The Sibylline Relationship Between Human Papillomavirus and Endometrial Cancer: Scarcity of Strong Evidence Linking Both Conditions
Abstract
1. Introduction
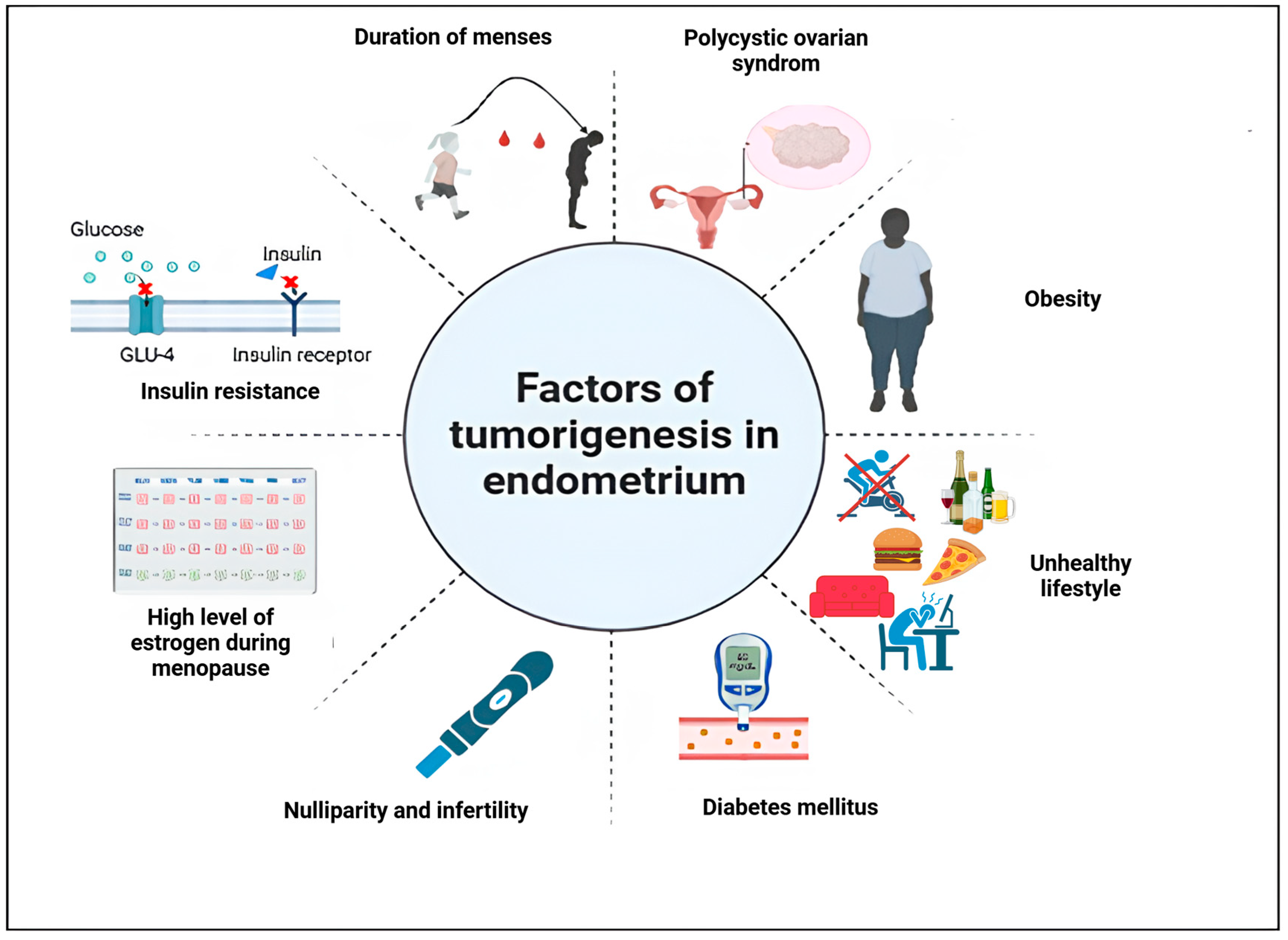
2. Risk Factors of Endometrial Cancer
3. Classification of Endometrial Cancer
4. FIGO Classification
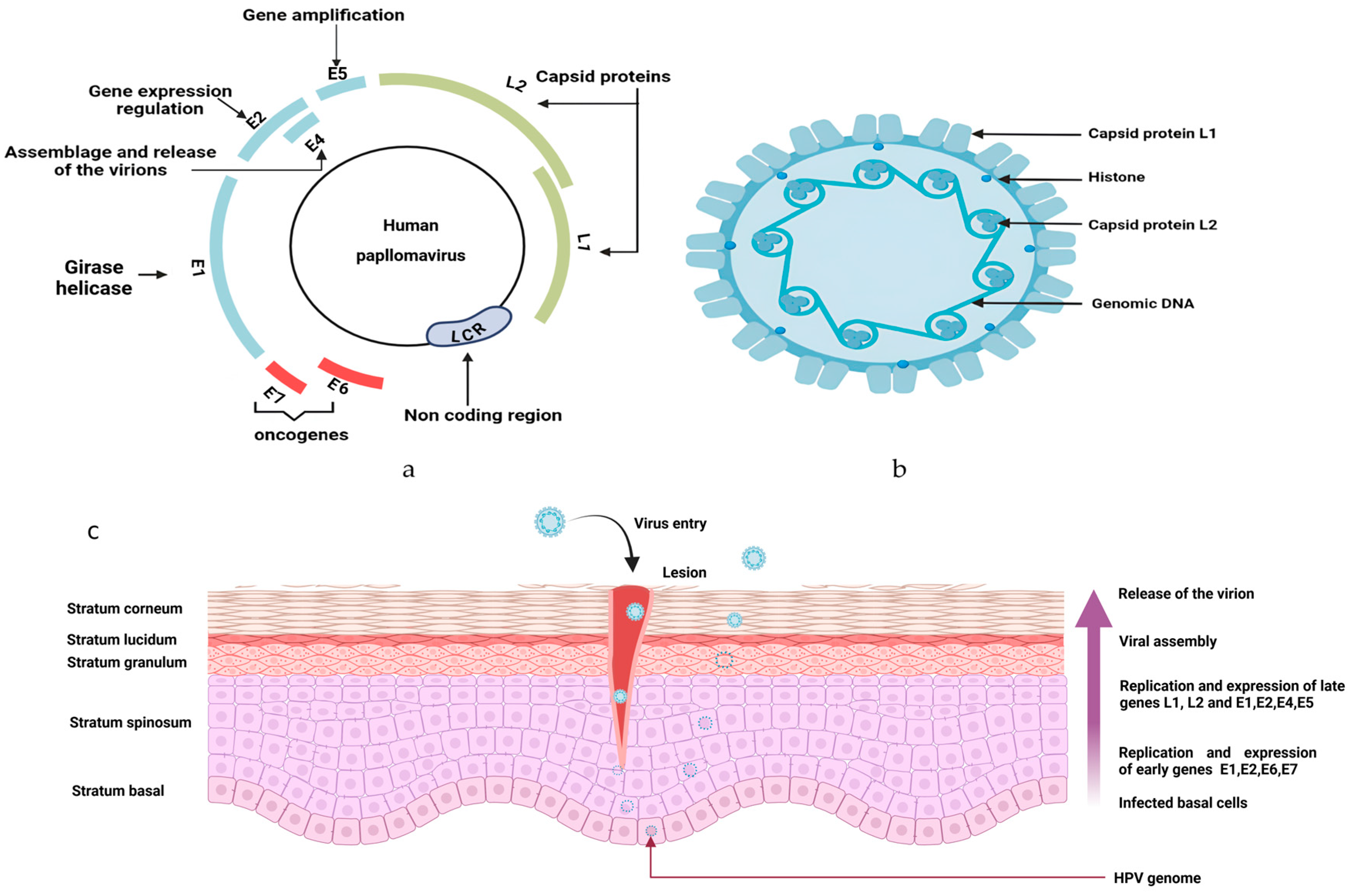
5. Human Papillomavirus (HPV)
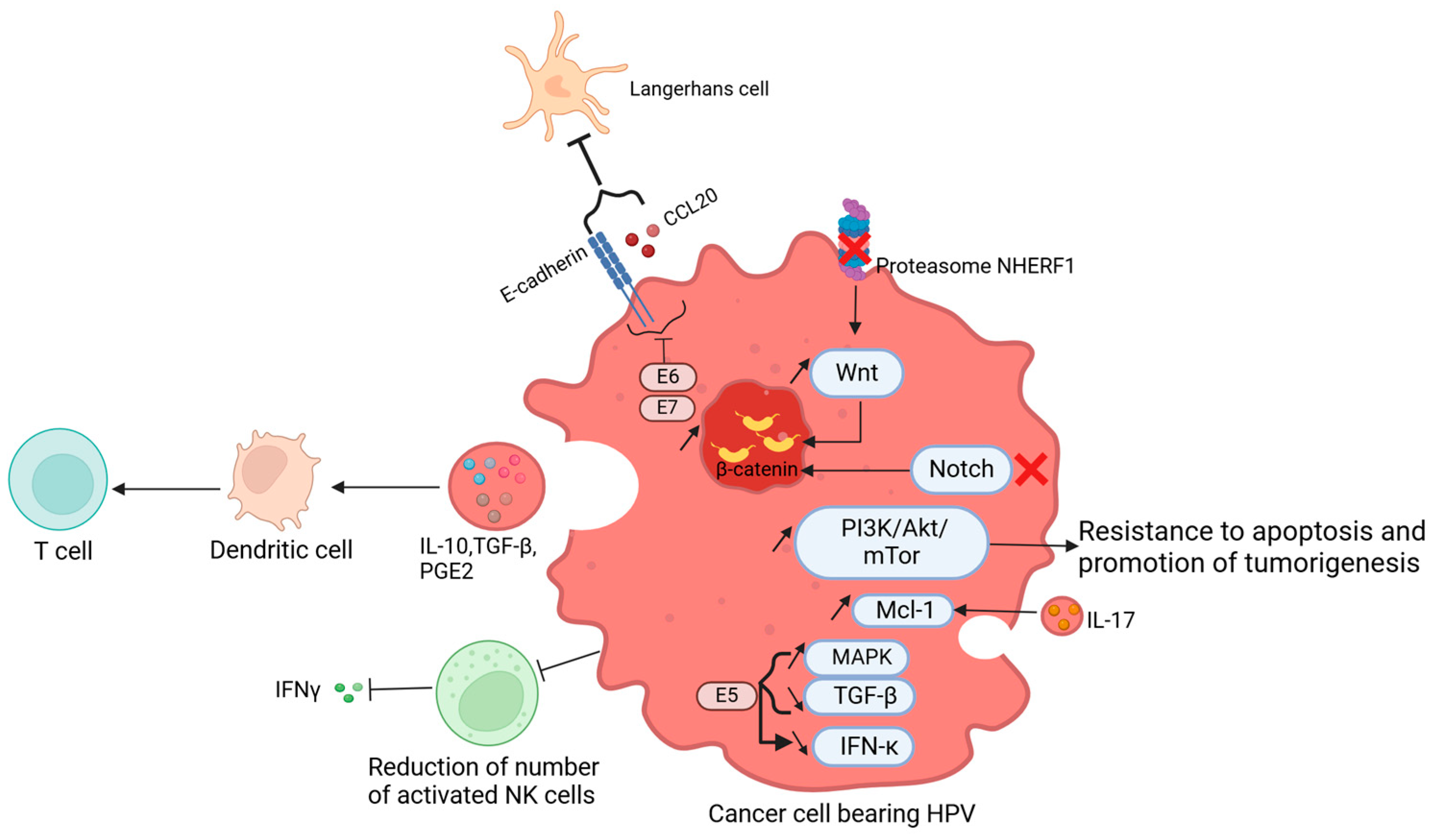
6. Human Papillomavirus in Cancer
7. Human Papillomavirus and Endometrial Cancer
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| CCL20 | CC chemokine ligand 20 |
| DNA | Desoxyribonucleic acid |
| EC | Endometrial cancer |
| E6-AP | E6-associated protein |
| FADD | Fas-associated protein with death domain |
| FIGO | International federation of gynecology and obstetrics |
| HPV | Human papillomavirus |
| HR-HPV | High-risk human papillomavirus |
| IFN | Interferon |
| IL | Interleukin |
| LR-HPV | Low-risk human papillomavirus |
| LVM | Low-volume metastasis |
| LVSI | Lymphovascular space invasion |
| MAPK | Mitogen-activated protein kinase |
| MDSC | Myeloid-derived suppressive cell |
| MHC | Major histocompatibility complex |
| MMRd | Mismatch repair deficiency |
| NCID | Notch intracellular domain |
| NK | Natural killer cell |
| NSMP | No specific molecular profile |
| p53 abn | p53 abnormal |
| PCR | Polymerase chain reaction |
| PGE2 | Prostaglandin E2 |
| POLE mut | POLE mutated |
| pRB | Retinoblastoma protein |
| PRR | pathogen recognition receptor |
| RT-PCR | Real-time polymerase chain reaction |
| SHBG | Sex-hormone-binding globulin |
| SLN | Sentinel lymph node |
| TAM | Tumor-associated macrophage |
| TCGA | The Cancer Genome Atlas |
| TGF | Transforming growth factor |
| TNF | Tumor necrosis factor |
| VEGF | Vascular endothelial growth factor |
| WHO | World Health Organization |
References
- Sahoo, S.S.; Zhang, X.D.; Hondermarck, H.; Tanwar, P.S. The Emerging Role of the Microenvironment in Endometrial Cancer. Cancers 2018, 10, 408. [Google Scholar] [CrossRef]
- Vanderstraeten, A.; Tuyaerts, S.; Amant, F. The immune system in the normal endometrium and implications for endometrial cancer development. J. Reprod. Immunol. 2015, 109, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Endometrial Cancer Statistics. World Cancer Research Fund International. WCRF International. Available online: https://www.wcrf.org/cancer-trends/endometrial-cancer-statistics/ (accessed on 11 July 2024).
