Abstract
Flaviviruses are a group of viruses transmitted mainly by mosquitoes and ticks, causing severe diseases in humans. Examples include dengue, Zika, West Nile virus, and yellow fever. They primarily affect individuals in tropical and subtropical regions, causing public health problems such as epidemic outbreaks and significant economic burdens due to hospitalizations and treatments. They share antigens, leading to cross-reactivity where antibodies generated against one flavivirus can react with others, complicating the accurate diagnosis of individual infections and making the development of treatments or vaccines more challenging. The role of T cells in the immune response to flaviviruses is a complex topic debated by scientists. On one hand, T cells help control infection by eliminating infected cells and protecting against disease. However, there is evidence that an excessive or dysregulated T cell response can cause tissue damage and worsen the disease, as seen in severe dengue cases. This duality underscores the complexity of the immune response to flavivirus infections, posing a significant challenge for researchers. Gaining a deeper understanding of the immune response at the cellular level, particularly the role of T follicular helper cells, can reveal new avenues of investigation that could lead to novel strategies for disease management. This review explores the dynamics of T cell responses, focusing on circulatory T follicular helper cells (cTFH), to enhance our understanding of flavivirus immunity and inform future interventions.
1. Dengue Virus Overview
Flaviviruses originated in 1984 when differences in viral structure, genome, and biology were discovered among togaviruses, and similarities in vectors were present. The genus Flavivirus comprises primarily dengue (DENV), yellow fever (YFV), West Nile (WN), Zika (ZIKV), hepatitis C (HCV), and Japanese encephalitis (JE) viruses, among others. These flaviviruses are classified as arboviruses due to their shared transmission by arthropod vectors [1]. The structural viral proteins encoded by this RNA genome include a membrane (M), envelope (E), and seven other non-structural (NS) proteins. The viral particle is surrounded by an outer layer of envelope (E) glycoproteins arranged as dimers, which mediate the initial attachment of the virus to the host cell. Each E protein consists of three structural domains that undergo pH-dependent conformational changes to expose a fusion loop, enabling the fusion of viral and host membranes and facilitating the release of viral RNA into the host cell. The M proteins, which help form the outer membrane of the viral particle along with E proteins, are derived from precursor membrane (prM) proteins. The presence of both mature M proteins and residual prM proteins on the viral surface contributes to the heterogeneity of viral particles and indicates viral maturity and infectivity. This variation can influence the degree of infectivity and the immune system’s ability to recognize the virus. Among the non-structural proteins, NS1 is secreted and contributes to immune evasion and vascular leakage. NS2A plays a role in RNA replication and interferon antagonism, while NS2B serves as a cofactor for the NS3 serine protease. NS3 has multiple enzymatic activities, including nucleoside triphosphatase (NTPase), RNA helicase, and protease functions. NS4A supports the formation of replication vesicles, and NS4B suppresses interferon-beta (IFN-β) and interferon-gamma (IFN-γ) signaling, thereby weakening the host’s antiviral response. NS5, the largest and most conserved protein, contains RNA-dependent RNA polymerase (RdRp) and methyltransferase domains, both essential for viral RNA synthesis and immune evasion. The prM, E, and NS1 proteins are key targets for viral neutralization and pathogenesis studies [2,3,4].
Dengue is the most common mosquito-borne viral disease caused by four different but closely related serotypes (DENV1-4), which share 65% homology but typically manifest with the same clinical presentation. Recently, DENV5 was discovered in Malaysia, but now it is rare [5,6]. The pathophysiology of the disease manifests through the attachment of the envelope protein to the host receptors that recognize the envelope protein epitopes, as well as through the recognition of anti-DENV antibodies through the FC receptor that binds the antibody isotype, leading to uptake of the virus, and eventual release of the positive-sense RNA, leading to the direct utilization and translation of viral components for maturation and release by the use of host translational machinery and the use of the host protein processing apparatus [7].
The different serotypes share the same clinical manifestations. However, there are differences between serotypes in disease severity, epidemic potential, transmissivity, and dynamics related to the mosquito vector and immune status of the patient. There is a consensus that DENV-2 may lead to higher rates of dengue hemorrhagic fever [8,9]. Some studies detail how DENV-2 of Asian origin replicates to higher titers in hosts at a higher rate than American DENV-2 strains. DENV-4 introduced in Puerto Rico in epidemics in 1982, 1986, and 1998 depicted clade changes in the circulating virus that contributed to increased transmission efficiency, where similar behavior has been seen in DENV-2 in the South Pacific and DENV-3 in Sri Lanka, with amino acid changes expressed in NS proteins. Based on observations, DENV serotypes may not be inherently virulent. However, they may be conditionally virulent based on repeat exposure, augmented by mutants or mutations with differing degrees of antibody-dependent enhancement (ADE) interactions and, thus, exhibiting different infectivity rates [8,9]. The phenomenon of antigenic sin also occurs with the dengue virus due to the cross-reactivity between serotypes. This can result in more severe disease upon subsequent exposure to a different serotype or even another flavivirus. This effect is largely associated with the envelope (E) protein and its three structural domains. Antigenic sin arises when the immune system, after initial exposure to one dengue serotype, mounts a memory response that is preferentially directed against that first serotype. Upon later infection with a different serotype, the immune system relies on this existing memory rather than generating a new, serotype-specific response. This leads to suboptimal neutralization and immune protection, contributing to enhanced disease severity—an effect similar to that seen with the influenza virus [4]. In this sense, viral immunity is achieved by forming antibodies to the antigen in the host’s humoral system. In the case of Flaviviridae, this may be achieved by the production of efficacious neutralizing antibodies (nAbs) through vaccine-mediated immunity, as is the case with YFV and the YFV vaccine (YF-17D), with attenuated (weakened) organisms introduced via injection for a long-lived response [10,11]. A major challenge in dengue vaccine development is generating nAbs that provide protection against all four serotypes without triggering antibody-dependent enhancement (ADE). This requires activating immune cells capable of recognizing a broad range of viral particles.
2. Dengue Clinical Manifestations
The typical clinical presentation of dengue fever presents as a patient who has an acute onset of fever, headache (or retroorbital pain), body aches, and rash spreading from the trunk, typically in patients who live in endemic areas or have traveled to endemic areas two weeks before the onset of symptoms. If a patient presents with this clinical picture, dengue is a nationally notifiable disease that should be reported to the local health department [12].
According to the updated WHO classification of 2009, dengue fever illness is categorized as 1-dengue without warning signs (previously acute dengue fever, also known as D-W), 2-dengue with warning signs (previously dengue hemorrhagic fever or DHF, now known as D+W), and 3-severe dengue (previously dengue shock syndrome or DSS, now known as SDF) [13]. D-W is present in patients who have traveled near or reside within a dengue-endemic area and present with a fever that lasts anywhere from 2–7 days in duration and has experienced one of the following: nausea, vomiting, rash, aches, or pains, a positive tourniquet test or leukopenia which require case and outpatient management on supportive treatment such as IV fluid repletion to manage volume depletion and acetaminophen as needed to manage fever [14]. D+W may present with the previous criteria around the time the fever and the body temperature decrease, and the patient also exhibits one of the following: severe abdominal pain or tenderness, vomiting, edema, orthostatic hypotension, mucosal bleeding, lethargy, restlessness, hepatomegaly, or a concurrent increase in hematocrit with a rapid decrease in platelets. At this point, hospital and observational admission are recommended, and blood transfusions may be given based on the severity of the bleeding observed [12]. SDF meets the criteria for dengue with warning signs and may also have one of the following: severe plasma leakage leading to shock or edema with respiratory distress, severe bleeding from the gastrointestinal tract or vaginal requiring IV fluid resuscitation or emergent blood transfusion, or severe organ impairment as evidenced by elevated transaminases ≥ 1000 IU/L, impaired consciousness, or heart impairment, in which case ICU admission is required [15].
