Attenuation of a Novel Goose Parvovirus Strain NMG21 via Serial Cell Passage
Abstract
1. Introduction
2. Materials and Methods
2.1. Virus, Animals and Ethics Statement
2.2. Adaptation and Serial Passages in DEFs
2.3. Virus Titration of Different Passages
2.4. Animal Experiment Design
2.5. Sample Collection
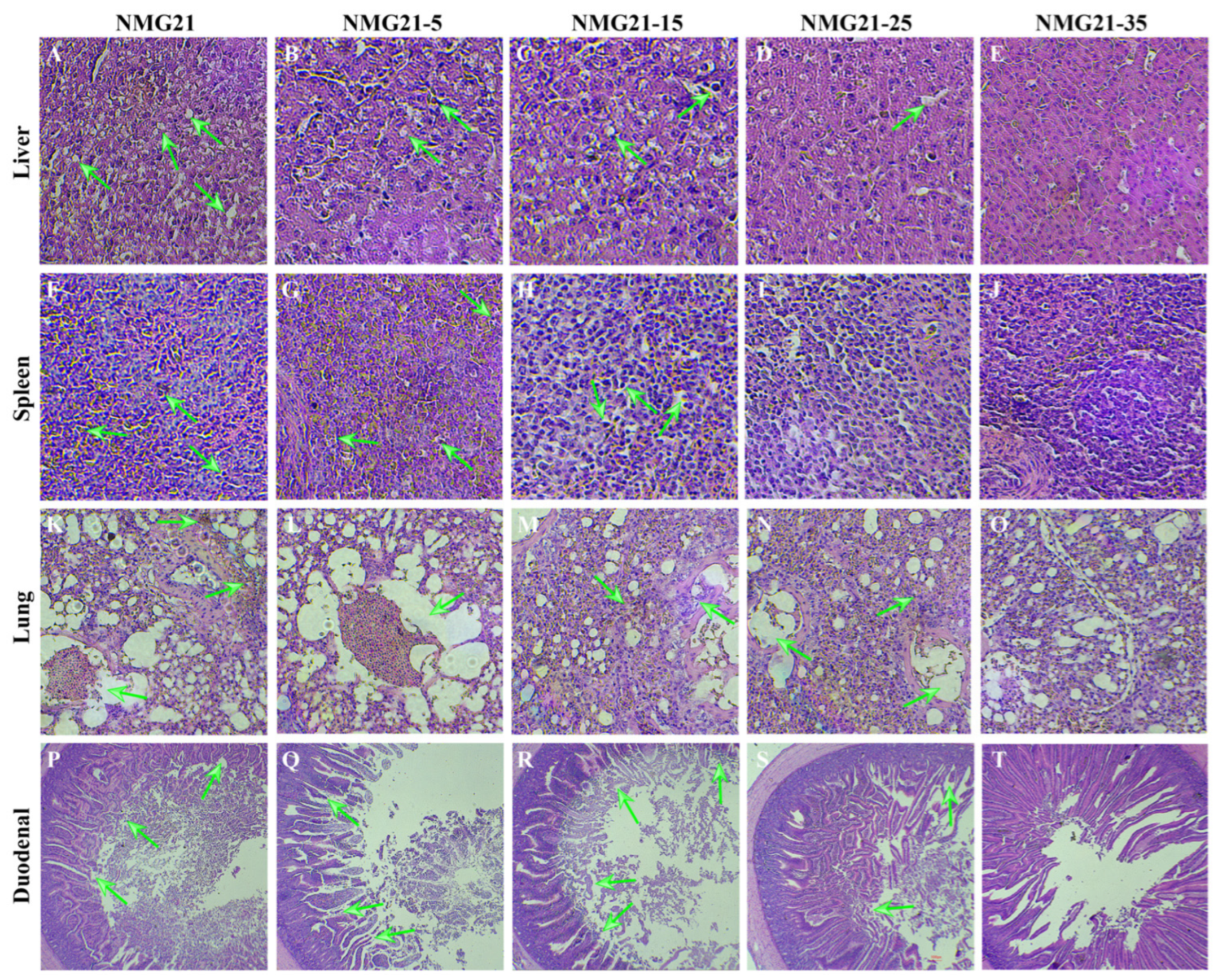
2.6. Histopathology
2.7. DNA Extraction and Quantitative Reverse Transcription PCR (qRT-PCR) Analysis
2.8. Serology
2.9. Statistical Analysis
3. Results
3.1. Attenuated by Serial Passage
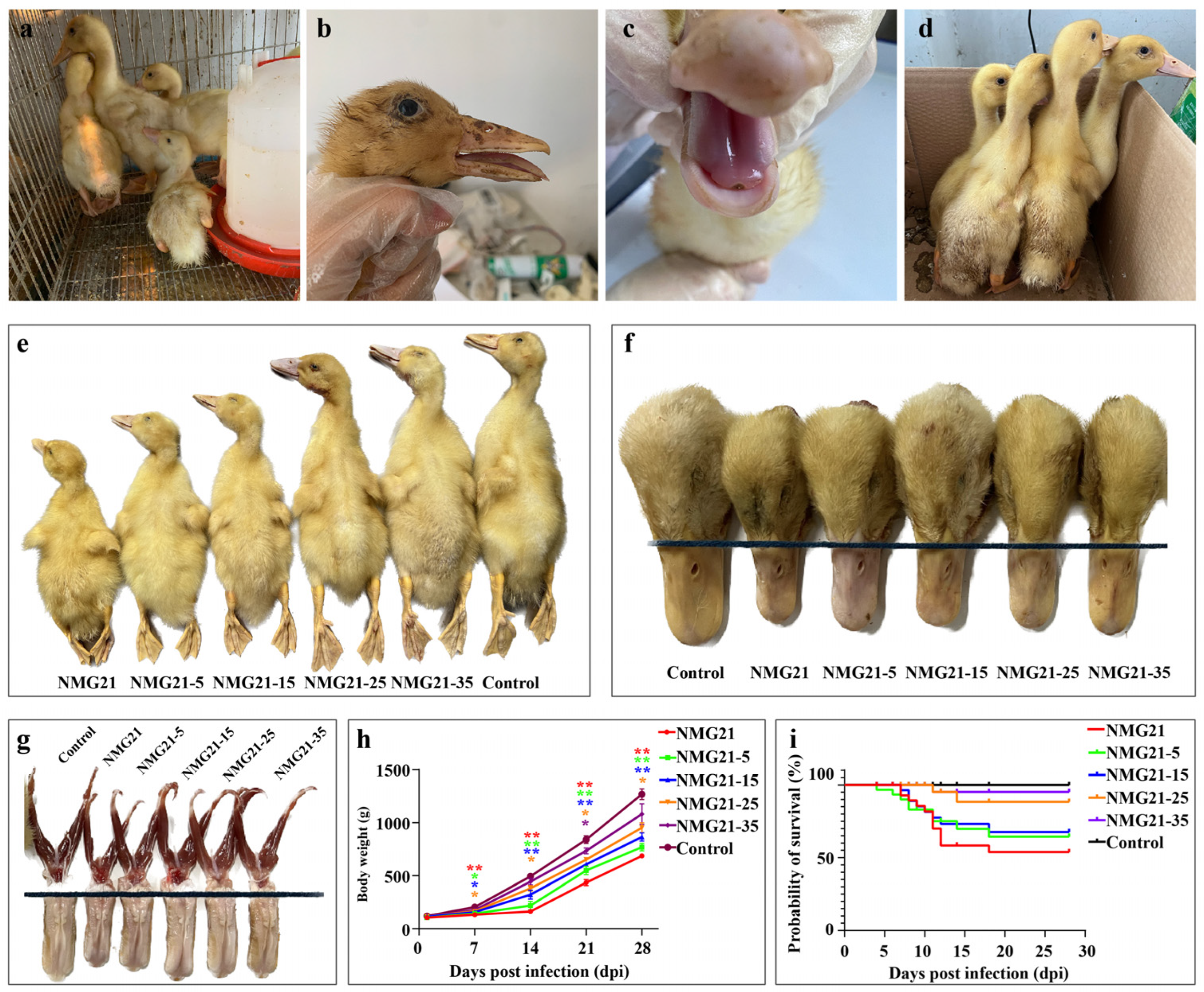
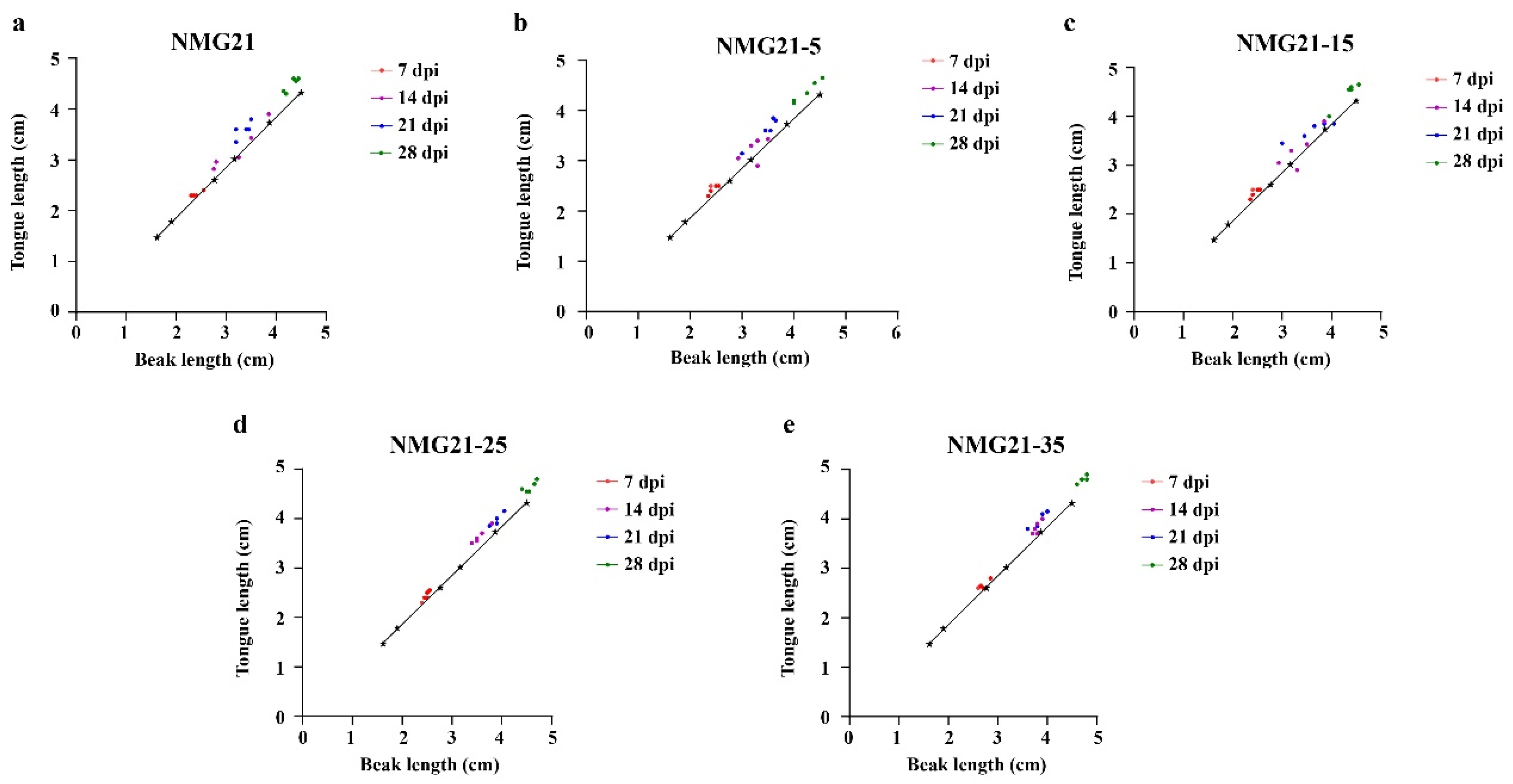
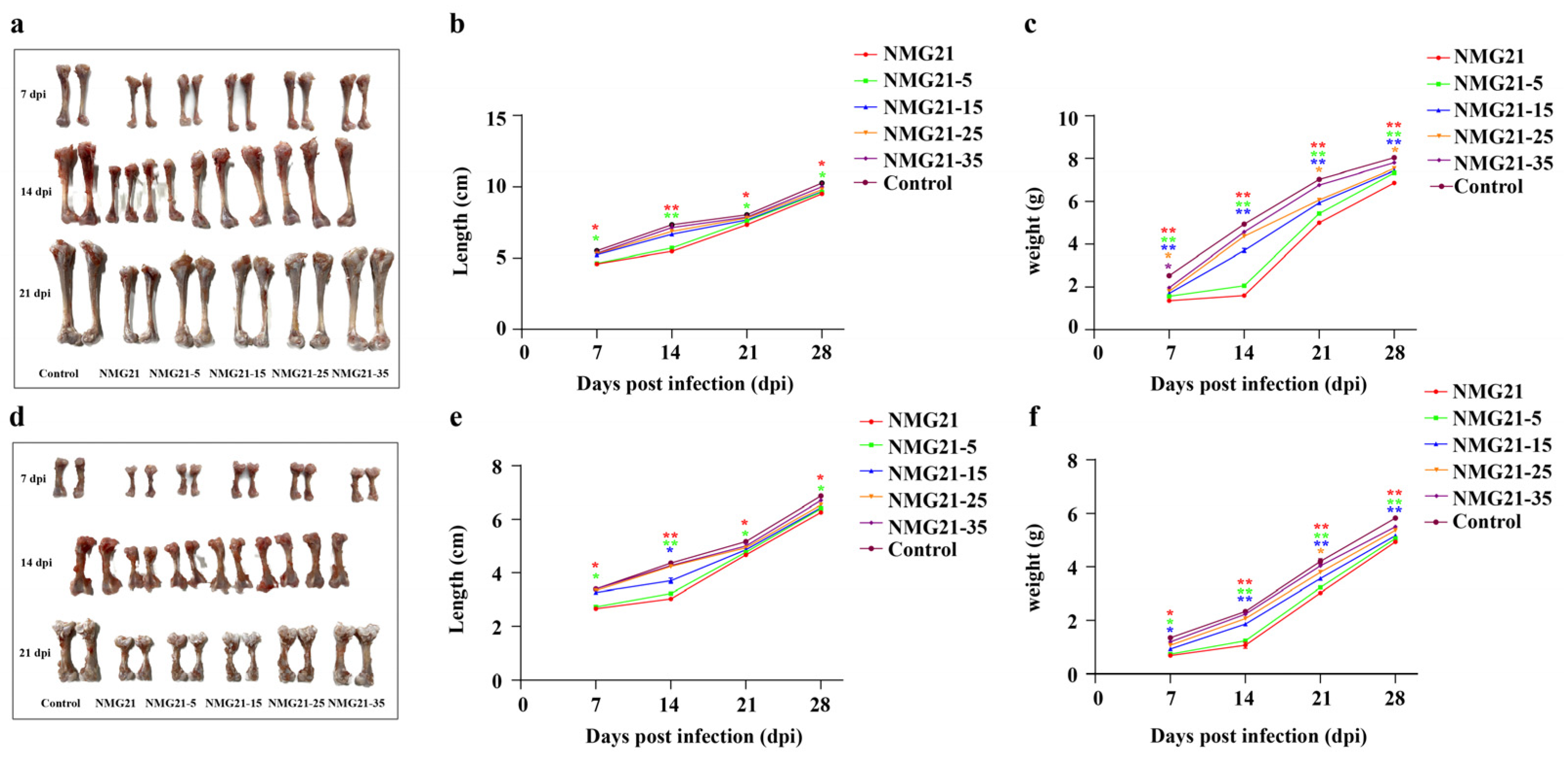
3.2. Clinical Signs
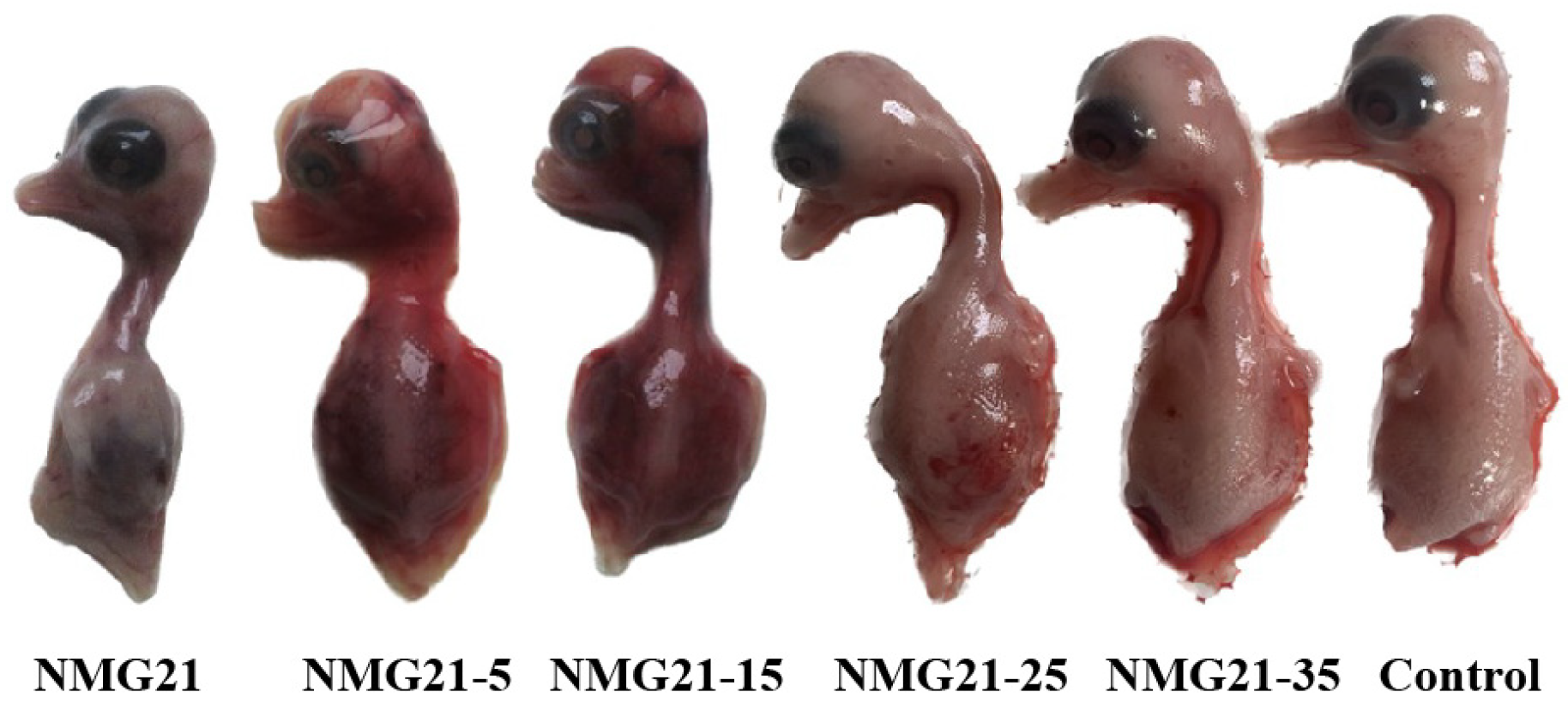
3.3. Gross Lesions
3.4. Histopathology
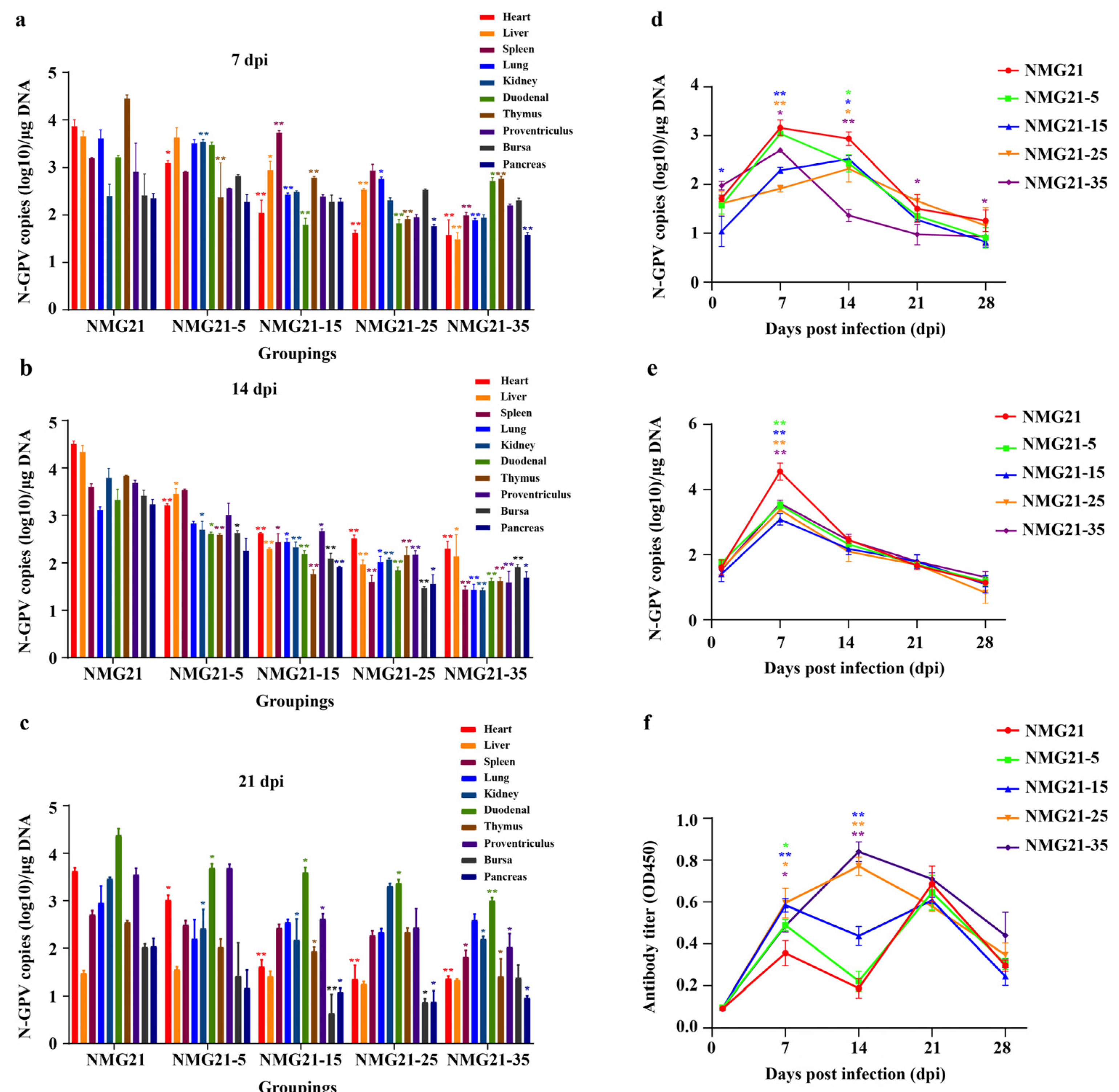
3.5. Viral Loads in Tissues
3.6. Viral Shedding in the Blood and Cloaca
3.7. Antibody Response