- Gaber, C.; Meza, R.; Ruterbusch, J.J.; Cote, M.L. Endometrial Cancer Trends by Race and Histology in the USA: Projecting the Number of New Cases from 2015 to 2040. J. Racial Ethn. Health Disparit. 2016, 4, 895–903. [Google Scholar] [CrossRef]
- Sherman, M.E.; Devesa, S.S. Analysis of racial differences in incidence, survival, and mortality for malignant tumors of the uterine corpus. Cancer 2003, 98, 176–186. [Google Scholar] [CrossRef] [PubMed]
- Madison, T.; Schottenfeld, D.; James, S.A.; Schwartz, A.G.; Gruber, S.B. Endometrial cancer: Socioeconomic status and racial/ethnic differences in stage at diagnosis, treatment, and survival. Am. J. Public Health 2004, 94, 2104–2111. [Google Scholar] [CrossRef]
- Shaw, E.; Farris, M.; McNeil, J.; Friedenreich, C. Obesity and Endometrial Cancer. In Obesity and Cancer; Recent Results in Cancer Research; Pischon, T., Nimptsch, K., Eds.; Springer: Cham, Switzerland, 2016; Volume 208, pp. 107–136. [Google Scholar] [CrossRef]
- Frias-Gomez, J.; Alemany, L.; Benavente, Y.; Clarke, M.A.; de Francisco, J.; De Vivo, I.; Du, M.; Goodman, M.T.; Lacey, J.; Liao, L.M.; et al. Night shift work, sleep duration and endometrial cancer risk: A pooled analysis from the Epidemiology of Endometrial Cancer Consortium (E2C2). Sleep Med. Rev. 2023, 72, 101848. [Google Scholar] [CrossRef] [PubMed]
- Oyouni, A.A.A. Human papillomavirus in cancer: Infection, disease transmission, and progress in vaccines. J. Infect. Public Health 2023, 16, 626–631. [Google Scholar] [CrossRef] [PubMed]
- Araldi, R.P.; Sant’Ana, T.A.; Módolo, D.G.; de Melo, T.C.; Spadacci-Morena, D.D.; de Cassia Stocco, R.; Cerutti, J.M.; de Souza, E.B. The human papillomavirus (HPV)-related cancer biology: An overview. Biomed. Pharmacother. 2018, 106, 1537–1556. [Google Scholar] [CrossRef] [PubMed]
- Obesity: Preventing and Managing the Global Epidemic: Report of a WHO Consultation; WHO Consultation on Obesity (1999: Geneva, Switzerland) & World Health Organization. 2000. Available online: https://iris.who.int/handle/10665/42330 (accessed on 11 July 2024).
