Molecular Epidemiology of Travel-Associated and Locally Acquired Dengue Virus Infections in Catalonia, Spain, 2019
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Viral Genome Detection, Dengue Serotyping and Sequencing
2.3. Phylogenetic Analysis
2.4. Data Analysis and Graphs
3. Results
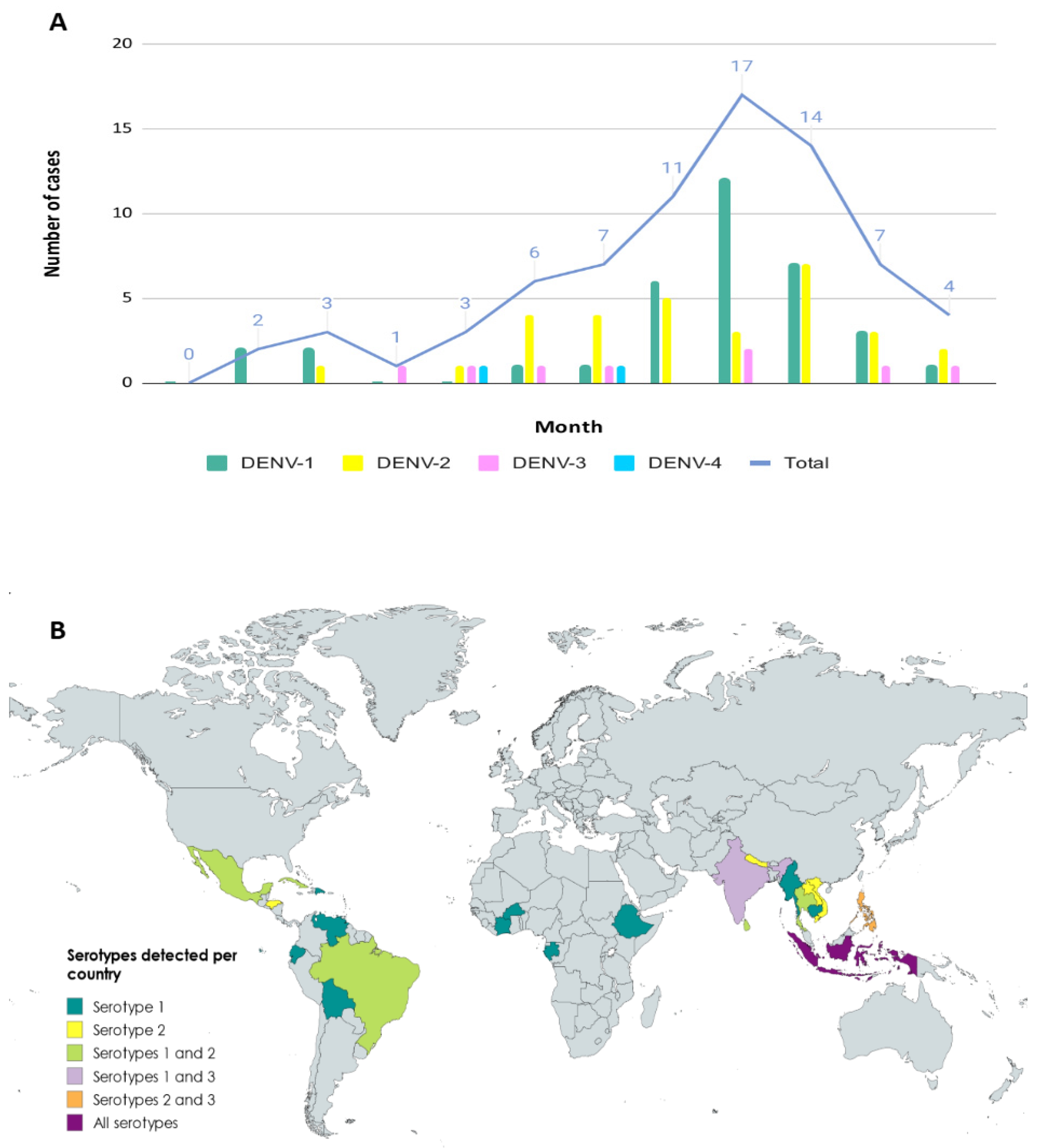
3.1. Epidemiology of Imported Cases
3.2. Serotyping and Genotyping of Imported Cases
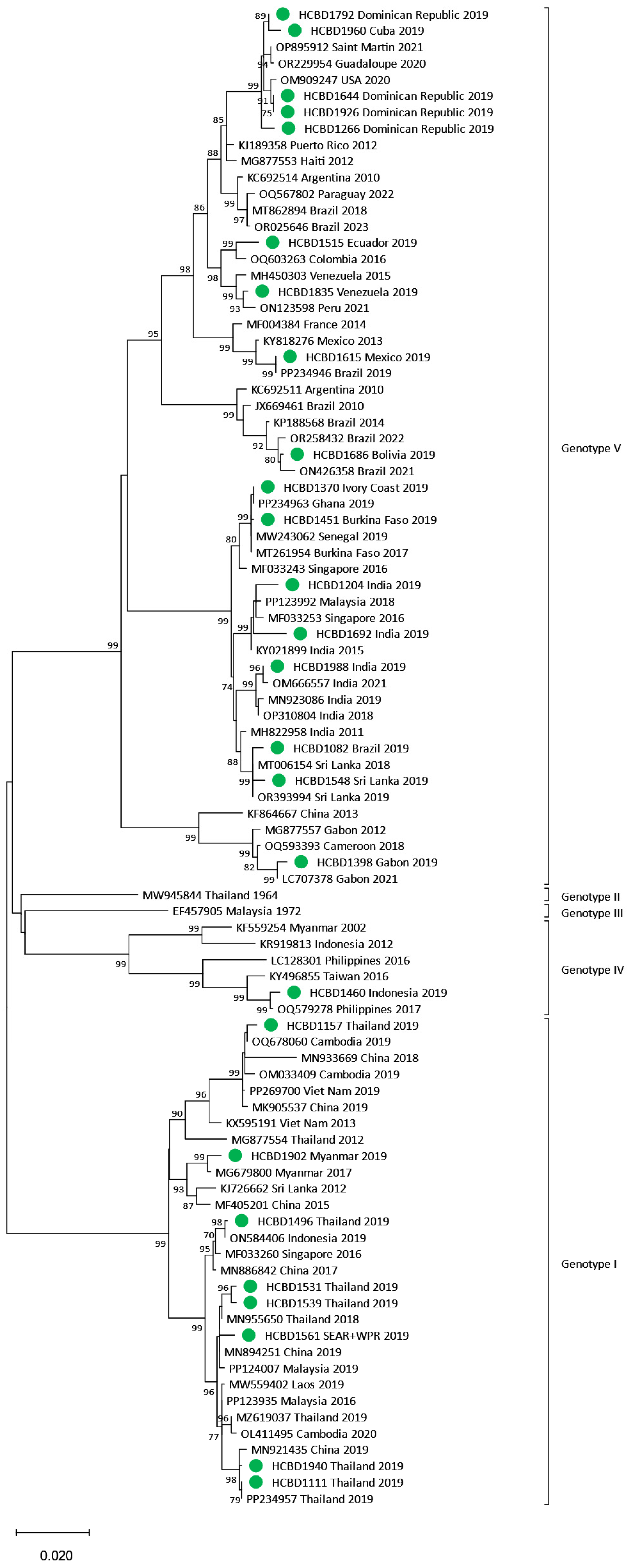
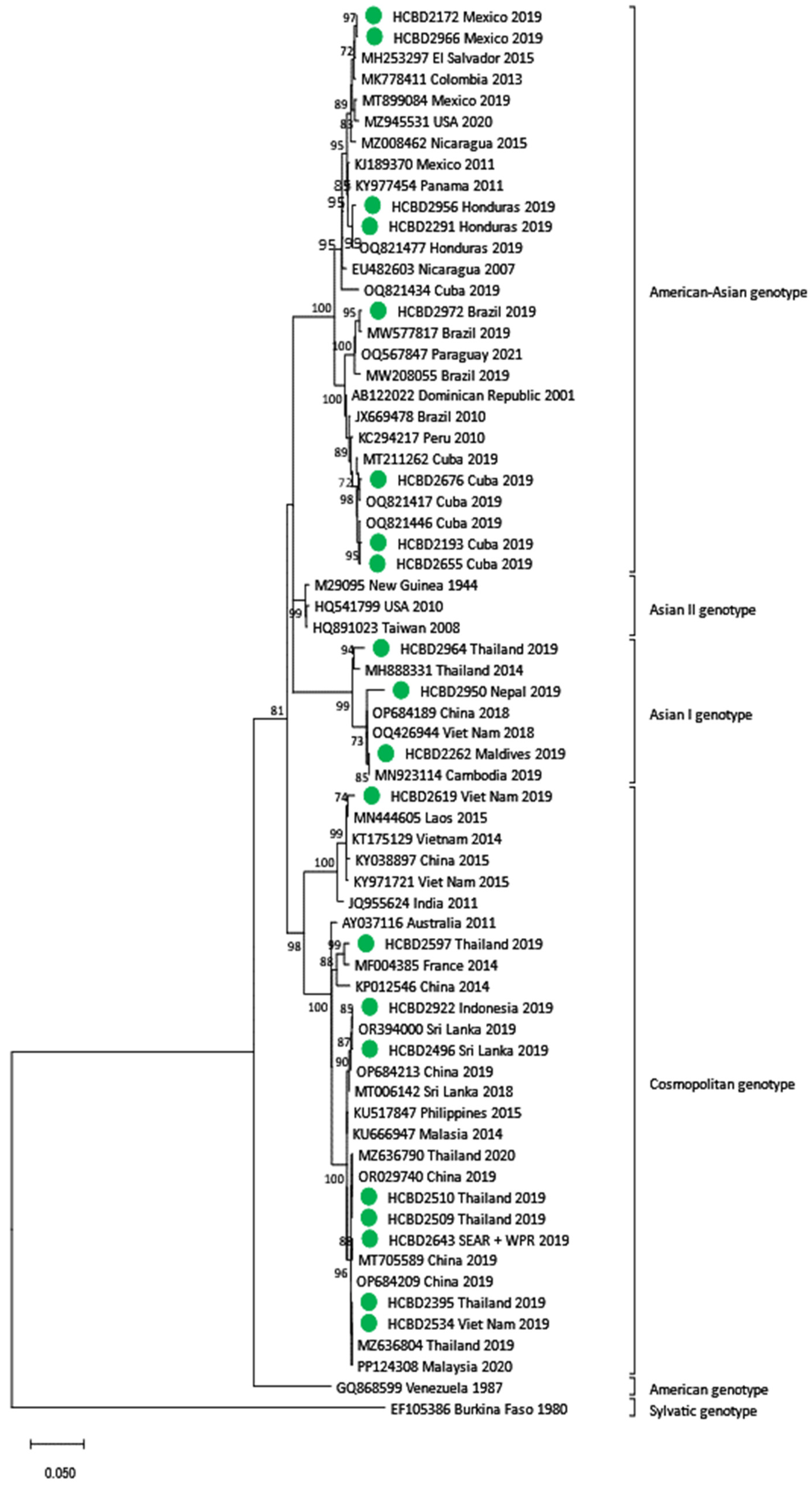
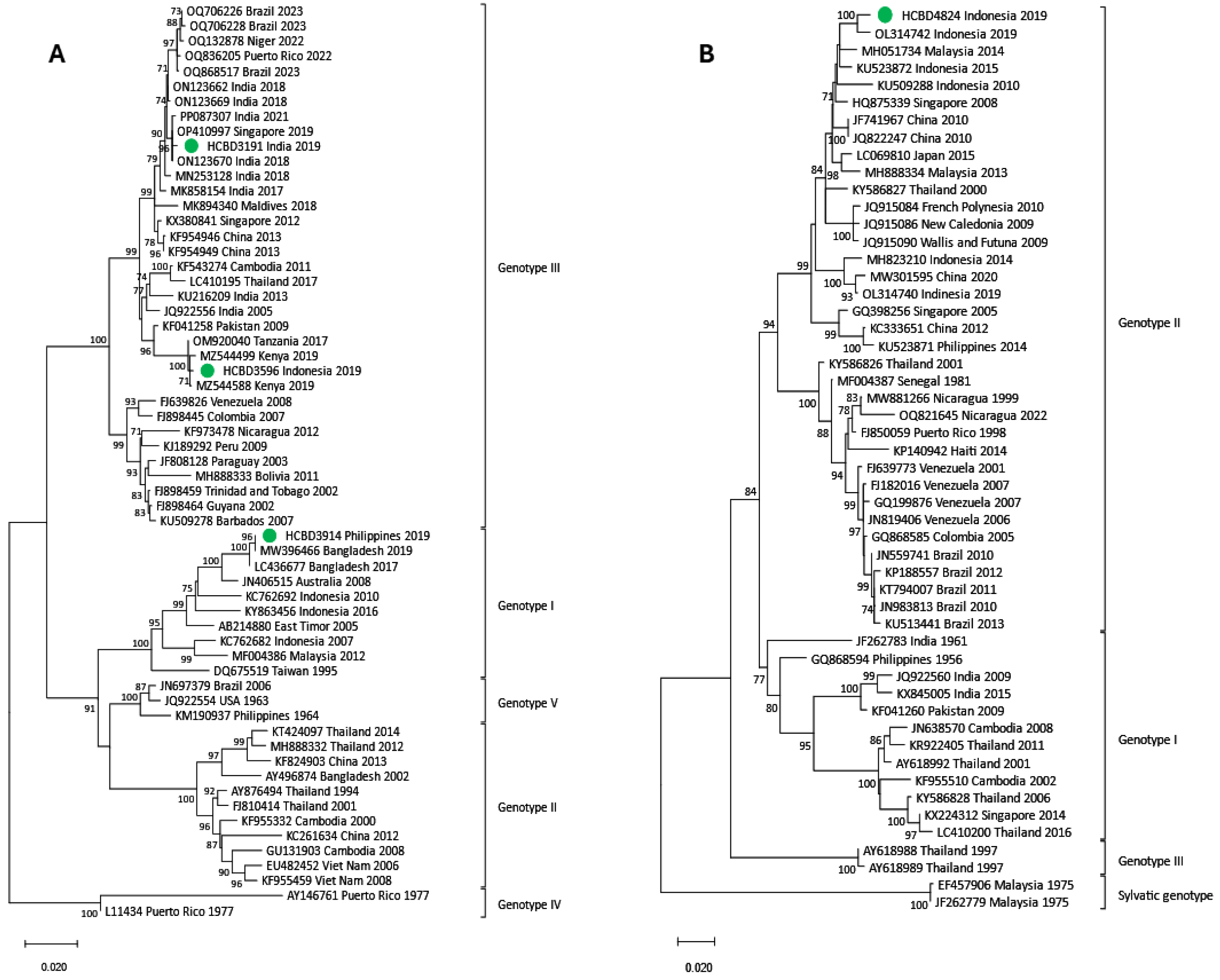
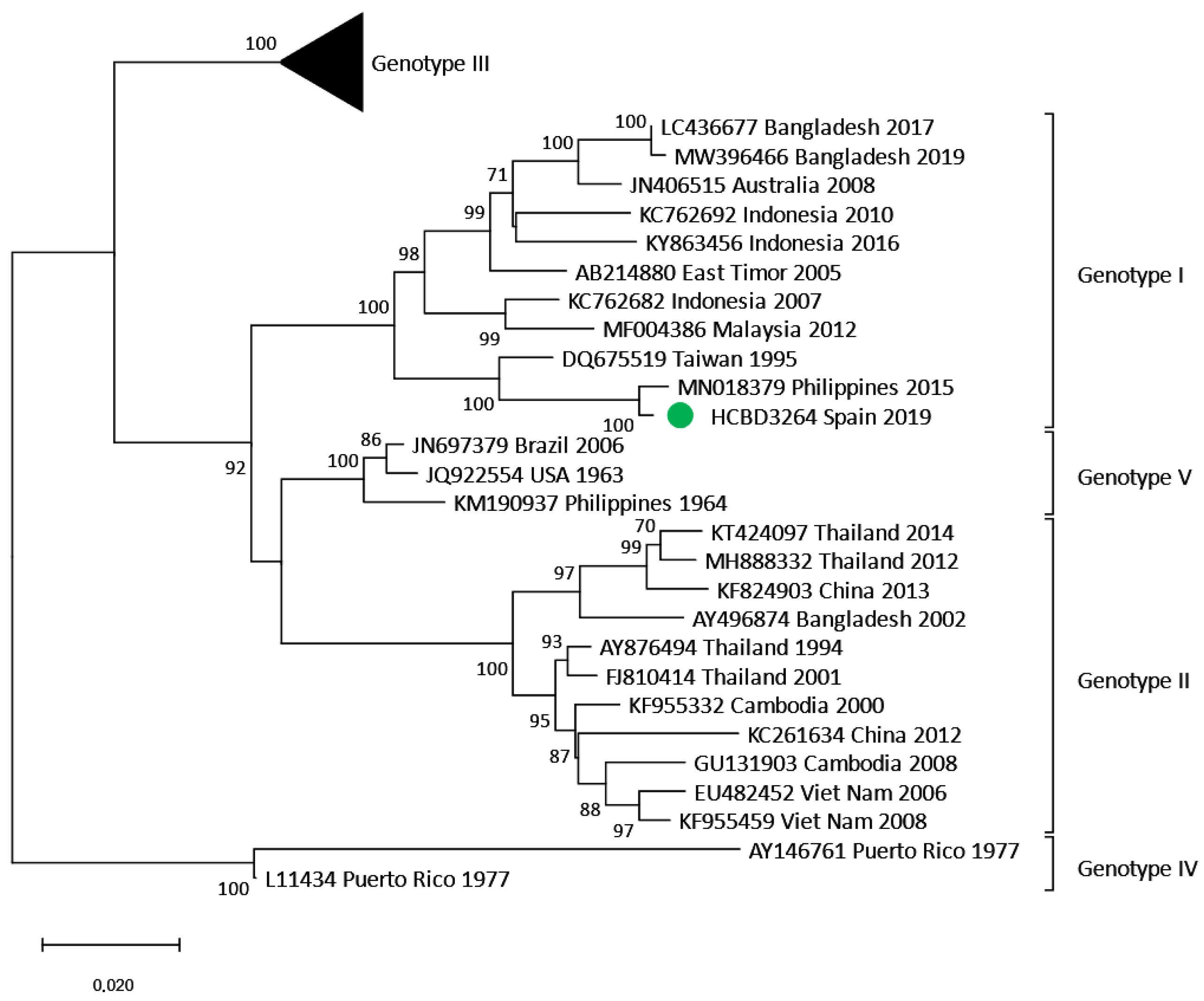
3.3. Phylogenetic Analysis of DENV Imported Strains
3.4. Local Transmission Events