The disease is thought to develop in three distinct phases, composed of a febrile phase, a critical phase, and a recovery phase. In both classification schemes, acute dengue fever manifests with febrile and recovery phases. In contrast, the moderate hemorrhagic presentation with warning signs and severe shock syndrome variants exhibits a febrile phase, a critical phase, and a recovery phase [16]. The symptoms mentioned above for the acute presentation in both classification schemes can describe the febrile phase, in which a patient rarely experiences a remission in fever and may recur for 1–2 days (bi-phasic fever) [14,17,18]. A physical exam with a tourniquet test screening for petechiae and a CBC with liver enzymes will be able to screen for the criteria mentioned above and for intervention if the patient needs correction [19,20]. It is recommended that the progression to plasma leakage in a patient, especially on days 3 and 7 of illness, be monitored using ultrasonography of the chest and abdomen and radiography of the chest [21,22]. The critical phase may occur more than 18 months after the initial infection of dengue after resolution or in children under one year of age with low levels of maternal antibodies, and the child may experience the infection as a primary infection and progress directly to the critical phase [16]. This may also be seen in patients with significant immunosuppressive comorbidities. The critical phase itself lasts from 24–48 h. It should involve carefully monitoring blood pressure with attention to careful resuscitation, as the patient may experience rapid-onset hypotension that may lead to shock. The recovery phase is marked by a resolution of plasma leakage and bleeding, resorption of excess fluids, and vital sign stabilization. However, an additional rash lasting at most five days may manifest after the resolution of the fever, and the patient may experience fatigue for days or weeks after recovery. The recovery phase lasts approximately 2–3 days [12].
3. Contribution of CD8+ T Cells to the Immune Response Against Dengue Virus
In recent years, significant advancements have been made in understanding the role of T cells in the immune response to dengue virus (DENV). Both CD8+ cytotoxic T cells and CD4+ helper T cells are indispensable in controlling DENV infection. The immune response is characterized by robust antiviral T cell activation, with CD8+ T cells targeting non-structural proteins like NS3, NS4b, and NS5, while CD4+ T cells focus on structural proteins such as the capsid, envelope, and secreted non-structural protein NS1 [23]. This complementary targeting ensures a coordinated immune defense: CD8+ T cells act as effectors that eliminate infected cells, and CD4+ T cells amplify the antiviral response by activating other immune cells and enhancing antibody production. The activation of CD8+ T cells is initiated when viral peptides, typically 9–10 amino acids in length, are processed and presented by MHC class I molecules on infected cells. CD8+ T cells recognize these peptide-MHC complexes via their T cell receptors (TCR), triggering the release of cytotoxic granules containing perforin and granzymes. These granules induce apoptosis in infected cells, thereby limiting viral replication and contributing to immune protection [23,24]. Beyond cytotoxicity, CD8+ T cells secrete antiviral cytokines such as IFN-γ and tumor necrosis factor-alpha (TNF-α), which activate macrophages and dendritic cells and establish a systemic antiviral state that curtails viral spread [25,26].
Memory CD8+ T cells generated during primary infections play a pivotal role in protecting against subsequent infections by the same DENV serotype. These memory cells enable a rapid and robust immune response that reduces disease severity and prevents progression to severe disease manifestations [25,26]. An intriguing aspect of CD8+ T cell responses in dengue is their skin-homing phenotype. During acute infection, CD8+ T cells express cutaneous lymphocyte antigen (CLA), which indicates that the skin is a critical site for immune surveillance and may be linked to dengue-associated dermatological symptoms [27]. CD4+ helper T cells complement this response by recognizing longer viral peptides (12–15 amino acids) presented by MHC class II molecules on antigen-presenting cells (APCs) such as dendritic cells and macrophages. These helper T cells enhance immune defense by promoting the activation of B cells, aiding in antibody production, and supporting CD8+ T cell responses [28]. CD4+ T cells are further categorized into subsets—Th1, Th2, Th17, and Treg—each contributing uniquely to the immune response. Th1 cells, for instance, secrete IFN-γ to promote cell-mediated immunity, while Th2 cells produce interleukin (IL)-4 and IL-5 to support humoral responses [23,29]. Interestingly, CD4+ T cells also display direct cytotoxicity akin to CD8+ T cells, killing infected cells and producing inflammatory cytokines to control the infection [25].
Recent studies have identified highly polarized CX3CR1+ cytotoxic CD4+ T cells in dengue patients, which exhibit potent antiviral activity and could serve as targets for novel vaccine strategies [30,31]. However, as with CD8+ T cells, CD4+ T cell responses must be tightly regulated to prevent immunopathogenesis. Dysregulated T cell responses during secondary infections may exacerbate disease severity, particularly in the context of ADE, where preexisting non-neutralizing antibodies facilitate viral entry into host cells [32,33]. Balancing the protective and potentially pathogenic roles of T cells is essential for vaccine development. The dual roles of CD8+ and CD4+ T cells in protection and immunopathogenesis underscore the complexity of dengue immunity. Effective vaccines must elicit robust and balanced T cell responses that provide cross-serotype protection while minimizing the risk of severe disease. Current research focuses on optimizing epitope selection and inducing memory T cell responses to achieve these goals [26,31].
4. CD4+ T Cells and Their Contribution to Dengue Virus Immunity
CD4+ T cells play a central role in orchestrating the immune response against dengue virus (DENV) infection. They recognize antigens presented by major histocompatibility complex (MHC) class II molecules on the surface of antigen-presenting cells (APCs) such as dendritic cells, macrophages, and B cells. This recognition not only activates CD4+ T cells but also enables them to facilitate the activation of other immune cells and produce cytokines that modulate the immune response, ensuring efficient immune functioning [23,28]. CD4+ T cells are functionally divided into subsets, including Th1, Th2, Th17, and Treg cells. Each subset has a distinct role in immune regulation. Th1 cells secrete IFN-γ, which activates macrophages and promotes cytotoxic T cell responses, forming the backbone of cell-mediated immunity. Th2 cells release IL-4 and IL-5, aiding B cells in producing antibodies and enhancing humoral immunity. Th17 cells specialize in combating extracellular bacteria and fungi, while Treg cells maintain immune tolerance, preventing autoimmune damage [29]. These subsets collectively ensure a balanced immune response.
In dengue, CD4+ T cells primarily recognize peptides of 12–15 amino acids presented by APCs on MHC class II molecules. This interaction is critical for eliciting robust humoral responses, including the production of high-affinity nAbs against DENV. Furthermore, CD4+ T cells contribute to establishing immunological memory by assisting B cells in producing long-lived plasma cells and supporting CD8+ T cells in generating memory T cells. These mechanisms enhance the immune system’s ability to respond more efficiently to subsequent DENV exposures [27,31]. In addition to their helper roles, CD4+ T cells produce pro-inflammatory cytokines such as IFN-γ and TNF-α. These cytokines reinforce the antiviral state in infected cells and amplify immune responses by recruiting and activating other immune cells. Importantly, CD4+ T cells also exhibit direct cytotoxic activity, a capability previously thought to be restricted to CD8+ T cells. By killing virus-infected cells, CD4+ T cells provide an additional immune defense against DENV [28,30].
Recent studies have uncovered the significance of highly polarized CX3CR1+ cytotoxic CD4+ T cells in dengue. These cells demonstrate potent antiviral activity by eliminating infected cells and secreting inflammatory cytokines to control the infection. A study by Weiskopf et al. revealed that these polarized T cells are critical for protective immunity and represent promising targets for vaccine development [30,31]. Inducing similar T cell responses could enhance vaccine efficacy by leveraging both cytotoxic and helper functions of CD4+ T cells. Despite their protective roles, CD4+ T cells can contribute to immunopathogenesis during secondary DENV infections. Imbalanced responses, particularly in the presence of ADE, can exacerbate disease severity, leading to complications such as DHF or DSS [32,33]. Balancing protective immunity with the prevention of adverse immune responses is essential for managing dengue and optimizing vaccine strategies. The dual role of CD4+ T cells in protection and immunopathogenesis underscores the challenges of dengue vaccine development. Vaccines must elicit protective immune responses without triggering severe disease outcomes. Future strategies, such as epitope-based vaccines and modulation of T cell subsets, aim to achieve this balance and improve the prospects of long-term immunity against DENV [27,31].
