Zika Virus: A Review of Biology, Clinical Impacts, and Coinfections
Abstract
1. Introduction
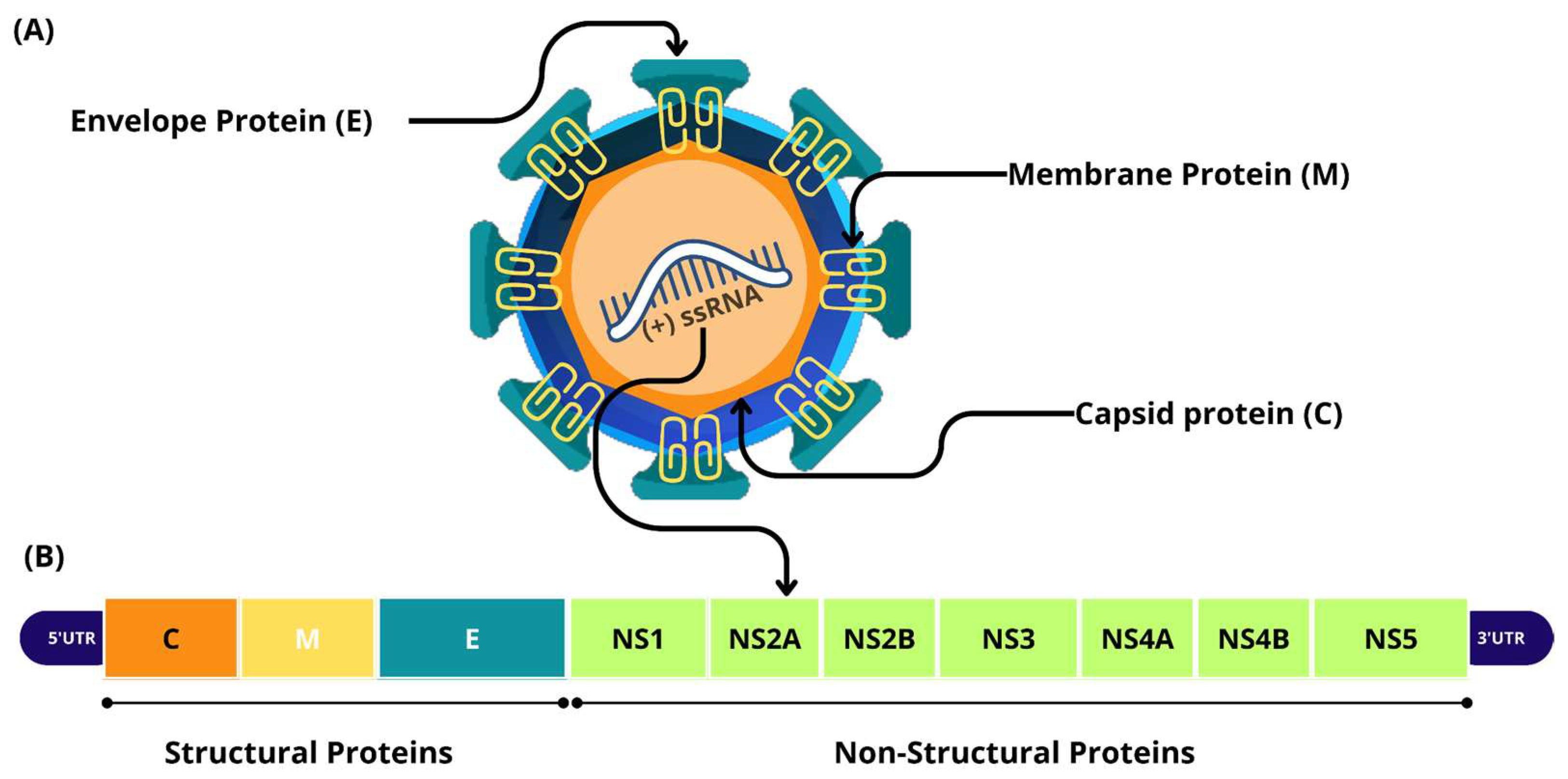
2. Biology of ZIKV
2.1. Infection and Immune Response
2.2. Blood–Brain Barrier and ZIKV Infection
2.3. Congenital Zika Syndrome
2.4. Neurological Manifestations in Adults
3. Cross-Reactive Immunity Among Primary and Secondary Infections
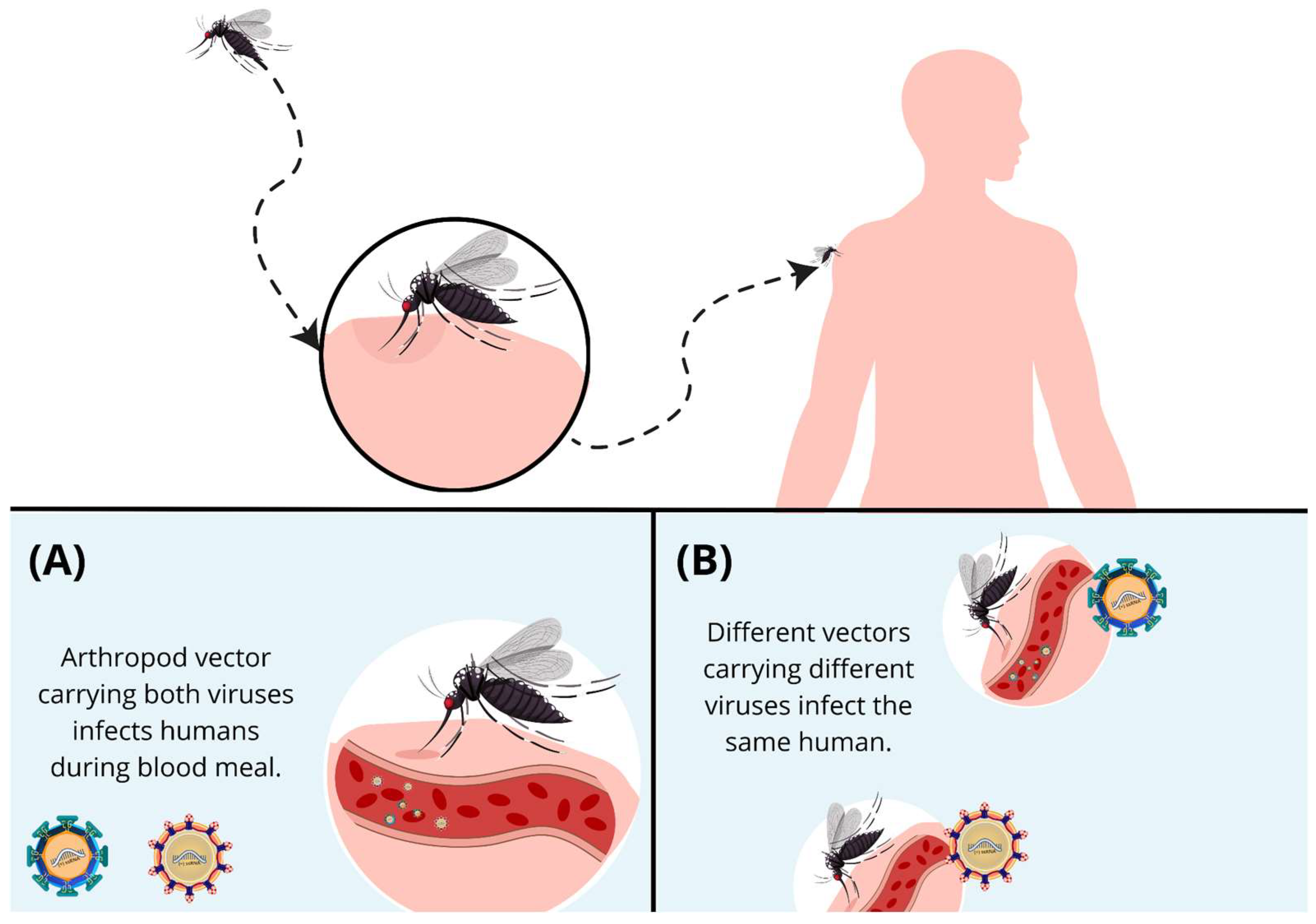
4. Coinfections
4.1. ZIKV and DENV Coinfection: Immune Response and Clinical Outcomes
4.2. ZIKV and CHIKV Coinfection
4.3. ZIKV and HIV-1 Coinfections
4.4. Herpesviridae: HSV-1, EBV, HHV6, and CMV
4.5. Other Viruses: OROV, MAYV, B19V, and SARS-CoV-2
4.6. Other Pathogens: Schistosoma mansoni, Toxoplasma gondii, Leptospira spp., Plasmodium spp., and Candida tropicalis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Souza, A.D.; Abreu, M.C.; Oliveira-Júnior, J.F. Impact of Climate Change on Human Infectious Diseases: Dengue. Braz. Arch. Biol. Technol. 2021, 64, e21190502. [Google Scholar] [CrossRef]
- Tajudeen, Y.A.; Oladunjoye, I.O.; Mustapha, M.O.; Mustapha, S.T.; Ajide-Bamigboye, N.T. Tackling the Global Health Threat of Arboviruses: An Appraisal of the Three Holistic Approaches to Health. Health Promot. Perspect. 2021, 11, 371–381. [Google Scholar] [CrossRef] [PubMed]
- Alves, V.S.; Leite-Aguiar, R.; da Silva, J.P.; Coutinho-Silva, R.; Savio, L.E.B. Purinergic Signaling in Infectious Diseases of the Central Nervous System. Brain. Behav. Immun. 2020, 89, 480–490. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Pant, P.; Bhagat, R.; Seth, P. Zika Virus E Protein Modulates Functions of Human Brain Microvascular Endothelial Cells and Astrocytes: Implications on Blood-Brain Barrier Properties. Front. Cell. Neurosci. 2023, 17, 1173120. [Google Scholar] [CrossRef]
- Dick, G.W.A.; Kitchen, S.F.; Haddow, A.J. Zika Virus (I). Isolations and Serological Specificity. Trans. R. Soc. Trop. Med. Hyg. 1952, 46, 509–520. [Google Scholar] [CrossRef]
- MacNamara, F.N. Zika Virus: A Report on Three Cases of Human Infection during an Epidemic of Jaundice in Nigeria. Trans. R. Soc. Trop. Med. Hyg. 1954, 48, 139–145. [Google Scholar] [CrossRef]
- Abrams, R.P.M.; Solis, J.; Nath, A. Therapeutic Approaches for Zika Virus Infection of the Nervous System. Neurotherapeutics 2017, 14, 1027–1048. [Google Scholar] [CrossRef]
- Counotte, M.J.; Kim, C.R.; Wang, J.; Bernstein, K.; Deal, C.D.; Broutet, N.J.N.; Low, N. Sexual Transmission of Zika Virus and Other Flaviviruses: A Living Systematic Review. PLoS Med. 2018, 15, e1002611. [Google Scholar] [CrossRef]
- Plourde, A.R.; Bloch, E.M. A Literature Review of Zika Virus. Emerg. Infect. Dis. 2016, 22, 1185–1192. [Google Scholar] [CrossRef]
- Raphael, L.M.S.; De Mello, I.S.; Gómez, M.M.; Ribeiro, I.P.; Furtado, N.D.; Lima, N.S.; Dos Santos, A.A.C.; Fernandes, D.R.; Da Cruz, S.O.D.; Damasceno, L.S.; et al. Phenotypic and Genetic Variability of Isolates of ZIKV-2016 in Brazil. Microorganisms 2022, 10, 854. [Google Scholar] [CrossRef]
- Cerbino-Neto, J.; Mesquita, E.C.; Souza, T.M.L.; Parreira, V.; Wittlin, B.B.; Durovni, B.; Lemos, M.C.F.; Vizzoni, A.; Bispo De Filippis, A.M.; Sampaio, S.A.; et al. Clinical Manifestations of Zika Virus Infection, Rio de Janeiro, Brazil, 2015. Emerg. Infect. Dis. 2016, 22, 1318–1320. [Google Scholar] [CrossRef] [PubMed]
- Musso, D.; Ko, A.I.; Baud, D. Zika Virus Infection—After the Pandemic. N. Engl. J. Med. 2019, 381, 1444–1457. [Google Scholar] [CrossRef] [PubMed]
- Rawal, G.; Yadav, S.; Kumar, R. Zika Virus: An Overview. J. Fam. Med. Prim. Care 2016, 5, 523. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, R.; Oo, H.H.; Balne, P.K.; Ng, L.; Tong, L.; Leo, Y.S. Zika Virus and the Eye. Ocul. Immunol. Inflamm. 2018, 26, 654–659. [Google Scholar] [CrossRef] [PubMed]
- Martins, M.M.; Medronho, R.D.A.; Cunha, A.J.L.A.D. Zika Virus in Brazil and Worldwide: A Narrative Review. Paediatr. Int. Child Health 2021, 41, 28–35. [Google Scholar] [CrossRef]
- Wimalasiri-Yapa, B.M.C.R.; Yapa, H.E.; Huang, X.; Hafner, L.M.; Kenna, T.J.; Frentiu, F.D. Zika Virus and Arthritis/Arthralgia: A Systematic Review and Meta-Analysis. Viruses 2020, 12, 1137. [Google Scholar] [CrossRef]