3.4.1. Human-to-Mosquito Transmission
3.4.2. Locally Acquired Dengue Case
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Health Topics. Dengue and Severe Dengue. Updated 23 April 2024. Available online: https://www.who.int/news-room/fact-sheets/detail/dengue-and-severe-dengue (accessed on 11 September 2024).
- Guzman, M.G.; Gubler, D.J.; Izquierdo, A.; Martinez, E.; Halstead, S.B. Dengue infection. Nat. Rev. Dis. Primers 2016, 2, 16055. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Vasilakis, N. Dengue—Quo tu et quo vadis? Viruses 2011, 3, 1562–1608. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pollett, S.; Melendrez, M.C.; Maljkovic Berry, I.; Duchêne, S.; Salje, H.; Cummings, D.A.T.; Jarman, R. Understanding dengue virus evolution to support epidemic surveillance and counter-measure development. Infect. Genet. Evol. 2018, 62, 279–295. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yacoub, S.; Mongkolsapaya, J.; Screaton, G. Recent advances in understanding dengue. F1000Research 2016, 5, 78. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Guzman, M.G.; Halstead, S.B.; Artsob, H.; Buchy, P.; Farrar, J.; Gubler, D.J.; Hunsperger, E.; Kroeger, A.; Margolis, H.S.; Martínez, E.; et al. Dengue: A continuing global threat. Nat. Rev. Microbiol. 2010, 8 (Suppl. 12), S7–S16. