Chemical Peeling Therapy Using Phenol for the Cervico-Vaginal Intraepithelial Neoplasia
Abstract
:1. Introduction
2. Materials and Methods
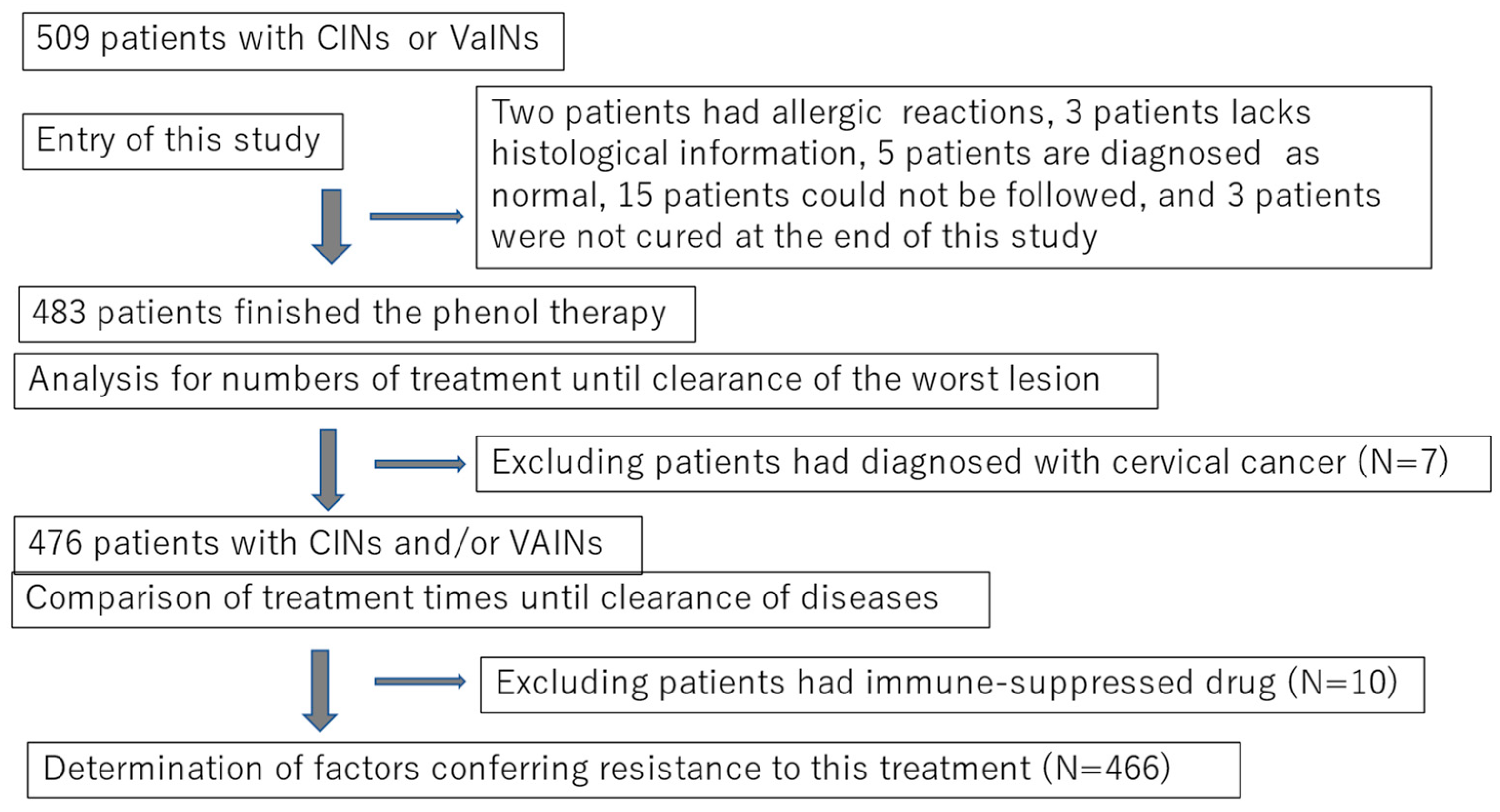
2.1. Subjects and Study Design