- Zhang, Y.; Liu, H.; Yang, S.; Zhang, J.; Qian, L.; Chen, X. Overweight, Obesity and Endometrial Cancer Risk: Results from a Systematic Review and Meta-Analysis. Int. J. Biol. Markers 2014, 29, e21–e29. [Google Scholar] [CrossRef]
- Fader, A.N.; Arriba, L.N.; Frasure, H.E.; von Gruenigen, V.E. Endometrial cancer and obesity: Epidemiology, biomarkers, prevention and survivorship. Gynecol. Oncol. 2009, 114, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Bakkum-Gamez, J.N.; Gonzalez-Bosquet, J.; Laack, N.N.; Mariani, A.; Dowdy, S.C. Current Issues in the Management of Endometrial Cancer. Mayo Clin. Proc. 2008, 83, 97–112. [Google Scholar] [CrossRef]
- Mu, N.; Zhu, Y.; Wang, Y.; Zhang, H.; Xue, F. Insulin resistance: A significant risk factor of endometrial cancer. Gynecol. Oncol. 2012, 125, 751–757. [Google Scholar] [CrossRef]
- Sağnıç, S. Obesity and Endometrial Cancer. In Role of Obesity in Human Health and Disease; IntechOpen: London, UK, 2021. [Google Scholar] [CrossRef]
- Wang, Y.; Zeng, X.M.; Tan, J.; Xu, Y.M.; Yi, C. Diabetes mellitus and endometrial carcinoma: Risk factors and etiological links. Medicine 2022, 101, e30299. [Google Scholar] [CrossRef]
- Chan, J.C.; Lim, L.L.; Wareham, N.J.; Shaw, J.E.; Orchard, T.J.; Zhang, P.; Lau, E.S.H.; Eliasson, B.; Kong, A.P.S.; Ezzati, M.; et al. The Lancet Commission on diabetes: Using data to transform diabetes care and patient lives. Lancet 2020, 396, 2019–2082. Available online: https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(20)32374-6/abstract (accessed on 11 July 2024). [CrossRef]
- Zhang, Y.; Liu, Z.; Yu, X.; Zhang, X.; Lü, S.; Chen, X.; Lü, B. The association between metabolic abnormality and endometrial cancer: A large case-control study in China. Gynecol. Oncol. 2010, 117, 41–46. [Google Scholar] [CrossRef]
- Byrne, F.L.; Martin, A.R.; Kosasih, M.; Caruana, B.T.; Farrell, R. The Role of Hyperglycemia in Endometrial Cancer Pathogenesis. Cancers 2020, 12, 1191. [Google Scholar] [CrossRef] [PubMed]
- Memarzadeh, S.; Zong, Y.; Janzen, D.M.; Goldstein, A.S.; Cheng, D.; Kurita, T.; Schafenacker, A.M.; Huang, J.; Witte, O.N. Cell-autonomous activation of the PI3-kinase pathway initiates endometrial cancer from adult uterine epithelium. Proc. Natl. Acad. Sci. USA 2010, 107, 17298–17303. [Google Scholar] [CrossRef]
- Weigelt, B.; Banerjee, S. Molecular targets and targeted therapeutics in endometrial cancer. Curr. Opin. Oncol. 2012, 24, 554–563. [Google Scholar] [CrossRef]
- Nissim, H.A.Y. Reprogramming glucose metabolism in cancer: Can it be exploited for cancer therapy? Nat. Rev. Cancer 2016, 16, 635–649. Available online: https://www.nature.com/articles/nrc.2016.77 (accessed on 15 July 2024).
- Yasin, H.K.; Taylor, A.H.; Ayakannu, T. A Narrative Review of the Role of Diet and Lifestyle Factors in the Development and Prevention of Endometrial Cancer. Cancers 2021, 13, 2149. [Google Scholar] [CrossRef] [PubMed]
- McTiernan, A. Mechanisms linking physical activity with cancer. Nat. Rev. Cancer 2008, 8, 205–211. [Google Scholar] [CrossRef]
- Lafferty, F.W.; Helmuth, D.O. Post-menopausal estrogen replacement: The prevention of osteoporosis and systemic effects. Maturitas 1985, 7, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Weiss, N.S.; Szekely, D.R.; Austin, D.F. Increasing Incidence of Endometrial Cancer in the United States. N. Engl. J. Med. 1976, 294, 1259–1262. [Google Scholar] [CrossRef] [PubMed]
- Key, T.J.; Pike, M.C. The dose-effect relationship between ‘unopposed’ oestrogens and endometrial mitotic rate: Its central role in explaining and predicting endometrial cancer risk. Br. J. Cancer 1988, 57, 205–212. [Google Scholar] [CrossRef]
- Kaaks, R.; Lukanova, A.; Kurzer, M.S. Obesity, endogenous hormones, and endometrial cancer risk: A synthetic review. Cancer Epidemiol. Biomark. Prev. 2002, 11, 1531–1543. [Google Scholar]
- Barry, J.A.; Azizia, M.M.; Hardiman, P.J. Risk of endometrial, ovarian and breast cancer in women with polycystic ovary syndrome: A systematic review and meta-analysis. Hum. Reprod. Updat. 2014, 20, 748–758. [Google Scholar] [CrossRef]
- Pillay, O.; Fong, L.W.T.; Crow, J.; Benjamin, E.; Mould, T.; Atiomo, W.; Menon, P.; Leonard, A.; Hardiman, P. The association between polycystic ovaries and endometrial cancer. Hum. Reprod. 2005, 21, 924–929. [Google Scholar] [CrossRef] [PubMed]
- Purwar, A.; Nagpure, S. Insulin Resistance in Polycystic Ovarian Syndrome. Cureus 2022, 14, e30351. [Google Scholar] [CrossRef]
- Lukanova, A.; Lundin, E.; Micheli, A.; Arslan, A.; Ferrari, P.; Rinaldi, S.; Krogh, V.; Lenner, P.; Shore, R.E.; Biessy, C.; et al. Circulating levels of sex steroid hormones and risk of endometrial cancer in postmenopausal women. Int. J. Cancer 2003, 108, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Pettersson, B.; Adami, H.; Bergström, R.; Johansson, E.D.B. Menstruation Span—A Time-Limited Risk Factor for Endometrial Carcinoma. Acta Obstet. Gynecol. Scand. 1986, 65, 247–255. [Google Scholar] [CrossRef]
- Felix, A.S.; Cook, L.S.; Gaudet, M.M.; Rohan, T.E.; Schouten, L.J.; Setiawan, V.W.; Wise, L.A.; Anderson, K.E.; Bernstein, L.; De Vivo, I.; et al. The etiology of uterine sarcomas: A pooled analysis of the epidemiology of endometrial cancer consortium. Br. J. Cancer 2013, 108, 727–734. [Google Scholar] [CrossRef]
- Wu, Y.; Sun, W.; Liu, H.; Zhang, D. Age at Menopause and Risk of Developing Endometrial Cancer: A Meta-Analysis. BioMed Res. Int. 2019, 2019, 8584130. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Tong, M.; Guo, F.; Lau, S.; Zhao, M. Parity Correlates with the Timing of Developing Endometrial Cancer, But Not Subtype of Endometrial Cancer. J. Cancer 2015, 6, 1087–1092. [Google Scholar] [CrossRef]
- Pfeiffer, R.M.; Mitani, A.; Landgren, O.; Ekbom, A.; Kristinsson, S.Y.; Björkholm, M.; Biggar, R.J.; Brinton, L.A. Timing of births and endometrial cancer risk in Swedish women. Cancer Causes Control 2009, 20, 1441–1449. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.P.; Cook, L.S.; Weiderpass, E.; Adami, H.-O.; Anderson, K.E.; Cai, H.; Cerhan, J.R.; Clendenen, T.V.; Felix, A.S.; Friedenreich, C.M.; et al. Infertility and incident endometrial cancer risk: A pooled analysis from the epidemiology of endometrial cancer consortium (E2C2). Br. J. Cancer 2015, 112, 925–933. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.T. Reproductive Factors and the Risk of Endometrial Cancer. Int. J. Gynecol. Cancer 2014, 24, 384–393. [Google Scholar] [CrossRef]
- Bokhman, J.V. Two pathogenetic types of endometrial carcinoma. Gynecol. Oncol. 1983, 15, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Herrington, S. WHO classification of tumours editorial board female genital tumours. Int. Agency Res. Cancer 2000, 5. [Google Scholar]
- WHO Classification of Tumours of Female Reproductive Organs. WHO Classification of Tumours, 4th Edition, Volume 6. Available online: https://publications.iarc.fr/Book-And-Report-Series/Who-Classification-Of-Tumours/WHO-Classification-Of-Tumours-Of-Female-Reproductive-Organs-2014 (accessed on 10 October 2024).