5. The Role of Circulatory T Follicular Helper Cells
Interactions between B and T cells are required to effectively generate protective nAbs and immunological memory [34]. This interaction primarily occurs in the germinal centers of secondary lymphoid organs [35]. During the germinal center reaction, a subset of cognate CD4+ T cells, the T follicular helper (TFH) cells, help activate and differentiate B cells and help in antibody production during infection (Figure 1) [36]. As a result, the chosen high-affinity B cells could differentiate into plasmablasts, plasma cells, or memory B cells. In secondary lymphoid tissues, TFH cells enhance CXCR5 expression while reducing CCR7 expression, allowing them to migrate to the follicles and aid B cells [37]. The secretion of IL-21 by TFH cells stimulates the differentiation and class-switching of B cells [38]. Studying these interactions in vivo in humans with a realistic microenvironment is challenging with existing experimental systems and is invasive for the study participants. However, circulating CD4+ T cells expressing CXCR5, named circulating TFH (cTFH) cells, have been suggested as a peripheral counterpart of TFH cells with similar functions and are easier to study in the context of immune responses to pathogens (Figure 1) [39].
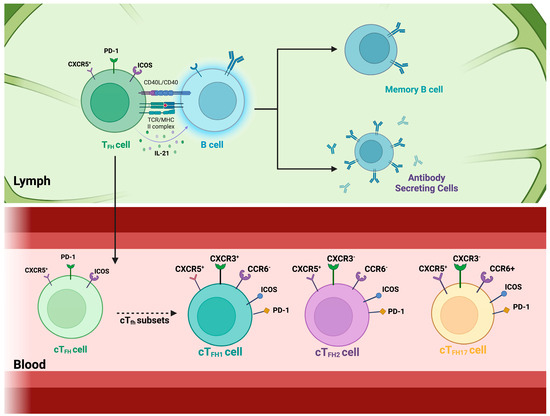
Figure 1.
T follicular helper cells and their circulating counterparts. Top panel: T follicular helper (TFH) cells are a specialized subset of CD4+ T cells that support B cell activation and antibody production. These cells reside in secondary lymphoid organs, such as the tonsils, spleen, and lymph nodes, where they localize to B cell zones. By interacting closely with B cells, TFH cells facilitate their activation and differentiation into antibody-secreting plasma cells and memory B cells, ensuring long-lasting immunity. This process is driven by TFH cell-mediated co-stimulation through CD40 ligand (CD40L) and cytokine secretion, particularly IL-21, which promotes B cell proliferation. TFH cells are defined by the transcription factor B-cell lymphoma 6 (Bcl6) and express surface markers such as CXCR5, PD-1, and ICOS. Bottom panel: Circulating T follicular helper (cTFH) cells represent a subset of TFH cells that can be detected in the bloodstream. These cells are classified based on their expression of chemokine receptors CXCR3 and CCR6 into three major subsets: cTFH1 (CXCR3+CCR6−), cTFH2 (CXCR3−CCR6−), and cTFH17 (CXCR3−CCR6+). Among these, cTFH cells expressing PD-1 and ICOS have been identified as the most efficient in providing B cell help, highlighting their potential role in shaping immune responses.
Circulatory T follicular helper cells express the surface protein PD-1, enabling them to receive signals from B cells via the PD-1 ligands (PD-L), which regulate the activity of cTFH cells [40,41]. The cTFH cells express the inducible costimulator (ICOS), a co-stimulatory molecule critical in many aspects, including development, follicle migration, and functions [41]. These cTFH cells also help B cells during the germinal center reaction by re-entering the lymph nodes in the case of re-infection [42]. This interaction between T follicular helper cells and B cells is essential for generating high-affinity antibodies and developing effective immune responses [43]. The cTFH cells are divided into different subsets. The combinations of these markers ultimately define them: CXCR5, CXCR3, CCR6, PD-1, and ICOS [11,39,44,45]. The combination of these markers defines the cTFH subsets, where mainly the combination of the markers is CXCR5+ CXCR3+ CCR6- cTFH1 cells, CXCR5+ CXCR3- CCR6- cTFH2 cells, and CXCR5+ CXCR3- CCR6+ cTFH17 cells [44,46]. These subsets exhibit distinct cytokine profiles and play different roles in immune responses. The ICOS and PD-1 expressions on these cells present different capacity levels at which these cells can help B cells [47]. A growing body of evidence has indicated that CXCR3−cTFH cells in human blood, comprising cTFH2 and cTFH17 subsets, serve as crucial functional equivalents for GC TFH cells because these cells effectively stimulate naïve B cells to generate antibodies in vitro; hence, they are known as “efficient helper cells”. On the other hand, CXCR3+ cTFH cells, comprising the cTFH1, do not possess this capability, which is why they are known as “non-efficient helper cells”. However, this “efficiency” in helping B cells seems to vary depending on the pathogen causing the infection.
The CXCR5+, CXCR3+, CCR6-, PD-1+, and ICOS+ cTFH1 cells are associated with promoting B cell responses by helping the memory B cells (MBCs), but not the naïve B cells, in becoming antibody-producing cells and producing IgG antibodies [48,49]. The cTFH1 produces the cytokines IFN-γ and IL-21, which help activate B cells and promote class switching. What promotes cTFH1 differentiation is IL-12, IFN gamma by activating the transcription factor (TF) T-bet, IL-2 deprivation, TCR signaling, ICOS signal, and CD28 signal [50,51]. The transcription factors responsible for promoting cTFH1 activation are T-bet and Bcl6 [52,53]. The CXCR5+, CXCR3-, CCR6-, PD-1+, and ICOS+ cTFH2 cells promote B cell responses characterized by the production of IgE and IgG antibodies, and they produce IL-4, IL-5, and IL-13 [51]. The cTFH2 differentiation occurs by IL-4 by activating TF GATA3, ICOS and CD28, IL-2 deprivation, IL-6, and IL-21. The transcription factors that promote cTFH2 activation are GATA3 and Bcl6 [47,54]. The CXCR5+, CXCR3-, CCR6+, PD-1+, and ICOS+ cTFH17 cells are associated with promoting B cell responses characterized by the production of IgA antibodies [41]. They produce the cytokines IL-17 and IL-21, which help activate B cells and support class switching to IgA and IgG. This cTFH17 differentiation is mediated through IL-6 and TGF-β (together activate the TF RORγt), IL-21, ICOS, CD28, and IL-23 (produced by APCs, which promote TF RORγt) [54,55]. RORγt is a master regulator of cTFH17 differentiation and promotes IL-17 and IL-22 production [51]. In summary, the cTFH cells are a specialized subset of CD4+ T cells that assist B cells in producing high-affinity antibodies, crucial for effective immunity. Originating from naïve T cells and influenced by factors like IL-21 and Bcl-6, they interact with B cells in germinal centers and secrete cytokines such as IL-21, which is important when helping B-cells. Their study is essential for understanding autoimmune diseases, improving cancer immunotherapies, and managing infectious diseases. Research on cTFH cells focuses on their molecular mechanisms, clinical applications, and potential as biomarkers, aiming to harness their role in immune regulation and long-term immunity. However, more research is needed to fully understand their functions and therapeutic potential.
