The Individual and Combined Entomopathogenic Activity of a Spodoptera frugiperda Multiple Nucleopolyhedrovirus and a Type I Spodoptera frugiperda Granulovirus on S. frugiperda Larvae
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Rearing
2.2. Virus Propagation
2.3. Bioassays
2.4. Experimental Design and Statistical Analysis
3. Results
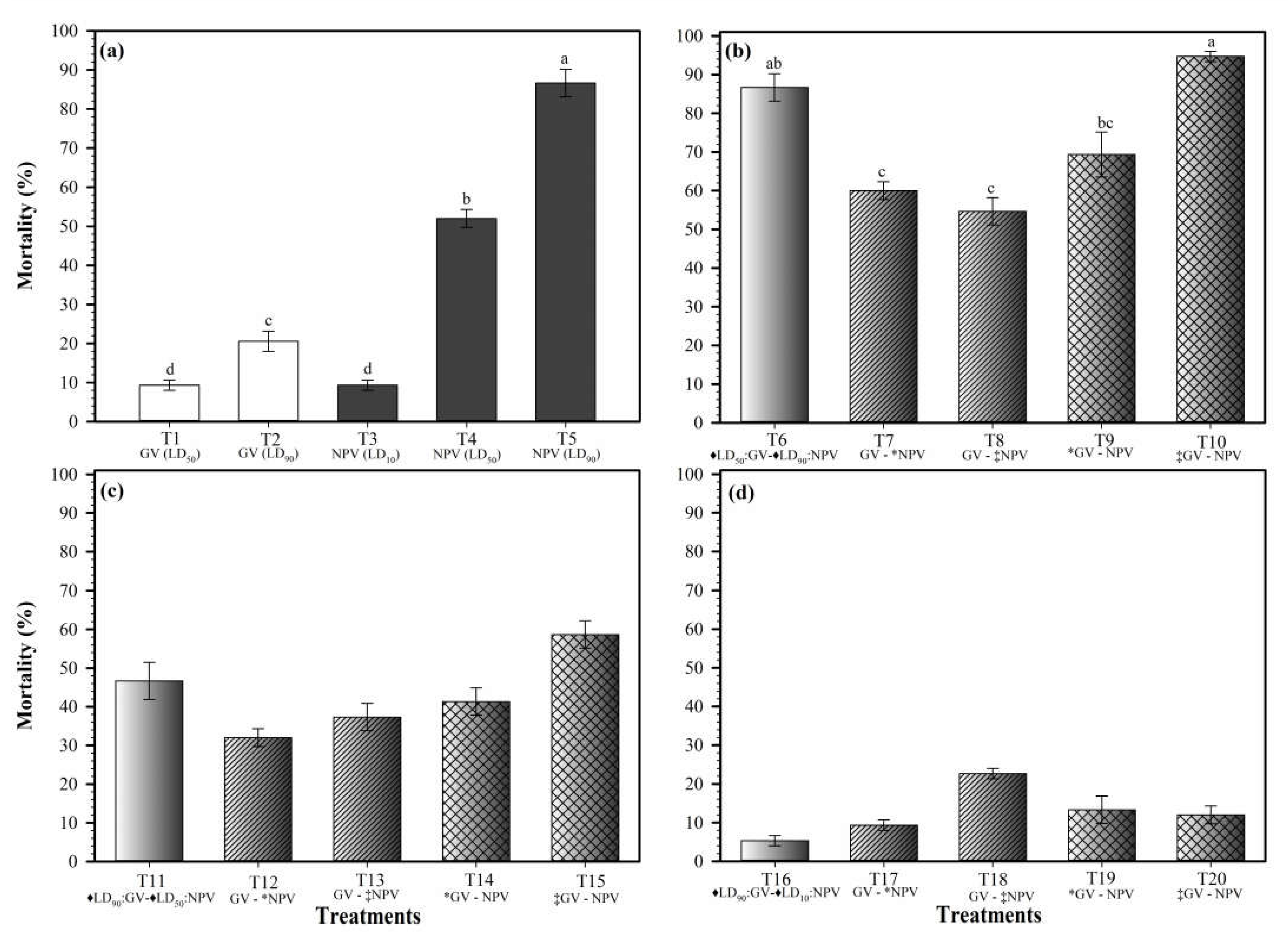
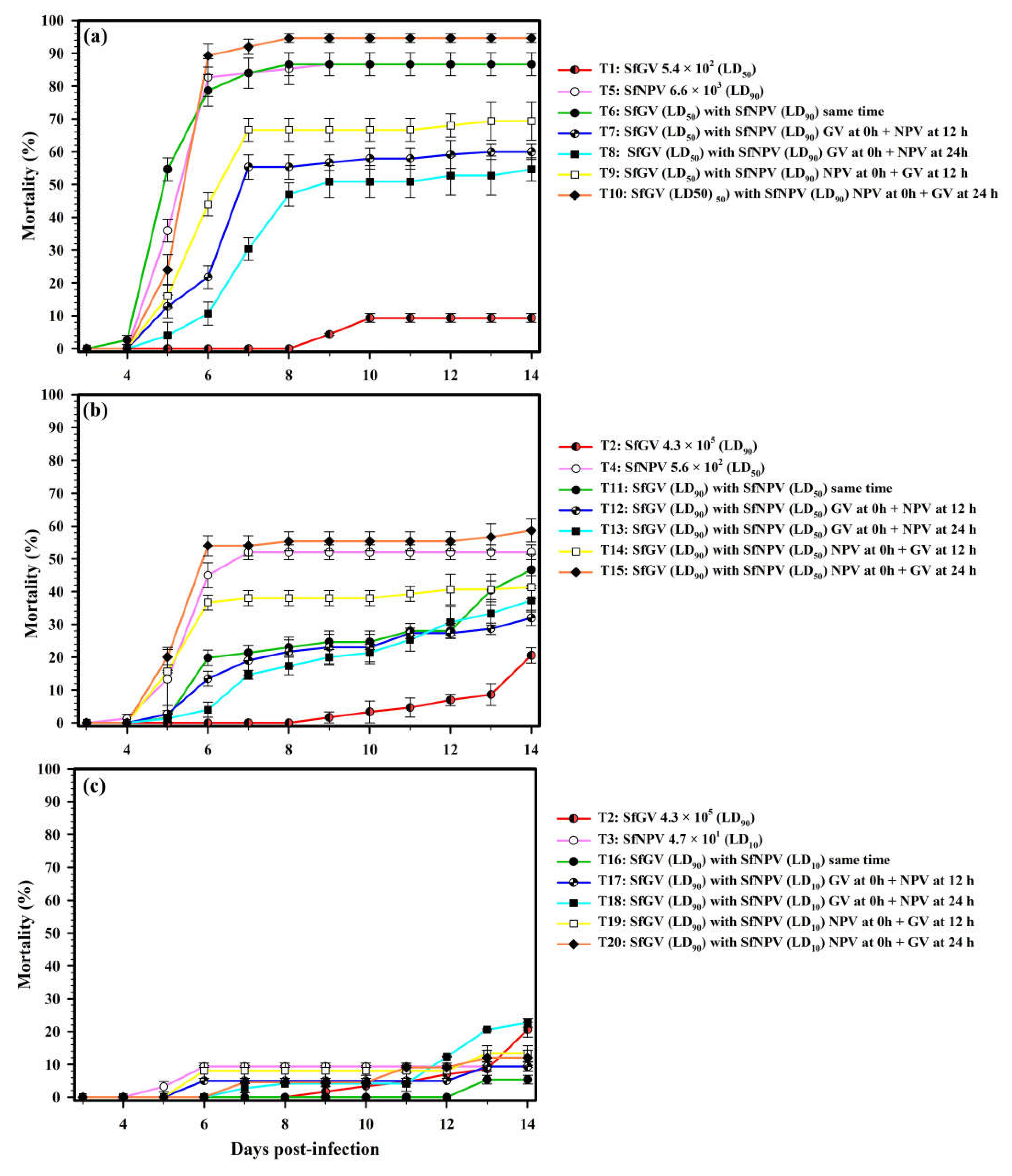
3.1. The Individual Insecticidal Activity of the Baculovirus
3.2. The Insecticidal Activity of the Baculovirus Mixtures
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SfMNPV | Spodoptera frugiperda multiple nucleopolyhedrovirus |
| LD10 | Lethal dose that kills 10% |
| LD50 | Median lethal dose |
| LD90 | Lethal dose that kills 90% |
| SfGV | Spodoptera frugiperda granulovirus |
| NPV | Nucleopolyhedrovirus |
| GV | Granulovirus |
| FAW | Fall armyworm |
| OBs | Occlusion bodies |
| RH | Relative humidity |
| SDW | Sterile distilled water |
| SDS | Sodium dodecyl sulphate |
| Dpi | Days post-infection |
| AgseNPV-B | Agrotis segetum nucleopolyhedrovirus B |
| AgseGV | Agrotis segetum granulovirus |
| HaGV | Helicoverpa armigera granulovirus |
| HzSNPV | Helicoverpa zea nucleopolyhedrovirus |
| SporNPV | Spodoptera ornithogalli nucleopolyhedrovirus |
| SporGV | Spodoptera ornithogalli granulovirus |
| XcGV | Xestia c-nigrum granulovirus |
| PhopGV | Phthorimaea operculella granulovirus |
| AgMNPV | Anticarsia gemmatalis nucleopolyhedrovirus |
| EpapGV | Epinotia aporema granulovirus |
| SpfrMNPV | Spodoptera frugiperda multiple nucleopolyhedrovirus |
| SpfrGV | Spodoptera frugiperda granulovirus |
References
- Goergen, G.; Kumar, P.L.; Sankung, S.B.; Togola, A.; Tamò, M. First report of outbreaks of the fall armyworm Spodoptera frugiperda (JE Smith) (Lepidoptera, Noctuidae), a new alien invasive pest in West and Central Africa. PLoS ONE 2016, 11, e0165632. [Google Scholar] [CrossRef] [PubMed]
- Rijal, S. Highlights on Appropriate Management Practices Against Fall Army Worm (Spodoptera Frugiperda) in the Context of Nepal. Environ. Contam. Rev. (ECR) 2019, 2, 13–14. [Google Scholar] [CrossRef]