- Makker, V.; MacKay, H.; Ray-Coquard, I.; Levine, D.A.; Westin, S.N.; Aoki, D.; Oaknin, A. Endometrial cancer. Nat. Rev. Dis. Prim. 2021, 7, 88. [Google Scholar] [CrossRef] [PubMed]
- Cancer Genome Atlas Research Network; Kandoth, C.; Schultz, N.; Cherniack, A.D.; Akbani, R.; Liu, Y.; Shen, H.; Robertson, A.G.; Pashtan, I.; Shen, R.; et al. Integrated genomic characterization of endometrial carcinoma. Nature 2013, 497, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Jamieson, A.; McAlpine, J.N. Molecular Profiling of Endometrial Cancer from TCGA to Clinical Practice. J. Natl. Compr. Cancer Netw. 2023, 21, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Kommoss, S.; McConechy, M.K.; Kommoss, F.; Leung, S.; Bunz, A.; Magrill, J.; Britton, H.; Grevenkamp, F.; Karnezis, A.; Yang, W.; et al. Final validation of the ProMisE molecular classifier for endometrial carcinoma in a large population-based case series. Ann. Oncol. 2018, 29, 1180–1188. [Google Scholar] [CrossRef] [PubMed]
- Talhouk, A.; McConechy, M.K.; Leung, S.; Yang, W.; Lum, A.; Senz, J.; Boyd, N.; Pike, J.; Anglesio, M.; Kwon, J.S.; et al. Confirmation of ProMisE: A simple, genomics-based clinical classifier for endometrial cancer. Cancer 2017, 123, 802–813. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wu, S.F.; Bao, W. Molecular subtypes of endometrial cancer: Implications for adjuvant treatment strategies. Int. J. Gynecol. Obstet. 2023, 164, 436–459. [Google Scholar] [CrossRef] [PubMed]
- Bonneville, R.; Krook, M.A.; Kautto, E.A.; Miya, J.; Wing, M.R.; Chen, H.Z.; Reeser, J.W.; Yu, L.; Roychowdhury, S. Landscape of microsatellite instability across 39 cancer types. JCO Precis. Oncol. 2017, 1, 1–15. [Google Scholar] [CrossRef]
- León-Castillo, A.; Britton, H.; McConechy, M.K.; McAlpine, J.N.; Nout, R.; Kommoss, S.; Brucker, S.Y.; Carlson, J.W.; Epstein, E.; Rau, T.T.; et al. Interpretation of somatic POLE mutations in endometrial carcinoma. J. Pathol. 2019, 250, 323–335. [Google Scholar] [CrossRef] [PubMed]
- Tunnage, I.; Stasenko, M.; Ashley, C.; Rubinstein, M.; Latham, A.; Mueller, J.; Leitao, M.; Friedman, C.; Makker, V.; Soslow, R.; et al. Clinical outcomes of patients with pole mutated endometrioid endometrial cancer. Gynecol. Oncol. 2019, 153, 194–202. [Google Scholar] [CrossRef]
- Piulats, J.M.; Matias-Guiu, X. Immunotherapy in Endometrial Cancer: In the Nick of Time. Clin. Cancer Res. 2016, 22, 5623–5625. [Google Scholar] [CrossRef] [PubMed]
- Stelloo, E.; Nout, R.A.; Osse, E.M.; Jürgenliemk-Schulz, I.J.; Jobsen, J.J.; Lutgens, L.C.; van der Steen-Banasik, E.M.; Nijman, H.W.; Putter, H.; Bosse, T.; et al. Improved Risk Assessment by Integrating Molecular and Clinicopathological Factors in Early-stage Endometrial Cancer—Combined Analysis of the PORTEC Cohorts. Clin. Cancer Res. 2016, 22, 4215–4224. [Google Scholar] [CrossRef]
- Mahdy, H.; Casey, M.J.; Crotzer, D. Endometrial Cancer; StatPearls Publishing: Treasure Island, FL, USA, 2023; Available online: http://europepmc.org/books/NBK525981 (accessed on 22 July 2024).
- Moreira, I.; Bartosch, C.; Teixeira, M.; Ferreira, M. Molecular Classification of Endometrial Carcinoma: Protocol for a Cohort Study. JMIR Res. Protoc. 2022, 11, e34461. [Google Scholar] [CrossRef] [PubMed]
- Costigan, D.C.; Dong, F.; Nucci, M.R.; Howitt, B.E. Clinicopathologic and Immunohistochemical Correlates of CTNNB1 Mutated Endometrial Endometrioid Carcinoma. Int. J. Gynecol. Pathol. 2020, 39, 119–127. [Google Scholar] [CrossRef]
- Berek, J.S.; Matias-Guiu, X.; Creutzberg, C.; Fotopoulou, C.; Gaffney, D.; Kehoe, S.; Lindemann, K.; Mutch, D.; Concin, N. FIGO staging of endometrial cancer: 2023. Int. J. Gynecol. Obstet. 2023, 162, 383–394. [Google Scholar] [CrossRef] [PubMed]
- Bosse, T.; Peters, E.E.; Creutzberg, C.L.; Jürgenliemk-Schulz, I.M.; Jobsen, J.J.; Mens, J.W.M.; Lutgens, L.C.; van der Steen-Banasik, E.M.; Smit, V.T.; Nout, R.A. Substantial lymph-vascular space invasion (LVSI) is a significant risk factor for recurrence in endometrial cancer—A pooled analysis of PORTEC 1 and 2 trials. Eur. J. Cancer 2015, 51, 1742–1750. [Google Scholar] [CrossRef]
- Ogunmuyiwa, J.; Williams, V. Emerging Advances in Endometrial Cancer: Integration of Molecular Classification into Staging for Enhanced Prognostic Accuracy and Implications for Racial Disparities. Cancers 2024, 16, 1172. [Google Scholar] [CrossRef]
- McCluggage, W.G. Pathologic Staging of Endometrial Carcinomas: Selected Areas of Difficulty. Adv. Anat. Pathol. 2018, 25, 71–84. [Google Scholar] [CrossRef]
- Zheng, W. Molecular Classification of Endometrial Cancer and the 2023 FIGO Staging: Exploring the Challenges and Opportunities for Pathologists. Cancers 2023, 15, 4101. [Google Scholar] [CrossRef] [PubMed]
- Female Genital Tumours. WHO Classification of Tumours, 5th Edition, Volume 4. WHO Classification of Tumours Editorial Board. 2020. Available online: https://publications.iarc.fr/Book-And-Report-Series/Who-Classification-Of-Tumours/Female-Genital-Tumours-2020 (accessed on 16 July 2024).
- Kim, C.H.; Soslow, R.A.; Park, K.J.; Barber, E.L.; Khoury-Collado, F.; Barlin, J.N.; Sonoda, Y.; Hensley, M.L.; Barakat, R.R.; Abu-Rustum, N.R. Pathologic Ultrastaging Improves Micrometastasis Detection in Sentinel Lymph Nodes During Endometrial Cancer Staging. Int. J. Gynecol. Cancer 2013, 23, 964–970. [Google Scholar] [CrossRef]
- WHO. Human Papillomavirus and Cancer. 2014. Available online: https://www.who.int/news-room/fact-sheets/detail/human-papilloma-virus-and-cancer (accessed on 17 July 2024).