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Germeraad, E.A.; Sanders, P.; Hagenaars, T.J.; de Jong, M.C.M.; Beerens, N.; Gonzales, J.L. Virus Shedding of Avian Influenza in Poultry: A Systematic Review and Meta-Analysis. Viruses 2019, 11, 812. [Google Scholar] [CrossRef] [PubMed]
- Andreychev, A.; Boyarova, E.; Brandler, O.; Tukhbatullin, A.; Kapustina, S. Terrestrial and Subterranean Mammals as Reservoirs of Zoonotic Diseases in the Central Part of European Russia. Diversity 2023, 15, 39. [Google Scholar] [CrossRef]
- Chen, H.; Dou, Y.; Tang, Y.; Zheng, X.; Niu, X.; Yang, J.; Yu, X.; Diao, Y. Experimental Reproduction of Beak Atrophy and Dwarfism Syndrome by Infection in Cherry Valley Ducklings with a Novel Goose Parvovirus-Related Parvovirus. Vet. Microbiol. 2016, 183, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Tang, Y.; Dou, Y.; Zheng, X.; Diao, Y. Evidence for Vertical Transmission of Novel Duck-Origin Goose Parvovirus-Related Parvovirus. Transbound. Emerg. Dis. 2016, 63, 243–247. [Google Scholar] [CrossRef]