6. Characterizing Circulating T Follicular Helper Cells in Flavivirus-Induced Immune Responses
Studies regarding cTFH cells suggest that these cells promote antibody responses across viral infections, including influenza, HIV-1, and SARS-CoV-2 [42,56,57]. These subsets of cTFH cells, characterized by markers like ICOS, PD-1, and CXCR5, interact with B cells to activate, proliferate, and differentiate them into antibody-producing cells. The frequency and functionality of cTFH cells correlate with the quality and durability of antibody responses against these viruses, underscoring their importance in immunity. Additionally, these studies emphasized the significance of nAbs in the immune response against viruses, with specialized subsets of TFH cells, such as ICOS+PD-1+CXCR3+ cTFH1 cells, contributing to their generation by promoting B cell activation and differentiation [42,46,56,57]. The nAbs antibodies specifically bind to flaviviruses and block their ability to infect cells, being crucial for protection and the main objective of vaccines [58]. Non-neutralizing antibodies, although they mark the virus for immunological recognition, do not prevent infection and may contribute to ADE, increasing the severity of the disease. Flaviviral vaccine development focuses on inducing strong nAb responses using live-attenuated, inactivated, and recombinant vaccines while addressing challenges like ADE and ensuring durable immunity. However, scientists must also concentrate on peptide-based vaccines, which offer a promising and safe approach to developing immunity against flaviviruses by using specific viral protein sequences to elicit targeted immune responses. Understanding these cell interactions and how the cTFH helps in the nAbs development could be crucial for effectively developing vaccines and therapeutic interventions to combat viral diseases.
Current research on flaviviruses focuses primarily on studying circulating T follicular helper (cTFH) cell populations following vaccination. For example, a study by Sandberg et al. [59] investigating the immune response following YFV 17D vaccination brought significant findings across various immune components to light. Th1-polarized CXCR3+ cTFH cells exhibited heightened activation on day 14, suggesting that robust germinal center activity is essential for generating high-quality antibodies. Plasmablast expansion, particularly the IgG1 subset, peaked on day 14, correlating positively with the subsequent development of nAbs on day 90. This linkage between cTFH1, plasmablast frequencies, and protective immunity highlights the potential of these cells as early indicators of vaccine efficacy. Moreover, YFV-E-specific IgG1 plasmablasts, detectable by day seven and peaking at day 14, emphasize the vaccine’s ability to induce antigen-specific B cell responses. This comprehensive understanding of cTFH subsets, plasmablast dynamics, memory B cell responses, and their correlations with antibody production contributes valuable insights into the mechanisms underpinning protective immunity elicited by YFV 17D vaccination [59]. Studies like this one, exploring the diverse subsets of cTFH cells, provide valuable insights into how individual immune responses are tailored to combat specific pathogens based on the pathogen’s characteristics and the epitopes’ specificity. By investigating the distinct phenotypic and functional attributes of cTFH subsets, researchers can elucidate how these cells modulate B cell activation and antibody production in response to various flaviviruses.
An important finding from another study on yellow fever vaccination dynamics is the correlation between the frequencies of cTFH cell subsets and the strength of the nAb response post-vaccination by Huber et al. Specifically, the study revealed that the frequencies of cTFH1-polarized cells positively correlated with the level of nAb activity, whereas the frequencies of cTFH17 cells exhibited an inverse correlation. This could suggest that the composition and polarization of cTFH cell subsets, particularly the cTFH1 subset, may serve as a predictive biomarker for the magnitude of the nAb response following yellow fever vaccination. The dominance of the cTFH1 subgroup, characterized by CXCR3 expression and IFN-γ secretion, was associated with higher neutralizing activity in the serum of vaccinees. At the same time, the cTFH17 subset, known for IL-17 production, showed a negative correlation with neutralizing antibody levels [11]. This correlation underscores the significance of cTFH cell subset dynamics in shaping the humoral immune response, particularly regarding antibody production and vaccine-induced immunity.
In the context of human leukocyte antigens (HLA; the human major histocompatibility complex) molecules and HLA restriction, the study provides insights into their significant role in the function of cTFH cells. By recognizing antigenic peptides presented by MHC class II molecules, cTFH cells can specifically target and interact with antigen-presenting cells. The study identifies the frequencies of cTFH1 cells as a predictive biomarker for the level of neutralizing antibody responses, suggesting that the interaction between HLA molecules and cTFH cells may influence the strength and quality of the antibody response post-YFV-17D vaccination. Through their engagement with HLA-presented viral epitopes, cTFH1 cells help B cells, driving their differentiation into antibody-secreting cells and promoting high-affinity antibodies to neutralize the virus [11]. Together, this can help us understand the relationship between cTFH cell subsets, HLA restriction, and antibody production and can provide valuable insights for optimizing vaccination strategies. Specifically, these observations suggest that the cTFH1 subset effectively supports B cells in producing antibodies targeting flavivirus infections by recognizing pathogen-specific epitopes presented by HLA molecules.
Research regarding DENV, for example, by Izmirly et al. [45], investigated the role of cTFH cells in the immune response pre- and post-vaccination with the TV003 vaccine, mainly focusing on their correlation with antibody production and vaccine efficacy. The study deepens into the role of circulating cTFH cells in the immune response before and after vaccination, revealing several interesting insights. First, higher baseline/pre-vaccination frequencies of total cTFH cells are positively correlated with neutralizing antibody titers and the breadth of the vaccine response, indicating the predictive value of cTFH cells for dengue vaccine outcomes. This association extends to subjects’ PBMCs, where the frequency of total cTFH cells predicts vaccine outcome and correlates with protection against all four dengue serotypes elicited by the vaccine. Additionally, a significant positive correlation exists between cTFH frequencies at baseline and plasmablast frequencies post-vaccination, further emphasizing the influence of cTFH cells on the immune response. The study’s implications extend to understanding natural infection with dengue virus, where prior exposure shapes cTFH responses, influencing immune memory, serotype-specific immunity, and vaccine efficacy in previously infected individuals. Conducting the study in an endemic area adds clinical relevance, providing insights applicable to populations at high risk of dengue infection and enhancing the understanding of immune responses to vaccination in diverse settings. Overall, the study’s findings underscore the intricate relationship between cTFH cells, B cells, and antibodies, emphasizing the importance of cTFH frequencies in predicting and influencing vaccine outcomes in dengue vaccination [45].
Building on these findings, it is evident that cTFH cells play a significant role in shaping the immune response after vaccination with YFV17 and the dengue vaccine TV003. However, this raises the question of how the cTFH cell population behaves during natural flavivirus infection. Will the same patterns observed after vaccination also apply? In a study by Haltaufderhyde et al. [40], during acute DENV infection, there was a significant increase in the activation of cTFH cells, with the cTFH1 subset (CXCR3+CCR6−) showing the highest activation, particularly during the critical phase of illness. Activation was indicated by elevated expression of PD-1 and co-expression of ICOS. This activation suggests that cTFH1 cells also have a role in the immune response during acute DENV infection, potentially influencing antibody production and overall disease outcome. Additionally, there was a positive correlation between the frequency of activated cTFH cells and the frequency of plasmablasts, indicating that cTFH activation may contribute to the induction of plasmablasts during acute DENV infection. Since cTFH cells have a role in promoting antibody production by providing help to B cells, their activation during acute DENV infection suggests their involvement in the generation of DENV-specific antibodies. Understanding the activation of cTFH cells and their role in antibody production is essential for elucidating the immune response to DENV infection. Moreover, further research on cTFH cells could better understand this interaction and how these cTFH subsets are involved in plasmablast activation and nAb production during a DENV infection, and for how long these nAbs last [40].