- de Martel, C.; Georges, D.; Bray, F.; Ferlay, J.; Clifford, G.M. Global burden of cancer attributable to infections in 2018: A worldwide incidence analysis. Lancet Glob. Health 2020, 8, e180–e190. [Google Scholar] [CrossRef] [PubMed]
- de Villiers, E.-M. Cross-roads in the classification of papillomaviruses. Virology 2013, 445, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Burd, E.M. Human Papillomavirus and Cervical Cancer. Clin. Microbiol. Rev. 2003, 16, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Szymonowicz, K.A.; Chen, J. Biological and clinical aspects of HPV-related cancers. Cancer Biol. Med. 2020, 17, 864–878. [Google Scholar] [CrossRef]
- Nguyen, H.P.; Ramírez-Fort, M.K.; Rady, P.L. The Biology of Human Papillomaviruses. In Current Problems in Dermatology; Ramírez-Fort, M.K., Khan, F., Rady, P.L., Tyring, S.K., Eds.; S. Karger: Basel, Switzerland, 2014; Volume 45, pp. 19–32. [Google Scholar] [CrossRef]
- Giles, S. Transmission of HPV. CMAJ Can. Med. Assoc. J. 2003, 168, 1391. [Google Scholar]
- Stubenraucha, F.; Laimins, L.A. Human papillomavirus life cycle: Active and latent phases. Semin. Cancer Biol. 1999, 9, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Pyeon, D.; Pearce, S.M.; Lank, S.M.; Ahlquist, P.; Lambert, P.F. Establishment of Human Papillomavirus Infection Requires Cell Cycle Progression. PLOS Pathog. 2009, 5, e1000318. [Google Scholar] [CrossRef] [PubMed]
- Dyson, N.; Howley, P.M.; Münger, K.; Harlow, E. The Human Papilloma Virus-16 E7 Oncoprotein Is Able to Bind to the Retinoblastoma Gene Product. Science 1989, 243, 934–937. [Google Scholar] [CrossRef] [PubMed]
- Caldeira, S.; Dong, W.; Tommasino, M. Analysis of E7/Rb associations. Methods Mol. Med. 2005, 119, 363–379. [Google Scholar] [CrossRef] [PubMed]
- Jensen, J.E.; Becker, G.L.; Jackson, J.B.; Rysavy, M.B. Human Papillomavirus and Associated Cancers: A Review. Viruses 2024, 16, 680. [Google Scholar] [CrossRef]
- Doorbar, J.; Quint, W.; Banks, L.; Bravo, I.G.; Stoler, M.; Broker, T.R.; Stanley, M.A. The Biology and Life-Cycle of Human Papillomaviruses. Vaccine 2012, 30 (Suppl. S5), F55–F70. [Google Scholar] [CrossRef] [PubMed]
- Rommel, O.; Dillner, J.; Fligge, C.; Bergsdorf, C.; Wang, X.; Selinka, H.; Sapp, M. Heparan sulfate proteoglycans interact exclusively with conformationally intact HPV L1 assemblies: Basis for a virus-like particle ELISA. J. Med. Virol. 2004, 75, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Couchman, J.R.; Chen, L.; Woods, A. Syndecans and cell adhesion. Int. Rev. Cytol. 2001, 207, 113–150. [Google Scholar] [CrossRef] [PubMed]
- Shafti-Keramat, S.; Handisurya, A.; Kriehuber, E.; Meneguzzi, G.; Slupetzky, K.; Kirnbauer, R. Different Heparan Sulfate Proteoglycans Serve asCellular Receptors for HumanPapillomaviruses. J. Virol. 2003, 77, 13125–13135. [Google Scholar] [CrossRef]
- Yang, Y.; Yaccoby, S.; Liu, W.; Langford, J.K.; Pumphrey, C.Y.; Theus, A.; Epstein, J.; Sanderson, R.D. Soluble syndecan-1 promotes growth of myeloma tumors in vivo. Blood 2002, 100, 610–617. [Google Scholar] [CrossRef] [PubMed]
- Asuthkar, S.; Velpula, K.K.; Nalla, A.K.; Gogineni, V.R.; Gondi, C.S.; Rao, J.S. Irradiation-induced angiogenesis is associated with an MMP-9-miR-494-syndecan-1 regulatory loop in medulloblastoma cells. Oncogene 2013, 33, 1922–1933. [Google Scholar] [CrossRef]
- Oh, J.-H.; Lee, H.-S.; Park, S.-H.; Ryu, H.-S.; Min, C.K. Syndecan-1 Overexpression Promotes Tumor Growth and Angiogenesis in an Endometrial Cancer Xenograft Model. Int. J. Gynecol. Cancer 2010, 20, 751–756. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S.A.; Yip, G.W.; Stock, C.; Pan, J.; Neubauer, C.; Poeter, M.; Pupjalis, D.; Koo, C.Y.; Kelsch, R.; Schüle, R.; et al. Targeting of syndecan-1 by microRNA miR-10b promotes breast cancer cell motility and invasiveness via a Rho-GTPase- and E-cadherin-dependent mechanism. Int. J. Cancer 2012, 131, E884–E896. [Google Scholar] [CrossRef] [PubMed]
- Szatmári, T.; Mundt, F.; Kumar-Singh, A.; Möbus, L.; Ötvös, R.; Hjerpe, A.; Dobra, K. Molecular targets and signaling pathways regulated by nuclear translocation of syndecan-1. BMC Cell Biol. 2017, 18, 34. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S.A.; Hassan, H.; Vilardo, L.; Kumar, S.K.; Kumar, A.V.; Kelsch, R.; Schneider, C.; Kiesel, L.; Eich, H.T.; Zucchi, I.; et al. Syndecan-1 (CD138) Modulates Triple-Negative Breast Cancer Stem Cell Properties via Regulation of LRP-6 and IL-6-Mediated STAT3 Signaling. PLoS ONE 2013, 8, e85737. [Google Scholar] [CrossRef]
- Doorbar, J.; Egawa, N.; Griffin, H.; Kranjec, C.; Murakami, I. Human papillomavirus molecular biology and disease association. Rev. Med. Virol. 2015, 25 (Suppl. S1), 2–23. [Google Scholar] [CrossRef] [PubMed]
- Joyce, J.G.; Tung, J.-S.; Przysiecki, C.T.; Cook, J.C.; Lehman, E.D.; Sands, J.A.; Jansen, K.U.; Keller, P.M. The L1 Major Capsid Protein of Human Papillomavirus Type 11 Recombinant Virus-like Particles Interacts with Heparin and Cell-surface Glycosaminoglycans on Human Keratinocytes. J. Biol. Chem. 1999, 274, 5810–5822. [Google Scholar] [CrossRef] [PubMed]
- Kines, R.C.; Thompson, C.D.; Lowy, D.R.; Schiller, J.T.; Day, P.M. The initial steps leading to papillomavirus infection occur on the basement membrane prior to cell surface binding. Proc. Natl. Acad. Sci. USA 2009, 106, 20458–20463. [Google Scholar] [CrossRef]
- DiGiuseppe, S.; Bienkowska-Haba, M.; Hilbig, L.; Sapp, M. The nuclear retention signal of HPV16 L2 protein is essential for incoming viral genome to transverse the trans-Golgi network. Virology 2014, 458–459, 93–105. [Google Scholar] [CrossRef] [PubMed]
- McBride, A.A. The papillomavirus E2 proteins. Virology 2013, 445, 57–79. [Google Scholar] [CrossRef] [PubMed]
- Ashrafi, G.H.; Haghshenas, M.R.; Marchetti, B.; O’Brien, P.M.; Campo, M.S. E5 protein of human papillomavirus type 16 selectively downregulates surface HLA class I. Int. J. Cancer 2004, 113, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Miura, S.; Kawana, K.; Schust, D.J.; Fujii, T.; Yokoyama, T.; Iwasawa, Y.; Nagamatsu, T.; Adachi, K.; Tomio, A.; Tomio, K.; et al. CD1d, a Sentinel Molecule Bridging Innate and Adaptive Immunity, Is Downregulated by the Human Papillomavirus (HPV) E5 Protein: A Possible Mechanism for Immune Evasion by HPV. J. Virol. 2010, 84, 11614–11623. [Google Scholar] [CrossRef]
- Zhang, B.; Li, P.; Wang, E.; Brahmi, Z.; Dunn, K.W.; Blum, J.S.; Roman, A. The E5 protein of human papillomavirus type 16 perturbs MHC class II antigen maturation in human foreskin keratinocytes treated with interferon-γ. Virology 2003, 310, 100–108. [Google Scholar] [CrossRef]
- Scott, M.L.; Woodby, B.L.; Ulicny, J.; Raikhy, G.; Orr, A.W.; Songock, W.K.; Bodily, J.M. Human Papillomavirus 16 E5 Inhibits Interferon Signaling and Supports Episomal Viral Maintenance. J. Virol. 2020, 94, e01582-19. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.-M.; Kim, S.-H.; Cho, E.-A.; Song, Y.-S.; Kim, W.-H.; Juhnn, Y.-S. Human papillomavirus type 16 E5 protein inhibits hydrogen peroxide-induced apoptosis by stimulating ubiquitin-proteasome-mediated degradation of Bax in human cervical cancer cells. Carcinog. 2009, 31, 402–410. [Google Scholar] [CrossRef] [PubMed]
- Kabsch, K.; Mossadegh, N.; Kohl, A.; Komposch, G.; Schenkel, J.; Alonso, A.; Tomakidi, P. The HPV-16 E5 Protein Inhibits TRAIL- and FasL-Mediated Apoptosis in Human Keratinocyte Raft Cultures. Intervirology 2004, 47, 48–56. [Google Scholar] [CrossRef]