- Zdori, Z.; Stefancsik, R.; Rauch, T.; Kisary, J. Analysis of the Complete Nucleotide Sequences of Goose and Muscovy Duck Pervoviruses Indicates Common Ancestral Origin with Adeno-Associated Virus 2. Virology 1995, 212, 562–573. [Google Scholar] [CrossRef]
- Smith, D.H.; Ward, P.; Linden, R.M. Comparative Characterization of Rep Proteins from the Helper-Dependent Adeno-Associated Virus Type 2 and the Autonomous Goose Parvovirus. J. Virol. 1999, 73, 2930–2937. [Google Scholar] [CrossRef]
- Chen, H.; Dou, Y.; Tang, Y.; Zhang, Z.; Zheng, X.; Niu, X.; Yang, J.; Yu, X.; Diao, Y. Isolation and Genomic Characterization of a Duck-Origin GPV-Related Parvovirus from Cherry Valley Ducklings in China. PLoS ONE 2015, 10, e0140284. [Google Scholar] [CrossRef]
- Jin, M.; Feng, C.; Wang, X.; Zhang, D. Molecular Evidence of Goose-Parvovirus-Related Abnormal Molting in Pekin Ducks. Arch. Virol. 2019, 164, 2837–2841. [Google Scholar] [CrossRef]
- He, D.; Wang, F.; Zhao, L.; Jiang, X.; Zhang, S.; Wei, F.; Wu, B.; Wang, Y.; Diao, Y.; Tang, Y. Epidemiological Investigation of Infectious Diseases in Geese on Mainland China during 2018–2021. Transbound. Emerg. Dis. 2022, 69, 3419–3432. [Google Scholar] [CrossRef]
- Zhou, J.; Li, C.; Tang, A.; Li, H.; Yu, Z.; Chen, Z.; Guo, X.; Liu, G. Immunogenicity of an Inactivated Novel Goose Parvovirus Vaccine for Short Beak and Dwarfism Syndrome in Cherry Valley Ducks. Arch. Virol. 2022, 167, 881–889. [Google Scholar] [CrossRef]
- Li, C.; Li, Q.; Chen, Z.; Liu, G. Novel Duck Parvovirus Identified in Cherry Valley Ducks (Anas platyrhynchos domesticus), China. Infect. Genet. Evol. 2016, 44, 278–280. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Sun, Z.; Shen, T.; Xu, D.; Huang, K.; Zhou, J.; Song, S.; Yan, L. Analysis of Evolutionary Processes of Species Jump in Waterfowl Parvovirus. Front. Microbiol. 