- Krauer, F.; Riesen, M.; Reveiz, L.; Oladapo, O.T.; Martínez-Vega, R.; Porgo, T.V.; Haefliger, A.; Broutet, N.J.; Low, N.; WHO Zika Causality Working Group. Zika Virus Infection as a Cause of Congenital Brain Abnormalities and Guillain–Barré Syndrome: Systematic Review. PLoS Med. 2017, 14, e1002203. [Google Scholar] [CrossRef]
- Lobkowicz, L.; Ramond, A.; Sanchez Clemente, N.; Ximenes, R.A.D.A.; Miranda-Filho, D.D.B.; Montarroyos, U.R.; Martelli, C.M.T.; De Araújo, T.V.B.; Brickley, E.B. The Frequency and Clinical Presentation of Zika Virus Coinfections: A Systematic Review. BMJ Glob. Health 2020, 5, e002350. [Google Scholar] [CrossRef]
- Beaver, J.T.; Lelutiu, N.; Habib, R.; Skountzou, I. Evolution of Two Major Zika Virus Lineages: Implications for Pathology, Immune Response, and Vaccine Development. Front. Immunol. 2018, 9, 1640. [Google Scholar] [CrossRef]
- Kazmi, S.S.; Ali, W.; Bibi, N.; Nouroz, F. A Review on Zika Virus Outbreak, Epidemiology, Transmission and Infection Dynamics. J. Biol. Res.-Thessalon. 2020, 27, 5. [Google Scholar] [CrossRef]
- Pettersson, J.H.-O.; Eldholm, V.; Seligman, S.J.; Lundkvist, Å.; Falconar, A.K.; Gaunt, M.W.; Musso, D.; Nougairède, A.; Charrel, R.; Gould, E.A.; et al. How Did Zika Virus Emerge in the Pacific Islands and Latin America? mBio 2016, 7, e01239-16. [Google Scholar] [CrossRef] [PubMed]
- Sheridan, M.A.; Balaraman, V.; Schust, D.J.; Ezashi, T.; Roberts, R.M.; Franz, A.W.E. African and Asian Strains of Zika Virus Differ in Their Ability to Infect and Lyse Primitive Human Placental Trophoblast. PLoS ONE 2018, 13, e0200086. [Google Scholar] [CrossRef] [PubMed]
- Sampaio, G.D.S.; Brites, C.; Drexler, J.F.; Moreira-Soto, A.; Miranda, F.; Martins, E. Expansão da circulação do vírus Zika da África à América, 1947-2018: Revisão da literatura*. Epidemiol. E Serviços Saúde 2019, 28, 1–23. [Google Scholar] [CrossRef]
- Ayres, C.F.J.; Guedes, D.R.D.; Paiva, M.H.S.; Morais-Sobral, M.C.; Krokovsky, L.; Machado, L.C.; Melo-Santos, M.A.V.; Crespo, M.; Oliveira, C.M.F.; Ribeiro, R.S.; et al. Zika Virus Detection, Isolation and Genome Sequencing through Culicidae Sampling during the Epidemic in Vitória, Espírito Santo, Brazil. Parasit. Vectors 2019, 12, 220. [Google Scholar] [CrossRef]
- White, M.K.; Wollebo, H.S.; David Beckham, J.; Tyler, K.L.; Khalili, K. Zika Virus: An Emergent Neuropathological Agent. Ann. Neurol. 2016, 80, 479–489. [Google Scholar] [CrossRef]
- Latanova, A.; Starodubova, E.; Karpov, V. Flaviviridae Nonstructural Proteins: The Role in Molecular Mechanisms of Triggering Inflammation. Viruses 2022, 14, 1808. [Google Scholar] [CrossRef]
- Nicholls, C.M.R.; Sevvana, M.; Kuhn, R.J. Structure-Guided Paradigm Shifts in Flavivirus Assembly and Maturation Mechanisms. In Advances in Virus Research; Elsevier: Amsterdam, The Netherlands, 2020; Volume 108, pp. 33–83. ISBN 978-0-12-820761-1. [Google Scholar]
- Miner, J.J.; Diamond, M.S. Zika Virus Pathogenesis and Tissue Tropism. Cell Host Microbe 2017, 21, 134–142. [Google Scholar] [CrossRef]
- Valdespino-Vázquez, M.Y.; Sevilla-Reyes, E.E.; Lira, R.; Yocupicio-Monroy, M.; Piten-Isidro, E.; Boukadida, C.; Hernández-Pando, R.; Soriano-Jimenez, J.D.; Herrera-Salazar, A.; Figueroa-Damián, R.; et al. Congenital Zika Syndrome and Extra-Central Nervous System Detection of Zika Virus in a Pre-Term Newborn in Mexico. Clin. Infect. Dis. 2019, 68, 903–912. [Google Scholar] [CrossRef]
- Tan, L.Y.; Komarasamy, T.V.; James, W.; Balasubramaniam, V.R.M.T. Host Molecules Regulating Neural Invasion of Zika Virus and Drug Repurposing Strategy. Front. Microbiol. 2022, 13, 743147. [Google Scholar] [CrossRef]
- Berglund, G.; Lennon, C.D.; Badu, P.; Berglund, J.A.; Pager, C.T. Zika Virus Infection in a Cell Culture Model Reflects the Transcriptomic Signatures in Patients. bioRxiv Prepr. Serv. Biol. 2024, 2024.05.25.595842. [Google Scholar] [CrossRef]
- De Sales-Neto, J.M.; Madruga Carvalho, D.C.; Arruda Magalhães, D.W.; Araujo Medeiros, A.B.; Soares, M.M.; Rodrigues-Mascarenhas, S. Zika Virus: Antiviral Immune Response, Inflammation, and Cardiotonic Steroids as Antiviral Agents. Int. Immunopharmacol. 2024, 127, 111368. [Google Scholar] [CrossRef] [PubMed]
- Serman, T.M.; Gack, M.U. Evasion of Innate and Intrinsic Antiviral Pathways by the Zika Virus. Viruses 2019, 11, 970. [Google Scholar] [CrossRef] [PubMed]