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- European Centre for Disease Prevention and Control (ECDC). Autochthonous Transmission of Dengue Virus in EU/EEA, 2010–Present. Available online: https://www.ecdc.europa.eu/en/all-topics-z/dengue/surveillance-and-disease-data/autochthonous-transmission-dengue-virus-eueea (accessed on 4 September 2024).
- Duong, V.; Lambrechts, L.; Paul, R.E.; Ly, S.; Lay, R.S.; Long, K.C.; Huy, R.; Tarantola, A.; Scott, T.W.; Sakuntabhai, A.; et al. Asymptomatic humans transmit dengue virus to mosquitoes. Proc. Natl. Acad. Sci. USA 2015, 112, 14688–14693. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Aranda, C.; Martínez, M.J.; Montalvo, T.; Eritja, R.; Navero-Castillejos, J.; Herreros, E.; Marqués, E.; Escosa, R.; Corbella, I.; Bigas, E.; et al. Arbovirus surveillance: First dengue virus detection in local Aedes albopictus mosquitoes in Europe, Catalonia, Spain, 2015. Eurosurveillance 2018, 23, 1700837. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Centro de Coordinación de Alertas y Emergencias Sanitarias. Primeros casos de Dengue Autóctono en España (In Spanish: First Autochthonous Cases of Dengue in Spain); 2018; Ministerio de Sanidad, Consumo y Bienestar Social. Available online: https://www.sanidad.gob.es/profesionales/saludPublica/ccayes/alertasActual/docs/ERR_Dengue_autoctono_Espana.pdf (accessed on 20 May 2024).