2017, 8, 421. [Google Scholar] [CrossRef]
- He, D.; Jiang, X.; Tian, M.; Niu, X.; Wei, F.; Wu, B.; Gao, L.; Tang, Y.; Diao, Y. Pathogenicity of Goose Astrovirus Genotype 2 in Chickens. Poult. Sci. 2023, 102, 102808. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, J.; Wang, Z.; Chen, L.; Diao, Y.; Tang, Y. Research Note: Development of an ELISA to Distinguish between Goose Parvovirus Infection and Vaccine Immunization Antibodies. Poult. Sci. 2020, 99, 1332–1340. [Google Scholar] [CrossRef] [PubMed]
- Glávits, R.; Zolnai, A.; Szabó, E.; Ivanics, E.; Zarka, P.; Palya, T.M. V Comparative Pathological Studies on Domestic Geese (Anser anser domestica) and Muscovy Ducks (Cairina moschata) Experimentally Infected with Parvovirus Strains of Goose and Muscovy Duck Origin. Acta Vet. Hung. 2005, 53, 73–89. [Google Scholar] [CrossRef]
- Yang, J.; Chen, H.; Wang, Z.; Yu, X.; Niu, X.; Tang, Y.; Diao, Y. Development of a Quantitative Loop-Mediated Isothermal Amplification Assay for the Rapid Detection of Novel Goose Parvovirus. Front. Microbiol. 2017, 8, 2472. [Google Scholar] [CrossRef] [PubMed]
- He, D.; Zhang, X.; Chen, L.; Tang, Y.; Diao, Y. Development of an Attenuated Live Vaccine Candidate of Duck Tembusu Virus Strain. Vet. Microbiol. 2019, 231, 218–225. [Google Scholar] [CrossRef]
- He, Y.; Wang, X.; Guo, J.; Mao, L.; Zhang, S.; Hu, T.; Wang, M.; Jia, R.; Zhu, D.; Liu, M.; et al. Replication/Assembly Defective Avian Flavivirus With Internal Deletions in the Capsid Can Be Used as an Approach for Living Attenuated Vaccine. Front. Immunol. 2021, 12, 694959. [Google Scholar] [CrossRef]
- Yang, L.; Liang, T.; Lv, J.; Qu, S.; Meng, R.; Yang, B.; Feng, C.; Li, Q.; Wang, X.; Zhang, D. A Quasispecies in a BHK-21 Cell-Derived Virulent Tembusu Virus Strain Contains Three Groups of Variants with Distinct Virulence Phenotypes. Vet. Microbiol. 