.F.; Smits, T.H.M.; Loessner, M.J.; Fieseler, L. Spontaneous Resistance of Erwinia amylovora Against Bacteriophage Y2 Affects Infectivity of Multiple Phages. Front. Microbiol. 2022, 13, 908346. [Google Scholar] [CrossRef]
- Falkenstein, H.; Bellemann, P.; Walter, S.; Zeller, W.; Geider, K. Identification of Erwinia amylovora, the Fireblight Pathogen, by Colony Hybridization with DNA from Plasmid pEA29. Appl. Environ. Microbiol. 1988, 54, 2798–2802. [Google Scholar] [CrossRef]
- Gill, J.J.; Svircev, A.M.; Smith, R.; Castle, A.J. Bacteriophages of Erwinia amylovora. Appl. Environ. Microbiol. 2003, 69, 2133–2138. [Google Scholar] [CrossRef]
- Adams, M.H. Bacteriophages; Interscience Publishers: New York, NY, USA, 1959. [Google Scholar]
- Kutter, E. Phage host range and efficiency of plating. Methods Mol. Biol. 2009, 501, 141–149. [Google Scholar] [PubMed]
- Kropinski, A.M.; Mazzocco, A.; Waddell, T.E.; Lingohr, E.; Johnson, R.P. Enumeration of bacteriophages by double agar overlay plaque assay. Methods Mol. Biol. 2009, 501, 69–76. [Google Scholar]
- Gayder, S.; Parcey, M.; Castle, A.J.; Svircev, A.M. Host Range of Bacteriophages Against a World-Wide Collection of Erwinia amylovora Determined Using a Quantitative PCR Assay. Viruses 2019, 11, 910. [Google Scholar] [CrossRef] [PubMed]
- Gayder, S.; Parcey, M.; Nesbitt, D.; Castle, A.J.; Svircev, A.M. Population Dynamics between Erwinia amylovora, Pantoea agglomerans and Bacteriophages: Exploiting Synergy and Competition to Improve Phage Cocktail Efficacy. Microorganisms 2020, 8, 1449. [Google Scholar] [CrossRef]
- Born, Y.; Fieseler, L.; Marazzi, J.; Lurz, R.; Duffy, B.; Loessner, M.J. Novel virulent and broad-host-range Erwinia amylovora bacteriophages reveal a high degree of mosaicism and a relationship to Enterobacteriaceae phages. Appl. Environ. Microbiol. 2011, 77, 5945–5954. [Google Scholar] [CrossRef]
- Makalatia, K.; Kakabadze, E.; Wagemans, J.; Grdzelishvili, N.; Bakuradze, N.; Natroshvili, G.; Macharashvili, N.; Sedrakyan, A.; Arakelova, K.; Ktsoyan, Z.; et al. Characterization of Salmonella Isolates from Various Geographical Regions of the Caucasus and Their Susceptibility to Bacteriophages. Viruses 2020, 12, 1418. [Google Scholar] [CrossRef]
- Koderi Valappil, S.; Shetty, P.; Deim, Z.; Terhes, G.; Urban, E.; Vaczi, S.; Patai, R.; Polgar, T.; Pertics, B.Z.; Schneider, G.; et al. Survival Comes at a Cost: A Coevolution of Phage and Its Host Leads to Phage Resistance and Antibiotic Sensitivity of Pseudomonas aeruginosa Multidrug Resistant Strains. Front. Microbiol. 2021, 12, 783722. [Google Scholar] [CrossRef]
- Duarte, J.; Pereira, C.; Costa, P.; Almeida, A. Bacteriophages with Potential to Inactivate Aeromonas hydrophila in Cockles: In Vitro and In Vivo Preliminary Studies. Antibiotics 2021, 10, 710. [Google Scholar] [CrossRef]