- Drews, C.M.; Case, S.; Pol, S.B.V. E6 proteins from high-risk HPV, low-risk HPV, and animal papillomaviruses activate the Wnt/β-catenin pathway through E6AP-dependent degradation of NHERF1. PLOS Pathog. 2019, 15, e1007575. [Google Scholar] [CrossRef] [PubMed]
- Borggrefe, T.; Liefke, R. Fine-tuning of the intracellular canonical Notch signaling pathway. Cell Cycle 2012, 11, 264–276. [Google Scholar] [CrossRef]
- Bray, S. Notch signalling: A simple pathway becomes complex. Nat. Rev. Mol. Cell Biol. 2006, 7, 678–689. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Xue, C.; Zeng, Y.; Yuan, X.; Chu, Q.; Jiang, S.; Wang, J.; Zhang, Y.; Zhu, D.; Li, L. Notch signaling pathway in cancer: From mechanistic insights to targeted therapies. Signal Transduct. Target. Ther. 2024, 9, 1–37. [Google Scholar] [CrossRef] [PubMed]
- Talora, C.; Sgroi, D.C.; Crum, C.P.; Dotto, G.P. Specific down-modulation of Notch1 signaling in cervical cancer cells is required for sustained HPV-E6/E7 expression and late steps of malignant transformation. Genes Dev. 2002, 16, 2252–2263. [Google Scholar] [CrossRef] [PubMed]
- Rangarajan, A.; Syal, R.; Selvarajah, S.; Chakrabarti, O.; Sarin, A.; Krishna, S. Activated Notch1 Signaling Cooperates with Papillomavirus Oncogenes in Transformation and Generates Resistance to Apoptosis on Matrix Withdrawal through PKB/Akt. Virology 2001, 286, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Nyman, P.E.; Buehler, D.; Lambert, P.F. Loss of Function of Canonical Notch Signaling Drives Head and Neck Carcinogenesis. Clin. Cancer Res. 2018, 24, 6308–6318. [Google Scholar] [CrossRef] [PubMed]
- Altomare, D.A.; Khaled, A.R. Homeostasis and the Importance for a Balance Between AKT/mTOR Activity and Intracellular Signaling. Curr. Med. Chem. 2012, 19, 3748–3762. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.M.; Fuhrman, C.B.; Planelles, V.; Peltier, M.R.; Gaffney, D.K.; Soisson, A.P.; Dodson, M.K.; Tolley, H.D.; Green, C.L.; Zempolich, K.A. Phosphatidylinositol 3-Kinase Inhibition by LY294002 Radiosensitizes Human Cervical Cancer Cell Lines. Clin. Cancer Res. 2006, 12, 250–256. [Google Scholar] [CrossRef]
- Chang, Y.; Yu, C.; Lai, L.; Tsao, C.; Ho, K.; Yang, S.; Lee, H.; Cheng, Y.; Wu, T.; Shiau, M. Up-regulation of interleukin-17 expression by human papillomavirus type 16 E6 in nonsmall cell lung cancer. Cancer 2010, 116, 4800–4809. [Google Scholar] [CrossRef] [PubMed]
- Antonsson, A.; Forslund, O.; Ekberg, H.; Sterner, G.; Hansson, B.G. The Ubiquity and Impressive Genomic Diversity of Human Skin Papillomaviruses Suggest a Commensalic Nature of These Viruses. J. Virol. 2000, 74, 11636–11641. [Google Scholar] [CrossRef]
- Haedicke, J.; Iftner, T. Human papillomaviruses and cancer. Radiother. Oncol. 2013, 108, 397–402. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.-Q.; Yu, J.; Wang, R.-Q.; Sang, L. Clinical and epidemiological features of high-risk human papillomavirus infection in patients with cervical intraepithelial lesions. BMC Women’s Health 2023, 23, 468. [Google Scholar] [CrossRef]
- Davis, K.S.; Byrd, J.K.; Mehta, V.; Chiosea, S.I.; Kim, S.; Ferris, R.L.; Johnson, J.T.; Duvvuri, U. Occult Primary Head and Neck Squamous Cell Carcinoma: Utility of Discovering Primary Lesions. Otolaryngol. Neck Surg. 2014, 151, 272–278. [Google Scholar] [CrossRef]
- von Knebel Doeberitz, M.; Rittmüller, C.; Hausen, H.Z.; Dürst, M. Inhibition of tumorigenicity of cervical cancer cells in nude mice by HPV E6-E7 anti-sense RNA. Int. J. Cancer 1992, 51, 831–834. [Google Scholar] [CrossRef] [PubMed]
- Veldhuijzen, N.J.; Braunstein, S.L.; Vyankandondera, J.; Ingabire, C.; Ntirushwa, J.; Kestelyn, E.; Tuijn, C.; Wit, F.W.; Umutoni, A.; Uwineza, M.; et al. The epidemiology of human papillomavirus infection in HIV-positive and HIV-negative high-risk women in Kigali, Rwanda. BMC Infect. Dis. 2011, 11, 333. [Google Scholar] [CrossRef] [PubMed]
- Chin-Hong, P.V. Human Papillomavirus in Kidney Transplant Recipients. Semin. Nephrol. 2016, 36, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Bienkowska-Haba, M.; Patel, H.D.; Sapp, M. Target Cell Cyclophilins Facilitate Human Papillomavirus Type 16 Infection. PLOS Pathog. 2009, 5, e1000524. [Google Scholar] [CrossRef] [PubMed]
- Steinbach, A.; Riemer, A.B. Immune evasion mechanisms of human papillomavirus: An update. Int. J. Cancer 2018, 142, 224–229. [Google Scholar] [CrossRef]
- Hong, S.; Laimins, L.A. Manipulation of the innate immune response by human papillomaviruses. Virus Res. 2017, 231, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Cosper, P.F.; Bradley, S.; Luo, Q.; Kimple, R.J.; Luo, L. Biology of HPV Mediated Carcinogenesis and Tumor Progression. Semin. Radiat. Oncol. 2021, 31, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Klicznik, M.; Szenes-Nagy, A.; Campbell, D.; Gratz, I. Taking the lead—How keratinocytes orchestrate skin T cell immunity. Immunol. Lett. 2018, 200, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Tummers, B.; Van der Burg, S.H. High-Risk Human Papillomavirus Targets Crossroads in Immune Signaling. Viruses 2015, 7, 2485–2506. [Google Scholar] [CrossRef] [PubMed]
- Polz-Dacewicz, M.; Strycharz-Dudziak, M.; Dworzański, J.; Stec, A.; Kocot, J. Salivary and serum IL-10, TNF-α, TGF-β, VEGF levels in oropharyngeal squamous cell carcinoma and correlation with HPV and EBV infections. Infect. Agents Cancer 2016, 11, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Bermúdez-Morales, V.H.; Peralta-Zaragoza, O.; Alcocer-González, J.M.; Moreno, J.; Madrid-Marina, V. IL-10 expression is regulated by HPV E2 protein in cervical cancer cells. Mol. Med. Rep. 2011, 4, 369–375. [Google Scholar] [CrossRef][Green Version]
- Dai, W.; Gui, L.; Du, H.; Li, S.; Wu, R. The association of cervicovaginal Langerhans cells with clearance of human papillomavirus. Front. Immunol. 2022, 13, 918190. [Google Scholar] [CrossRef]
- Herfs, M.; Herman, L.; Hubert, P.; Minner, F.; Arafa, M.; Roncarati, P.; Henrotin, Y.; Boniver, J.; Delvenne, P. High expression of PGE2 enzymatic pathways in cervical (pre)neoplastic lesions and functional consequences for antigen-presenting cells. Cancer Immunol. Immunother. 2008, 58, 603–614. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, A.; Weinberg, V.; Darragh, T.; Smith-McCune, K. Evolving immunosuppressive microenvironment during human cervical carcinogenesis. Mucosal immunology 2008, 1, 412–420. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, L.; Li, Y.; Zhao, X. Research Progress on Tumor-Associated Macrophages and Inflammation in Cervical Cancer. BioMed Res. Int. 2020, 2020, 6842963. [Google Scholar] [CrossRef] [PubMed]
- Costa, N.L.; Valadares, M.C.; Souza, P.P.C.; Mendonça, E.F.; Oliveira, J.C.; Silva, T.A.; Batista, A.C. Tumor-associated macrophages and the profile of inflammatory cytokines in oral squamous cell carcinoma. Oral Oncol. 2012, 49, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Jianyi, D.; Haili, G.; Bo, Y.; Meiqin, Y.; Baoyou, H.; Haoran, H.; Fang, L.; Qingliang, Z.; Lingfei, H. Myeloid-derived suppressor cells cross-talk with B10 cells by BAFF/BAFF-R pathway to promote immunosuppression in cervical cancer. Cancer Immunol. Immunother. 2022, 72, 73–85. [Google Scholar] [CrossRef]
