Abstract
The transcription and replication of the genome of influenza A virus (IAV) take place in the nucleus of infected cells, which is catalyzed by the viral ribonucleoprotein (vRNP) complex. The nuclear import of the vRNP complex and its component proteins is essential for the efficient replication of IAV and is therefore prone to be targeted by host restriction factors. Herein, we found that host cellular protein keratin 6A (KRT6A) is a negative regulator of IAV replication because siRNA-mediated knockdown of KRT6A expression increased the growth titers of IAV, whereas exogenous overexpression of KRT6A reduced viral yields. The nuclear import of incoming vRNP complexes and newly synthesized nucleoprotein (NP) was significantly impaired when KRT6A was overexpressed. Further studies showed that KRT6A interacts with the four vRNP complex proteins—polymerase basic protein 1 (PB1), polymerase basic protein 2 (PB2), polymerase acidic protein (PA), and NP. Notably, the interaction between KRT6A and vRNP complex proteins had no effect on the nuclear import of PB2 or the PB1-PA heterodimer but impaired the interaction between NP and the nuclear import adaptor importin α3, thereby inhibiting the nuclear import of incoming vRNP complexes and newly synthesized NP. Moreover, KRT6A was further shown to suppress the assembly of the vRNP complex and consequently reduce viral polymerase activity. Together, our data uncover a novel role of KRT6A in counteracting the nuclear import and functions of the vRNP complex, thereby restricting the replication of IAV.
1. Introduction
Influenza A virus (IAV) is an important zoonotic pathogen that belongs to the family Orthomyxoviridae. The genome of IAV consists of eight single-stranded negative-sense RNA segments, encoding ten essential proteins and up to eight accessory proteins [1]. Based on the antigenicity of the two surface glycoproteins, hemagglutinin (HA) and neuraminidase (NA), IAV is classified into different subtypes. So far, 19 HA subtypes and 11 NA subtypes of IAV have been identified [2,3]. Since 1918, only H1N1, H2N2, H3N2, and H1N2 subtypes of IAV have circulated in humans [4], causing seasonal epidemics and/or occasional pandemics. However, the widespread global circulation of avian influenza viruses in recent years has led to the appearance of more and more subtypes of IAV (i.e., H5N1, H5N8, H7N9, H9N2, H3N8, and H10N8) capable of infecting or even killing humans [5,6,7,8,9,10,11,12,13].
The three viral polymerases [polymerase basic protein 1 (PB1), polymerase basic protein 2 (PB2), and polymerase acidic protein (PA)], along with nucleoprotein (NP) and viral RNA, together constitute the viral ribonucleoprotein (vRNP) complex, which is responsible for the transcription and replication of the viral genome. In the replication cycle of IAV, upon the completion of the uncoating step, the incoming vRNP complex is released into the cytoplasm and subsequently delivered into the nucleus through the classical nuclear import pathway [14,15]. The translocation of the vRNP complex into the nucleus is mediated by viral NP protein, which harbors two nuclear localization signals (NLSs); one is an unconventional NLS located between amino acids 3–13 at the N terminus [16,17], and the other is a bipartite NLS that sits between amino acids 198 and 216 [18]. The NLSs of NP bind to isoforms of importin α, which further recruits importin β, leading to the ultimate transport of the incoming vRNP complex or NP itself into the nucleus through the nuclear pore complex [14,15].
The vRNP complex lies in the center of the IAV replication cycle. Within the vRNP complex, NP is the most abundant component protein and serves as the carrier of viral RNA [19]. Several amino acid mutations in NP (e.g., A286V, T437M, and Q357K [20,21]) have been identified to be important for the pathogenicity of IAV. To efficiently replicate in the host, IAV uses NP to interact with proviral host factors. For example, the association between NP and Bcl10-interacting protein with CARD 1 (BinCARD1) enhances the binding between NP and importin α7, thereby facilitating the nuclear import of the vRNP complex and newly synthesized NP [22]; Tat stimulatory factor 1 (Tat-SF1) interacts with free NP but not NP bound to RNA, and acts as a molecular chaperone to promote the formation of NP–RNA complexes [23]; DEAD-box RNA helicase U2AF65-associated protein (UAP56) associates with NP and enhances viral RNA synthesis [24]; high-mobility group box 1 protein (HMGB1) interacts with NP in the nucleus to promote the recruitment of the vRNP complex at chromatin transcriptional active sites, thus enhancing viral polymerase activity and growth [25]; and fragile X mental retardation protein (FMRP) binds NP to promote the assembly and nuclear export of the vRNP complex [26]. On the other hand, the crucial role of NP in the replication cycle of IAV also makes it an important target of the host restriction factors in combating IAV infection. Among them, Moloney Leukemia virus 10 (MOV10) and phospholipid scramblase 1 (PLSCR1) interact with NP and interfere with its binding with importin α or the further recruitment of importin β, thereby suppressing the nuclear import of the NP/vRNP complex and inhibiting the replication of IAV [27,28]; tripartite motif-containing 4 (TRIM4), TRIM14, TRIM22, and TRIM41 interact with and mediate polyubiquitination of NP, leading to its proteasomal degradation [29,30,31,32].
Keratin proteins are the predominant subgroup of intermediate filaments (IFs) [33], and consist of two types: type I (28 members) and type II (26 members) [34]. All type I keratin genes, except the gene encoding KRT18, are clustered on the long arm of chromosome 17, whereas all type II keratin genes, together with the gene encoding KRT18, are located on the long arm of chromosome 12 [35]. KRT6A is a member of type II keratins [34]. It is reported that KRT6A modulates the migration of keratinocytes in response to injury [36]. The expression of KRT6A has been shown to be significantly upregulated in non-small cell lung cancer (NSCLC) and colorectal cancer [37,38], and its expression promotes cell proliferation and invasion in NSCLC and nasopharyngeal carcinoma [39,40]. Of importance, the high expression level of KRT6A in NSCLC is correlated with poor patient prognosis [37]. However, whether KRT6A plays a role in virus infection is still largely unknown.
In the present study, we demonstrated that KRT6A is a novel restriction factor for the replication of IAV. KRT6A interacted with the four vRNP complex proteins of IAV, and the interaction between KRT6A and NP specifically impaired the association between NP and importin α3, thereby inhibiting the nuclear import of the incoming vRNP complex and NP itself. Furthermore, the expression of KRT6A also hindered the assembly of the vRNP complex and consequently reduced the viral polymerase activity. Our study thus unveiled that KRT6A is a negative regulator of IAV replication by modulating the nuclear import and function of the vRNP complex.
2. Materials and Methods
2.1. Cells and Virus
A549, HEK293T, HeLa, and MDCK cells were cultured in F12K (Gibco, Grand Island, NY, USA) with 10% fetal bovine serum (FBS, Gibco) (A549), DMEM (Sigma-Aldrich, St. Louis, MO, USA) containing 10% FBS (HEK293T, HeLa), and DMEM containing 5% bovine calf serum (BCS, Sigma-Aldrich) (MDCK). All media were supplemented with 100 units/mL penicillin and 100 μg/mL streptomycin (Gibco). All cells were cultured at 37 °C with 5% CO2. A/WSN/1933 (WSN, H1N1) virus was propagated in MDCK cells, as previously described [41].
2.2. Plasmids and Small Interfering RNAs
The open reading frame (ORF) of KRT6A was inserted into the pCDNA3.1 vector with a Myc tag at the N-terminus. The ORFs of PB2, PB1, PA, and NP derived from the WSN (H1N1) virus were cloned into the mammalian expression vector pCAGGS as described previously [27]. Plasmids pCAGGS-V5-WSNPB1, pCAGGS-V5-WSNPA, pCAGGS-V5-WSNNP, pCAGGS-Flag-importin α1, pCAGGS-Flag-importin α3, pCAGGS-Flag-importin α5, and pCAGGS-Flag-importin α7 were generated by inserting the ORFs of PB1, PA, NP of WSN (H1N1) virus, importin α1, importin α3, importin α5, and importin α7 fused with a V5 or Flag tag sequence at the N-terminus into the pCAGGS vector. Plasmids pCAGGS-WSNPA-Flag and pCAGGS-WSNNP-Flag were generated by inserting the ORF of PA and NP of WSN (H1N1) virus fused with a C-terminal Flag tag sequence into the pCAGGS vector. The generation of pHH21-SC09NS-F-Luc, used to produce virus-like negative-sense RNA harboring a firefly luciferase reporter gene, has been described previously [27]. All plasmid constructs were confirmed by sequencing. The small interfering RNA targeting KRT6A (si_KRT6A, sense: 5′-CCAGCAGGAAGAGCUAUATT-3′; antisense: 5′-UAUAGCUCUUCCUGCUGGTT-3′) and a scrambled siRNA (si_control, sense: 5′-UUCUCCGAACGUGUCACGUTT-3′; antisense: 5′-ACGUGACACGUUCGGAGAATT-3′) were purchased from GenePharma (Shanghai, China).
2.3. Antibodies
Mouse monoclonal antibodies (mAbs) and rabbit polyclonal antibodies (pAbs) against the following IAV proteins were generated in our laboratory: PB2, PB1, PA, and NP [42]. Mouse anti-V5 mAb (SAB2702199), mouse anti-Myc mAb (M4439), and rabbit anti-Flag pAb (F7425) were from Sigma Aldrich. Rabbit anti-Myc pAb (16286-1-AP), mouse anti-KRT6A mAb (66685-1-lg), rabbit anti-KRT6A pAb (10590-1-AP), rabbit anti-GAPDH pAb (10494-1-AP), mouse anti-GAPDH mAb (60004-1-Ig), and rabbit anti-LaminB1 pAb (12987-1-AP) were from Proteintech (Wuhan, China). Rabbit anti-V5 pAb (AB3792) and mouse anti-Flag mAb (B3111) were from Millipore (Darmstadt, Germany). Alexa Fluor 633 goat anti-mouse IgG (H + L) (A21050), Alexa Fluor 633 goat anti-rabbit IgG (H + L) (A21071), Alexa Fluor 488 goat anti-rabbit IgG (H + L) (A11034), and Alexa Fluor 488 goat anti-mouse IgG (H + L) (A11029) from Life Technologies (Grand Island, NY, USA) were used for confocal microscopy. The secondary antibodies used for western blotting—DyLight 800 goat anti-mouse IgG (H + L) (RS23910) and DyLight 680 goat anti-rabbit IgG (H + L) (RS23720)—were obtained from Immunoway (Plano, TX, USA).