- Kim, S.G.; Jun, J.W.; Giri, S.S.; Yun, S.; Kim, H.J.; Kim, S.W.; Kang, J.W.; Han, S.J.; Jeong, D.; Park, S.C. Isolation and characterisation of pVa-21, a giant bacteriophage with anti-biofilm potential against Vibrio alginolyticus. Sci. Rep. 2019, 9, 6284. [Google Scholar] [CrossRef]
- Jo, S.J.; Kim, S.G.; Lee, Y.M.; Giri, S.S.; Kang, J.W.; Lee, S.B.; Jung, W.J.; Hwang, M.H.; Park, J.; Cheng, C.; et al. Evaluation of the Antimicrobial Potential and Characterization of Novel T7-Like Erwinia Bacteriophages. Biology 2023, 12, 180. [Google Scholar] [CrossRef]
- Erol, H.B.; Kaskatepe, B.; Ozturk, S.; Safi Oz, Z. The comparison of lytic activity of isolated phage and commercial Intesti bacteriophage on ESBL producer E. coli and determination of Ec_P6 phage efficacy with in vivo Galleria mellonella larvae model. Microb. Pathog. 2022, 167, 105563. [Google Scholar] [CrossRef]
- Peng, Q.; Ma, Z.; Han, Q.; Xiang, F.; Wang, L.; Zhang, Y.; Zhao, Y.; Li, J.; Xian, Y.; Yuan, Y. Characterization of bacteriophage vB_KleM_KB2 possessing high control ability to pathogenic Klebsiella pneumoniae. Sci. Rep. 2023, 13, 9815. [Google Scholar] [CrossRef]
- Knecht, L.E.; Heinrich, N.; Born, Y.; Felder, K.; Pelludat, C.; Loessner, M.J.; Fieseler, L. Bacteriophage S6 requires bacterial cellulose for Erwinia amylovora infection. Environ. Microbiol. 2022, 24, 3436–3450. [Google Scholar] [CrossRef] [PubMed]
- Merabishvili, M.; Vandenheuvel, D.; Kropinski, A.M.; Mast, J.; De Vos, D.; Verbeken, G.; Noben, J.P.; Lavigne, R.; Vaneechoutte, M.; Pirnay, J.P. Characterization of newly isolated lytic bacteriophages active against Acinetobacter baumannii. PLoS ONE 2014, 9, e104853. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Kim, B.; Song, S.; Lee, Y.W.; Roh, E. Isolation of Nine Bacteriophages Shown Effective against Erwinia amylovora in Korea. Plant Pathol. J. 2022, 38, 248–253. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Lee, G.M.; Kim, D.; Park, D.H.; Oh, C.S. Characterization of the Lytic Bacteriophage phiEaP-8 Effective against Both Erwinia amylovora and Erwinia pyrifoliae Causing Severe Diseases in Apple and Pear. Plant Pathol. J. 2018, 34, 445–450. [Google Scholar] [CrossRef]
- Luo, J.; Xie, L.; Yang, M.; Liu, M.; Li, Q.; Wang, P.; Fan, J.; Jin, J.; Luo, C. Synergistic Antibacterial Effect of Phage pB3074 in Combination with Antibiotics Targeting Cell Wall against Multidrug-Resistant Acinetobacter baumannii In Vitro and Ex Vivo. Microbiol. Spectr. 2023, 11, e0034123. [Google Scholar] [CrossRef]
- Jo, S.J.; Kim, S.G.; Park, J.; Lee, Y.M.; Giri, S.S.; Lee, S.B.; Jung, W.J.; Hwang, M.H.; Park, J.H.; Roh, E.; et al. Optimizing the formulation of Erwinia bacteriophages for improved UV stability and adsorption on apple leaves. Heliyon 2023, 9, e22034. [Google Scholar] [CrossRef]