2021, 263, 109252. [Google Scholar] [CrossRef]
- Zhang, L.; Sun, M.; Zhang, Q.; Wang, J.; Cao, Y.; Cui, S.; Su, J. Long-Term Passage of Duck Tembusu Virus in BHK-21 Cells Generates a Completely Attenuated and Immunogenic Population with Increased Genetic Diversity. Vaccine 2020, 38, 933–941. [Google Scholar] [CrossRef]
- Yang, Y.; Sui, N.; Zhang, R.; Lan, J.; Li, P.; Lian, C.; Li, H.; Xie, Z.; Jiang, S. Coinfection of Novel Goose Parvovirus–Associated Virus and Duck Circovirus in Feather Sacs of Cherry Valley Ducks with Feather Shedding Syndrome. Poult. Sci. 2020, 99, 4227–4234. [Google Scholar] [CrossRef] [PubMed]

| Generation | P0 (NMG21) | P5 (NMG21) | P15 (NMG21) | P25 (NMG21) | P35 (NMG21) |
|---|---|---|---|---|---|
| EID50 (0.2 mL) | 10−7 | 10−5 | 10−4.7 | 10−4.2 | 10−3.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.; He, D.; Wu, B.; Yan, Y.; Zhang, Y.; Zhou, J.; Wei, F.; Diao, Y. Attenuation of a Novel Goose Parvovirus Strain NMG21 via Serial Cell Passage. Viruses 2025, 17, 618. https://doi.org/10.3390/v17050618
Yang J, He D, Wu B, Yan Y, Zhang Y, Zhou J, Wei F, Diao Y. Attenuation of a Novel Goose Parvovirus Strain NMG21 via Serial Cell Passage. Viruses. 2025; 17(5):618. https://doi.org/10.3390/v17050618
Chicago/Turabian StyleYang, Jing, Dalin He, Bingrong Wu, Yun Yan, Yupei Zhang, Jiaping Zhou, Feng Wei, and Youjiang Diao. 2025. "Attenuation of a Novel Goose Parvovirus Strain NMG21 via Serial Cell Passage" Viruses 17, no. 5: 618. https://doi.org/10.3390/v17050618
APA StyleYang, J., He, D., Wu, B., Yan, Y., Zhang, Y., Zhou, J., Wei, F., & Diao, Y. (2025). Attenuation of a Novel Goose Parvovirus Strain NMG21 via Serial Cell Passage. Viruses, 17(5), 618. https://doi.org/10.3390/v17050618