- Plociennikowska, A.; Frankish, J.; Moraes, T.; Del Prete, D.; Kahnt, F.; Acuna, C.; Slezak, M.; Binder, M.; Bartenschlager, R. TLR3 Activation by Zika Virus Stimulates Inflammatory Cytokine Production Which Dampens the Antiviral Response Induced by RIG-I-like Receptors. J. Virol. 2021, 95, e01050-20. [Google Scholar] [CrossRef] [PubMed]
- Komarasamy, T.V.; Adnan, N.A.A.; James, W.; Balasubramaniam, V.R.M.T. Zika Virus Neuropathogenesis: The Different Brain Cells, Host Factors and Mechanisms Involved. Front. Immunol. 2022, 13, 773191. [Google Scholar] [CrossRef]
- Lee, J.K.; Kim, J.-A.; Oh, S.-J.; Lee, E.-W.; Shin, O.S. Zika Virus Induces Tumor Necrosis Factor-Related Apoptosis Inducing Ligand (TRAIL)-Mediated Apoptosis in Human Neural Progenitor Cells. Cells 2020, 9, 2487. [Google Scholar] [CrossRef]
- Beys-da-Silva, W.O.; Rosa, R.L.; Santi, L.; Berger, M.; Park, S.K.; Campos, A.R.; Terraciano, P.; Varela, A.P.M.; Teixeira, T.F.; Roehe, P.M.; et al. Zika Virus Infection of Human Mesenchymal Stem Cells Promotes Differential Expression of Proteins Linked to Several Neurological Diseases. Mol. Neurobiol. 2019, 56, 4708–4717. [Google Scholar] [CrossRef]
- Mufrrih, M.; Chen, B.; Chan, S.-W. Zika Virus Induces an Atypical Tripartite Unfolded Protein Response with Sustained Sensor and Transient Effector Activation and a Blunted BiP Response. mSphere 2021, 6, e00361-21. [Google Scholar] [CrossRef]
- Hoffmann, H.-H.; Schneider, W.M.; Blomen, V.A.; Scull, M.A.; Hovnanian, A.; Brummelkamp, T.R.; Rice, C.M. Diverse Viruses Require the Calcium Transporter SPCA1 for Maturation and Spread. Cell Host Microbe 2017, 22, 460–470.e5. [Google Scholar] [CrossRef]
- Chen, X.; Yan, Y.; Liu, Z.; Yang, S.; Li, W.; Wang, Z.; Wang, M.; Guo, J.; Li, Z.; Zhu, W.; et al. In Vitro and in Vivo Inhibition of the Host TRPC4 Channel Attenuates Zika Virus Infection. EMBO Mol. Med. 2024, 16, 1817–1839. [Google Scholar] [CrossRef]
- Helms, H.C.; Abbott, N.J.; Burek, M.; Cecchelli, R.; Couraud, P.-O.; Deli, M.A.; Förster, C.; Galla, H.J.; Romero, I.A.; Shusta, E.V.; et al. In Vitro Models of the Blood–Brain Barrier: An Overview of Commonly Used Brain Endothelial Cell Culture Models and Guidelines for Their Use. J. Cereb. Blood Flow Metab. 2016, 36, 862–890. [Google Scholar] [CrossRef]
- Ayala-Nunez, N.V.; Gaudin, R. A Viral Journey to the Brain: Current Considerations and Future Developments. PLoS Pathog. 2020, 16, e1008434. [Google Scholar] [CrossRef] [PubMed]
- Vhp, L.; Aragão, M.; Pinho, R.; Hazin, A.; Paciorkowski, A.; Penalva De Oliveira, A.; Masruha, M.R. Congenital Zika Virus Infection: A Review with Emphasis on the Spectrum of Brain Abnormalities. Curr. Neurol. Neurosci. Rep. 2020, 20, 49. [Google Scholar] [CrossRef] [PubMed]
- Ades, A.E.; Soriano-Arandes, A.; Alarcon, A.; Bonfante, F.; Thorne, C.; Peckham, C.S.; Giaquinto, C. Vertical Transmission of Zika Virus and Its Outcomes: A Bayesian Synthesis of Prospective Studies. Lancet Infect. Dis. 2021, 21, 537–545. [Google Scholar] [CrossRef] [PubMed]
- Shapiro-Mendoza, C.K.; Rice, M.E.; Galang, R.R.; Fulton, A.C.; VanMaldeghem, K.; Prado, M.V.; Ellis, E.; Anesi, M.S.; Simeone, R.M.; Petersen, E.E.; et al. Pregnancy Outcomes After Maternal Zika Virus Infection During Pregnancy—U.S. Territories, January 1, 2016–April 25, 2017. MMWR Morb. Mortal. Wkly. Rep. 2017, 66, 615–621. [Google Scholar] [CrossRef]
- Coutinho, C.; Negrini, S.; Araujo, D.; Teixeira, S.; Amaral, F.; Moro, M.; Fernandes, J.; Da Motta, M.; Negrini, B.; Caldas, C.; et al. Early Maternal Zika Infection Predicts Severe Neonatal Neurological Damage: Results from the Prospective Natural History of Zika Virus Infection in Gestation Cohort Study. BJOG Int. J. Obstet. Gynaecol. 2021, 128, 317–326. [Google Scholar] [CrossRef]
- Cunha, A.J.L.A.D.; De Magalhães-Barbosa, M.C.; Lima-Setta, F.; Medronho, R.D.A.; Prata-Barbosa, A. Microcephaly Case Fatality Rate Associated with Zika Virus Infection in Brazil: Current Estimates. Pediatr. Infect. Dis. J. 2017, 36, 528–530. [Google Scholar] [CrossRef]
- Antoniou, E.; Orovou, E.; Andronikidi, P.E.; Orovas, C.; Rigas, N.; Palaska, E.; Sarella, A.; Iatrakis, G.; Voyiatzaki, C. Congenital Zika Infection and the Risk of Neurodevelopmental, Neurological, and Urinary Track Disorders in Early Childhood. A Systematic Review. Viruses 2021, 13, 1671. [Google Scholar] [CrossRef]