- European Centre for Disease Prevention and Control. Annual Epidemiological Report for 2019. 2021. Available online: https://www.ecdc.europa.eu/sites/default/files/documents/AER-dengue-2019.pdf (accessed on 20 May 2024).
- Domingo, C.; Niedrig, M.; Gascón, J.; Palacios, G.; Reyes, N.; Malo, M.J.; Wichmann, O.; Ruiz, J.; Schultze, D.; Schunk, M.; et al. Molecular surveillance of circulating dengue genotypes through European travelers. J. Travel Med. 2011, 18, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Franco, L.; Pagan, I.; Serre Del Cor, N.; Schunk, M.; Neumayr, A.; Molero, F.; Potente, A.; Hatz, C.; Wilder-Smith, A.; Sánchez-Seco, M.; et al. Molecular epidemiology suggests Venezuela as the origin of the dengue outbreak in Madeira, Portugal in 2012–2013. Clin. Microbiol. Infect. 2015, 21, 713.e5–713.e8. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Martínez, B.; Pampaka, D.; Suárez-Sánchez, P.; Figuerola, J.; Sierra, M.J.; León-Gomez, I.; del Aguila, J.; Gómez-Barroso, D. Spatial analysis for risk assessment of dengue in Spain. Enferm. Infecc. Microbiol. Clin. (Engl. Ed.) 2024, 42, 406–413. [Google Scholar] [CrossRef] [PubMed]
- Montalvo, T.; Fernández, L.; Franco, S.; Peracho, V. El programa de vigilancia y control de mosquitos en Barcelona (In Spanish: Mosquito surveillance and control program in Barcelona). Viure Salut. 2016, 105, 15–16. Available online: https://www.mosquitoalert.com/wp-content/uploads/Viure_en_Salut_105.pdf (accessed on 2 April 2025).
- Navero-Castillejos, J.; Benitez, R.; Torner, N.; Muñoz, J.; Camprubí-Ferrer, D.; Peiró-Mestres, A.; Sulleiro, E.; Silgado, A.; Gonzalo, V.; Falgueras, T.; et al. Molecular Characterization of Imported and Autochthonous Dengue in Northeastern Spain. Viruses 2021, 13, 1910. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Moureau, G.; Temmam, S.; Gonzalez, J.P.; Charrel, R.N.; Grard, G.; de Lamballerie, X. A real-time RT-PCR method for the universal detection and identification of flaviviruses. Vector Borne Zoonotic Dis. 2007, 7, 467–477. [Google Scholar] [CrossRef] [PubMed]
- Torina, A.; La Russa, F.; Blanda, V.; Peralbo-Moreno, A.; Casades-Martí, L.; Di Pasquale, L.; Bongiorno, C.; Badaco, V.V.; Toma, L.; Ruiz-Fons, F. Modelling time-series Aedes albopictus abundance as a forecasting tool in urban environments. Ecol. Indic. 2023, 150, 110232. [Google Scholar] [CrossRef]
- European Centre for Diesase Prevention and Control. Aedes albopictus—Factsheet for Experts. 2016. Available online: https://www.ecdc.europa.eu/en/disease-vectors/facts/mosquito-factsheets/aedes-albopictus (accessed on 20 May 2024).