A study by Wijesinghe et al. [60] sheds light on the complex dynamics between cTFH cells, plasmablasts, and antibodies during acute dengue infection, aiming to discern their impact on disease severity and clinical outcomes. Several key findings emerged. Notably, cTFH cell expansion was more pronounced in cases of severe dengue and in secondary infections, indicating an activated immune profile. This activation was marked by elevated PD-1 expression, a known indicator of T cell activation. Further phenotypic analysis showed a significant increase in cTFH cells co-expressing PD-1 and ICOS, suggesting enhanced capacity for B cell help and a potential role in modulating the humoral immune response during more severe manifestations of the disease. Functionally, IL-21-producing cTFH cells surged in acute dengue, correlating strongly with plasmablasts expansion and thus driving antibody production. Plasmablast expansion, notably elevated in DHF, correlated positively with cTFH cell frequency, particularly the IL-21 producers. Interestingly, cTFH cells and plasmablasts frequencies inversely correlated with viral loads, indicating a role in viral control, while plasmablasts expansion correlated with DENV-specific IgG titers, especially in DHF. Nonetheless, Neut50 titers significantly correlated with cTFH cell and plasmablast frequencies, notably in DHF, exhibiting a marked increase throughout convalescence, particularly in DENV2 infections. These findings could denote that the cTFH cells have a role in helping B cells drive antibody responses in acute dengue infection, particularly emphasizing the significance of IL-21-producing cTFH cells in generating robust nAbs. This study discusses the intricate interplay between cellular and humoral immune responses in dengue pathogenesis [60].
7. T Cell Function in Dengue Immune Response: Implications and Challenges for Vaccine Development
Developing a vaccine for the dengue virus (DENV) has proven exceptionally challenging due to the co-circulation of four distinct serotypes (DENV-1 to DENV-4), which share approximately 70% sequence homology. In endemic regions, individuals are frequently exposed to multiple serotypes over their lifetime. While infection with one serotype confers lifelong immunity to that specific serotype, it simultaneously increases the risk of severe dengue upon subsequent infection with a different serotype. This increased risk is attributed to ADE, wherein non-nAbs from a prior infection bind to, but fail to effectively neutralize, the secondary infecting serotype. This mechanism facilitates viral entry into host cells, amplifying infection severity [32,61]. The role of T cells in dengue immunity adds another layer of complexity. While T cells are critical in orchestrating protective immune responses and targeting infected cells, their dysregulation can contribute to immunopathogenesis. Vaccines must elicit a balanced immune response that provides broad protection without predisposing individuals to severe disease upon secondary infection. Achieving immunity against all four serotypes simultaneously is particularly difficult, as ADE and inappropriate T cell responses can exacerbate disease severity. Ensuring the long-term safety of vaccines is also crucial, as memory T cells may contribute to enhanced immunopathology in future exposures to DENV [27,33].
Researchers are exploring innovative strategies to address these challenges. One promising approach is T cell epitope mapping, which identifies conserved T cell epitopes across all DENV serotypes that elicit protective immune responses without triggering harmful immunopathology. These epitope-based vaccine designs rely on HLA-binding studies to select immunodominant epitopes that are safe and cross-reactive. Broadly protective vaccines, designed to induce durable immunity against all four serotypes, are also under investigation. Specific adjuvants and vaccine formulations are being developed to modulate immune responses and minimize ADE-associated risks. For example, some formulations favor a Th1-skewed response, which is less likely to exacerbate disease severity [28,31,62]. CD4+ T cells are particularly crucial in this context. Their activation involves antigen uptake, presentation on HLA complexes, and recognition by T cell receptors (TCRs) on naïve CD4+ T cells. This process initiates a cascade of events, including cytokine secretion and costimulatory receptor engagement, which stimulate B cells to produce nAbs. However, the complexity lies in understanding how specific CD4+ T cell subsets, such as follicular helper T cells (cTFH), contribute to either protective immunity or immunopathology. Dysregulation of these cells can drive ADE and other pathological outcomes [25,27].
Another challenge is the variability in immune responses across geographical regions. Individuals with the same HLA allele types may exhibit different magnitudes of DENV-specific CD4+ T cell activation, influenced by regional factors such as serotype prevalence and HLA polymorphism. Discrepancies between predicted and observed epitopes highlight the importance of refining HLA-restriction studies to identify universally protective epitope candidates. Comparative analyses across flaviviruses, including DENV, may also reveal conserved epitopes useful for cross-protective vaccine development [25,31]. To overcome these obstacles, researchers are integrating multiple approaches, including advanced epitope mapping, adjuvant optimization, and in-depth analysis of T cell subset dynamics. These strategies aim to enhance protective T cell responses while mitigating immunopathological risks. By improving our understanding of the intricate interplay between DENV and the immune system, it is possible to create safer and more effective vaccines. This multifaceted approach is essential to reduce the global burden of dengue and develop lasting solutions to this significant public health challenge.
8. Conclusions and Perspectives
Flaviviruses are a global concern, affecting millions of individuals in tropical and sub-tropical regions and causing thousands of deaths yearly. However, no completely effective treatments or vaccines currently protect entirely and safely against specific flaviviruses such as DENV. Not to mention that the vaccine currently approved for DENV by the US FDA has some limitations, including the risk of ADE in individuals without prior dengue infection, restricted efficacy across serotypes, and the need for pre-vaccination testing. In the U.S., its use is limited to children aged 9–16 who live in dengue-endemic areas and have laboratory-confirmed prior DENV infection [63,64,65]. Therefore, more research is needed to develop effective prevention methods and treatments. This review article highlights the critical role of cTFH cells and antibodies in developing robust immune responses against flaviviruses, particularly DENV. Studies highlight the role of cTFH cells, characterized by markers such as CXCR5, ICOS, and PD-1, in promoting antibody production and shaping the quality of immune responses to combat infections. As mentioned above, research on yellow fever 17D vaccination and DENV infection demonstrates the value of cTFH cells for vaccine outcomes as a predictive method, with higher baseline frequencies correlating with improved antibody responses (Figure 2). The importance of nAbs in conferring protection against flaviviruses is emphasized, with specific cTFH cell subsets, particularly those polarized toward a Th1 phenotype, playing a critical role in their generation. While significant progress has been made in understanding the functions of cTFH cells, more knowledge still needs to be gained regarding certain aspects of cTFH immunity, particularly in flavivirus infections. For example, it would be interesting to characterize if there is any cross-reactivity or cross-protection with the cTFH cells and other flaviviruses, how long this response of the cTFH lasts, the memory of the different subsets, the immunogenicity, and how the antigen presentation influences their response. While many studies have focused on correlating cTFH cell fluctuations with immune responses—which is valuable—we also need functional experiments. For instance, co-culturing different cTFH subsets with B cells could reveal their roles in driving B cell differentiation and the production of virus-specific and neutralizing antibodies. Additionally, assessing the cytokine profiles these cells express upon stimulation with specific peptides would help clarify the magnitude and quality of their T cell responses. Also, the role of HLA molecules in presenting viral antigens to cTFH cells and in shaping the immune response warrants further investigation, as HLA polymorphisms may influence individual susceptibility to DENV infection and responses to vaccines. Closing these knowledge gaps regarding understanding the meticulous dynamics of cTFH cells and their interactions with B cells will be essential to have more strategies and insights to combat flavivirus infections and improve global public health outcomes.
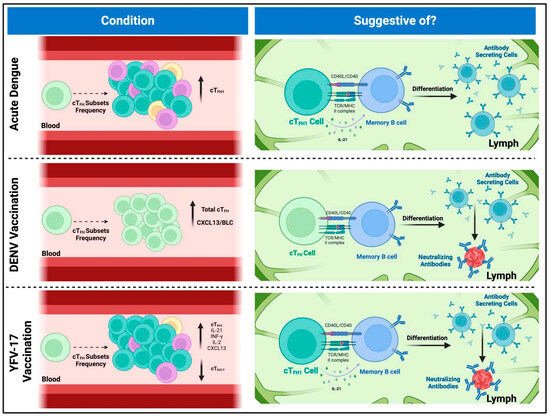
Figure 2.