2.4. Transfection and Virus Titration
A549 cells were allowed to grow to approximately 70–80% confluency in 12-well plates and then transfected with the indicated plasmids for protein expression by using Lipofectamine 2000 reagent (Invitrogen, Carlsbad, CA, USA). Si_KRT6A or scrambled siRNA at a final concentration of 30 nM was transfected into approximately 5 × 10⁵ A549 cells seeded in 12-well plates by using the Lipofectamine RNAiMAX transfection reagent (Invitrogen). The siRNA knockdown efficiency of KRT6A was confirmed by means of RT-qPCR. Plasmid- or siRNA-transfected A549 cells grown in 12-well plates were infected with WSN (H1N1) virus at an MOI of 0.1. After virus adsorption for 1 h at 37 °C, the cells were washed with phosphate-buffered saline (PBS) and then incubated in F12K medium containing 0.125 μg/mL L-1-tosylamide-2-phenylmethyl chloromethyl ketone (TPCK)-treated trypsin (Worthington, Lakewood, NJ, USA) at 37 °C. Supernatants were collected at 24 and 48 h post-infection (p.i.), and virus titers were determined by means of plaque assays on MDCK cells, as described previously [43].
2.5. Co-Immunoprecipitation Assay
HEK293T cells were grown to approximately 70–80% confluency in 6-well plates and then transfected with the indicated plasmids by using the Lipofectamine LTX and Plus Reagents (Invitrogen). At 48 h post-transfection, the cells were lysed with IP lysis buffer (Pierce, Rockford, IL, USA) containing protease inhibitor cocktail (Roche, GmbH, Mannheim, Germany) on ice for 30 min and then centrifuged at 12,000 rpm at 4 °C for 10 min. Supernatants were incubated with the corresponding primary antibodies at 4 °C overnight, followed by the addition of protein A/G agarose (Roche) and incubation at 4 °C with rotation. After 6 h, the beads were washed three times with cold PBS. The immunoprecipitated proteins were then separated by SDS-PAGE and transferred onto nitrocellulose for western blotting.
2.6. Indirect Immunofluorescence Assay
A549 or HeLa cells grown on glass-bottom dishes were transfected with plasmids or siRNA, or further infected with WSN (H1N1) virus as indicated. The cells were fixed with 4% paraformaldehyde (PFA) for 30 min, permeabilized with 0.2% Triton X-100 for 30 min, and blocked with 1% BSA for 1 h at room temperature (RT). Incubation with the corresponding primary antibodies was carried out at 4 °C overnight, followed by further incubation with secondary antibodies conjugated to Alexa Fluor 488 or Alexa Fluor 633 for 1 h at RT. After three washes, the cells were incubated with DAPI (4’,6-diamidino-2-phenylindole; Thermo Fisher Scientific, Waltham, MA, USA) for 15 min at RT to stain the nuclei. Images were visualized using an LSM 980 with AiryScan confocal microscope (Zeiss, Oberkochen, Germany).
2.7. RNA Quantification
Total RNA of A549 cells was extracted by using an RNAsimple Total RNA Kit (Tiangen, Beijing, China) and was used to synthesize first-strand cDNA with a cDNA synthesis kit (Vazyme, Nanjing, China). Real-time PCR was performed by using ChamQ SYBR qPCR Master Mix (Vazyme). Relative RNA quantities were determined by using the ΔΔCt method and were normalized to the expression of the cellular GAPDH gene.
2.8. Nuclear and Cytoplasmic Fractionation
NE-PER Nuclear and Cytoplasmic Extraction Reagents (Thermo Fisher Scientific) were used for the separation of cytoplasmic and nuclear extracts. Briefly, the cell pellet was incubated with ice-cold CER I on ice for 10 min, followed by the addition of ice-cold CER II. After centrifugation at 12,000 rpm for 10 min at 4 °C, the supernatants were collected and used as cytoplasmic proteins. The insoluble fraction was suspended in ice-cold NER and vortexed at the highest setting for 15 s. The sample was then placed on ice and vortexed for 15 s every 10 min, for a total of 40 min. The extractions were centrifuged at 12,000 rpm for 15 min at 4 °C, and the supernatants were collected as the nuclear proteins.
2.9. Western Blotting
Protein samples were separated by SDS-PAGE using 10% or 12.5% polyacrylamide gel (EpiZyme), with 20 µL of protein extract loaded per well. Subsequently, the fractionated proteins were transferred onto nitrocellulose membranes (GE Healthcare) for Western blotting analysis. After being blocked with 5% skim milk in PBS, the membranes were incubated with primary antibodies diluted in PBS containing 0.5% BSA overnight at 4 °C and then washed three times with PBST. Following incubation with DyLight 800 goat anti-mouse IgG (H + L) or DyLight 680 goat anti-rabbit IgG (H + L) at RT for 1 h, the membranes were visualized using an Odyssey CLX infrared imaging system (LI-COR Biosciences).
2.10. Dual-Luciferase Reporter Assay
HEK293T cells were transfected with either si_KRT6A or scrambled siRNA. At 24 h post-transfection, the cells were further transfected with the plasmids of the minireplicon system, including the four RNP complex protein expression plasmids from the WSN (H1N1) virus (pCAGGS-PB2, pCAGGS-PB1, pCAGGS-PA, and pCAGGS-NP; 0.5 μg of each), the construct pHH21-SC09NS F-Luc (0.1 μg), and an internal control pRL-TK (0.1 μg). In a separate experiment, HEK293T cells were directly transfected with plasmids of the minireplicon system, together with a gradually increasing amount of Myc-KRT6A-expressing plasmids. Forty-eight hours later, cell lysates were prepared by using a dual-luciferase reporter assay system (Promega, Madison, WI, USA), with luciferase activities measured on a GloMax 96 microplate luminometer (Promega).
2.11. Cell Viability Assay
Cell viability was assessed by using a CellTiter-Glo luminescent cell viability assay, as described previously [43]. Briefly, A549 cells were treated with the indicated siRNA for 48 h. A total of 100 μL of CellTiter-Glo reagent (Promega) was then added directly into each well for 10 min at RT to lyse the cells. The luminescence of the cell lysates was measured using a GloMax 96 microplate luminometer.
2.12. Statistical Analysis
Data were statistically analyzed using a two-tailed unpaired Student’s t-test with GraphPad Prism 7.0 software (San Diego, CA, USA). A p value of <0.05 was considered to be statistically significant.
3. Results
3.1. KRT6A Negatively Regulates the Replication of IAV
KRT6A was identified as a potential regulator for the replication of IAV in a preliminary mass spectrometry screen. To confirm this finding, we analyzed the impact of siRNA-mediated KRT6A knockdown on the growth of IAV. Quantitative reverse transcription PCR (RT-qPCR) analysis showed that the level of KRT6A mRNA was reduced in A549 cells treated with KRT6A-specific siRNA (si_KRT6A) but not in scrambled siRNA (si_control)-treated cells (Figure 1A). Meanwhile, si_KRT6A treatment had no cytotoxic effects on A549 cells, as measured using a luminescent cell viability assay (Figure 1B). The siRNA-treated cells were infected with A/WSN/1933 (WSN, H1N1) virus (MOI = 0.1), and the viral growth titers in the supernatants were determined by plaque assay on MDCK cells. We found that KRT6A downregulation by siRNA treatment resulted in 3.3-/5.6-fold increases in virus titers in A549 cells at 24 and 48 h p.i. (Figure 1C), indicating that KRT6A plays a role in restricting the efficient replication of IAV.
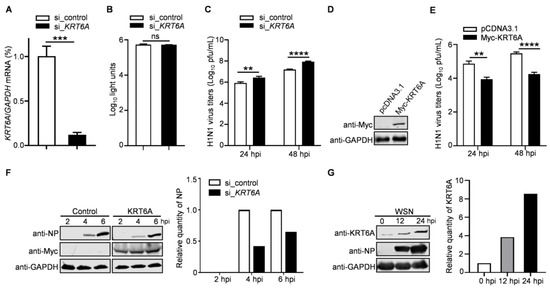
Figure 1.
KRT6A negatively regulates the replication of IAV. (A) A549 cells were transfected with KRT6A siRNA (si_KRT6A) or with scrambled siRNA (si_control) for 48 h. Whole cell lysates were collected and analyzed by RT-qPCR. ***, p < 0.001. (B) Cell viability of si_KRT6A- or si_control-treated A549 cells as measured by using a CellTiter-Glo assay. ns, not significant. (C) A549 cells treated with si_KRT6A or si_control were infected with WSN (H1N1) virus at an MOI of 0.1. Supernatants were collected at 24 and 48 h p.i. and titrated for infectious viruses by means of plaque assay on MDCK cells. **, p < 0.01; ****, p < 0.0001. (D) A549 cells were transfected with plasmids expressing Myc-KRT6A or pCDNA3.1 vector, and the overexpression of Myc-KRT6A was confirmed by western blotting with a mouse anti-Myc mAb. (E) The Myc-KRT6A-overexpressing or control A549 cells were infected with WSN (H1N1) virus at an MOI of 0.1. Supernatants were collected at 24 and 48 h p.i. and titrated for infectious viruses by means of plaque assay on MDCK cells. **, p < 0.01; ****, p < 0.0001. (F) A549 cells were transfected with plasmids expressing Myc-KRT6A or pCDNA3.1 vector. At 48 h post-transfection, the cells were infected with WSN (H1N1) virus at an MOI of 5, and cell lysates were western blotted with a mouse anti-NP mAb at the indicated time points. (G) A549 cells were infected with WSN (H1N1) virus at an MOI of 0.1. Cell lysates were collected at 0, 12, and 24 h p.i. and western blotted with a mouse anti-KRT6A mAb.