- Pereira, H.V.F.S.; Dos Santos, S.P.; Amâncio, A.P.R.L.; De Oliveira-Szejnfeld, P.S.; Flor, E.O.; De Sales Tavares, J.; Ferreira, R.V.B.; Tovar-Moll, F.; De Amorim, M.M.R.; Melo, A. Neurological Outcomes of Congenital Zika Syndrome in Toddlers and Preschoolers: A Case Series. Lancet Child Adolesc. Health 2020, 4, 378–387. [Google Scholar] [CrossRef]
- Cicuto Ferreira Rocha, N.A.; De Campos, A.C.; Cicuto Ferreira Rocha, F.; Pereira Dos Santos Silva, F. Microcephaly and Zika Virus: Neuroradiological Aspects, Clinical Findings and a Proposed Framework for Early Evaluation of Child Development. Infant Behav. Dev. 2017, 49, 70–82. [Google Scholar] [CrossRef]
- Coffey, L.L.; Keesler, R.I.; Pesavento, P.A.; Woolard, K.; Singapuri, A.; Watanabe, J.; Cruzen, C.; Christe, K.L.; Usachenko, J.; Yee, J.; et al. Intraamniotic Zika Virus Inoculation of Pregnant Rhesus Macaques Produces Fetal Neurologic Disease. Nat. Commun. 2018, 9, 2414. [Google Scholar] [CrossRef]
- Janssens, S.; Schotsaert, M.; Karnik, R.; Balasubramaniam, V.; Dejosez, M.; Meissner, A.; García-Sastre, A.; Zwaka, T.P. Zika Virus Alters DNA Methylation of Neural Genes in an Organoid Model of the Developing Human Brain. mSystems 2018, 3, e00219-17. [Google Scholar] [CrossRef] [PubMed]
- Halani, S.; Tombindo, P.E.; O’Reilly, R.; Miranda, R.N.; Erdman, L.K.; Whitehead, C.; Bielecki, J.M.; Ramsay, L.; Ximenes, R.; Boyle, J.; et al. Clinical Manifestations and Health Outcomes Associated with Zika Virus Infections in Adults: A Systematic Review. PLoS Negl. Trop. Dis. 2021, 15, e0009516. [Google Scholar] [CrossRef] [PubMed]
- Filgueiras, I.S.; Torrentes De Carvalho, A.; Cunha, D.P.; Mathias Da Fonseca, D.L.; El Khawanky, N.; Freire, P.P.; Cabral-Miranda, G.; Schimke, L.F.; Camara, N.O.S.; Ochs, H.D.; et al. The Clinical Spectrum and Immunopathological Mechanisms Underlying ZIKV-Induced Neurological Manifestations. PLoS Negl. Trop. Dis. 2021, 15, e0009575. [Google Scholar] [CrossRef]
- Muñoz, L.S.; Parra, B.; Pardo, C.A. Neuroviruses Emerging in the Americas Study Neurological Implications of Zika Virus Infection in Adults. J. Infect. Dis. 2017, 216, S897–S905. [Google Scholar] [CrossRef]
- Robbiani, D.F.; Olsen, P.C.; Costa, F.; Wang, Q.; Oliveira, T.Y.; Nery, N.; Aromolaran, A.; Do Rosário, M.S.; Sacramento, G.A.; Cruz, J.S.; et al. Risk of Zika Microcephaly Correlates with Features of Maternal Antibodies. J. Exp. Med. 2019, 216, 2302–2315. [Google Scholar] [CrossRef] [PubMed]
- Charniga, K.; Cucunubá, Z.M.; Walteros, D.M.; Mercado, M.; Prieto, F.; Ospina, M.; Nouvellet, P.; Donnelly, C.A. Descriptive Analysis of Surveillance Data for Zika Virus Disease and Zika Virus-Associated Neurological Complications in Colombia, 2015–2017. PLoS ONE 2021, 16, e0252236. [Google Scholar] [CrossRef]
- Akrami, K.M.; de Nogueira, B.M.F.; do Rosário, M.S.; de Moraes, L.; Cordeiro, M.T.; Haddad, R.; Gomes, L.N.; de Pádua Carvalho, I.; Dos Reis Pimentel, E.; de Jesus Silva, J.; et al. The Re-Emergence of Zika in Brazil in 2020: A Case of Guillain Barré Syndrome during the Low Season for Arboviral Infections. J. Travel Med. 2020, 27, taaa165. [Google Scholar] [CrossRef]
- Berry, N.; Ferguson, D.; Ham, C.; Hall, J.; Jenkins, A.; Giles, E.; Devshi, D.; Kempster, S.; Rose, N.; Dowall, S.; et al. High Susceptibility, Viral Dynamics and Persistence of South American Zika Virus in New World Monkey Species. Sci. Rep. 2019, 9, 14495. [Google Scholar] [CrossRef]
- Pantoja, P.; Pérez-Guzmán, E.X.; Rodríguez, I.V.; White, L.J.; González, O.; Serrano, C.; Giavedoni, L.; Hodara, V.; Cruz, L.; Arana, T.; et al. Zika Virus Pathogenesis in Rhesus Macaques Is Unaffected by Pre-Existing Immunity to Dengue Virus. Nat. Commun. 2017, 8, 15674. [Google Scholar] [CrossRef]
- Oliveira, R.A.; De Oliveira-Filho, E.F.; Fernandes, A.I.; Brito, C.A.; Marques, E.T.; Tenório, M.C.; Gil, L.H. Previous Dengue or Zika Virus Exposure Can Drive to Infection Enhancement or Neutralisation of Other Flaviviruses. Mem. Inst. Oswaldo Cruz 2019, 114, e190098. [Google Scholar] [CrossRef]
- Thomas, S.; Smatti, M.K.; Ouhtit, A.; Cyprian, F.S.; Almaslamani, M.A.; Thani, A.A.; Yassine, H.M. Antibody-Dependent Enhancement (ADE) and the Role of Complement System in Disease Pathogenesis. Mol. Immunol. 2022, 152, 172–182. [Google Scholar] [CrossRef] [PubMed]