- Born, Y.; Bosshard, L.; Duffy, B.; Loessner, M.J.; Fieseler, L. Protection of Erwinia amylovora bacteriophage Y2 from UV-induced damage by natural compounds. Bacteriophage 2015, 5, e1074330. [Google Scholar] [CrossRef]
- Fu, J.; Li, Y.; Zhao, L.; Wu, C.; He, Z. Characterization of vB_ValM_PVA8, a broad-host-range bacteriophage infecting Vibrio alginolyticus and Vibrio parahaemolyticus. Front. Microbiol. 2023, 14, 1105924. [Google Scholar] [CrossRef]
- Tom, E.F.; Molineux, I.J.; Paff, M.L.; Bull, J.J. Experimental evolution of UV resistance in a phage. PeerJ 2018, 6, e5190. [Google Scholar] [CrossRef] [PubMed]
- Sabri, M.; El Handi, K.; Valentini, F.; De Stradis, A.; Achbani, E.H.; Benkirane, R.; Resch, G.; Elbeaino, T. Identification and Characterization of Erwinia Phage IT22: A New Bacteriophage-Based Biocontrol against Erwinia amylovora. Viruses 2022, 14, 2455. [Google Scholar] [CrossRef] [PubMed]
- Brok-Volchanskaya, V.S.; Kadyrov, F.A.; Sivogrivov, D.E.; Kolosov, P.M.; Sokolov, A.S.; Shlyapnikov, M.G.; Kryukov, V.M.; Granovsky, I.E. Phage T4 SegB protein is a homing endonuclease required for the preferred inheritance of T4 tRNA gene region occurring in co-infection with a related phage. Nucleic Acids Res. 2008, 36, 2094–2105. [Google Scholar] [CrossRef] [PubMed]
- Chung, K.M.; Liau, X.L.; Tang, S.S. Bacteriophages and Their Host Range in Multidrug-Resistant Bacterial Disease Treatment. Pharmaceuticals 2023, 16, 1467. [Google Scholar] [CrossRef]



| Ea | Strain Collection and Origin | Isolation Source |
|---|---|---|
| Ea B 01272 | National Collection of Agricultural and Industrial Microorganisms (Hungary) | Apple |
| Ea B 01616 | “ | Unknown (plant) |
| Ea B 01728 | “ | Apple |
| Ea B 01729 | “ | Apple |
| Ea B 01731 | “ | Apple |
| Ea B 01733 | “ | Apple |
| Ea B 01734 | “ | Pear |
| Ea B 01735 | “ | Quince |
| Ea B 01738 | “ | Medlar |
| Ea B 01756 | “ | Apple |
| Ea B 01757 | “ | Apple |
| Ea B 01840 | “ | Crataegus sp. |
| Ea B 01843 | “ | Apple |
| Ea B 01844 | “ | Pear |
| Ea B 01853 | “ | Cotoneaster sp. |
| Ea B 01855 | “ | Pyracantha sp. |
| Ea B 01896 | “ | Unknown (plant) |
| Ea B 01898 | “ | Apple |
| Ea B 01901 | “ | Apple |
| Ea B 01903 | “ | Apple |
| Ea B 01905 | “ | Apple |
| Ea B 01906 | “ | Apple |
| Ea B 01960 | “ | Apple |
| Ea B 01961 | “ | Apple |
| Ea B 01962 | “ | Apple |
| Ea B 01963 | “ | Apple |
| Ea B 01964 | “ | Apple |
| Ea B 01971 | “ | Apple |
| Ea B 01972 | “ | Pear |
| Ea B 01973 | “ | Pear |
| Ea B 01975 | “ | Pear |
| Ea B 01978 | “ | Pear |
| Ea B 01980 | “ | Apple |
| Ea B 01983 | “ | Quince |
| Ea G254 | Government Office of Baranya Country, Hungary | Apple |