We next evaluated the role of KRT6A in the replication of IAV in KRT6A-overexpressing or control cells. A549 cells were transfected with plasmids expressing Myc-KRT6A or empty pCDNA3.1 vector for 48 h (Figure 1D), followed by infection with WSN (H1N1) virus (MOI = 0.1). The culture supernatants were subjected to virus titration by plaque assay. We found that virus titers in KRT6A-overexpressing A549 cells were reduced by 11.1-/16.9-fold compared with those of control cells at 24 and 48 h p.i. (Figure 1E). These data further demonstrate that KRT6A negatively regulates IAV replication.
To corroborate the above findings, the KRT6A-overexpressing or control A549 cells were infected with WSN (H1N1) virus at an MOI of 5, and the expression of viral NP protein was detected by western blotting at 2, 4, and 6 h p.i. We found that viral NP levels in cells overexpressing KRT6A were remarkably reduced compared with those in cells transfected with empty vector (Figure 1F), indicating KRT6A may impair the early stage of the IAV replication cycle.
To further determine the biological importance of KRT6A in the replication of IAV, we also examined the expression level of KRT6A in response to IAV infection. A549 cells were infected with WSN (H1N1) virus at an MOI of 0.1, and the levels of KRT6A were determined by western blotting at 0, 12, and 24 h p.i. We found that along with the infection of IAV, the expression of KRT6A was gradually induced (Figure 1G). These data indicate that KRT6A plays a biological role in the course of IAV infection.
3.2. KRT6A Suppresses the Nuclear Import of Incoming vRNP Complex and Newly Synthesized NP
To investigate the stage of the IAV replication cycle in which KRT6A functions, A549 cells were transfected with plasmids expressing Myc-KRT6A or empty pCDNA3.1 vector. At 48 h post-transfection, the cells were infected with WSN (H1N1) virus at an MOI of 5. The cellular localization of viral NP (indicative of the localization of the vRNP complex) and KRT6A was visualized by means of confocal microscopy at 3, 4, and 5 h p.i. (Figure 2A,B). We found that KRT6A was predominantly localized in the cytoplasm of Myc-KRT6A-overexpressing cells across the three time points p.i. Notably, viral NP was clearly accumulated in the nucleus of 32%, 80%, and 45% of control cells at 3, 4, and 5 h p.i., respectively. By contrast, only 4%, 10%, and 27% of KRT6A-overexpressing cells exhibited visible nuclear accumulation of NP at the same time points. These results indicate that the overexpression of Myc-KRT6A significantly inhibits the early stage of the IAV replication cycle.
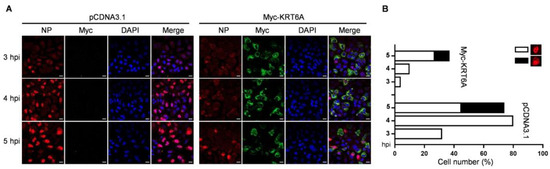
Figure 2.
KRT6A inhibits the early stage of the IAV replication cycle. (A) A549 cells were transiently transfected with pCDNA3.1 vector or Myc-KRT6A-expressing plasmids. At 48 h post-transfection, the cells were infected with WSN (H1N1) virus at an MOI of 5. At 3, 4, and 5 h p.i., the cells were fixed and stained with a mouse anti-NP mAb and a rabbit anti-Myc pAb, followed by incubation with Alexa Fluor 488 goat anti-rabbit IgG (H + L) (green) and Alexa Fluor 633 goat anti-mouse IgG (H + L) (red). The nuclei were stained with DAPI (blue). Scale bar, 20 μm. (B) Statistical analysis of NP localization in virus-infected A549 cells as indicated in (A). The localization of NP after nuclear import was classified into two types: clear nuclear localization and predominant nuclear localization. The results are counted from one hundred cells observed under a confocal microscope with a 60× objective lens.
The nuclear import of the vRNP complex was mediated by the interaction of NP with the classical nuclear import pathway. We then investigated whether KRT6A regulates the nuclear import of vRNP during IAV infection. To this end, A549 cells were treated with si_control, si_KRT6A, or siRNA targeting vATPase subunit ATP6V1B2 gene (si_ATP6V1B2) for 48 h, followed by infection with WSN (H1N1) virus at an MOI of 50 in the presence of cycloheximide (CHX) to inhibit the synthesis of new proteins. At 4 h p.i., the nuclear import of the incoming vRNP complex was visualized by confocal microscopy. We found that following si_control treatment, viral NP was simultaneously detected in the nucleus and cytoplasm in 85.8% of cells, and was clearly accumulated in the nucleus in 14.2% of cells, indicating that the nuclear import of the incoming vRNP complex had occurred. By contrast, due to the inhibition of the viral uncoating step caused by ATP6V1B2 downregulation, the vRNP complex was predominantly retained in the cytoplasm of si_ATP6V1B2-treated cells. However, 41.4% of si_KRT6A-treated cells showed apparent nuclear accumulation of viral NP, which was much higher than that of the si_control-treated cells (Figure 3A). Since the only source of NP protein was derived from the incoming vRNP complex under the treatment of CHX, these results demonstrate that KRT6A directly inhibits the nuclear import of the incoming vRNP complex during the early stage of the virus replication cycle.
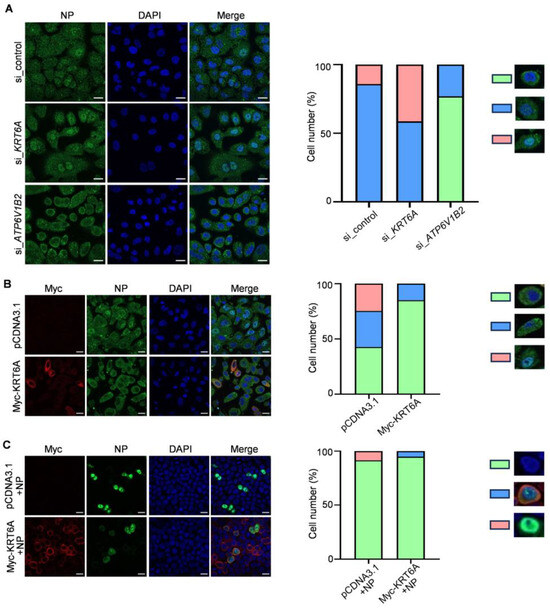
Figure 3.
KRT6A inhibits the nuclear import of the incoming vRNP complex and newly synthesized NP. (A) A549 cells were transfected with si_control, si_KRT6A, or si_ATP6V1B2. At 48 h post-transfection, the cells were incubated with 50 μg/mL CHX for 1 h to inhibit protein synthesis. The treated cells were infected with WSN (H1N1) virus at an MOI of 50 on ice, followed by incubation in a culture medium containing CHX for another 4 h at 37 °C. The cells were then fixed, incubated with a mouse anti-NP mAb, and stained with Alexa Fluor 488 goat anti-mouse IgG (H + L). (B) A549 cells were transfected with plasmids expressing Myc-KRT6A or pCDNA3.1 vector. At 48 h post-transfection, the cells were treated with 50 μg/mL CHX for 1 h. The treated cells were infected with WSN (H1N1) virus at an MOI of 50 on ice, followed by incubation in a culture medium containing CHX for another 5 h at 37 °C. The cells were then fixed, incubated with a mouse anti-NP mAb and a rabbit anti-Myc pAb, and stained with Alexa Fluor 488 goat anti-mouse IgG (H + L) and Alexa Fluor 633 goat anti-rabbit IgG (H + L). (C) HeLa cells grown on glass bottom dishes were co-transfected with plasmids expressing WSNNP and Myc-KRT6A or pcDNA3.1 vector. At 48 h post-transfection, the cells were fixed, incubated with a mouse anti-NP mAb and a rabbit anti-Myc pAb, and stained with Alexa Fluor 488 goat anti-mouse IgG (H + L) and Alexa Fluor 633 goat anti-rabbit IgG (H + L). The nuclei were stained with DAPI (A–C). Scale bar, 20 μm.
We also examined the effect of KRT6A overexpression on the nuclear import of the incoming vRNP complex. A549 cells were transfected with plasmids expressing Myc-KRT6A or pCDNA3.1 vector. At 48 h post-transfection, the cells were infected with WSN (H1N1) virus at an MOI of 50 in the presence of CHX treatment. At 5 h p.i., the nuclear import of the incoming vRNP complex was visualized by confocal microscopy. We found that viral NP was accumulated in the nucleus in 24.6% of control cells; in contrast, viral NP was predominantly distributed in the cytoplasm of Myc-KRT6A-overexpressing cells (Figure 3B). These data confirmed that KRT6A functions to inhibit the nuclear import of the incoming vRNP complex.
We further determined the effect of KRT6A on the cellular localization of viral NP when they were co-expressed. To this end, Hela cells were used for confocal microscopy to exclude the possibility of cell type-specific effect. The cells were transfected with plasmids expressing viral NP and Myc-KRT6A individually or in combination, and the distribution of NP and KRT6A was examined by confocal microscopy at 48 h post-transfection. We found that NP was dominantly accumulated in the nucleus of the control cells, whereas its nuclear accumulation was dramatically inhibited when KRT6A was co-expressed (Figure 3C). These data, together with the above results, demonstrate that KRT6A inhibits the nuclear import of the incoming vRNP complexes and newly synthesized NP, thereby suppressing the virus replication cycle of IAV.