2.2. Demographic Characteristics of Subjects
2.3. Procedure of the Phenol Therapy
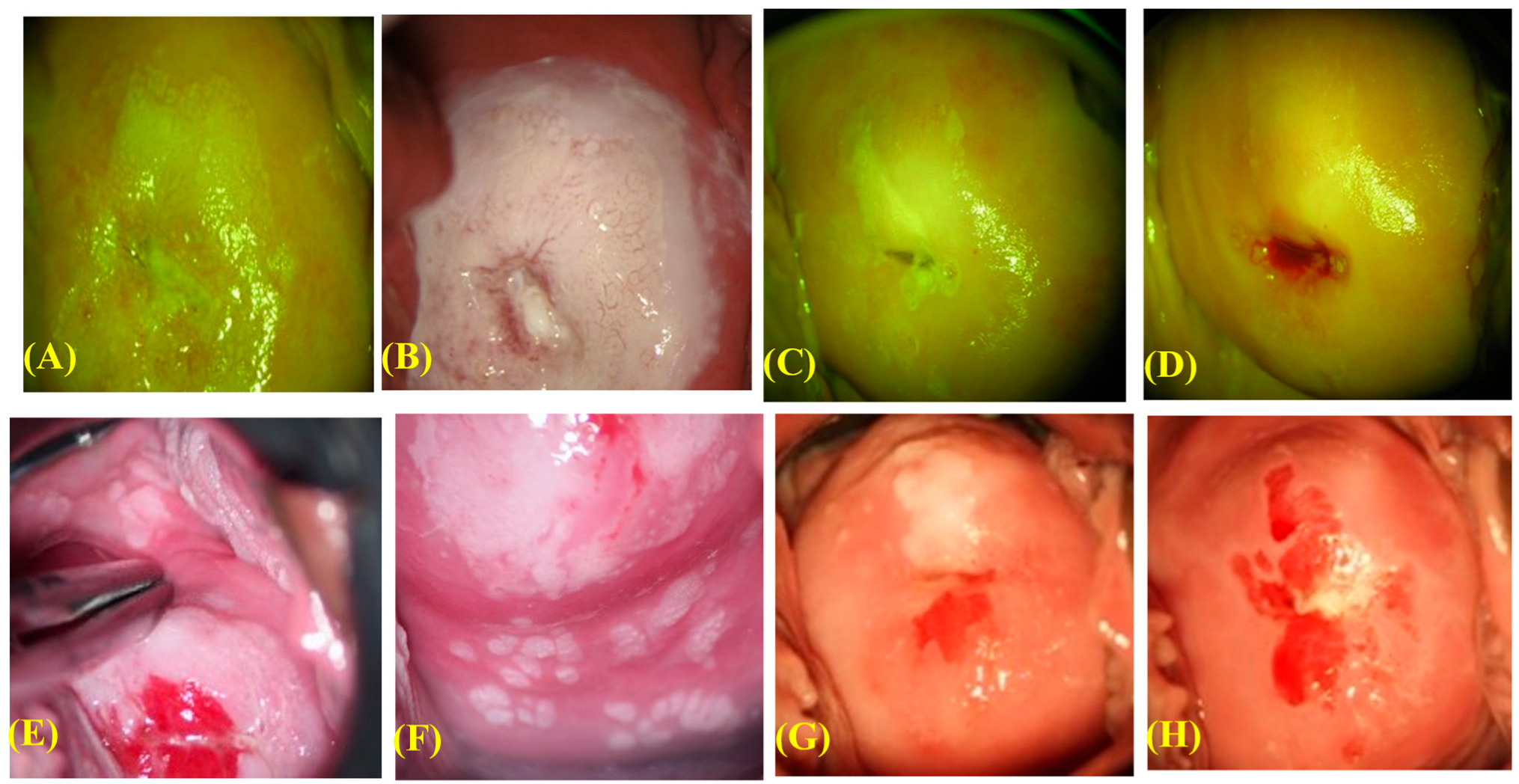
2.4. Pathological Diagnosis, HPV Testing, and Confirmation of Disease Clearance
2.5. Statistical Analysis
3. Results