Implications of circulating T follicular helper cell frequencies in flaviviral infections and vaccine responses. Top panel: During acute dengue virus (DENV) infection, cTFH1 cells, characterized by the expression of PD-1, ICOS, and IL-21 production, are present at high frequencies. The frequency of these cells correlates with the presence of antibody-secreting cells. Although cTFH1 cells are typically classified as “non-efficient helper cells” due to their limited capacity to activate and differentiate naïve B cells, they have been shown to support the activation and differentiation of memory B cells (MBCs) into antibody-secreting plasma cells and plasmablasts. This suggests that, during acute dengue infection, cTFH1 cells are among the first responders, facilitating the differentiation of MBCs into antibody-producing cells upon their re-entry into secondary lymphoid organs. Middle panel: In the context of DENV TV003 vaccination, higher baseline frequencies of cTFH cells are associated with more robust neutralizing antibody responses and broader vaccine-induced immunity. Also, baseline cTFH levels correlate with post-vaccination frequencies of antibody-secreting cells, suggesting a direct role of cTFH cells in driving the immune response. Elevated levels of CXCL13/BLC correlate with increased cTFH frequencies, further complicating the relationship between cTFH cells, B cell activation, and antibody production in dengue vaccination. These findings imply that cTFH cells contribute to the differentiation of MBCs into neutralizing antibody-producing cells post-vaccination. Bottom panel: Research on yellow fever vaccination shows that cTFH1 cells peak in activation post-vaccination, coinciding with strong germinal center activity and the generation of high-quality antibodies. The secretion of cytokines such as IFN-γ, IL-2, and IL-21 by cTFH1 cells is linked to enhanced antibody responses. Moreover, the expansion of antibody-secreting cells like plasmablasts correlates with the development of neutralizing antibodies, indicating that cTFH1 cells serve as early indicators of vaccine efficacy. The composition of cTFH subsets, especially the dominance of cTFH1 cells, predicts the magnitude of neutralizing antibody responses, with a positive correlation between cTFH1 prevalence and neutralizing activity.
Author Contributions
Conceptualization, P.N.F.-P. and V.R.-A.; writing—original draft preparation, P.N.F.-P., J.A.C.-L. and F.A.R.-A.; writing—review and editing, V.R.-A.; visualization, P.N.F.-P.; supervision, V.R.-A.; project administration, V.R.-A.; funding acquisition, V.R.-A. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the National Institutes of Health (NIH) Minority Health and Health Disparities (MHD) Research Centers in Minority Institutions (RCMI) Center for Research Resources (CRR), grant number U54MD007579, and by the Centers for Disease Control and Prevention, grant number U01CK000580 (V.R.-A.). PNFP was supported by the National Institute of General Medical Sciences (T32GM144896).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the work; in the writing of the manuscript; or in the decision to publish.
References
- Holbrook, M.R. Historical Perspectives on Flavivirus Research. Viruses 2017, 9, 97. [Google Scholar] [CrossRef] [PubMed]
- Blaney, J.E.; Durbin, A.P.; Murphy, B.R.; Whitehead, S.S. Development of a live attenuated dengue virus vaccine using reverse genetics. Viral Immunol. 2006, 19, 10–32. [Google Scholar] [CrossRef] [PubMed]
- Nasar, S.; Rashid, N.; Iftikhar, S. Dengue proteins with their role in pathogenesis, and strategies for developing an effective anti-dengue treatment: A review. J. Med. Virol. 2020, 92, 941–955. [Google Scholar] [CrossRef]
- Rothman, A.L. Immunity to dengue virus: A tale of original antigenic sin and tropical cytokine storms. Nat. Rev. Immunol. 2011, 11, 532–543. [Google Scholar] [CrossRef] [PubMed]
- Education, N. Dengue Viruses. Available online: https://www.nature.com/scitable/topicpage/dengue-viruses-22400925/#:~:text=The%20Dengue%20Serotypes&text=These%20four%20viruses%20are%20called,there%20is%20some%20genetic%20variation (accessed on 15 February 2024).
- Zerfu, B.; Kassa, T.; Legesse, M. Epidemiology, biology, pathogenesis, clinical manifestations, and diagnosis of dengue virus infection, and its trend in Ethiopia: A comprehensive literature review. Trop. Med. Health 2023, 51, 11. [Google Scholar] [CrossRef] [PubMed]
- Chng, M.H.Y.; Lim, M.Q.; Rouers, A.; Becht, E.; Lee, B.; MacAry, P.A.; Lye, D.C.; Leo, Y.S.; Chen, J.; Fink, K.; et al. Large-Scale HLA Tetramer Tracking of T Cells during Dengue Infection Reveals Broad Acute Activation and Differentiation into Two Memory Cell Fates. Immunity 2019, 51, 1119–1135. [Google Scholar] [CrossRef]
- Guzman, M.G.; Gubler, D.J.; Izquierdo, A.; Martinez, E.; Halstead, S.B. Dengue infection. Nat. Rev. Dis. Primers 2016, 2, 16055. [Google Scholar] [CrossRef]
- Khan, M.B.; Yang, Z.S.; Lin, C.Y.; Hsu, M.C.; Urbina, A.N.; Assavalapsakul, W.; Wang, W.H.; Chen, Y.H.; Wang, S.F. Dengue overview: An updated systemic review. J. Infect. Public Health 2023, 16, 1625–1642. [Google Scholar] [CrossRef]
- Izmirly, A.M.; Alturki, S.O.; Alturki, S.O.; Connors, J.; Haddad, E.K. Challenges in Dengue Vaccines Development: Pre-existing Infections and Cross-Reactivity. Front. Immunol. 2020, 11, 1055. [Google Scholar] [CrossRef] [PubMed]
- Huber, J.E.; Ahlfeld, J.; Scheck, M.K.; Zaucha, M.; Witter, K.; Lehmann, L.; Karimzadeh, H.; Pritsch, M.; Hoelscher, M.; von Sonnenburg, F.; et al. Dynamic changes in circulating T follicular helper cell composition predict neutralising antibody responses after yellow fever vaccination. Clin. Transl. Immunol. 2020, 9, e1129. [Google Scholar] [CrossRef] [PubMed]
- Dengue: Guidelines for Diagnosis, Treatment, Prevention and Control: New Edition; WHO: Geneva, Switzerland, 2009.