3.3. KRT6A Interacts with RNP Complex Proteins of IAV
To determine whether KRT6A interacts with the component proteins of the RNP complex of IAV, HEK293T cells were transfected with plasmids expressing Myc-tagged KRT6A and NP, PB2, PB1, or V5-PA of WSN (H1N1) virus, either alone or in combination. At 48 h post-transfection, cell lysates were subject to immunoprecipitation with a mouse anti-NP, PB2, PB1, or PA monoclonal antibody (mAb), followed by western blotting with a rabbit anti-Myc polyclonal antibody (pAb) and a rabbit anti-NP, anti-PB2, anti-PB1, or anti-V5 pAb. We found that Myc-KRT6A was co-immunoprecipitated with WSN NP, PB2, PB1, or PA when they were co-expressed (Figure 4A,C,E,G). Meanwhile, WSN NP, PB2, PB1, or PA was also co-immunoprecipitated with Myc-KRT6A when the co-IP experiment was performed with a mouse anti-Myc mAb (Figure 4B,D,F,H). Together, these results indicate that KRT6A interacts with IAV NP, PB2, PB1, and PA in transiently transfected cells.
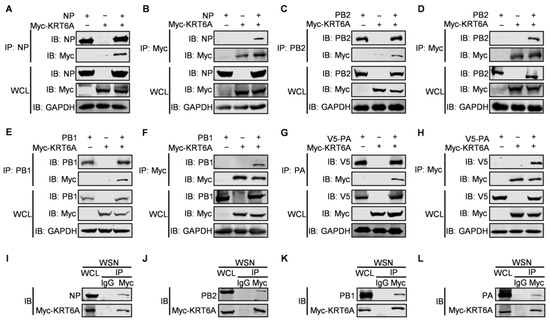
Figure 4.
KRT6A interacts with RNP complex proteins. (A-H) HEK293T cells were transfected individually or in combination with plasmids expressing Myc-KRT6A and WSNNP (A,B), WSNPB2 (C,D), WSNPB1 (E,F), or V5-WSNPA (G,H). Cell lysates were immunoprecipitated with a mouse anti-NP mAb (A), anti-PB2 mAb (C), anti-PB1 mAb (E), anti-PA mAb (G), or a mouse anti-Myc mAb (B,D,F,H). The immunoprecipitated proteins were western blotted with a rabbit anti-Myc pAb and a rabbit anti-NP pAb (A,B), anti-PB2 pAb (C,D), anti-PB1 pAb (E,F), or anti-V5 pAb (G,H). (I–L) HEK293T cells were transfected with Myc-KRT6A-expressing plasmids, and at 48 h post-transfection, the cells were infected with WSN (H1N1) virus at an MOI of 5. At 8 h p.i., cell lysates were immunoprecipitated with a mouse IgG or a mouse anti-Myc mAb, and the immunoprecipitated proteins were western blotted with a rabbit anti-Myc pAb and a rabbit anti-NP pAb (I), anti-PB2 pAb (J), anti-PB1 pAb (K), or anti-PA pAb (L).
To further investigate the interaction between RNP complex proteins and KRT6A during IAV infection, HEK293T cells were transfected to express Myc-KRT6A. At 48 h post-transfection, the cells were infected with WSN (H1N1) virus at an MOI of 5. After 8 h, the cell lysates were immunoprecipitated with a mouse anti-Myc mAb or mouse IgG, and the presence of RNP complex proteins and KRT6A in the immunoprecipitates was revealed by western blotting. We found that the four RNP complex proteins interacted with KRT6A in the course of IAV infection (Figure 4I–L).
3.4. KRT6A Has No Effect on the Nuclear Import of PB2 or PB1-PA Heterodimer
Given that KRT6A interacts with all four component proteins of the vRNP complex and suppresses the nuclear import of NP protein as well as the vRNP complex as a whole, we then asked whether KRT6A also affects the nuclear import of PB2, PB1, and PA protein. At first, we examined the effect of KRT6A on the nuclear import of the viral PB2 protein. HeLa cells were transfected with plasmids expressing WSN PB2 together with Myc-KRT6A-expressing plasmids or pCDNA3.1 vector and were subjected to confocal microscopy at 48 h post-transfection. We found that PB2 was similarly transported into the nucleus of both control cells and KRT6A-overexpressing cells (Figure 5A). To confirm this finding, we performed a nuclear and cytoplasmic fractionation assay in HEK293T cells that were co-transfected with plasmids expressing WSN PB2 and Myc-KRT6A or pCDNA3.1 vector. At 48 h post-transfection, nuclear and cytoplasmic proteins were separated and analyzed by western blotting, with LaminB1 and GAPDH used as nuclear and cytoplasmic marker proteins, respectively. As shown in Figure 5B, KRT6A was primarily localized in the cytoplasm and was less abundantly detected in the nucleus of KRT6A-overexpressing cells. Notably, the expression of PB2 in the cytoplasm and nucleus was comparable between control cells and KRT6A-overexpressing cells. These data further demonstrate that the nuclear import of PB2 is not affected by KRT6A.
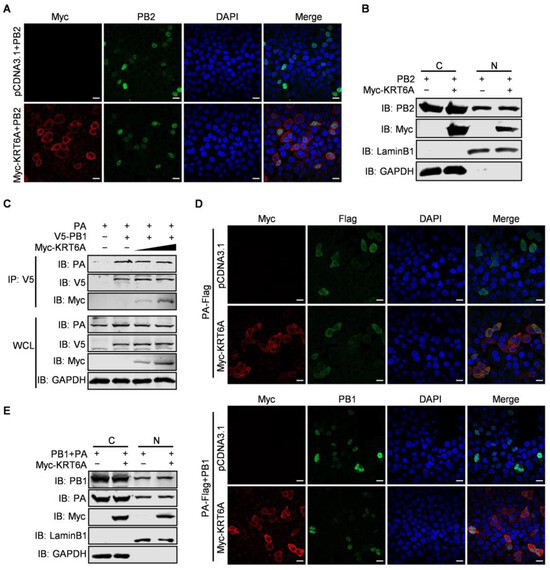
Figure 5.
KRT6A does not affect the nuclear import of PB2 or the PB1-PA heterodimer. (A) HeLa cells grown on glass bottom dishes were co-transfected with plasmids expressing WSNPB2 and Myc-KRT6A or pcDNA3.1 vector. At 48 h post-transfection, the cells were fixed, incubated with a mouse anti-PB2 mAb and a rabbit anti-Myc pAb, and stained with Alexa Fluor 488 goat anti-mouse IgG (H + L) and Alexa Fluor 633 goat anti-rabbit IgG (H + L). DAPI was used to stain the nuclei. (B) HEK293T cells were co-transfected with plasmids expressing WSNPB2 and Myc-KRT6A or pCDNA3.1 vector. Forty-eight hours later, the cells were separated into nuclear (N) and cytoplasmic (C) fractions. Each fraction was western blotted with a mouse anti-PB2 mAb and a rabbit anti-Myc pAb for protein detection. LaminB1 and GAPDH were used as nuclear and cytoplasmic marker proteins, respectively. (C) HEK293T cells were transfected with the indicated combinations of plasmids. At 48 h post-transfection, the cell lysates were immunoprecipitated with a mouse anti-V5 mAb, and the immunoprecipitated proteins were western blotted with a rabbit anti-PA pAb, anti-V5 pAb, and anti-Myc pAb. (D) HeLa cells grown on glass bottom dishes were transfected with the indicated combination of plasmids to express WSNPA-Flag together with or without WSNPB1, Myc-KRT6A, and empty pCDNA3.1 vector. At 48 h post-transfection, the cells were incubated with a rabbit anti-Myc pAb and a mouse anti-Flag mAb or a mouse anti-PB1 mAb, and stained with Alexa Fluor 633 goat anti-rabbit IgG (H + L) and Alexa Fluor 488 goat anti-mouse IgG (H + L). DAPI was used to stain the nuclei. (E) HEK293T cells were transfected with plasmids expressing WSNPB1, WSNPA, and Myc-KRT6A or pCDNA3.1 vector. Forty-eight hours later, the cells were separated into nuclear (N) and cytoplasmic (C) fractions. Each fraction was western blotted with a mouse anti-PB1 mAb, a mouse anti-PA mAb, and a rabbit anti-Myc pAb for protein detection.
In the course of IAV infection, newly synthesized PB1 and PA proteins form heterodimers in the cytoplasm before they are imported into the nucleus of infected cells [44]. To examine the effect of KRT6A on the nuclear import of the PB1-PA heterodimer, we first determined whether it affects the interaction between PB1 and PA. HEK293T cells were transfected with plasmids expressing V5-PB1 and PA of WSN (H1N1) virus, together with or without Myc-KRT6A-expressing plasmids. At 48 h post-transfection, the cell lysates were immunoprecipitated with a mouse anti-V5 mAb and then subjected to western blotting with a rabbit anti-V5 pAb, anti-PA pAb, or anti-Myc pAb. We found that the amount of PA co-immunoprecipitated with PB1 was similar in the absence or presence of two different doses of KRT6A (Figure 5C), indicating that co-expression of KRT6A had no effect on the formation of the PB1-PA heterodimer. Next, we performed a confocal microscopy assay to monitor the nuclear import of the PB1-PA dimer in the absence or presence of KRT6A. HeLa cells were transfected with plasmids to express PA-Flag together with or without PB1, Myc-KRT6A, and empty pCDNA3.1 vector. We found that at 48 h post-transfection, PA-Flag was dominantly localized in the cytoplasm when it was expressed alone, and its cellular localization was not affected by the co-expression of Myc-KRT6A (Figure 5D). When the localization of PB1 was visualized to indicate the nuclear import of PB1-PA dimer, we found that PB1 was accumulated in the nucleus of cells co-expressing PB1 and PA-Flag. Notably, the nuclear accumulation of PB1 in the presence of PA-Flag was not affected by the co-expression of Myc-KRT6A (Figure 5D). Nuclear and cytoplasmic fractionation assay in HEK293T cells also showed no differences in the protein levels of nucleus-localized PB1 and PA between KRT6A-overexpressing and control cells (Figure 5E). Together, these results demonstrate that KRT6A does not affect the nuclear import of the PB1-PA heterodimer in the replication cycle of IAV.