- Gossner, C.M.; Fournet, N.; Frank, C.; Fernández-Martínez, B.; Del Manso, M.; Gomes Dias, J.; de Valk, H. Dengue virus infections among European travellers, 2015 to 2019. Eurosurveillance 2022, 27, 2001937. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Messina, J.P.; Brady, O.J.; Scott, T.W.; Zou, C.; Pigott, D.M.; Duda, K.A.; Bhatt, S.; Katzelnick, L.; Howes, R.E.; Battle, K.E.; et al. Global spread of dengue virus types: Mapping the 70 year history. Trends Microbiol. 2014, 22, 138–146. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sang, S.; Liu-Helmersson, J.; Quam, M.B.M.; Zhou, H.; Guo, X.; Wu, H.; Liu, Q. The evolutionary dynamics of DENV 4 genotype I over a 60-year period. PLoS Negl. Trop. Dis. 2019, 13, e0007592. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rojas, A.; Shen, J.; Cardozo, F.; Bernal, C.; Caballero, O.; Ping, S.; Key, A.; Haider, A.; de Guillén, Y.; Langjahr, P.; et al. Characterization of Dengue Virus 4 Cases in Paraguay, 2019–2020. Viruses 2024, 16, 181. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sharmila, P.F.; Vanathy, K.; Rajamani, B.; Kaliaperumal, V.; Dhodapkar, R. Emergence of dengue virus 4 as the predominant serotype during the outbreak of 2017 in South India. Indian J. Med. Microbiol. 2019, 37, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Aryati, A.; Wrahatnala, B.J.; Yohan, B.; Fanny, M.; Hakim, F.K.N.; Sunari, E.; Zuroidah, N.; Wardhani, P.; Santoso, M.S.; Husada, D.; et al. Dengue Virus Serotype 4 Is Responsible for the Outbreak of Dengue in East Java City of Jember, Indonesia. Viruses 2020, 12, 913. [Google Scholar] [CrossRef]
- Poltep, K.; Phadungsombat, J.; Nakayama, E.E.; Kosoltanapiwat, N.; Hanboonkunupakarn, B.; Wiriyarat, W.; Shioda, T.; Leaungwutiwong, P. Genetic Diversity of Dengue Virus in Clinical Specimens from Bangkok, Thailand, during 2018–2020: Co-Circulation of All Four Serotypes with Multiple Genotypes and/or Clades. Trop. Med. Infect. Dis. 2021, 6, 162. [Google Scholar] [CrossRef]
- Maduranga, S.; Valencia, B.M.; Sigera, C.; Adikari, T.; Weeratunga, P.; Fernando, D.; Rajapakse, S.; Lloyd, A.R.; Bull, R.A.; Rodrigo, C. Genomic Surveillance of Recent Dengue Outbreaks in Colombo, Sri Lanka. Viruses 2023, 15, 1408. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vazquez, C.; Alcantara, L.C.J.; Fonseca, V.; Lima, M.; Xavier, J.; Adelino, T.; Fritsch, H.; Castro, E.; de Oliveira, C.; Schuab, G.; et al. Retrospective Spatio-Temporal Dynamics of Dengue Virus 1, 2 and 4 in Paraguay. Viruses 2023, 15, 1275. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Muthanje, E.M.; Kimita, G.; Nyataya, J.; Njue, W.; Mulili, C.; Mugweru, J.; Mutai, B.; Kituyi, S.N.; Waitumbi, J. March 2019 dengue fever outbreak at the Kenyan south coast involving dengue virus serotype 3, genotypes III and V. PLoS Glob. Public Health 2022, 2, e0000122. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kelly, M.E.; Msafiri, F.; Affara, M.; Gehre, F.; Moremi, N.; Mghamba, J.; Misinzo, G.; Thye, T.; Gatei, W.; Whistler, T.; et al. Molecular Characterization and Phylogenetic Analysis of Dengue Fever Viruses in Three Outbreaks in Tanzania Between 2017 and 2019. PLoS Negl. Trop. Dis. 2023, 17, e0011289. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- de Bruycker-Nogueira, F.; Mir, D.; Dos Santos, F.B.; Bello, G. Evolutionary history and spatiotemporal dynamics of DENV-1 genotype V in the Americas. Infect. Genet. Evol. 2016, 45, 454–460. [Google Scholar] [CrossRef]
- Tsegaye, M.M.; Mekonnen, A.T.; Gebretsion, D.T.; Gelanew, T.; Alemayehu, D.H.; Tefera, D.A.; Woldemichael, T.S.; Getaneh, B.A.; Abera, E.K.; Jebessa, G.G.; et al. Predominance of Dengue Virus Serotype-1/Genotype-I in Eastern and Southeastern Ethiopia. Viruses 2024, 16, 1334. [Google Scholar] [CrossRef]