- Ordóñez-García, M.; Rios-Velasco, C.; Ornelas-Paz, J.d.J.; Bustillos-Rodríguez, J.C.; Acosta-Muñiz, C.H.; Berlanga-Reyes, D.I.; Salas-Marina, M.Á.; Cambero-Campos, O.J.; Gallegos-Morales, G. Molecular and Morphological characterization of multiple nucleopolyhedrovirus from Mexico and their insecticidal activity against Spodoptera frugiperda (Lepidoptera: Noctuidae). J. Appl. Entomol. 2020, 144, 123–132. [Google Scholar] [CrossRef]
- Harrison, R.D.; Thierfelder, C.; Baudron, F.; Chinwada, P.; Midega, C.; Schaffner, U.; Van Den Berg, J. Agro-ecological options for fall armyworm (Spodoptera frugiperda JE Smith) management: Providing low-cost, smallholder friendly solutions to an invasive pest. J. Environ. Manag. 2019, 243, 318–330. [Google Scholar] [CrossRef]
- Nboyine, J.; Kusi, F.; Abudulai, M.; Badii, B.; Zakaria, M.; Adu, G.; Haruna, A.; Seidu, A.; Osei, V.; Alhassan, S. A new pest, Spodoptera frugiperda (JE Smith), in tropical Africa: Its seasonal dynamics and damage in maize fields in northern Ghana. Crop Prot. 2020, 127, 104960. [Google Scholar] [CrossRef]
- Sree, K.S.; Varma, A. An Introduction to Entomopathogenic Microorganisms. In Biocontrol of Lepidopteran Pests: Use of Soil Microbes and their Metabolites; Sree, K.S., Varma, A., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 1–10. [Google Scholar]
- Zimmermann, G. Review on safety of the entomopathogenic fungi Beauveria bassiana and Beauveria brongniartii. Biocontrol Sci. Technol. 2007, 17, 553–596. [Google Scholar] [CrossRef]
- Natra, N.T.; Kumar, M.; Apurava, K.; Thakur, V.V. Biopesticides: An effective and environmental friendly insect-pests inhibitor line of action. In Biofertilizers and Biopesticides in Sustainable Agriculture; Kaushik, B.D., Kumar, D., Shamim, M., Eds.; CRC Press: Boca Raton, FL, USA, 2015; pp. 71–90. [Google Scholar]
- Inceoglu, A.B.; Kamita, S.G.; Hammock, B.D. Genetically modified baculoviruses: A historical overview and future outlook. Adv. Virus Res. 2006, 68, 323–360. [Google Scholar]
- Sood, P.; Choudhary, A.; Prabhakar, C.S. Granuloviruses in insect pest management. In Microbes for Sustainable Insect Pest Management: An Eco-Friendly Approach; Khan, M.A., Ahmad, W., Eds.; Springer: Cham, Switzerland, 2019. [Google Scholar]