- Masel, J.; McCracken, M.K.; Gleeson, T.; Morrison, B.; Rutherford, G.; Imrie, A.; Jarman, R.G.; Koren, M.; Pollett, S. Does Prior Dengue Virus Exposure Worsen Clinical Outcomes of Zika Virus Infection? A Systematic Review, Pooled Analysis and Lessons Learned. PLoS Negl. Trop. Dis. 2019, 13, e0007060. [Google Scholar] [CrossRef] [PubMed]
- Tonnerre, P.; Melgaço, J.G.; Torres-Cornejo, A.; Pinto, M.A.; Yue, C.; Blümel, J.; De Sousa, P.S.F.; De Mello, V.D.M.; Moran, J.; De Filippis, A.M.B.; et al. Evolution of the Innate and Adaptive Immune Response in Women with Acute Zika Virus Infection. Nat. Microbiol. 2019, 5, 76–83. [Google Scholar] [CrossRef]
- Estofolete, C.F.; Terzian, A.C.B.; Colombo, T.E.; De Freitas Guimarães, G.; Ferraz, H.C.; Da Silva, R.A.; Greque, G.V.; Nogueira, M.L. Co-Infection between Zika and Different Dengue Serotypes during DENV Outbreak in Brazil. J. Infect. Public Health 2019, 12, 178–181. [Google Scholar] [CrossRef] [PubMed]
- Zambrana, J.V.; Hasund, C.M.; Aogo, R.A.; Bos, S.; Arguello, S.; Gonzalez, K.; Collado, D.; Miranda, T.; Kuan, G.; Gordon, A.; et al. Primary Exposure to Zika Virus Is Linked with Increased Risk of Symptomatic Dengue Virus Infection with Serotypes 2, 3, and 4, but Not 1. Sci. Transl. Med. 2024, 16, eadn2199. [Google Scholar] [CrossRef]
- Breitbach, M.E.; Newman, C.M.; Dudley, D.M.; Stewart, L.M.; Aliota, M.T.; Koenig, M.R.; Shepherd, P.M.; Yamamoto, K.; Crooks, C.M.; Young, G.; et al. Primary Infection with Dengue or Zika Virus Does Not Affect the Severity of Heterologous Secondary Infection in Macaques. PLOS Pathog. 2019, 15, e1007766. [Google Scholar] [CrossRef]
- Hassert, M.; Scroggins, S.; Coleman, A.K.; Shacham, E.; Brien, J.D.; Pinto, A.K. Heterologous Flavivirus Exposure Provides Varying Degrees of Cross-Protection from Zika Virus in a Mouse Model of Infection. bioRxiv 2020. [Google Scholar] [CrossRef]
- Ahmed, S.; Sultana, S.; Kundu, S.; Alam, S.S.; Hossan, T.; Islam, M.A. Global Prevalence of Zika and Chikungunya Coinfection: A Systematic Review and Meta-Analysis. Diseases 2024, 12, 31. [Google Scholar] [CrossRef]
- Wu, H.; Zhou, H.-Y.; Zheng, H.; Wu, A. Towards Understanding and Identification of Human Viral Co-Infections. Viruses 2024, 16, 673. [Google Scholar] [CrossRef]
- Cherabuddi, K.; Iovine, N.M.; Shah, K.; White, S.K.; Paisie, T.; Salemi, M.; Morris, J.G.; Lednicky, J.A. Zika and Chikungunya Virus Co-Infection in a Traveller Returning from Colombia, 2016: Virus Isolation and Genetic Analysis. JMM Case Rep. 2016, 3, e005072. [Google Scholar] [CrossRef]
- Alves, R.P.D.S.; Amorim, J.H. Editorial: Arboviruses: Co-Circulation, Co-Transmission, and Co-Infection. Front. Microbiol. 2023, 14, 1321166. [Google Scholar] [CrossRef] [PubMed]
- Rothan, H.A.; Bidokhti, M.R.M.; Byrareddy, S.N. Current Concerns and Perspectives on Zika Virus Co-Infection with Arboviruses and HIV. J. Autoimmun. 2018, 89, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Zhang, M.; Wang, G.; Zhang, D.; Zheng, X.; Li, Y. Biased Virus Transmission Following Sequential Coinfection of Aedes Aegypti with Dengue and Zika Viruses. PLoS Negl. Trop. Dis. 2024, 18, e0012053. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.C.-D.; Weng, S.-C.; Tsao, P.-N.; Chu, J.J.H.; Shiao, S.-H. Co-Infection of Dengue and Zika Viruses Mutually Enhances Viral Replication in the Mosquito Aedes Aegypti. Parasit. Vectors 2023, 16, 160. [Google Scholar] [CrossRef]
- Brustolin, M.; Pujhari, S.; Terradas, G.; Werling, K.; Asad, S.; Metz, H.C.; Henderson, C.A.; Kim, D.; Rasgon, J.L. In Vitro and In Vivo Coinfection and Superinfection Dynamics of Mayaro and Zika Viruses in Mosquito and Vertebrate Backgrounds. J. Virol. 2023, 97, e01778-22. [Google Scholar] [CrossRef]
- Frota, C.C.; Correia, F.G.S.; Alves Vasconcelos, L.R.; De Sousa, P.R.C.; Ferreira, M.L.D.S.; Saraiva, S.P.; Mota Ferreira, R.; Romcy, K.A.M.; Pinheiro, R.F.; De Oliveira, R.T.G.; et al. Positivity of Dengue, Chikungunya, and Zika Infections in Women in Northeast Brazil Post-Zika Epidemic. Pathog. Glob. Health 2023, 117, 485–492. [Google Scholar] [CrossRef]
- ELmojtaba, I.M.; Al-Maqrashi, K.; Al-Musalhi, F.; Al-Salti, N. Optimal Control and Cost Effectiveness Analysis of a Zika–Malaria Co-Infection Model. Partial Differ. Equ. Appl. Math. 2024, 11, 100754. [Google Scholar] [CrossRef]