3.5. KRT6A Weakens the Binding of NP with Importin α3
The nuclear import of NP is mediated by its association with importin α1, α3, α5, or α7 and the subsequent binding of importin β1 [27,28,45]. Given that KRT6A interacts with NP and hinders the nuclear import of the vRNP complex and newly synthesized NP, we hypothesized that KRT6A might affect the interaction between NP and importin α isoforms. To test this hypothesis, we first determined whether KRT6A interacts with importin α isoforms by performing co-IP assays. HEK293T cells were transfected with plasmids expressing Myc-KRT6A and Flag-tagged importin α1, α3, α5, or α7, individually or in combination. Cell lysates were immunoprecipitated with a mouse anti-Flag mAb, and then subjected to western blotting with a rabbit anti-Flag or anti-KRT6A pAb. As shown in Figure 6A–D, KRT6A did not interact with importin α1, α3, α5, or α7.
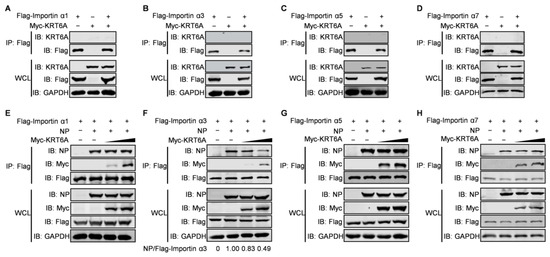
Figure 6.
KRT6A weakens the binding of NP to importin α3. (A–D) HEK293T cells were transfected individually or in combination with plasmids expressing Myc-KRT6A and Flag-tagged importin α1, α3, α5, or α7. Cell lysates were immunoprecipitated with a mouse anti-Flag mAb, and the immunoprecipitated proteins were western blotted with a rabbit anti-KRT6A pAb and anti-Flag pAb. (E–H) HEK293T cells were transfected with plasmids expressing WSNNP and Flag-tagged importin α1, α3, α5, or α7, in the absence or presence of gradually increasing amounts of Myc-KRT6A. Cell lysates were immunoprecipitated with a mouse anti-Flag mAb, and the immunoprecipitated proteins were western blotted with a rabbit anti-NP pAb, anti-Flag pAb, and anti-Myc pAb.
We further examined whether KRT6A affects complex formation between NP and importin α isoforms. HEK293T cells were transfected with plasmids expressing WSN NP and Flag-tagged importin α1, α3, α5, or α7, together with gradually increasing amounts of Myc-KRT6A-expressing plasmids. At 48 h post-transfection, the cell lysates were immunoprecipitated with a mouse anti-Flag mAb and then subjected to western blotting with a rabbit anti-Flag, anti-Myc, or anti-NP pAb. We found that the amount of NP co-immunoprecipitated with Flag-tagged importin α1, α5, or α7 was not affected in the presence of gradually increasing amounts of Myc-KRT6A (Figure 6E,G,H). By contrast, the co-expression of gradually increasing amounts of Myc-KRT6A reduced the amount of NP co-immunoprecipitated with importin α3 in a dose-dependent manner (Figure 6F). These results demonstrate that the presence of KRT6A specifically interferes with the formation of a complex between NP and importin α3, thereby inhibiting the nuclear import of the incoming vRNP complex and newly synthesized NP.
3.6. KRT6A Impairs vRNP Complex Assembly
IAV NP forms homo-oligomers and multiple copies of NP wrap around genomic RNA, which provides a structural framework for the assembly of the vRNP complex [19,46]. We therefore performed a co-IP assay to determine whether KRT6A affects the homo-oligomerization of NP. HEK293T cells were co-transfected with plasmids expressing V5-NP and NP-Flag of WSN (H1N1) virus, together with or without Myc-KRT6A-expressing plasmids. At 48 h post-transfection, cell lysates were immunoprecipitated with a mouse anti-Flag mAb and then subjected to western blotting with a rabbit anti-Flag, anti-V5, or anti-Myc pAb. We found that the co-expression of increasing amounts of Myc-KRT6A did not affect the interaction of V5-NP and NP-Flag (Figure 7A), indicating the lack of effect of KRT6A on the homo-oligomerization of NP.
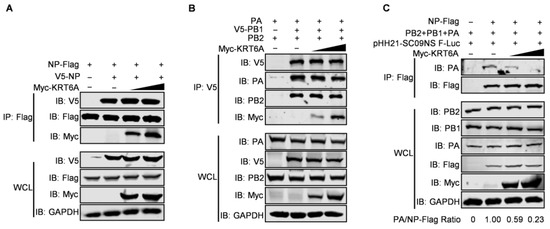
Figure 7.
KRT6A impairs vRNP complex assembly. (A) HEK293T cells were transfected with plasmids expressing WSNNP-Flag and V5-WSNNP, along with increasing amounts of Myc-KRT6A-expressing plasmids. At 48 h post-transfection, cell lysates were immunoprecipitated with a mouse anti-Flag mAb, and the immunoprecipitated proteins were western blotted with a rabbit anti-Flag pAb, anti-V5 pAb, and anti-Myc pAb. (B) HEK293T cells were transfected with plasmids expressing WSNPB2, V5-WSNPB1, and WSNPA, along with increasing amounts of Myc-KRT6A-expressing plasmids. At 48 h post-transfection, cell lysates were immunoprecipitated with a mouse anti-V5 mAb, and the immunoprecipitated proteins were western blotted with a rabbit anti-PB2 pAb, anti-V5 pAb, anti-PA pAb, and anti-Myc pAb. (C) HEK293T cells were transfected with plasmids expressing WSNPB2, WSNPB1, WSNPA, WSNNP-Flag, and pHH21-SC09NS F-Luc, along with increasing amounts of Myc-KRT6A-expressing plasmids. At 48 h post-transfection, cell lysates were immunoprecipitated with a mouse anti-Flag mAb, and the immunoprecipitated proteins were western blotted with a rabbit anti-PA pAb and anti-Flag pAb.
The interaction between KRT6A and the three polymerase subunits of IAV also prompted us to examine whether it affects the formation of the integral viral polymerase complex. The co-IP assay performed in HEK293T cells showed that the amounts of PB2 and PA protein co-immunoprecipitated with V5-PB1 were not affected by the gradual increase of KRT6A expression (Figure 7B), thereby indicating that KRT6A has no effect on the formation of the polymerase complex of IAV.
Given that the transcription and replication of the viral genome is catalyzed by the vRNP complex of IAV, we further determined whether the interaction between KRT6A and all four component proteins of the vRNP complex affects the formation of the vRNP complex. To this end, we performed a co-IP assay in HEK293T cells transfected with plasmids expressing PB2, PB1, PA, NP-Flag, and virus-like RNA to constitute the vRNP complex, together with a gradually increasing amount of KRT6A-expressing plasmids. We found that the amount of PA protein co-immunoprecipitated with NP was dramatically reduced along with the gradual increase of KRT6A expression (Figure 7C). Because viral PA does not directly interact with NP and can only be co-immunoprecipitated by NP in the vRNP complex [47], these results indicate that the expression of KRT6A impairs the formation of functional vRNP complexes of IAV.
3.7. KRT6A Reduces the Polymerase Activity of IAV
As the formation of a functional vRNP complex is required for the viral polymerase to catalyze the transcription and replication of the viral genome, we finally explored the impact of KRT6A on viral polymerase activity by performing a minigenome assay. HEK293T cells were transfected with plasmids expressing the four RNP complex proteins of WSN (H1N1) virus, the construct pHH21-SC09NS F-Luc for the synthesis of virus-like RNA, and the internal control pRL-TK, together with increasing amounts of Myc-KRT6A-expressing plasmids. We found that viral polymerase activity was reduced due to the overexpression of KRT6A in a dose-dependent manner (Figure 8A). Meanwhile, KRT6A overexpression did not affect the expression levels of PB2, PB1, PA, and NP (Figure 8B), indicating that KRT6A inhibits viral polymerase activity without affecting the expression levels of RNP component proteins. Conversely, we found that siRNA-mediated KRT6A knockdown led to increased viral polymerase activity without affecting the expression levels of RNP complex proteins (Figure 8C,D). Together, these results indicate that KRT6A suppresses the viral polymerase activity of IAV.
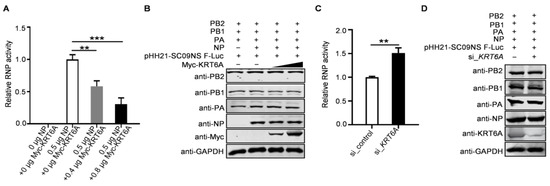
Figure 8.
KRT6A reduces the polymerase activity of IAV. (A,B) HEK293T cells were transfected with plasmids expressing the four RNP complex proteins (PB2, PB1, PA, and NP) of WSN (H1N1) virus, pHH21-SC09NS-F-Luc, pRL-TK, and Myc-KRT6A-expressing plasmids. At 48 h post-transfection, a dual-luciferase assay was performed, and the relative firefly luciferase activity was normalized to the value of Renilla luciferase activity (A). **, p < 0.01; ***, p < 0.001. The cell lysates were also western blotted with a mouse anti-PB2 mAb, anti-PB1 mAb, anti-PA mAb, anti-NP mAb, and anti-Myc mAb to detect the corresponding proteins (B). (C,D) HEK293T cells were transfected with si_KRT6A or scrambled siRNA. At 24 h post-transfection, the siRNA-treated cells were further transfected with plasmids expressing the four RNP complex proteins (PB2, PB1, PA, and NP) of WSN (H1N1) virus, pHH21-SC09NS-F-Luc, and pRL-TK. At 48 h post-transfection, a dual-luciferase assay was performed, and the relative firefly luciferase activity was normalized to the value of Renilla luciferase activity (C). **, p < 0.01. The cell lysates were also western blotted with a mouse anti-PB2 mAb, anti-PB1 mAb, anti-PA mAb, anti-NP mAb, and anti-KRT6A pAb to detect the corresponding proteins (D).