- Barrera, G.; Gómez, J.; Cuartas, P.; León, G.; Villamizar, L. Caracterización morfológica, biológica y genética de un aislamiento colombiano de granulovirus de Erinnyis ello (L.) (Lepidoptera: Sphingidae). Rev. Colomb. Biotecnol. 2014, 16, 129–140. [Google Scholar] [CrossRef]
- Bernal, A.; Williams, T.; Hernández-Suárez, E.; Carnero, A.; Caballero, P.; Simón, O. A native variant of Chrysodeixis chalcites nucleopolyhedrovirus: The basis for a promising bioinsecticide for control of C. chalcites on Canary Islands’ banana crops. Biol. Control 2013, 67, 101–110. [Google Scholar] [CrossRef][Green Version]
- Harrison, R.L.; Popham, H.J.; Breitenbach, J.E.; Rowley, D.L. Genetic variation and virulence of Autographa californica multiple nucleopolyhedrovirus and Trichoplusia ni single nucleopolyhedrovirus isolates. J. Invertebr. Pathol. 2012, 110, 33–47. [Google Scholar] [CrossRef]
- Jukes, M.D.; Knox, C.M.; Hill, M.P.; Moore, S.D. The isolation and genetic characterisation of a South African strain of Phthorimaea operculella granulovirus, PhopGV-SA. Virus Res. 2014, 183, 85–88. [Google Scholar] [CrossRef] [PubMed]
- Zamora-Avilés, N.; Murillo, R.; Lasa, R.; Pineda, S.; Figueroa, J.; Bravo-Patiño, A.; Díaz, O.; Corrales, J.; Martínez, A. Genetic and biological characterization of four nucleopolyhedrovirus isolates collected in Mexico for the control of Spodoptera exigua (Lepidoptera: Noctuidae). J. Econ. Entomol. 2017, 110, 1465–1475. [Google Scholar] [CrossRef] [PubMed]
- Barrera, G.; Simón, O.; Villamizar, L.; Williams, T.; Caballero, P. Spodoptera frugiperda multiple nucleopolyhedrovirus as a potential biological insecticide: Genetic and phenotypic comparison of field isolates from Colombia. Biol. Control 2011, 58, 113–120. [Google Scholar] [CrossRef]
- Behle, R.W.; Popham, H.J. Laboratory and field evaluations of the efficacy of a fast-killing baculovirus isolate from Spodoptera frugiperda. J. Invertebr. Pathol. 2012, 109, 194–200. [Google Scholar] [CrossRef]
- Martínez, A.M.; Pineda, S.; Figueroa, J.I.; Chavarrieta, J.M.; Williams, T. Los baculovirus como bioinsecticidas: Evaluación de un nucleopoliedrovirus para el combate de Spodoptera frugiperda (Lepidoptera: Noctuidae) en México y Honduras. Cienc. Nicolaita 2012, 35–47. [Google Scholar]