- Fontenille, D.; Powell, J.R. From Anonymous to Public Enemy: How Does a Mosquito Become a Feared Arbovirus Vector? Pathogens 2020, 9, 265. [Google Scholar] [CrossRef]
- Kuno, G.; Mackenzie, J.; Junglen, S.; Hubálek, Z.; Plyusnin, A.; Gubler, D. Vertebrate Reservoirs of Arboviruses: Myth, Synonym of Amplifier, or Reality? Viruses 2017, 9, 185. [Google Scholar] [CrossRef]
- García-Romero, C.; Carrillo Bilbao, G.A.; Navarro, J.-C.; Martin-Solano, S.; Saegerman, C. Arboviruses in Mammals in the Neotropics: A Systematic Review to Strengthen Epidemiological Monitoring Strategies and Conservation Medicine. Viruses 2023, 15, 417. [Google Scholar] [CrossRef]
- Liang, G.; Gao, X.; Gould, E.A. Factors Responsible for the Emergence of Arboviruses; Strategies, Challenges and Limitations for Their Control. Emerg. Microbes Infect. 2015, 4, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Khongwichit, S.; Chuchaona, W.; Vongpunsawad, S.; Poovorawan, Y. Molecular Surveillance of Arboviruses Circulation and Co-Infection during a Large Chikungunya Virus Outbreak in Thailand, October 2018 to February 2020. Sci. Rep. 2022, 12, 22323. [Google Scholar] [CrossRef] [PubMed]
- Sardi, S.I.; Somasekar, S.; Naccache, S.N.; Bandeira, A.C.; Tauro, L.B.; Campos, G.S.; Chiu, C.Y. Coinfections of Zika and Chikungunya Viruses in Bahia, Brazil, Identified by Metagenomic Next-Generation Sequencing. J. Clin. Microbiol. 2016, 54, 2348–2353. [Google Scholar] [CrossRef]
- Calvet, G.A.; Filippis, A.M.B.; Mendonça, M.C.L.; Sequeira, P.C.; Siqueira, A.M.; Veloso, V.G.; Nogueira, R.M.; Brasil, P. First Detection of Autochthonous Zika Virus Transmission in a HIV-Infected Patient in Rio de Janeiro, Brazil. J. Clin. Virol. Off. Publ. Pan Am. Soc. Clin. Virol. 2016, 74, 1–3. [Google Scholar] [CrossRef]
- de Araújo, P.S.R.; de Mélo Silva, M.L., Jr.; Tenório, M.; Santos, F.G.T.D. Co-Infection ZIKV and HSV-1 Associated with Meningoencephalitis: Case Report and Literature Review. J. Infect. Public Health 2019, 12, 97–100. [Google Scholar] [CrossRef]
- Martins-Luna, J.; Del Valle-Mendoza, J.; Silva-Caso, W.; Sandoval, I.; Del Valle, L.J.; Palomares-Reyes, C.; Carrillo-Ng, H.; Peña-Tuesta, I.; Aguilar-Luis, M.A. Oropouche Infection a Neglected Arbovirus in Patients with Acute Febrile Illness from the Peruvian Coast. BMC Res. Notes 2020, 13, 67. [Google Scholar] [CrossRef]
- De Souza Costa, M.C.; Siqueira Maia, L.M.; Costa De Souza, V.; Gonzaga, A.M.; Correa De Azevedo, V.; Ramos Martins, L.; Chavez Pavoni, J.H.; Gomes Naveca, F.; Dezengrini Slhessarenko, R. Arbovirus Investigation in Patients from Mato Grosso during Zika and Chikungunya Virus Introdution in Brazil, 2015–2016. Acta Trop. 2019, 190, 395–402. [Google Scholar] [CrossRef]
- Morais, V.D.S.; Reis Santana, L.M.; Bezerra, J.F.; Cruz, F.E.; Rocha De Souza, T.; Tahmasebi, R.; Alves Raposo, R.A.; Marcatti, R.; Garcia Barbosa, E.M.; Hefford, P.M.; et al. Detection of Coinfection with Primate Erythroparvovirus 1 and Arboviruses (DENV, CHIKV and ZIKV) in Individuals with Acute Febrile Illness in the State of Rio Grande Do Norte in 2016. PLoS Negl. Trop. Dis. 2023, 17, e0011701. [Google Scholar] [CrossRef]
- Gunturiz, M.L.; Cortés, L.; Cuevas, E.L.; Chaparro, P.; Ospina, M.L. Toxoplasmosis cerebral congénita e infección por el virus del Zika y del chikunguña: Reporte de un caso. Biomédica 2018, 38, 144–152. [Google Scholar] [CrossRef]
- Alves, L.S.; Estanislau, C.; Barreto, L.; Batista, F.; Toppa, N. Concomitant Testicular Infection by Zika Virus and Schistosoma Mansoni in a Brazilian Young Boy. Rev. Assoc. Médica Bras. 2017, 63, 500–503. [Google Scholar] [CrossRef]
- Otu, A.A.; Udoh, U.A.; Ita, O.I.; Hicks, J.P.; Ukpeh, I.; Walley, J. Prevalence of Zika and Malaria in Patients with Fever in Secondary Healthcare Facilities in South-Eastern Nigeria. Trop. Doct. 2020, 50, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Neaterour, P.; Rivera, A.; Galloway, R.L.; Negrón, M.G.; Rivera-Garcia, B.; Sharp, T.M. Fatal Leptospira Spp./Zika Virus Coinfection-Puerto Rico, 2016. Am. J. Trop. Med. Hyg. 2017, 97, 1085–1087. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.J.; Othman, M.S.; Hiu, J.; Wong, K.T.; Lai, S.K. Idiopathic Ileal Volvulus with Multiple Concomitant Infections in a Starving Man. Malays. J. Pathol. 2021, 43, 81–85. [Google Scholar]