- Kalayanarooj, S. Clinical Manifestations and Management of Dengue/DHF/DSS. Trop. Med. Health 2011, 39, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Trofa, A.F.; DeFraites, R.F.; Smoak, B.L.; Kanesa-thasan, N.; King, A.D.; Burrous, J.M.; MacArthy, P.O.; Rossi, C.; Hoke, C.H. Dengue fever in US military personnel in Haiti. JAMA 1997, 277, 1546–1548. [Google Scholar] [CrossRef] [PubMed]
- Ajlan, B.A.; Alafif, M.M.; Alawi, M.M.; Akbar, N.A.; Aldigs, E.K.; Madani, T.A. Assessment of the new World Health Organization’s dengue classification for predicting severity of illness and level of healthcare required. PLoS Negl. Trop. Dis. 2019, 13, e0007144. [Google Scholar] [CrossRef] [PubMed]
- Kalayanarooj, S.; Vaughn, D.W.; Nimmannitya, S.; Green, S.; Suntayakorn, S.; Kunentrasai, N.; Viramitrachai, W.; Ratanachu-eke, S.; Kiatpolpoj, S.; Innis, B.L.; et al. Early clinical and laboratory indicators of acute dengue illness. J. Infect. Dis. 1997, 176, 313–321. [Google Scholar] [CrossRef]
- Fukusumi, M.; Arashiro, T.; Arima, Y.; Matsui, T.; Shimada, T.; Kinoshita, H.; Arashiro, A.; Takasaki, T.; Sunagawa, T.; Oishi, K. Dengue Sentinel Traveler Surveillance: Monthly and Yearly Notification Trends among Japanese Travelers, 2006–2014. PLoS Negl. Trop. Dis. 2016, 10, e0004924. [Google Scholar] [CrossRef]
- Leder, K.; Torresi, J.; Brownstein, J.S.; Wilson, M.E.; Keystone, J.S.; Barnett, E.; Schwartz, E.; Schlagenhauf, P.; Wilder-Smith, A.; Castelli, F.; et al. Travel-associated illness trends and clusters, 2000–2010. Emerg. Infect. Dis. 2013, 19, 1049–1073. [Google Scholar] [CrossRef]
- Cao, X.T.; Ngo, T.N.; Wills, B.; Kneen, R.; Nguyen, T.T.; Ta, T.T.; Tran, T.T.; Doan, T.K.; Solomon, T.; Simpson, J.A.; et al. Evaluation of the World Health Organization standard tourniquet test and a modified tourniquet test in the diagnosis of dengue infection in Viet Nam. Trop. Med. Int. Health 2002, 7, 125–132. [Google Scholar]
- Wilder-Smith, A.; Schwartz, E. Dengue in travelers. N. Engl. J. Med. 2005, 353, 924–932. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, B.D.C.; Correia, D. Ultrasound Assessment of Hepatobiliary and Splenic Changes in Patients with Dengue and Warning Signs During the Acute and Recovery Phases. J. Ultrasound Med. 2019, 38, 2015–2024. [Google Scholar] [CrossRef]
- Srikiatkhachorn, A.; Krautrachue, A.; Ratanaprakarn, W.; Wongtapradit, L.; Nithipanya, N.; Kalayanarooj, S.; Nisalak, A.; Thomas, S.J.; Gibbons, R.V.; Mammen, M.P.; et al. Natural history of plasma leakage in dengue hemorrhagic fever: A serial ultrasonographic study. Pediatr. Infect. Dis. J. 2007, 26, 283–290; discussion 291–292. [Google Scholar] [CrossRef]
- Rivino, L. T cell immunity to dengue virus and implications for vaccine design. Expert Rev. Vaccines 2016, 15, 443–453. [Google Scholar] [CrossRef] [PubMed]
- Janeway, C.A., Jr.; Travers, P.; Walport, M.; Shlomchik, M.J. Immunobiology: The Immune System in Health and Disease, 5th ed.; Garland Science: New York, NY, USA, 2001. [Google Scholar]
- Weiskopf, D.; Angelo, A.M.; Azeredo, D.L.E.; Sidney, J.; Greenbaum, A.J.; Fernando, N.A.; Broadwater, A.; Kolla, V.R.; Silva, D.D.A.; Silva, D.M.A.; et al. Comprehensive analysis of dengue virus-specific responses supports an HLA-linked protective role for CD8+ T cells. Proc. Natl. Acad. Sci. USA 2013, 110, E2046–E2053. [Google Scholar] [CrossRef] [PubMed]
- Zellweger, M.R.L.; Shresta, S. Mouse Models to Study Dengue Virus Immunology and Pathogenesis. Front. Immunol. 2014, 5, 151. [Google Scholar]
- Rivino, L. Understanding the Human T Cell Response to Dengue Virus. In Advances in Experimental Medicine and Biology; Springer Nature: London, UK, 2018; Volume 1062, pp. 241–250. [Google Scholar]
- Ngono, E.A.; Shresta, S. Immune Response to Dengue and Zika. Annu. Rev. Immunol. 2018, 36, 279–308. [Google Scholar] [CrossRef]
- Fisher, G.R.; James, C.E., Jr.; Johnson, T.R.; Tang, Y.W.; Graham, B.S. Passive IgA Monoclonal Antibody Is No More Effective Than IgG at Protecting Mice from Mucosal Challenge with Respiratory Syncytial Virus. J. Infect. Dis. 1999, 180, 1324–1327. [Google Scholar] [CrossRef]
- Weiskopf, D.; Bangs, D.J.; Sidney, J.; Kolla, R.V.; Silva, D.D.A.; Silva, D.M.A.; Crotty, S.; Peters, B.; Sette, A. Dengue virus infection elicits highly polarized CX3CR1 + cytotoxic CD4 + T cells associated with protective immunity. Proc. Natl. Acad. Sci. USA 2015, 112, E4256–E4263. [Google Scholar]
- Grifoni, A.; Pham, J.; Sidney, J.; O’Rourke, H.P.; Paul, S.; Peters, B.; Martini, R.S.; Silva, D.D.A.; Ricciardi, J.M.; Magnani, M.D.; et al. Prior Dengue Virus Exposure Shapes T Cell Immunity to Zika Virus in Humans. J. Virol. 2017, 91, e01469-17. [Google Scholar] [CrossRef]
- Guzman, M.G.; Harris, E. Dengue. Lancet 2015, 385, 453–465. [Google Scholar] [CrossRef]
- St John, A.L.; Rathore, A.P.S. Adaptive immune responses to primary and secondary dengue virus infections. Nat. Rev. Immunol. 2019, 19, 218–230. [Google Scholar]
- Stebegg, M.; Kumar, S.D.; Silva-Cayetano, A.; Fonseca, V.R.; Linterman, M.A.; Graca, L. Regulation of the Germinal Center Response. Front. Immunol. 2018, 9, 2469. [Google Scholar] [CrossRef] [PubMed]
- Jogdand, G.M.; Mohanty, S.; Devadas, S. Regulators of Tfh Cell Differentiation. Front. Immunol. 2016, 7, 520. [Google Scholar]
- Vinuesa, C.G.; Cyster, J.G. How T cells earn the follicular rite of passage. Immunity 2011, 35, 671–680. [Google Scholar] [PubMed]
- Vinuesa, C.G.; Linterman, M.A.; Yu, D.; MacLennan, I.C. Follicular Helper T Cells. Annu. Rev. Immunol. 2016, 34, 335–368. [Google Scholar]
- Crotty, S. T Follicular Helper Cell Biology: A Decade of Discovery and Diseases. Immunity 2019, 50, 1132–1148. [Google Scholar]
- Locci, M.; Havenar-Daughton, C.; Landais, E.; Wu, J.; Kroenke, M.A.; Arlehamn, C.L.; Su, L.F.; Cubas, R.; Davis, M.M.; Sette, A.; et al. Human circulating PD-1 + CXCR3-CXCR5 + memory Tfh cells are highly functional and correlate with broadly neutralizing HIV antibody responses. Immunity 2013, 39, 758–769. [Google Scholar] [PubMed]
- Haltaufderhyde, K.; Srikiatkhachorn, A.; Green, S.; Macareo, L.; Park, S.; Kalayanarooj, S.; Rothman, A.L.; Mathew, A. Activation of Peripheral T Follicular Helper Cells During Acute Dengue Virus Infection. J. Infect. Dis. 2018, 218, 1675–1685. [Google Scholar]
- Gong, F.; Zheng, T.; Zhou, P. T Follicular Helper Cell Subsets and the Associated Cytokine IL-21 in the Pathogenesis and Therapy of Asthma. Front. Immunol. 2019, 10, 2918. [Google Scholar]