- Méndez, W.A.; Valle, J.; Ibarra, J.E.; Cisneros, J.; Penagos, D.I.; Williams, T. Spinosad and nucleopolyhedrovirus mixtures for control of Spodoptera frugiperda (Lepidoptera: Noctuidae) in maize. Biol. Control 2002, 25, 195–206. [Google Scholar] [CrossRef]
- Núñez, J.C.R.; Ramírez, M.F.V.; Castro, M.C.D.R. Caracterización biológica y molecular de cepas exóticas de Baculovirus SfNPV, con actividad bioinsecticida hacia una población mexicana del gusano cogollero del maíz Spodoptera frugiperda (Lepidóptera: Noctuidae). Interciencia 2014, 39, 320–326. [Google Scholar]
- Vieira, C.M.; Tuelher, E.S.; Valicente, F.H.; Wolff, J.L.C. Characterization of a Spodoptera frugiperda multiple nucleopolyhedrovirus isolate that does not liquefy the integument of infected larvae. J. Invertebr. Pathol. 2012, 111, 189–192. [Google Scholar] [CrossRef]
- Rios-Velasco, C.; Gallegos-Morales, G.; Del Rincón-Castro, M.C.; Cerna-Chávez, E.; Sánchez-Peña, S.R.; Siller, M.C. Insecticidal activity of native isolates of Spodoptera frugiperda multiple nucleopolyhedrovirus from soil samples in Mexico. Fla. Entomol. 2011, 94, 716–718. [Google Scholar] [CrossRef]
- Simón, O.; Williams, T.; López-Ferber, M.; Taulemesse, J.-M.; Caballero, P. Population genetic structure determines speed of kill and occlusion body production in Spodoptera frugiperda multiple nucleopolyhedrovirus. Biol. Control 2008, 44, 321–330. [Google Scholar] [CrossRef]
- Ordóñez-García, M.; Bustillos-Rodríguez, J.C.; de Jesús Ornelas-Paz, J.; Acosta-Muñiz, C.H.; Salas-Marina, M.Á.; Cambero-Campos, O.J.; Estrada-Virgen, M.O.; Morales-Ovando, M.A.; Rios-Velasco, C. Morphological, biological, and molecular characterization of Type I granuloviruses of Spodoptera frugiperda. Neotrop. Entomol. 2024, 53, 917–928. [Google Scholar] [CrossRef] [PubMed]
- Pidre, M.L.; Sabalette, K.B.; Romanowski, V.; Ferrelli, M.L. Identification of an Argentinean isolate of Spodoptera frugiperda granulovirus. Rev. Argent. Microbiol. 2019, 51, 381–385. [Google Scholar] [CrossRef] [PubMed]
- Federici, B.A. Baculovirus pathogenesis. In The Baculoviruses; Springer: Boston, MA, USA, 1997; pp. 33–59. [Google Scholar]