- Rückert, C.; Weger-Lucarelli, J.; Garcia-Luna, S.M.; Young, M.C.; Byas, A.D.; Murrieta, R.A.; Fauver, J.R.; Ebel, G.D. Impact of Simultaneous Exposure to Arboviruses on Infection and Transmission by Aedes Aegypti Mosquitoes. Nat. Commun. 2017, 8, 15412. [Google Scholar] [CrossRef]
- Villamil-Gómez, W.E.; Rodríguez-Morales, A.J.; Uribe-García, A.M.; González-Arismendy, E.; Castellanos, J.E.; Calvo, E.P.; Álvarez-Mon, M.; Musso, D. Zika, Dengue, and Chikungunya Co-Infection in a Pregnant Woman from Colombia. Int. J. Infect. Dis. IJID Off. Publ. Int. Soc. Infect. Dis. 2016, 51, 135–138. [Google Scholar] [CrossRef]
- Iovine, N.M.; Lednicky, J.; Cherabuddi, K.; Crooke, H.; White, S.K.; Loeb, J.C.; Cella, E.; Ciccozzi, M.; Salemi, M.; Morris, J.G. Coinfection With Zika and Dengue-2 Viruses in a Traveler Returning from Haiti, 2016: Clinical Presentation and Genetic Analysis. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2017, 64, 72–75. [Google Scholar] [CrossRef]
- Siqueira, C.; Féres, V.; Coutinho, L.; Junqueira, I.; Bento, L.; Montes, L.; Siqueira, J.B. Six Cases of Zika/Dengue Coinfection in a Brazilian Cohort, 2015–2019. Viruses 2020, 12, 1201. [Google Scholar] [CrossRef]
- Ngwe Tun, M.M.; Raini, S.K.; Fernando, L.; Gunawardene, Y.; Inoue, S.; Takamatsu, Y.; Urano, T.; Muthugala, R.; Hapugoda, M.; Morita, K. Epidemiological Evidence of Acute Transmission of Zika Virus Infection in Dengue Suspected Patients in Sri-Lanka. J. Infect. Public Health 2023, 16, 1435–1442. [Google Scholar] [CrossRef]
- Mapalagamage, M.; Weiskopf, D.; Sette, A.; De Silva, A.D. Current Understanding of the Role of T Cells in Chikungunya, Dengue and Zika Infections. Viruses 2022, 14, 242. [Google Scholar] [CrossRef]
- Valiant, W.G.; Mattapallil, M.J.; Higgs, S.; Huang, Y.-J.S.; Vanlandingham, D.L.; Lewis, M.G.; Mattapallil, J.J. Simultaneous Coinfection of Macaques with Zika and Dengue Viruses Does Not Enhance Acute Plasma Viremia but Leads to Activation of Monocyte Subsets and Biphasic Release of Pro-Inflammatory Cytokines. Sci. Rep. 2019, 9, 7877. [Google Scholar] [CrossRef]
- Wichit, S.; Gumpangseth, N.; Hamel, R.; Yainoy, S.; Arikit, S.; Punsawad, C.; Missé, D. Chikungunya and Zika Viruses: Co-Circulation and the Interplay between Viral Proteins and Host Factors. Pathogens 2021, 10, 448. [Google Scholar] [CrossRef] [PubMed]
- Silva, K.R.; Bica, B.E.R.G.; Pimenta, E.S.; Serafim, R.B.; Abreu, M.M.; Gonçalves, J.L.S.; Santana, L.d.S.; Cabral-Castro, M.J.; Peralta, J.M.; Cavalcanti, M.G. Fatal Human Case of Zika and Chikungunya Virus Co-Infection with Prolonged Viremia and Viruria. Diseases 2018, 6, 53. [Google Scholar] [CrossRef] [PubMed]
- Prata-Barbosa, A.; Cleto-Yamane, T.L.; Robaina, J.R.; Guastavino, A.B.; De Magalhães-Barbosa, M.C.; Brindeiro, R.D.M.; Medronho, R.A.; Da Cunha, A.J.L.A. Co-Infection with Zika and Chikungunya Viruses Associated with Fetal Death—A Case Report. Int. J. Infect. Dis. 2018, 72, 25–27. [Google Scholar] [CrossRef]
- Brito, C.A.A.; Azevedo, F.; Cordeiro, M.T.; Marques, E.T.A.; Franca, R.F.O. Central and Peripheral Nervous System Involvement Caused by Zika and Chikungunya Coinfection. PLoS Negl. Trop. Dis. 2017, 11, e0005583. [Google Scholar] [CrossRef]
- Villamil-Gómez, W.E.; Sánchez-Herrera, Á.R.; Hernández-Prado, H.; Hernández-Iriarte, J.; Díaz-Ricardo, K.; Vergara-Serpa, O.; Castellanos, J.; Portela-Gaviria, J.E.; Patiño-Valencia, S.; Vargas-Bedoya, D.C.; et al. Zika Virus and HIV Co-Infection in Five Patients from Two Areas of Colombia. J. Formos. Med. Assoc. 2018, 117, 856–858. [Google Scholar] [CrossRef]
- Bidokhti, M.R.M.; Dutta, D.; Madduri, L.S.V.; Woollard, S.M.; Norgren, R.; Giavedoni, L.; Byrareddy, S.N. SIV/SHIV-Zika Co-Infection Does Not Alter Disease Pathogenesis in Adult Non-Pregnant Rhesus Macaque Model. PLoS Negl. Trop. Dis. 2018, 12, e0006811. [Google Scholar] [CrossRef]
- Torres, L.R.; Capobianco, L.R.P.L.; De Souza, A.A.A.; De Almeida Ribeiro, C.R.; Cascabulho, C.; Garzoni, L.R.; Portari, E.A.; Gardel, M.A.; Meuser-Batista, M.; De Paula, V.S.; et al. ZIKV Replication Is Differential in Explants and Cells of Human Placental Which Is Suppressed by HSV-2 Coinfection. Virology 2022, 570, 45–56. [Google Scholar] [CrossRef]