4. Discussion
The replication cycle of IAV begins with binding to the sialic acid receptors on the surface of target cells [48]. After binding, the virus is internalized and delivered into early endosomes [49,50]. The pH decrease as the virus moves from the early to late endosome alters the conformation of the viral HA protein, resulting in the exposure of the fusion peptide and consequent fusion between the viral envelope and late endosomal membranes [51,52,53]. Further acidification of the interior of the virus particle finally uncoats the virion, resulting in the release of the vRNP complex into the cytoplasm [54,55]. vRNPs are subsequently imported into the nucleus of the infected cells via the classical nuclear import pathway, which is mediated by the interaction between the NLSs of NP and isoforms of importin α, i.e., α1, α3, α5, and α7 [27,28,45]. Upon entering the nucleus, the incoming vRNPs begin a primary round of transcription, producing mRNA from which progeny viral proteins, including PB2, PB1, PA, and NP, are translated [47]. PB1 and PA form heterodimers in the cytoplasm, which are transported to the nucleus with the aid of the nuclear import receptor Ran-binding protein 5 (RanBP5) [56]. By contrast, the NLSs of PB2 and NP bind isoforms of importin α to access the classical nuclear import pathway [17,57,58,59,60]. In the nucleus, the newly synthesized polymerase and NP proteins interact with nascent viral RNA to assemble into progeny vRNP complexes.
A couple of host cellular proteins are capable of promoting the nuclear import of vRNP complexes and/or newly synthesized component proteins through different mechanisms. For example, BinCARD1 can specifically enhance the binding between NP and importin α7 [22]; and Hsp40 binds to NP through its J domain and recruits importin α isoforms through its C-terminus, thereby facilitating NP–importin α interaction [61]. On the other hand, several other host factors can inhibit the nuclear import of the vRNP complex and/or its component proteins. Among them, PLSCR1 forms a trimeric complex with NP and importin α, thereby blocking the further recruitment of importin β [27]; eEF1D weakens the interactions of NP and importin α5 as well as PB1 and RanBP5 to impede the nuclear import of NP and the PB1-PA heterodimer [62]; MOV10 interacts with NP to prevent the further binding of NP with importin α [28]; and HCLS1-associated protein X1 (HAX1) interacts with the NLS domain of PA and impedes its nuclear import [63]. In the present study, we identified KRT6A as a negative regulator of the replication of IAV. siRNA-mediated knockdown of KRT6A mRNA level increased the replication of IAV, whereas overexpression of KRT6A reduced virus growth titers. We demonstrated that KRT6A interacted with viral PB2, PB1, PA, and NP proteins in both transfected and infected cells. Of note, KRT6A affected the nuclear import of incoming vRNP complexes and newly synthesized NP but had no effect on the nuclear import of the PB1-PA heterodimer or PB2 protein. Given that the vRNP complexes of IAV utilize the NLSs of NP for their nuclear import, we further explored the mechanism by which KRT6A affected the nuclear import of the vRNP complex and NP. We found that, among the different isoforms of importin α that interact with NP, KRT6A specifically interfered with the binding between importin α3 and NP. Further investigation also demonstrated that KRT6A impaired the assembly of the vRNP complex and reduced viral polymerase activity. Together, these negative effects of KRT6A on nuclear import and function of the vRNP complex contributed to suppressing the propagation of IAV.
Being one of the critical steps of the IAV replication cycle, the nuclear import of vRNP complexes becomes the key node where proviral and antiviral host factors function. Interestingly, some host factors such as Hsp40 and MOV10 indiscriminately affect the interaction between NP and different isoforms of importin α, whereas other host factors such as BinCARD1, eEF1D, and KRT6A differentially affect the interaction of NP with certain isoforms of importin α. Although different importin α members share general structural features, their sequence homology can be as low as 41% [64]. The finding that some host factors specifically affect the interaction between NP and certain importin α members may reflect the subtle difference of the importin α family members in their interaction with viral NP protein. Since KRT6A does not interact with importin α3, it may directly compete with importin α3 for the binding with NP, thereby inhibiting the nuclear import of vRNP complexes and NP.
At present, the role of keratins in IAV replication has been gradually uncovered. An influenza virus–host interactome screen by Watanabe et al. indicated that KRT14 plays an important role in early steps of the IAV life cycle, such as virus binding, internalization, and/or transport of vRNP complexes into the nucleus [65]. The replication of IAV has been shown to induce KRT8 phosphorylation, which enhances viral replication efficiency in A549 cells [66]. In addition, proteomic analyses in H9N2 avian influenza virus-infected A549 cells demonstrated that some keratins (i.e., KRT1, KRT10, KRT14, KRT16, KRT18, and KRT19) were differentially expressed [67,68], indicating that these cytoskeleton molecules may be involved in the replication cycle of IAV. In this study, we identified KRT6A as a restriction factor for the replication of IAV and elucidated its underlying functional mechanism. Our study thus clearly demonstrates the role of keratins in the replication of IAV.
In summary, our data demonstrate that KRT6A is a novel NP-binding protein that has a negative regulatory effect on IAV replication. KRT6A suppresses the nuclear import of the vRNP complex and newly synthesized NP, thereby impeding the replication cycle of IAV. Notably, we unveiled the underlying mechanism by which KRT6A functions, namely by impairing the formation of a complex between viral NP and the nuclear import adaptor importin α3. Furthermore, KRT6A also inhibits the assembly of functional vRNP complexes and consequently reduces viral polymerase activity. Our findings thus comprehensively reveal the role of KRT6A in the replication cycle of IAV, which advances our understanding of the interaction network between IAV and host cellular factors.
5. Conclusions
In summary, our results demonstrated that the KRT6A–NP interaction impairs the nuclear import of incoming vRNP complexes and newly synthesized NP. KRT6A specifically interferes with the interaction between NP and importin α3. Consequently, the inhibitory effect of KRT6A on the nuclear import of NP suppresses the assembly of vRNP complexes and viral polymerase activity.
Author Contributions
Conceptualization, Y.C., Z.S., L.J., H.C. and C.L.; methodology, Y.C. and Z.S.; validation, Y.C. and Z.S.; formal analysis, Y.C., Z.S., G.W., L.J., H.C. and C.L.; investigation, Y.C., Z.S., W.S., Q.L., Y.W. and B.W.; data curation, Y.C., Z.S., L.J., H.C. and C.L.; writing—original draft preparation, Y.C., L.J., H.C. and C.L.; writing—review and editing, Y.C., L.J., H.C. and C.L.; visualization, Y.C. and Z.S.; supervision, L.J., H.C. and C.L.; project administration, L.J., H.C. and C.L.; funding acquisition, G.W., L.J., H.C. and C.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (NSFC) [32172847 (L.J.), 32192453 (C.L.)], the National Key Research and Development Program of China [2021YFD1800203 (H.C.), 2021YFD1800204 (C.L.), 2024YFE0198700 (L.J.)], the Natural Science Foundation of Heilongjiang Province (JQ2023C006) (L.J.), the Innovation Program of the Chinese Academy of Agricultural Sciences (CAAS-CSLPDCP-202401) (C.L.), and the Youth Innovation Program of the Chinese Academy of Agricultural Sciences (Y2025QC21) (G.W.).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are included in the article. Further inquiries can be directed to the corresponding authors.