- Lee, S.-J.; Cho, Y.-S.; Cho, M.-C.; Shim, J.-H.; Lee, K.-A.; Ko, K.-K.; Choe, Y.K.; Park, S.-N.; Hoshino, T.; Kim, S.; et al. Both E6 and E7 Oncoproteins of Human Papillomavirus 16 Inhibit IL-18-Induced IFN-γ Production in Human Peripheral Blood Mononuclear and NK Cells. J. Immunol. 2001, 167, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Hajek, M.; Sewell, A.; Kaech, S.; Burtness, B.; Yarbrough, W.G.; Issaeva, N. TRAF3/CYLD mutations identify a distinct subset of human papillomavirus-associated head and neck squamous cell carcinoma. Cancer 2017, 123, 1778–1790. [Google Scholar] [CrossRef] [PubMed]
- Woodham, A.W.; Yan, L.; Skeate, J.G.; van der Veen, D.; Brand, H.E.; Wong, M.K.; Da Silva, D.M.; Kast, W.M. T cell ignorance is bliss: T cells are not tolerized by Langerhans cells presenting human papillomavirus antigens in the absence of costimulation. Papillomavirus Res. 2016, 2, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Leong, C.M.; Doorbar, J.; Nindl, I.; Yoon, H.-S.; Hibma, M.H. Loss of Epidermal Langerhans Cells Occurs in Human Papillomavirus α, γ, and μ but Not β Genus Infections. J. Investig. Dermatol. 2010, 130, 472–480. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-F.; Wang, S.-S.; Tang, Y.-J.; Chen, Y.; Zheng, M.; Tang, Y.-L.; Liang, X.-H. The Double-Edged Sword—How Human Papillomaviruses Interact with Immunity in Head and Neck Cancer. Front. Immunol. 2019, 10, 653. [Google Scholar] [CrossRef] [PubMed]
- zur Hausen, H. Papillomavirus infections—A major cause of human cancers. Biochim. Biophys. Acta (BBA) Rev. Cancer 1996, 1288, F55–F78. [Google Scholar] [CrossRef]
- Okunade, K.S. Human papillomavirus and cervical cancer. J. Obstet. Gynaecol. 2020, 40, 602–608. [Google Scholar] [CrossRef] [PubMed]
- De Martel, C.; Plummer, M.; Vignat, J.; Franceschi, S. Worldwide burden of cancer attributable to HPV by site, country and HPV type. Int. J. Cancer 2017, 141, 664–670. [Google Scholar] [CrossRef] [PubMed]
- Petrelli, F.; De Santi, G.; Rampulla, V.; Ghidini, A.; Mercurio, P.; Mariani, M.; Manara, M.; Rausa, E.; Lonati, V.; Viti, M.; et al. Human papillomavirus (HPV) types 16 and 18 infection and esophageal squamous cell carcinoma: A systematic review and meta-analysis. J. Cancer Res. Clin. Oncol. 2021, 147, 3011–3023. [Google Scholar] [CrossRef]
- Ragin, C.; Obikoya-Malomo, M.; Kim, S.; Chen, Z.; Flores-Obando, R.; Gibbs, D.; Koriyama, C.; Aguayo, F.; Koshiol, J.; Caporaso, N.E.; et al. HPV-associated lung cancers: An international pooled analysis. Carcinogenesis 2014, 35, 1267–1275. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, J.P.; González, C.; Parra, B.; Corvalán, A.H.; Tornesello, M.L.; Eizuru, Y.; Aguayo, F. Functional Interaction between Human Papillomavirus Type 16 E6 and E7 Oncoproteins and Cigarette Smoke Components in Lung Epithelial Cells. PLoS ONE 2012, 7, e38178. [Google Scholar] [CrossRef] [PubMed]
- Nogues, J.C.; Fassas, S.; Mulcahy, C.; Zapanta, P.E. Human Papillomavirus-Associated Head and Neck Cancer. J. Am. Board Fam. Med. 2021, 34, 832–837. [Google Scholar] [CrossRef]
- Forman, D.; de Martel, C.; Lacey, C.J.; Soerjomataram, I.; Lortet-Tieulent, J.; Bruni, L.; Vignat, J.; Ferlay, J.; Bray, F.; Plummer, M.; et al. Global Burden of Human Papillomavirus and Related Diseases. Vaccine 2012, 30 (Suppl. S5), F12–F23. [Google Scholar] [CrossRef]
- Wira, C.R.; Rodriguez-Garcia, M.; Patel, M.V. The role of sex hormones in immune protection of the female reproductive tract. Nat. Rev. Immunol. 2015, 15, 217–230. [Google Scholar] [CrossRef]
- Yarbrough, V.L.; Winkle, S.; Herbst-Kralovetz, M.M. Antimicrobial peptides in the female reproductive tract: A critical component of the mucosal immune barrier with physiological and clinical implications. Hum. Reprod. Updat. 2014, 21, 353–377. [Google Scholar] [CrossRef]
- Blaskewicz, C.D.; Pudney, J.; Anderson, D.J. Structure and Function of Intercellular Junctions in Human Cervical and Vaginal Mucosal Epithelia. Biol. Reprod. 2011, 85, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Daunter, B.; Counsilman, C. Cervical mucus: Its structure and possible biological functions. Eur. J. Obstet. Gynecol. Reprod. Biol. 1980, 10, 141–161. [Google Scholar] [CrossRef]
- Eckmann, L.; Kagnoff, M.F.; Fierer, J. Epithelial cells secrete the chemokine interleukin-8 in response to bacterial entry. Infect. Immun. 1993, 61, 4569–4574. [Google Scholar] [CrossRef] [PubMed]
- Fahey, J.; Schaefer, T.; Channon, J.; Wira, C. Secretion of cytokines and chemokines by polarized human epithelial cells from the female reproductive tract. Hum. Reprod. 2005, 20, 1439–1446. [Google Scholar] [CrossRef]
- Zhao, C.; Yan, S.; Song, Y.; Xia, X. Roles of Antimicrobial Peptides in Gynecological Cancers. Int. J. Mol. Sci. 2022, 23, 10104. [Google Scholar] [CrossRef] [PubMed]
- Critchley, H.O.D.; Maybin, J.A.; Armstrong, G.M.; Williams, A.R.W. Physiology of the Endometrium and Regulation of Menstruation. Physiol. Rev. 2020, 100, 1149–1179. [Google Scholar] [CrossRef] [PubMed]
- King, A.E.; Critchley, H.O.; Kelly, R.W. Innate immune defences in the human endometrium. Reprod. Biol. Endocrinol. 2003, 1, 116. [Google Scholar] [CrossRef] [PubMed]
- Hirata, T.; Osuga, Y.; Hamasaki, K.; Hirota, Y.; Nose, E.; Morimoto, C.; Harada, M.; Takemura, Y.; Koga, K.; Yoshino, O.; et al. Expression of toll-like receptors 2, 3, 4, and 9 genes in the human endometrium during the menstrual cycle. J. Reprod. Immunol. 2007, 74, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Perez-Muñoz, M.E.; Arrieta, M.-C.; Ramer-Tait, A.E.; Walter, J. A critical assessment of the “sterile womb” and “in utero colonization” hypotheses: Implications for research on the pioneer infant microbiome. Microbiome 2017, 5, 48. [Google Scholar] [CrossRef]
- Chen, C.; Song, X.; Wei, W.; Zhong, H.; Dai, J.; Lan, Z.; Li, F.; Yu, X.; Feng, Q.; Wang, Z.; et al. The microbiota continuum along the female reproductive tract and its relation to uterine-related diseases. Nat. Commun. 2017, 8, 875. [Google Scholar] [CrossRef]
- Fedrizzi, E.N.M.; Villa, L.L.; de Souza, I.V.M.; Sebastião, A.P.M.M.; Urbanetz, A.A.; De Carvalho, N.S. Does Human Papillomavirus Play a Role in Endometrial Carcinogenesis? Int. J. Gynecol. Pathol. 2009, 28, 322–327. [Google Scholar] [CrossRef] [PubMed]
- Spurgeon, M.E.; den Boon, J.A.; Horswill, M.; Barthakur, S.; Forouzan, O.; Rader, J.S.; Beebe, D.J.; Roopra, A.; Ahlquist, P.; Lambert, P.F. Human papillomavirus oncogenes reprogram the cervical cancer microenvironment independently of and synergistically with estrogen. Proc. Natl. Acad. Sci. USA 2017, 114, E9076–E9085. [Google Scholar] [CrossRef] [PubMed]
- Bergeron, C.; Shah, K.; Daniel, R.; Ferenczy, A. Search for human papillomaviruses in normal, hyperplastic, and neoplastic endometria. Obstet. Gynecol. 1988, 72, 383–387. [Google Scholar] [PubMed]
- Bouziyane, A.; Lamsisi, M.; Benaguida, H.; Benhessou, M.; Ennachit, M.; Karroumi, M.E.; Ennaji, M.M. Detection of Human Papilloma Virus in Endometrial Cancers among Moroccan Women. Teikyo Med. J. 2022, 45, 3818–3823. [Google Scholar]
- Karadayi, N.; Gecer, M.; Kayahan, S.; Yamuc, E.; Onak, N.K.; Korkmaz, T.; Yavuzer, D. Association between human papillomavirus and endometrial adenocarcinoma. Med. Oncol. 2013, 30, 597. [Google Scholar] [CrossRef] [PubMed]