- Rosenstierne, M.W.; Schaltz-Buchholzer, F.; Bruzadelli, F.; Có, A.; Cardoso, P.; Jørgensen, C.S.; Michiels, J.; Heyndrickx, L.; Ariën, K.K.; Fischer, T.K.; et al. Zika Virus IgG in Infants with Microcephaly, Guinea-Bissau, 2016. Emerg. Infect. Dis. 2018, 24, 948–950. [Google Scholar] [CrossRef]
- Lichs, G.G.C.; Fernandez, Z.D.C.; Nascimento, V.A.D.; Alcantara, D.M.C.; Lemos, E.F.; Carvalho, C.M.E.; Demarchi, L.H.F.; Gonçalves, C.C.M.; Naveca, F.G.; Favacho, A.R.D.M. Surveillance of Erythrovirus B19 (B19V) in Patients with Acute Febrile Illness Suspected of Arboviruses in Mato Grosso Do Sul State, Brazil. Front. Microbiol. 2024, 15, 1417434. [Google Scholar] [CrossRef]
- Fantinato, F.F.S.T.; Araújo, E.L.L.; Ribeiro, I.G.; Andrade, M.R.D.; Dantas, A.L.D.M.; Rios, J.M.T.; Silva, O.M.V.D.; Silva, M.D.S.D.; Nóbrega, R.V.; Batista, D.D.A.; et al. Descrição Dos Primeiros Casos de Febre Pelo Vírus Zika Investigados Em Municípios Da Região Nordeste Do Brasil, 2015. Epidemiol. E Serviços Saúde 2016, 25, 683–690. [Google Scholar] [CrossRef]
- Grayo, S. Is the ZIKV Congenital Syndrome and Microcephaly Due to Syndemism with Latent Virus Coinfection? Viruses 2021, 13, 669. [Google Scholar] [CrossRef] [PubMed]
- Vouga, M.; Baud, D.; Jolivet, E.; Najioullah, F.; Monthieux, A.; Schaub, B. Congenital Zika Virus Syndrome… what Else? Two Case Reports of Severe Combined Fetal Pathologies. BMC Pregnancy Childbirth 2018, 18, 356. [Google Scholar] [CrossRef] [PubMed]
- Nunez-Avellaneda, D.; Villagómez, F.R.; Villegas-Pineda, J.C.; Barrios-Palacios, J.; Salazar, M.I.; Machain-Williams, C.; Blitvich, B.J. Evidence of Coinfections between SARS-CoV-2 and Select Arboviruses in Guerrero, Mexico, 2020–2021. Am. J. Trop. Med. Hyg. 2022, 106, 896–899. [Google Scholar] [CrossRef]
- Silva, S.J.R.D.; Magalhães, J.J.F.D.; Pena, L. Simultaneous Circulation of DENV, CHIKV, ZIKV and SARS-CoV-2 in Brazil: An Inconvenient Truth. One Health 2021, 12, 100205. [Google Scholar] [CrossRef]
- Biron, A.; Cazorla, C.; Amar, J.; Pfannstiel, A.; Dupont-Rouzeyrol, M.; Goarant, C. Zika Virus Infection as an Unexpected Finding in a Leptospirosis Patient. JMM Case Rep. 2016, 3, e005033. [Google Scholar] [CrossRef]
| Sample/ZIKV Detection Method | Co-Infecting Pathogen (CP) | Sample/Detection Method of the CP | References |
|---|---|---|---|
| Serum/RT-qPCR | DENV-1 and DENV-2 | Serum/RT qPCR | [65] |
| Serum/RT-qPCR | DENV-3 and DENV-4 | Serum/RT qPCR | [83] |
| Serum/mNGS and RT qPCR | CHIKV | Serum/mNGS and RT PCR | [84] |
| Serum/RT-PCR and sequencing | HIV | NI * | [85] |
| Serum/RT-PCR | HSV-1 | Serum/RT-qPCR | [86] |
| Brain, thymus, lungs, kidneys, adrenal glands, and spleen/RT-qPCR | EBV | Brain and Liver/qPCR | [29] |
| HHV-6 | Thymus, kidneys, adrenal glands, and liver/qPCR | ||
| Serum/RT-qPCR | OROV | Serum/RT-PCR | [87] |
| Serum/RT-PCR | MAYV | Serum/RT-PCR and Nested PCR | [88] |
| Serum/RT-qPCR | B19V | Serum/RT-qPCR | [89] |
| Amniotic fluid/RT-qPCR | Toxoplasma gondii | Amniotic fluid/PCR | [90] |
| Blood/RT-PCR | Schistosoma mansoni | Testicle/biopsy | [91] |
| Serum/qualitative lateral flow immuno-chromographic cassettes for IgM and IgG | Plasmodium spp. | Blood/Microscopy of EDTA blood sample pieces | [92] |
| Serum/RT-PCR | Leptospira spp. | Serum/PCR | [93] |
| Blood, liver, lung, and tissue/RT PCR | Candida tropicalis | Blood, pus, peritoneal and pericardial fluid/NI * | [94] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santana, L.M.B.; de Moura, I.A.; Mouzinho Ramos Tanaka, Y.; França, R.F.d.O. Zika Virus: A Review of Biology, Clinical Impacts, and Coinfections. Viruses 2025, 17, 637. https://doi.org/10.3390/v17050637
Santana LMB, de Moura IA, Mouzinho Ramos Tanaka Y, França RFdO. Zika Virus: A Review of Biology, Clinical Impacts, and Coinfections. Viruses. 2025; 17(5):637. https://doi.org/10.3390/v17050637
Chicago/Turabian StyleSantana, Lucas Matheus Barreto, Ingrid Andrêssa de Moura, Yuri Mouzinho Ramos Tanaka, and Rafael Freitas de Oliveira França. 2025. "Zika Virus: A Review of Biology, Clinical Impacts, and Coinfections" Viruses 17, no. 5: 637. https://doi.org/10.3390/v17050637
APA StyleSantana, L. M. B., de Moura, I. A., Mouzinho Ramos Tanaka, Y., & França, R. F. d. O. (2025). Zika Virus: A Review of Biology, Clinical Impacts, and Coinfections. Viruses, 17(5), 637. https://doi.org/10.3390/v17050637