- Biedma, M.E.; Salvador, R.; Ferrelli, M.L.; Sciocco-Cap, A.; Romanowski, V. Effect of the interaction between Anticarsia gemmatalis multiple nucleopolyhedrovirus and Epinotia aporema granulovirus, on A. gemmatalis (Lepidoptera: Noctuidae) larvae. Biol. Control 2015, 91, 17–21. [Google Scholar]
- Espinel-Correal, C.; López-Ferber, M.; Zeddam, J.-L.; Villamizar, L.; Gómez, J.; Cotes, A.M.; Léry, X. Experimental mixtures of Phthorimaea operculella granulovirus isolates provide high biological efficacy on both Phthorimaea operculella and Tecia solanivora (Lepidoptera: Gelechiidae). J. Invertebr. Pathol. 2012, 110, 375–381. [Google Scholar] [CrossRef]
- Hackett, K.J.; Boore, A.; Deming, C.; Buckley, E.; Camp, M.; Shapiro, M. Helicoverpa armigera granulovirus interference with progression of H. zea nucleopolyhedrovirus disease in H. zea larvae. J. Invertebr. Pathol. 2000, 75, 99–106. [Google Scholar] [CrossRef]
- Cuartas-Otálora, P.E.; Gómez-Valderrama, J.A.; Ramos, A.E.; Barrera-Cubillos, G.P.; Villamizar-Rivero, L.F. Bio-Insecticidal Potential of Nucleopolyhedrovirus and Granulovirus Mixtures to Control the Fall Armyworm Spodoptera frugiperda (JE Smith, 1797) (Lepidoptera: Noctuidae). Viruses 2019, 11, 684. [Google Scholar] [CrossRef]
- Cheng, X.W.; Lynn, D.E. Baculovirus Interactions: In Vitro and In Vivo. In Advances in Applied Microbiology; Laskin, A.I., Sariaslani, S., Gadd, G.M., Eds.; Academic Press: Cambridge, MA, USA, 2009; pp. 217–239. [Google Scholar]
- Ishimwe, E.; Hodgson, J.J.; Passarelli, A.L. Expression of the Cydia pomonella granulovirus matrix metalloprotease enhances Autographa californica multiple nucleopolyhedrovirus virulence and can partially substitute for viral cathepsin. Virology 2015, 481, 166–178. [Google Scholar] [CrossRef]
- Hughes, P.; Wood, H. A synchronous peroral technique for the bioassay of insect viruses. J. Invertebr. Pathol. 1981, 37, 154–159. [Google Scholar] [CrossRef]
- Muñoz, D.; Martínez, A.M.; Pérez, R.M.; de Escudero Fuentemilla, I.R.; Vilaplana, L. Técnicas básicas para la caracterización de baculovirus. In Los Baculovirus y sus Aplicaciones Como Bioinsecticidas en el Control Biológico de Plagas; Caballero, P., Williams, T., López-Ferber, M., Eds.; Universidad Pública de Navarra-Phytoma: Valencia, Spain, 2001; pp. 479–518. [Google Scholar]