- Bentebibel, S.E.; Khurana, S.; Schmitt, N.; Kurup, P.; Mueller, C.; Obermoser, G.; Palucka, A.K.; Albrecht, R.A.; Garcia-Sastre, A.; Golding, H.; et al. ICOS(+)PD-1(+)CXCR3(+) T follicular helper cells contribute to the generation of high-avidity antibodies following influenza vaccination. Sci. Rep. 2016, 6, 26494. [Google Scholar]
- Bentebibel, S.E.; Lopez, S.; Obermoser, G.; Schmitt, N.; Mueller, C.; Harrod, C.; Flano, E.; Mejias, A.; Albrecht, R.A.; Blankenship, D.; et al. Induction of ICOS+CXCR3+CXCR5+ TH cells correlates with antibody responses to influenza vaccination. Sci. Transl. Med. 2013, 5, 176ra32. [Google Scholar]
- Byford, E.; Carr, M.; Piñon, L.; Ahearne, M.J.; Wagner, S.D. Isolation of CD4+ T-cells and Analysis of Circulating T-follicular Helper (cTfh) Cell Subsets from Peripheral Blood Using 6-color Flow Cytometry. J. Vis. Exp. 2019, e58431. [Google Scholar] [CrossRef]
- Izmirly, A.M.; Pelletier, A.N.; Connors, J.; Taramangalam, B.; Alturki, S.O.; Gordon, E.A.; Alturki, S.O.; Mell, J.C.; Swaminathan, G.; Karthik, V.; et al. Pre-vaccination frequency of circulatory Tfh is associated with robust immune response to TV003 dengue vaccine. PLoS Pathog. 2022, 18, e1009903. [Google Scholar] [CrossRef]
- Kudryavtsev, I.V.; Arsentieva, N.A.; Batsunov, O.K.; Korobova, Z.R.; Khamitova, I.V.; Isakov, D.V.; Kuznetsova, R.N.; Rubinstein, A.A.; Stanevich, O.V.; Lebedeva, A.A.; et al. Alterations in B Cell and Follicular T-Helper Cell Subsets in Patients with Acute COVID-19 and COVID-19 Convalescents. Curr. Issues Mol. Biol. 2021, 44, 194–205. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, N.; Bentebibel, S.E.; Ueno, H. Phenotype and functions of memory Tfh cells in human blood. Trends Immunol. 2014, 35, 436–442. [Google Scholar] [CrossRef] [PubMed]
- Byford, E.T.; Carr, M.; Ladikou, E.; Ahearne, M.J.; Wagner, S.D. Circulating Tfh1 (cTfh1) cell numbers and PD1 expression are elevated in low-grade B-cell non-Hodgkin’s lymphoma and cTfh gene expression is perturbed in marginal zone lymphoma. PLoS ONE 2018, 13, e0190468. [Google Scholar] [CrossRef] [PubMed]
- Ueno, H. Tfh cell response in influenza vaccines in humans: What is visible and what is invisible. Curr. Opin. Immunol. 2019, 59, 9–14. [Google Scholar] [CrossRef]
- Ghamar Talepoor, A.; Khosropanah, S.; Doroudchi, M. Functional subsets of circulating follicular helper T cells in patients with atherosclerosis. Physiol. Rep. 2020, 8, e14637. [Google Scholar] [CrossRef]
- Morita, R.; Schmitt, N.; Bentebibel, S.E.; Ranganathan, R.; Bourdery, L.; Zurawski, G.; Foucat, E.; Dullaers, M.; Oh, S.; Sabzghabaei, N.; et al. Human blood CXCR5(+)CD4(+) T cells are counterparts of T follicular cells and contain specific subsets that differentially support antibody secretion. Immunity 2011, 34, 108–121. [Google Scholar] [CrossRef]
- Johnston, R.J.; Poholek, A.C.; DiToro, D.; Yusuf, I.; Eto, D.; Barnett, B.; Dent, A.L.; Craft, J.; Crotty, S. Bcl6 and Blimp-1 are reciprocal and antagonistic regulators of T follicular helper cell differentiation. Science 2009, 325, 1006–1010. [Google Scholar] [CrossRef]
- Kroenke, M.A.; Eto, D.; Locci, M.; Cho, M.; Davidson, T.; Haddad, E.K.; Crotty, S. Bcl6 and Maf cooperate to instruct human follicular helper CD4 T cell differentiation. J. Immunol. 2012, 188, 3734–3744. [Google Scholar] [CrossRef]
- Boswell, K.L.; Paris, R.; Boritz, E.; Ambrozak, D.; Yamamoto, T.; Darko, S.; Wloka, K.; Wheatley, A.; Narpala, S.; McDermott, A.; et al. Loss of circulating CD4 T cells with B cell helper function during chronic HIV infection. PLoS Pathog. 2014, 10, e1003853. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.S.; Suryani, S.; Avery, D.T.; Chan, A.; Nanan, R.; Santner-Nanan, B.; Deenick, E.K.; Tangye, S.G. Early commitment of naïve human CD4(+) T cells to the T follicular helper (T(FH)) cell lineage is induced by IL-12. Immunol. Cell Biol. 2009, 87, 590–600. [Google Scholar] [CrossRef] [PubMed]
- Cui, D.; Tang, Y.; Jiang, Q.; Jiang, D.; Zhang, Y.; Lv, Y.; Xu, D.; Wu, J.; Xie, J.; Wen, C.; et al. Follicular Helper T Cells in the Immunopathogenesis of SARS-CoV-2 Infection. Front. Immunol. 2021, 12, 731100. [Google Scholar] [CrossRef] [PubMed]
- Baiyegunhi, O.; Ndlovu, B.; Ogunshola, F.; Ismail, N.; Walker, B.D.; Ndung’u, T.; Ndhlovu, Z.M. Frequencies of Circulating Th1-Biased T Follicular Helper Cells in Acute HIV-1 Infection Correlate with the Development of HIV-Specific Antibody Responses and Lower Set Point Viral Load. J. Virol. 2018, 92, e00659-18. [Google Scholar] [CrossRef] [PubMed]
- Bos, S.; Graber, A.L.; Cardona-Ospina, J.A.; Duarte, E.M.; Zambrana, J.V.; Ruíz Salinas, J.A.; Mercado-Hernandez, R.; Singh, T.; Katzelnick, L.C.; de Silva, A.; et al. The association of neutralizing antibodies with protection against symptomatic dengue virus infection varies by serotype, prior infection history, and assay condition. medRxiv 2023. [Google Scholar] [CrossRef]
- Sandberg, J.T.; Ols, S.; Löfling, M.; Varnaitė, R.; Lindgren, G.; Nilsson, O.; Rombo, L.; Kalén, M.; Loré, K.; Blom, K.; et al. Activation and Kinetics of Circulating T Follicular Helper Cells, Specific Plasmablast Response, and Development of Neutralizing Antibodies following Yellow Fever Virus Vaccination. J. Immunol. 2021, 207, 1033–1043. [Google Scholar] [CrossRef]
- Wijesinghe, A.; Gamage, J.; Goonewardena, H.; Gomes, L.; Jayathilaka, D.; Wijeratne, D.T.; de Alwis, R.; Jeewandara, C.; Wijewickrama, A.; Ogg, G.S.; et al. Phenotype and functionality of follicular helper T cells in patients with acute dengue infection. J. Biomed. Sci. 2020, 27, 50. [Google Scholar] [CrossRef]
- Halstead, B.S. Dengue Virus–Mosquito Interactions. Annu. Rev. Entomol. 2008, 53, 273–291. [Google Scholar] [CrossRef]
- Dempsey, A.L. Zika-neutralizing antibodies. Nat. Immunol. 2017, 18, 603. [Google Scholar] [CrossRef]
- Wilder-Smith, A.; Hombach, J.; Ferguson, N.; Selgelid, M.; O’Brien, K.; Vannice, K.; Barrett, A.; Ferdinand, E.; Flasche, S.; Guzman, M.; et al. Deliberations of the Strategic Advisory Group of Experts on Immunization on the use of CYD-TDV dengue vaccine. Lancet Infect. Dis. 2019, 19, e31–e38. [Google Scholar] [CrossRef]
- Shukla, R.; Ramasamy, V.; Shanmugam, R.K.; Ahuja, R.; Khanna, N. Antibody-Dependent Enhancement: A Challenge for Developing a Safe Dengue Vaccine. Front. Cell. Infect. Microbiol. 2020, 10, 572681. [Google Scholar] [CrossRef]
- Henein, S.; Swanstrom, J.; Byers, A.M.; Moser, J.M.; Shaik, S.F.; Bonaparte, M.; Jackson, N.; Guy, B.; Baric, R.; de Silva, A.M. Dissecting Antibodies Induced by a Chimeric Yellow Fever-Dengue, Live-Attenuated, Tetravalent Dengue Vaccine (CYD-TDV) in Naive and Dengue-Exposed Individuals. J. Infect. Dis. 2017, 215, 351–358. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).