- Olesen, T.B.; Svahn, M.F.; Faber, M.T.; Duun-Henriksen, A.K.; Junge, J.; Norrild, B.; Kjaer, S.K. Prevalence of Human Papillomavirus in endometrial cancer: A systematic review and meta-analysis. Gynecol. Oncol. 2014, 134, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Hording, U.; Daugaard, S.; Visfeldt, J. Adenocarcinoma of the cervix and adenocarcinoma of the endometrium: Distinction with PCR-mediated detection of HPV DNA. APMIS 1997, 105, 313–316. [Google Scholar] [CrossRef]
- Mahmoud, M.M.; Rifat, A.G. Relation of Human Papilloma Virus Infection with Pre Malignant and Malignant Endometrial Lesions. Medico-Legal Updat. 2021, 21, 490–496. [Google Scholar] [CrossRef]
- Grabarek, B.O.; Ossowski, P.; Czarniecka, J.; Ożóg, M.; Prucnal, J.; Dziuba, I.; Ostenda, A.; Dziobek, K.; Boroń, D.; Peszek, W.; et al. Detection and Genotyping of Human Papillomavirus (HPV16/18), Epstein–Barr Virus (EBV), and Human Cytomegalovirus (HCMV) in Endometrial Endometroid and Ovarian Cancers. Pathogens 2023, 12, 397. [Google Scholar] [CrossRef] [PubMed]
- Giatromanolaki, A.; Sivridis, E.; Papazoglou, D.; Koukourakis, M.I.; Maltezos, E. Human Papillomavirus in Endometrial Adenocarcinomas: Infectious Agent or a Mere “Passenger”? Infect. Dis. Obstet. Gynecol. 2007, 2007, 60549. [Google Scholar] [CrossRef]
- Ibragimova, M.K.; Kokorina, E.V.; Tsyganov, M.M.; Churuksaeva, O.N.; Litviakov, N.V. Human papillomavirus and endometrial cancer (review of literature and meta-analysis). Tumors Female Reprod. Syst. 2021, 16, 91–99. [Google Scholar] [CrossRef]
- Abu-Lubad, M.A.; Jarajreh, D.A.; Helaly, G.F.; Alzoubi, H.M.; Haddadin, W.J.; Dabobash, M.D.; Albataineh, E.M.; Aqel, A.A.; Alnawaiseh, N.A. Human papillomavirus as an independent risk factor of invasive cervical and endometrial carcinomas in Jordan. J. Infect. Public Health 2019, 13, 613–618. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.-J.; Tsai, S.C.-S.; Huang, J.-Y.; Lee, M.-S.; Wang, P.-H.; Lin, F.C.-F. From Infection to Malignancy: Tracing the Impact of Human Papillomavirus on Uterine Endometrial Cancer in a Nationwide Population-Based Cohort Study. Viruses 2023, 15, 2314. [Google Scholar] [CrossRef] [PubMed]
- Fujita, M.; Shroyer, K.R.; Markham, N.; Inoue, M.; Iwamoto, S.; Kyo, S.; Enomoto, T. Association of human papillomavirus with malignant and premalignant lesions of the uterine endometrium. Hum. Pathol. 1995, 26, 650–658. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.-I.; Francoeur, A.A.; Kapp, D.S.; Caesar, M.A.P.; Huh, W.K.; Chan, J.K. Trends in Human Papillomavirus–Associated Cancers, Demographic Characteristics, and Vaccinations in the US, 2001-2017. JAMA Netw. Open 2022, 5, e222530. [Google Scholar] [CrossRef]
- Venuti, A.; Paolini, F. HPV Detection Methods in Head and Neck Cancer. Head Neck Pathol. 2012, 6, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.V.; Hwang, S.; Lee, S.W.; Jin, E.; Lee, M.-H. Detection of HPV 16 and 18 L1 genes by a nucleic acid amplification-free electrochemical biosensor powered by CRISPR/Cas9. Bioelectrochemistry 2024, 162, 108861. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Dou, Z.; Yu, S.; Wu, X.; Zhang, J.; Li, Z.; Zhang, L. Hepatitis B virus infection and the risk of gynecologic cancers: A systematic review and meta-analysis. Discov. Oncol. 2024, 15, 340. [Google Scholar] [CrossRef] [PubMed]
- Bai, W.; Wu, X.; Zhao, S.; Yu, Y.; Wang, Z.; Li, X.; Zhou, N. Incidence and risk factors of hepatitis E virus infection in women with gynecological tumors in Eastern China. PeerJ 2024, 12, e18747. [Google Scholar] [CrossRef]
- Benharroch, D.; Klinkovich, I.; Piura, B.; Shaco-Levy, R.; Gopas, J. Evidence of measles virus antigens and RNA in endometrial cancer. Eur. J. Obstet. Gynecol. Reprod. Biol. 2009, 147, 206–209. [Google Scholar] [CrossRef]
- Liu, J.; Qu, Y.; Li, Y.-Y.; Xu, Y.-L.; Yan, Y.-F.; Qin, H. Exploring prognostic microbiota markers in patients with endometrial carcinoma: Intratumoral insights. Heliyon 2024, 10, e27879. [Google Scholar] [CrossRef] [PubMed]
- Semertzidou, A.; Whelan, E.; Smith, A.; Ng, S.; Roberts, L.; Brosens, J.J.; Marchesi, J.R.; Bennett, P.R.; MacIntyre, D.A.; Kyrgiou, M. Microbial signatures and continuum in endometrial cancer and benign patients. Microbiome 2024, 12, 118. [Google Scholar] [CrossRef] [PubMed]
| Aggressiveness | Histological Type |
|---|---|
| Non-aggressive type | Endometrioid endometrial carcinoma (grades I and II) (EEC) |
| Aggressive types | Endometrioid endometrial carcinoma (grade III) (EEC) |
| Serous carcinoma (SC) | |
| Undifferentiated carcinoma (UC) | |
| Clear cell carcinoma (CCC) | |
| Carcinosarcoma (CS) | |
| Mixed carcinoma (MC) | |
| Other unusual types, mesonephric-like adenocarcinoma | |
| Gastrointestinal mucinous type carcinoma |
| Molecular Subgroup | Characteristics of the Subgroup | Prognosis |
|---|---|---|
| POLE ultra mutated subtype |
| Favorable prognosis |
| Microsatellite instability hypermutated subtype (MSI) |
| Intermediate prognosis |
| Copy-number low subtype |
| Intermediate prognosis |
| Copy-number high subtype |
| Worst prognosis |
| ProMisE Subgroup | Concordant TCGA Subgroup | Characteristics of the ProMisE Subgroup | References |
|---|---|---|---|
| MMR deficient subgroup (MMRd) | TCGA «MSI» subgroup |
| [51,52,53] |
| POLE mut subgroup | TCGA «POLE ultramutated» subgroup |
| [54,55] |
| p53 abn subgroup | TCGA «copy-number high» subgroup |
| [50,56,57] |
| No specific molecular profile subgroup (NSMP) | TCGA «copy-number low» subgroup |
| [58,59] |
| Aggressive Cancer | Endometrioid Endometrial Carcinomas (EECs) | Low-Grade Endometrioid Carcinoma (Grade I and II) | Characterized by Excessive Estrogen Exposure |
|---|---|---|---|
| Non-aggressive cancer | Non-endometrioid endometrial carcinomas (NEECs) | High-grade endometrioid carcinoma (grade III), serous carcinoma (SC), clear cell carcinoma (CCC), mixed carcinoma (MC), undifferentiated carcinoma (UC), carcinosarcoma (CS), other rare forms, including mesonephric-like, and gastrointestinal mucinous carcinomas | Characterized by the absence of excessive estrogen exposure |
| Lymph Node Status | Size | Prognosis |
|---|---|---|
| Micrometastasis | 0.2–2 mm and/or >200 cells | Unclear |
| Isolated tumor cells | ≥0.2 mm and ≤200 cells | Unclear |
| Macrometastasis | >2 mm | Unfavorable |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bichri, K.; El Ghanmi, A.; Kouhen, F.; Hamdi, S.; Fichtali, K.; El Mansouri, F.; El Bakkouri, J.; Ghazi, B. The Sibylline Relationship Between Human Papillomavirus and Endometrial Cancer: Scarcity of Strong Evidence Linking Both Conditions. Viruses 2025, 17, 607. https://doi.org/10.3390/v17050607
Bichri K, El Ghanmi A, Kouhen F, Hamdi S, Fichtali K, El Mansouri F, El Bakkouri J, Ghazi B. The Sibylline Relationship Between Human Papillomavirus and Endometrial Cancer: Scarcity of Strong Evidence Linking Both Conditions. Viruses. 2025; 17(5):607. https://doi.org/10.3390/v17050607
Chicago/Turabian StyleBichri, Khadija, Adil El Ghanmi, Fadila Kouhen, Salsabil Hamdi, Karima Fichtali, Fadoua El Mansouri, Jalila El Bakkouri, and Bouchra Ghazi. 2025. "The Sibylline Relationship Between Human Papillomavirus and Endometrial Cancer: Scarcity of Strong Evidence Linking Both Conditions" Viruses 17, no. 5: 607. https://doi.org/10.3390/v17050607
APA StyleBichri, K., El Ghanmi, A., Kouhen, F., Hamdi, S., Fichtali, K., El Mansouri, F., El Bakkouri, J., & Ghazi, B. (2025). The Sibylline Relationship Between Human Papillomavirus and Endometrial Cancer: Scarcity of Strong Evidence Linking Both Conditions. Viruses, 17(5), 607. https://doi.org/10.3390/v17050607