- Finney, D.J. Probit Analysis, 3rd ed.; Cambridge University Press: New York, NY, USA, 1971; p. 333. [Google Scholar]
- SAS Institute Incorporated. SAS User’s Guide; SAS Institute Incorporated: Cary, NC, USA, 2002. [Google Scholar]
- Koppenhöfer, A.M.; Kaya, H.K. Additive and synergistic interaction between entomopathogenic nematodes and bacillus thuringiensis for scarab grub control. Biol. Control 1997, 8, 131–137. [Google Scholar] [CrossRef]
- Du Plessis, H.; Schlemmer, M.-L.; Van den Berg, J. The effect of temperature on the development of Spodoptera frugiperda (Lepidoptera: Noctuidae). Insects 2020, 11, 228. [Google Scholar] [CrossRef]
- Wang, Y.; Choi, J.Y.; Roh, J.Y.; Woo, S.D.; Jin, B.R.; Je, Y.H. Molecular and phylogenetic characterization of Spodoptera litura granulovirus. J. Microbiol. 2008, 46, 704–708. [Google Scholar] [CrossRef] [PubMed]
- Wennmann, J.T.; Köhler, T.; Gueli Alletti, G.; Jehle, J.A. Mortality of cutworm larvae is not enhanced by Agrotis segetum granulovirus and Agrotis segetum nucleopolyhedrovirus B coinfection relative to single infection by either virus. Appl. Environ. Microbiol. 2015, 81, 2893–2899. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dias-Vasconcelos, S. Ecología de los baculovirus. In Los Baculovirus y sus Aplicaciones Como Bioinsecticidas en el Control Biológico de Plagas; Caballero, P., Williams, T., López-Ferber, M., Eds.; Universidad Pública de Navarra-Phytoma: Valencia, Spain, 2001; pp. 143–201. [Google Scholar]
- Sciocco-Cap, A. Biología y patogénesis de los baculovirus. In Los Baculovirus y sus Aplicaciones como Bioinsecticidas en el Control Biológico de Plagas; Caballero, P., Williams, T., López-Ferber, M., Eds.; Universidad Pública de Navarra-Phytoma: Valencia, Spain, 2001; pp. 47–72. [Google Scholar]
- Ferrelli, M.L.; Salvador, R. Effects of mixed baculovirus infections in biological control: A comprehensive historical and technical analysis. Viruses 2023, 15, 1838. [Google Scholar] [CrossRef]
- Barrera, G.P.; Villamizar, L.F.; Araque, G.A.; Gómez, J.A.; Guevara, E.J.; Cerrudo, C.S.; Belaich, M.N. Natural coinfection between novel species of baculoviruses in Spodoptera Ornithogalli larvae. Viruses 2021, 13, 2520. [Google Scholar] [CrossRef]