- Akelew, Y.; Pareyn, M.; Lemma, M.; Negash, M.; Bewket, G.; Derbew, A.; Belay, G.; Pollmann, J.; Adriaensen, W.; Peeters, M.; et al. Aetiologies of acute undifferentiated febrile illness at the emergency ward of the University of Gondar Hospital, Ethiopia. Trop. Med. Int. Health 2022, 27, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Alfsnes, K.; Eldholm, V.; Gaunt, M.W.; de Lamballerie, X.; Gould, E.A.; Pettersson, J.H. Tracing and tracking the emergence, epidemiology and dispersal of dengue virus to Africa during the 20th century. One Health 2021, 13, 100337. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Letizia, A.G.; Pratt, C.B.; Wiley, M.R.; Fox, A.T.; Mosore, M.; Agbodzi, B.; Yeboah, C.; Kumordjie, S.; Di Paola, N.; Assana, K.C.; et al. Retrospective Genomic Characterization of a 2017 Dengue Virus Outbreak, Burkina Faso. Emerg. Infect. Dis. 2022, 28, 1198–1210. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Agbodzi, B.; Sado Yousseu, F.B.; Nemg Simo, F.B.; Kumordjie, S.; Yeboah, C.; Mosore, M.T.; Bentil, R.E.; Coatsworth, H.G.; Attram, N.; Nimo-Paintsil, S.; et al. Whole genome sequencing of outbreak strains from 2017 to 2018 reveals an endemic clade of dengue 1 virus in Cameroon. Emerg. Microbes Infect. 2023, 12, 2281352. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ushijima, Y.; Abe, H.; Mbadinga, M.J.V.M.; Ondo, G.N.; Bikangui, R.; Agnandji, S.T.; Lell, B.; Yasuda, J. Re-emergence of dengue, chikungunya, and Zika viruses in 2021 after a 10-year gap in Gabon. IJID Reg. 2022, 5, 68–71. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]


| DENV Serotype | DENV Genotype | Number of Cases |
|---|---|---|
| DENV-1 (n = 34) | I | 13 |
| IV | 1 | |
| V | 20 | |
| DENV-2 (n = 26) | American–Asian | 11 |
| Asian I | 3 | |
| Cosmopolitan | 12 | |
| DENV-3 (n = 6) | I | 3 |
| III | 3 | |
| DENV-4 (n = 2) | I | 1 |
| II | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Navero-Castillejos, J.; Sánchez-Montalvá, A.; Sulleiro, E.; Silgado, A.; Montalvo, T.; Barahona, L.; Busquets, N.; Muñoz, J.; Camprubí-Ferrer, D.; Valdivia, M.; et al. Molecular Epidemiology of Travel-Associated and Locally Acquired Dengue Virus Infections in Catalonia, Spain, 2019. Viruses 2025, 17, 621. https://doi.org/10.3390/v17050621
Navero-Castillejos J, Sánchez-Montalvá A, Sulleiro E, Silgado A, Montalvo T, Barahona L, Busquets N, Muñoz J, Camprubí-Ferrer D, Valdivia M, et al. Molecular Epidemiology of Travel-Associated and Locally Acquired Dengue Virus Infections in Catalonia, Spain, 2019. Viruses. 2025; 17(5):621. https://doi.org/10.3390/v17050621
Chicago/Turabian StyleNavero-Castillejos, Jéssica, Adrián Sánchez-Montalvá, Elena Sulleiro, Aroa Silgado, Tomás Montalvo, Laura Barahona, Núria Busquets, José Muñoz, Daniel Camprubí-Ferrer, Manuel Valdivia, and et al. 2025. "Molecular Epidemiology of Travel-Associated and Locally Acquired Dengue Virus Infections in Catalonia, Spain, 2019" Viruses 17, no. 5: 621. https://doi.org/10.3390/v17050621
APA StyleNavero-Castillejos, J., Sánchez-Montalvá, A., Sulleiro, E., Silgado, A., Montalvo, T., Barahona, L., Busquets, N., Muñoz, J., Camprubí-Ferrer, D., Valdivia, M., Martínez, A., Bou-Monclús, M. A., Martínez-Calleja, I., Jané, M., Rius, C., Vargas-Leguas, H., Escudero-Pérez, B., Albarracín, R., Navarro, A., ... Martínez, M. J. (2025). Molecular Epidemiology of Travel-Associated and Locally Acquired Dengue Virus Infections in Catalonia, Spain, 2019. Viruses, 17(5), 621. https://doi.org/10.3390/v17050621