Acknowledgments
We thank Yoshihiro Kawaoka (University of Wisconsin–Madison) for the gift of the pCAGGS vector.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Yamayoshi, S.; Watanabe, M.; Goto, H.; Kawaoka, Y. Identification of a Novel Viral Protein Expressed from the PB2 Segment of Influenza A Virus. J. Virol. 2016, 90, 444–456. [Google Scholar] [CrossRef] [PubMed]
- Tong, S.; Zhu, X.; Li, Y.; Shi, M.; Zhang, J.; Bourgeois, M.; Yang, H.; Chen, X.; Recuenco, S.; Gomez, J.; et al. New world bats harbor diverse influenza A viruses. PLoS Pathog. 2013, 9, e1003657. [Google Scholar] [CrossRef]
- Karakus, U.; Mena, I.; Kottur, J.; El Zahed, S.S.; Seoane, R.; Yildiz, S.; Chen, L.; Plancarte, M.; Lindsay, L.; Halpin, R.; et al. H19 influenza A virus exhibits species-specific MHC class II receptor usage. Cell Host Microbe 2024, 32, 1089–1102.e10. [Google Scholar] [CrossRef]
- Neumann, G.; Chen, H.; Gao, G.F.; Shu, Y.; Kawaoka, Y. H5N1 influenza viruses: Outbreaks and biological properties. Cell Res. 2010, 20, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Gu, W.; Shi, J.; Cui, P.; Yan, C.; Zhang, Y.; Wang, C.; Zhang, Y.; Xing, X.; Zeng, X.; Liu, L.; et al. Novel H5N6 reassortants bearing the clade 2.3.4.4b HA gene of H5N8 virus have been detected in poultry and caused multiple human infections in China. Emerg. Microbes Infect. 2022, 11, 1174–1185. [Google Scholar] [CrossRef]
- Lai, S.; Qin, Y.; Cowling, B.J.; Ren, X.; Wardrop, N.A.; Gilbert, M.; Tsang, T.K.; Wu, P.; Feng, L.; Jiang, H.; et al. Global epidemiology of avian influenza A H5N1 virus infection in humans, 1997–2015: A systematic review of individual case data. Lancet Infect. Dis. 2016, 16, e108–e118. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.; Cao, B.; Hu, Y.; Feng, Z.; Wang, D.; Hu, W.; Chen, J.; Jie, Z.; Qiu, H.; Xu, K.; et al. Human infection with a novel avian-origin influenza A (H7N9) virus. N. Engl. J. Med. 2013, 368, 1888–1897. [Google Scholar] [CrossRef]
- Shi, J.; Deng, G.; Kong, H.; Gu, C.; Ma, S.; Yin, X.; Zeng, X.; Cui, P.; Chen, Y.; Yang, H.; et al. H7N9 virulent mutants detected in chickens in China pose an increased threat to humans. Cell Res. 2017, 27, 1409–1421. [Google Scholar] [CrossRef]
- Li, C.; Chen, H. H7N9 Influenza Virus in China. Cold Spring Harb. Perspect. Med. 2021, 11, a038349. [Google Scholar] [CrossRef]
- Shi, J.Z.; Zeng, X.Y.; Cui, P.F.; Yan, C.; Chen, H.L. Alarming situation of emerging H5 and H7 avian influenza and effective control strategies. Emerg. Microbes Infect. 2023, 12, 2155072. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, J. H9N2 influenza virus in China: A cause of concern. Protein Cell 2015, 6, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Bao, P.; Liu, Y.; Zhang, X.; Fan, H.; Zhao, J.; Mu, M.; Li, H.; Wang, Y.; Ge, H.; Li, S.; et al. Human infection with a reassortment avian influenza A H3N8 virus: An epidemiological investigation study. Nat. Commun. 2022, 13, 6817. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Yuan, H.; Gao, R.; Zhang, J.; Wang, D.; Xiong, Y.; Fan, G.; Yang, F.; Li, X.; Zhou, J.; et al. Clinical and epidemiological characteristics of a fatal case of avian influenza A H10N8 virus infection: A descriptive study. Lancet 2014, 383, 714–721. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, R.E.; Jaskunas, R.; Blobel, G.; Palese, P.; Moroianu, J. Nuclear import of influenza virus RNA can be mediated by viral nucleoprotein and transport factors required for protein import. J. Biol. Chem. 1995, 270, 22701–22704. [Google Scholar] [CrossRef]
- Martin, K.; Helenius, A. Transport of incoming influenza virus nucleocapsids into the nucleus. J. Virol. 1991, 65, 232–244. [Google Scholar] [CrossRef]
- Neumann, G.; Castrucci, M.R.; Kawaoka, Y. Nuclear import and export of influenza virus nucleoprotein. J. Virol. 1997, 71, 9690–9700. [Google Scholar] [CrossRef]
- Wang, P.; Palese, P.; O’Neill, R.E. The NPI-1/NPI-3 (karyopherin alpha) binding site on the influenza a virus nucleoprotein NP is a nonconventional nuclear localization signal. J. Virol. 1997, 71, 1850–1856. [Google Scholar] [CrossRef]
- Weber, F.; Kochs, G.; Gruber, S.; Haller, O. A classical bipartite nuclear localization signal on Thogoto and influenza A virus nucleoproteins. Virology 1998, 250, 9–18. [Google Scholar] [CrossRef]
- Ye, Q.; Krug, R.M.; Tao, Y.J. The mechanism by which influenza A virus nucleoprotein forms oligomers and binds RNA. Nature 2006, 444, 1078–1082. [Google Scholar] [CrossRef]
- Zhu, W.; Feng, Z.; Chen, Y.; Yang, L.; Liu, J.; Li, X.; Liu, S.; Zhou, L.; Wei, H.; Gao, R.; et al. Mammalian-adaptive mutation NP-Q357K in Eurasian H1N1 Swine Influenza viruses determines the virulence phenotype in mice. Emerg. Microbes Infect. 2019, 8, 989–999. [Google Scholar] [CrossRef]
- Ma, S.; Zhang, B.; Shi, J.; Yin, X.; Wang, G.; Cui, P.; Liu, L.; Deng, G.; Jiang, Y.; Li, C.; et al. Amino Acid Mutations A286V and T437M in the Nucleoprotein Attenuate H7N9 Viruses in Mice. J. Virol. 2020, 94, e01530-19. [Google Scholar] [CrossRef]
- Wang, X.; Jiang, L.; Wang, G.; Shi, W.; Hu, Y.; Wang, B.; Zeng, X.; Tian, G.; Deng, G.; Shi, J.; et al. Influenza A virus use of BinCARD1 to facilitate the binding of viral NP to importin alpha7 is counteracted by TBK1-p62 axis-mediated autophagy. Cell. Mol. Immunol. 2022, 19, 1168–1184. [Google Scholar] [CrossRef] [PubMed]
- Naito, T.; Kiyasu, Y.; Sugiyama, K.; Kimura, A.; Nakano, R.; Matsukage, A.; Nagata, K. An influenza virus replicon system in yeast identified Tat-SF1 as a stimulatory host factor for viral RNA synthesis. Proc. Natl. Acad. Sci. USA 2007, 104, 18235–18240. [Google Scholar] [CrossRef]
- Momose, F.; Basler, C.F.; O’Neill, R.E.; Iwamatsu, A.; Palese, P.; Nagata, K. Cellular splicing factor RAF-2p48/NPI-5/BAT1/UAP56 interacts with the influenza virus nucleoprotein and enhances viral RNA synthesis. J. Virol. 2001, 75, 1899–1908. [Google Scholar] [CrossRef] [PubMed]
- Moisy, D.; Avilov, S.V.; Jacob, Y.; Laoide, B.M.; Ge, X.Y.; Baudin, F.; Naffakh, N.; Jestin, J.L. HMGB1 Protein Binds to Influenza Virus Nucleoprotein and Promotes Viral Replication. J. Virol. 2012, 86, 9122–9133. [Google Scholar] [CrossRef]
- Zhang, X.X.; Pu, J.; Sun, Y.P.; Bi, Y.H.; Jiang, Z.M.; Xu, G.L.; Zhang, H.Y.; Cao, J.; Chang, K.C.; Liu, J.H.; et al. Neurovirulence of Avian Influenza Virus Is Dependent on the Interaction of Viral NP Protein with FMRP in the Murine Brain. J. Virol. 2021, 95, e01272-20. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Zhang, J.; Liang, L.; Wang, G.; Li, Q.; Zhu, P.; Zhou, Y.; Li, J.; Zhao, Y.; Sun, N.; et al. Phospholipid scramblase 1 interacts with influenza A virus NP, impairing its nuclear import and thereby suppressing virus replication. PLoS Pathog. 2018, 14, e1006851. [Google Scholar] [CrossRef]
- Zhang, J.S.; Huang, F.; Tan, L.K.; Bai, C.; Chen, B.; Liu, J.; Liang, J.R.; Liu, C.; Zhang, S.Y.; Lu, G.; et al. Host Protein Moloney Leukemia Virus 10 (MOV10) Acts as a Restriction Factor of Influenza A Virus by Inhibiting the Nuclear Import of the Viral Nucleoprotein. J. Virol. 2016, 90, 3966–3980. [Google Scholar] [CrossRef]
- Di Pietro, A.; Kajaste-Rudnitski, A.; Oteiza, A.; Nicora, L.; Towers, G.J.; Mechti, N.; Vicenzi, E. TRIM22 inhibits influenza A virus infection by targeting the viral nucleoprotein for degradation. J. Virol. 2013, 87, 4523–4533. [Google Scholar] [CrossRef]
- Patil, G.; Zhao, M.; Song, K.; Hao, W.; Bouchereau, D.; Wang, L.; Li, S. TRIM41-Mediated Ubiquitination of Nucleoprotein Limits Influenza A Virus Infection. J. Virol. 2018, 92, e00905-18. [Google Scholar] [CrossRef]
- Wu, X.; Wang, J.; Wang, S.; Wu, F.; Chen, Z.; Li, C.; Cheng, G.; Qin, F.X. Inhibition of Influenza A Virus Replication by TRIM14 via Its Multifaceted Protein-Protein Interaction with NP. Front. Microbiol. 2019, 10, 344. [Google Scholar] [CrossRef]
- Shi, W.J.; Shan, Z.B.; Jiang, L.; Wang, G.W.; Wang, X.Y.; Chang, Y.; Hu, Y.Z.; Wang, B.; Li, Q.B.; Wang, Y.H.; et al. ABTB1 facilitates the replication of influenza A virus by counteracting TRIM4-mediated degradation of viral NP protein. Emerg. Microbes Infect. 2023, 12, 2270073. [Google Scholar] [CrossRef]
- Toivola, D.M.; Boor, P.; Alam, C.; Strnad, P. Keratins in health and disease. Curr. Opin. Cell Biol. 2015, 32, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Jacob, J.T.; Coulombe, P.A.; Kwan, R.; Omary, M.B. Types I and II Keratin Intermediate Filaments. Cold Spring Harb. Perspect. Biol. 2018, 10, a018275. [Google Scholar] [CrossRef] [PubMed]