- Guo, H.; Fang, J.; Wang, J.; Zhong, W.; Liu, B. Interaction of Xestia c-nigrum granulovirus with peritrophic matrix and Spodoptera litura nucleopolyhedrovirus in Spodoptera litura. J. Econ. Entomol. 2007, 100, 20–25. [Google Scholar] [CrossRef]
- Lasa, R.; Caballero, P.; Williams, T. Juvenile hormone analogs greatly increase the production of a nucleopolyhedrovirus. Biol. Control 2007, 41, 389–396. [Google Scholar] [CrossRef]
- Hatem, A.E.-S.; Aldebis, H.K.; Osuna, E.V. Effects of the Spodoptera littoralis granulovirus on the development and reproduction of cotton leafworm S. littoralis. Biol. Control 2011, 59, 192–199. [Google Scholar] [CrossRef]

| Isolate | Lethal Doses | Doses (OBs/Larva) | Fiducial Limits (95%) | χ2 | df | Slope ± (SE) | Intercept ± (SE) | |
|---|---|---|---|---|---|---|---|---|
| Lower | Upper | |||||||
| SfGV-CH13 | LD50 | 5.4 × 102 | 3.1 × 102 | 9.5 × 102 | 4.5 | 4 | 0.4 ± 0.04 | −1.2 ± 0.13 |
| LD90 | 4.3 × 105 | 1.3 × 105 | 2.3 × 106 | 4.5 | 4 | 0.4 ± 0.04 | −1.2 ± 0.13 | |
| SfNPV-CH32 | LD10 | 4.7 × 101 | 2.6 × 101 | 6.7 × 101 | 4.9 | 3 | 1.2 ± 0.08 | −3.3 ± 0.23 |
| LD50 | 5.6 × 102 | 4.3 × 102 | 7.4 × 102 | 4.9 | 3 | 1.2 ± 0.08 | −3.3 ± 0.23 | |
| LD90 | 6.6 × 103 | 4.4 × 103 | 1.1 × 104 | 4.9 | 3 | 1.2 ± 0.08 | −3.3 ± 0.23 | |
| Dose OBs/Larva | Inoculation of Viral Isolates | Interaction Type | ||
|---|---|---|---|---|
| Treatment | SfGV-CH13 | SfNPV-CH32 | ||
| T1 | 5.4 × 102 (LD50) | -- | Alone | -- |
| T2 | 4.3 × 105 (LD90) | -- | Alone | -- |
| T3 | -- | 4.7 × 101 (LD10) | Alone | -- |
| T4 | -- | 5.6 × 102 (LD50) | Alone | -- |
| T5 | -- | 6.6 × 103 (LD90) | Alone | -- |
| T6 | ♦ LD50 | ♦ LD90 | Same time | Antagonism |
| T7 | LD50 | * LD90 | GV at 0 h + NPV at 12 h | Antagonism |
| T8 | LD50 | ‡ LD90 | GV at 0 h + NPV at 24 h | Antagonism |
| T9 | * LD50 | LD90 | NPV at 0 h + GV at 12 h | Antagonism |
| T10 | ‡ LD50 | LD90 | NPV at 0 h + GV at 24 h | Synergism |
| T11 | ♦ LD90 | ♦ LD50 | Same time | Antagonism |
| T12 | LD90 | * LD50 | GV at 0 h + NPV at 12 h | Antagonism |
| T13 | LD90 | ‡ LD50 | GV at 0 h + NPV at 24 h | Antagonism |
| T14 | * LD90 | LD50 | NPV at 0 h + GV at 12 h | Antagonism |