| Ea G255 | Government Office of Baranya Country, Hungary | Apple |
| Ea B 0118T | National Collection of Agricultural and Industrial Microorganisms (Hungary) | Pear |
| CFBP 1430 | CIRM-CFBP French Collection for Plant-Associated Bacteria | Crataegus sp. |
| Ea 4/82 | Egypt, 1982 [33] | Pear |
| Ea Strains | Lysis Activity | EOP | ||
|---|---|---|---|---|
| Ea PF 7 | Ea PF 9 | Ea PF 7 | Ea PF 9 | |
| Ea B 01272 | 3 | 0 | 0.5 | 0 |
| Ea B 01616 | 2 | 3 | 0.7 | 0.8 |
| Ea B 01728 | 4 | 3 | 0.7 | 0.7 |
| Ea B 01729 | 4 | 1 | 0.7 | 0.3 |
| Ea B 01731 | 2 | 1 | 1 | 0.1 |
| Ea B 01733 | 4 | 2 | 1 | 0.4 |
| Ea B 01734 | 4 | 4 | 0.9 | 0.9 |
| Ea B 01735 | 4 | 4 | 0.9 | 0.8 |
| Ea B 01738 | 4 | 3 | 0.9 | 0.9 |
| Ea B 01756 | 4 | 4 | 1 | 1 |
| Ea B 01757 | 4 | 4 | 1 | 1.1 |
| Ea B 01840 | 4 | 4 | 1 | 0.9 |
| Ea B 01843 | 3 | 3 | 0.6 | 0.2 |
| Ea B 01844 | 3 | 3 | 0.6 | 0.5 |
| Ea B 01853 | 3 | 4 | 0.7 | 0.8 |
| Ea B 01855 | 3 | 3 | 0.7 | 0.8 |
| Ea B 01896 | 2 | 3 | 0.7 | 0.9 |
| Ea B 01898 | 3 | 3 | 0.7 | 0.4 |
| Ea B 01901 | 4 | 3 | 1.1 | 1 |
| Ea B 01903 | 3 | 3 | 0.9 | 0.9 |
| Ea B 01905 | 3 | 3 | 0.9 | 1 |
| Ea B 01906 | 4 | 2 | 1.2 | 0.7 |
| Ea B 01960 | 3 | 4 | 0.8 | 1.1 |
| Ea B 01961 | 2 | 2 | 0.7 | 0.7 |
| Ea B 01962 | 4 | 4 | 1 | 0.9 |
| Ea B 01963 | 4 | 4 | 1 | 1 |
| Ea B 01964 | 4 | 4 | 0.9 | 1.1 |
| Ea B 01971 | 3 | 4 | 0.7 | 0.9 |
| Ea B 01972 | 3 | 3 | 0.8 | 0.8 |
| Ea B 01973 | 4 | 4 | 0.9 | 1.1 |
| Ea B 01975 | 3 | 3 | 0.7 | 0.9 |
| Ea B 01978 | 4 | 3 | 0.8 | 0.7 |
| Ea B 01980 | 1 | 4 | 0.2 | 1.1 |
| Ea B 01983 | 3 | 3 | 1 | 0.9 |
| Ea G254 | 3 | 4 | 0.8 | 1 |
| Ea G255 | 3 | 4 | 0.8 | 1 |
| Ea B 0118T | 0 | 2 | 0 | 1.1 |
| CFBP 1430 | 3 | 3 | 0.8 | 0.7 |
| Ea 4/82 | 4 | 4 | 1 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lomadze, E.; Schneider, G.; Papp, S.; Bali, D.; Princz-Tóth, R.; Kovács, T. Characterizations of Newly Isolated Erwinia amylovora Loessnervirus-like Bacteriophages from Hungary. Viruses 2025, 17, 677. https://doi.org/10.3390/v17050677
Lomadze E, Schneider G, Papp S, Bali D, Princz-Tóth R, Kovács T. Characterizations of Newly Isolated Erwinia amylovora Loessnervirus-like Bacteriophages from Hungary. Viruses. 2025; 17(5):677. https://doi.org/10.3390/v17050677
Chicago/Turabian StyleLomadze, Elene, György Schneider, Szilvia Papp, Dominika Bali, Roberta Princz-Tóth, and Tamás Kovács. 2025. "Characterizations of Newly Isolated Erwinia amylovora Loessnervirus-like Bacteriophages from Hungary" Viruses 17, no. 5: 677. https://doi.org/10.3390/v17050677
APA StyleLomadze, E., Schneider, G., Papp, S., Bali, D., Princz-Tóth, R., & Kovács, T. (2025). Characterizations of Newly Isolated Erwinia amylovora Loessnervirus-like Bacteriophages from Hungary. Viruses, 17(5), 677. https://doi.org/10.3390/v17050677