- Hesse, M.; Zimek, A.; Weber, K.; Magin, T.M. Comprehensive analysis of keratin gene clusters in humans and rodents. Eur. J. Cell Biol. 2004, 83, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Chen, S.; Liu, H.B.; Parent, C.A.; Coulombe, P.A. Keratin 6 regulates collective keratinocyte migration by altering cell-cell and cell-matrix adhesion. J. Cell Biol. 2018, 217, 4314–4330. [Google Scholar] [CrossRef]
- Zhou, J.Z.; Jiang, G.Q.; Xu, E.W.; Zhou, J.X.; Liu, L.L.; Yang, Q.Y. Identification of SRXN1 and KRT6A as Key Genes in Smoking-Related Non-Small-Cell Lung Cancer Through Bioinformatics and Functional Analyses. Front. Oncol. 2022, 11, 810301. [Google Scholar] [CrossRef]
- Wang, H.J.; Liu, J.; Li, J.S.; Zang, D.; Wang, X.H.; Chen, Y.Y.; Gu, T.T.; Su, W.; Song, N. Identification of gene modules and hub genes in colon adenocarcinoma associated with pathological stage based on WGCNA analysis. Cancer Genet. 2020, 242, 1–7. [Google Scholar] [CrossRef]
- Chen, C.; Shan, H. Keratin 6A gene silencing suppresses cell invasion and metastasis of nasopharyngeal carcinoma via the beta-catenin cascade. Mol. Med. Rep. 2019, 19, 3477–3484. [Google Scholar]
- Yang, B.; Zhang, W.; Zhang, M.M.; Wang, X.H.; Peng, S.Z.; Zhang, R.S. KRT6A Promotes EMT and Cancer Stem Cell Transformation in Lung Adenocarcinoma. Technol. Cancer Res. Treat. 2020, 19, 1533033820921248. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, J.; Kong, F.; Li, Q.; Wang, J.; Ma, S.; Zhao, Y.; Liang, L.; Li, J.; Sun, N.; et al. Generation and application of replication-competent Venus-expressing H5N1, H7N9, and H9N2 influenza A viruses. Sci. Bull. 2018, 63, 176–186. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wen, X.; Li, Q.; Jiang, L.; Wang, G.; Liang, L.; Wang, X.; Chen, H.; Li, C. Generation and application of two monoclonal antibodies targeting conserved linear epitopes in the NP protein of influenza A virus. J. Integr. Agric. 2021, 21, 2095–2105. [Google Scholar] [CrossRef]
- Zhu, P.; Liang, L.; Shao, X.; Luo, W.; Jiang, S.; Zhao, Q.; Sun, N.; Zhao, Y.; Li, J.; Wang, J.; et al. Host Cellular Protein TRAPPC6ADelta Interacts with Influenza A Virus M2 Protein and Regulates Viral Propagation by Modulating M2 Trafficking. J. Virol. 2017, 91, e01757-16. [Google Scholar] [CrossRef] [PubMed]
- Fodor, E.; Smith, M. The PA subunit is required for efficient nuclear accumulation of the PB1 subunit of the influenza A virus RNA polymerase complex. J. Virol. 2004, 78, 9144–9153. [Google Scholar] [CrossRef]
- Gabriel, G.; Klingel, K.; Otte, A.; Thiele, S.; Hudjetz, B.; Arman-Kalcek, G.; Sauter, M.; Shmidt, T.; Rother, F.; Baumgarte, S.; et al. Differential use of importin-alpha isoforms governs cell tropism and host adaptation of influenza virus. Nat. Commun. 2011, 2, 156. [Google Scholar] [CrossRef] [PubMed]
- Elton, D.; Medcalf, L.; Bishop, K.; Harrison, D.; Digard, P. Identification of amino acid residues of influenza virus nucleoprotein essential for RNA binding. J. Virol. 1999, 73, 7357–7367. [Google Scholar] [CrossRef]
- Mondal, A.; Dawson, A.R.; Potts, G.K.; Freiberger, E.C.; Baker, S.F.; Moser, L.A.; Bernard, K.A.; Coon, J.J.; Mehle, A. Influenza virus recruits host protein kinase C to control assembly and activity of its replication machinery. Elife 2017, 6, e26910. [Google Scholar] [CrossRef]
- Rogers, G.N.; Pritchett, T.J.; Lane, J.L.; Paulson, J.C. Differential sensitivity of human, avian, and equine influenza A viruses to a glycoprotein inhibitor of infection: Selection of receptor specific variants. Virology 1983, 131, 394–408. [Google Scholar] [CrossRef]
- Rust, M.J.; Lakadamyali, M.; Zhang, F.; Zhuang, X. Assembly of endocytic machinery around individual influenza viruses during viral entry. Nat. Struct. Mol. Biol. 2004, 11, 567–573. [Google Scholar] [CrossRef]
- Ni, Z.X.; Wang, J.L.; Yu, X.F.; Wang, Y.F.; Wang, J.F.; He, X.J.; Li, C.J.; Deng, G.H.; Shi, J.Z.; Kong, H.H.; et al. Influenza virus uses mGluR2 as an endocytic receptor to enter cells. Nat. Microbiol. 2024, 9, 1764–1777. [Google Scholar] [CrossRef]
- Lakadamyali, M.; Rust, M.J.; Babcock, H.P.; Zhuang, X. Visualizing infection of individual influenza viruses. Proc. Natl. Acad. Sci. USA 2003, 100, 9280–9285. [Google Scholar] [CrossRef] [PubMed]
- Skehel, J.J.; Wiley, D.C. Receptor binding and membrane fusion in virus entry: The influenza hemagglutinin. Annu. Rev. Biochem. 2000, 69, 531–569. [Google Scholar] [CrossRef]
- Hu, Y.Z.; Jiang, L.; Wang, G.W.; Song, Y.M.; Shan, Z.B.; Wang, X.Y.; Deng, G.H.; Shi, J.Z.; Tian, G.B.; Zeng, X.Y.; et al. M6PR interacts with the HA2 subunit of influenza A virus to facilitate the fusion of viral and endosomal membranes. Sci. China Life Sci. 2024, 67, 579–595. [Google Scholar] [CrossRef]
- Miyake, Y.; Keusch, J.J.; Decamps, L.; Ho-Xuan, H.; Iketani, S.; Gut, H.; Kutay, U.; Helenius, A.; Yamauchi, Y. Influenza virus uses transportin 1 for vRNP debundling during cell entry. Nat. Microbiol. 2019, 4, 578–586. [Google Scholar] [CrossRef]
- Larson, G.P.; Tran, V.; Yu, S.; Cai, Y.; Higgins, C.A.; Smith, D.M.; Baker, S.F.; Radoshitzky, S.R.; Kuhn, J.H.; Mehle, A. EPS8 Facilitates Uncoating of Influenza A Virus. Cell Rep. 2019, 29, 2175–2183.e4. [Google Scholar] [CrossRef]
- Deng, T.; Engelhardt, O.G.; Thomas, B.; Akoulitchev, A.V.; Brownlee, G.G.; Fodor, E. Role of ran binding protein 5 in nuclear import and assembly of the influenza virus RNA polymerase complex. J. Virol. 2006, 80, 11911–11919. [Google Scholar] [CrossRef]
- Tarendeau, F.; Boudet, J.; Guilligay, D.; Mas, P.J.; Bougault, C.M.; Boulo, S.; Baudin, F.; Ruigrok, R.W.; Daigle, N.; Ellenberg, J.; et al. Structure and nuclear import function of the C-terminal domain of influenza virus polymerase PB2 subunit. Nat. Struct. Mol. Biol. 2007, 14, 229–233. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, G.; Herwig, A.; Klenk, H.D. Interaction of polymerase subunit PB2 and NP with importin alpha1 is a determinant of host range of influenza A virus. PLoS Pathog. 2008, 4, e11. [Google Scholar] [CrossRef] [PubMed]
- Melen, K.; Fagerlund, R.; Franke, J.; Kohler, M.; Kinnunen, L.; Julkunen, I. Importin alpha nuclear localization signal binding sites for STAT1, STAT2, and influenza A virus nucleoprotein. J. Biol. Chem. 2003, 278, 28193–28200. [Google Scholar] [CrossRef]
- Resa-Infante, P.; Jorba, N.; Zamarreno, N.; Fernandez, Y.; Juarez, S.; Ortin, J. The host-dependent interaction of alpha-importins with influenza PB2 polymerase subunit is required for virus RNA replication. PLoS ONE 2008, 3, e3904. [Google Scholar] [CrossRef]
- Batra, J.; Tripathi, S.; Kumar, A.; Katz, J.M.; Cox, N.J.; Lal, R.B.; Sambhara, S.; Lal, S.K. Human Heat shock protein 40 (Hsp40/DnaJB1) promotes influenza A virus replication by assisting nuclear import of viral ribonucleoproteins. Sci. Rep. 2016, 6, 19063. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Yang, C.; Ren, C.; Zhang, S.; Gao, X.; Jin, M.; Chen, H.; Ma, W.; Zhou, H. Eukaryotic Translation Elongation Factor 1 Delta Inhibits the Nuclear Import of the Nucleoprotein and PA-PB1 Heterodimer of Influenza A Virus. J. Virol. 2020, 95, e01391-20. [Google Scholar] [CrossRef]
- Hsu, W.B.; Shih, J.L.; Shih, J.R.; Du, J.L.; Teng, S.C.; Huang, L.M.; Wang, W.B. Cellular protein HAX1 interacts with the influenza A virus PA polymerase subunit and impedes its nuclear translocation. J. Virol. 2013, 87, 110–123. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, Y.; Yamada, K.; Yoneda, Y. Importin alpha: A key molecule in nuclear transport and non-transport functions. J. Biochem. 2016, 160, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Kawakami, E.; Shoemaker, J.E.; Lopes, T.J.; Matsuoka, Y.; Tomita, Y.; Kozuka-Hata, H.; Gorai, T.; Kuwahara, T.; Takeda, E.; et al. Influenza virus-host interactome screen as a platform for antiviral drug development. Cell Host Microbe 2014, 16, 795–805. [Google Scholar] [CrossRef]
- De Conto, F.; Conversano, F.; Razin, S.V.; Belletti, S.; Arcangeletti, M.C.; Chezzi, C.; Calderaro, A. Host-cell dependent role of phosphorylated keratin 8 during influenza A/NWS/33 virus (H1N1) infection in mammalian cells. Virus Res. 2021, 295, 198333. [Google Scholar] [CrossRef]
- Yu, G.; Liang, W.; Liu, J.; Meng, D.; Wei, L.; Chai, T.; Cai, Y. Proteomic Analysis of Differential Expression of Cellular Proteins in Response to Avian H9N2 Virus Infection of A549 Cells. Front. Microbiol. 2016, 7, 1962. [Google Scholar] [CrossRef]
- Liu, N.; Song, W.; Wang, P.; Lee, K.; Chan, W.; Chen, H.; Cai, Z. Proteomics analysis of differential expression of cellular proteins in response to avian H9N2 virus infection in human cells. Proteomics 2008, 8, 1851–1858. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).