| T15 | ‡ LD90 | LD50 | NPV at 0 h + GV at 24 h | Antagonism |
| T16 | ♦ LD90 | ♦ LD10 | Same time | Antagonism |
| T17 | LD90 | * LD10 | GV at 0 h + NPV at 12 h | Antagonism |
| T18 | LD90 | ‡ LD10 | GV at 0 h + NPV at 24 h | Antagonism |
| T19 | * LD90 | LD10 | NPV at 0 h + GV at 12 h | Antagonism |
| T20 | ‡ LD90 | LD10 | NPV at 0 h + GV at 24 h | Antagonism |
| T21 | Un-infected larvae | Control | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ordóñez-García, M.; Bustillos-Rodríguez, J.C.; Ornelas-Paz, J.d.J.; Salas-Marina, M.Á.; Cambero-Campos, O.J.; Acosta-Muñiz, C.H.; Berlanga-Reyes, D.I.; Rios-Velasco, C. The Individual and Combined Entomopathogenic Activity of a Spodoptera frugiperda Multiple Nucleopolyhedrovirus and a Type I Spodoptera frugiperda Granulovirus on S. frugiperda Larvae. Viruses 2025, 17, 674. https://doi.org/10.3390/v17050674
Ordóñez-García M, Bustillos-Rodríguez JC, Ornelas-Paz JdJ, Salas-Marina MÁ, Cambero-Campos OJ, Acosta-Muñiz CH, Berlanga-Reyes DI, Rios-Velasco C. The Individual and Combined Entomopathogenic Activity of a Spodoptera frugiperda Multiple Nucleopolyhedrovirus and a Type I Spodoptera frugiperda Granulovirus on S. frugiperda Larvae. Viruses. 2025; 17(5):674. https://doi.org/10.3390/v17050674
Chicago/Turabian StyleOrdóñez-García, Magali, Juan Carlos Bustillos-Rodríguez, José de Jesús Ornelas-Paz, Miguel Ángel Salas-Marina, Octavio Jhonathan Cambero-Campos, Carlos Horacio Acosta-Muñiz, David Ignacio Berlanga-Reyes, and Claudio Rios-Velasco. 2025. "The Individual and Combined Entomopathogenic Activity of a Spodoptera frugiperda Multiple Nucleopolyhedrovirus and a Type I Spodoptera frugiperda Granulovirus on S. frugiperda Larvae" Viruses 17, no. 5: 674. https://doi.org/10.3390/v17050674
APA StyleOrdóñez-García, M., Bustillos-Rodríguez, J. C., Ornelas-Paz, J. d. J., Salas-Marina, M. Á., Cambero-Campos, O. J., Acosta-Muñiz, C. H., Berlanga-Reyes, D. I., & Rios-Velasco, C. (2025). The Individual and Combined Entomopathogenic Activity of a Spodoptera frugiperda Multiple Nucleopolyhedrovirus and a Type I Spodoptera frugiperda Granulovirus on S. frugiperda Larvae. Viruses, 17(5), 674. https://doi.org/10.3390/v17050674