3.1. Clinical Course of Cervico-Vaginal Abnormal Lesions with Phenol Therapy
3.2. Management of Therapy-Resistant and Recurrent Cases
3.3. Obstetric Outcomes in Subsequent Pregnancies
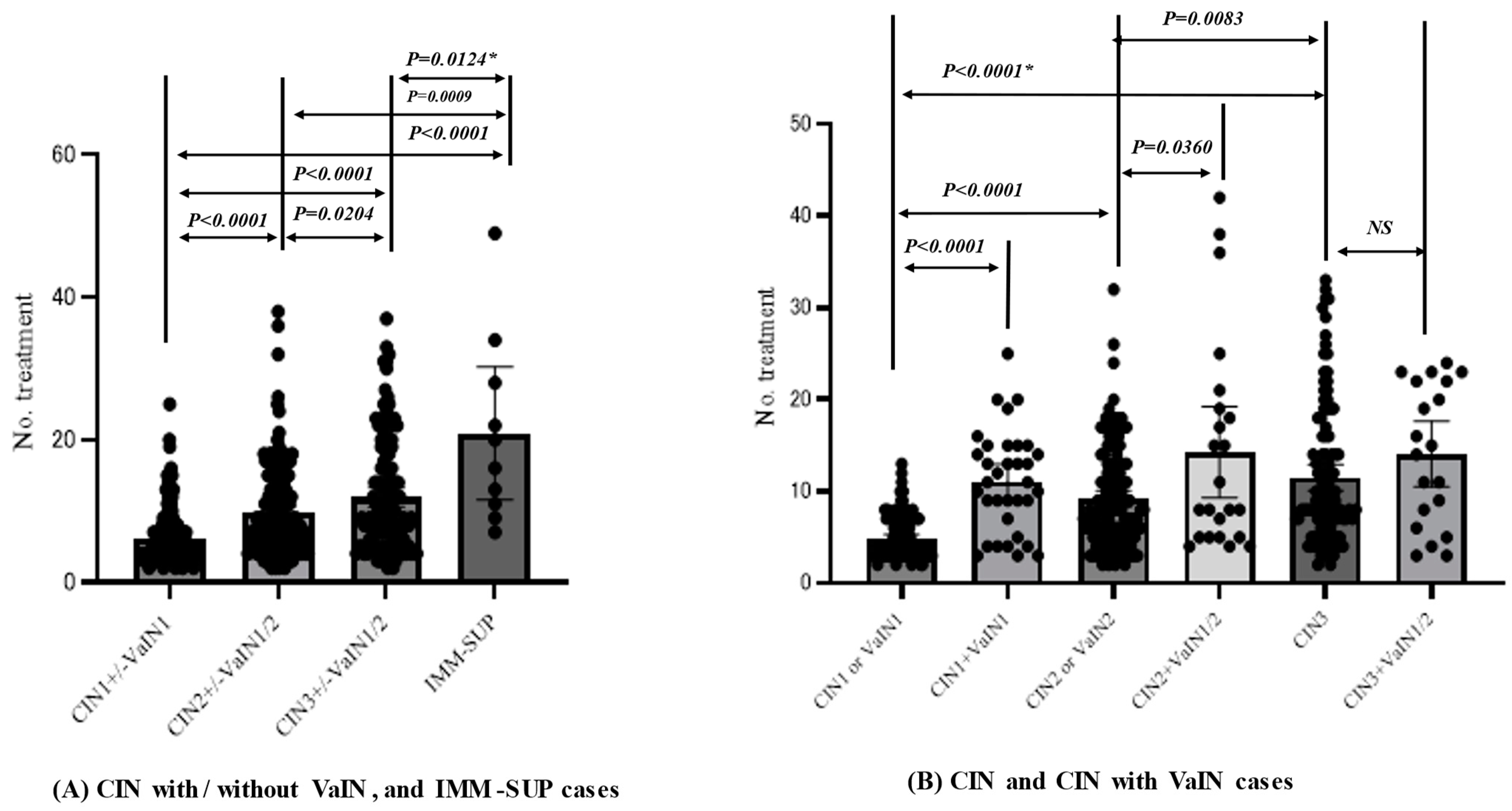
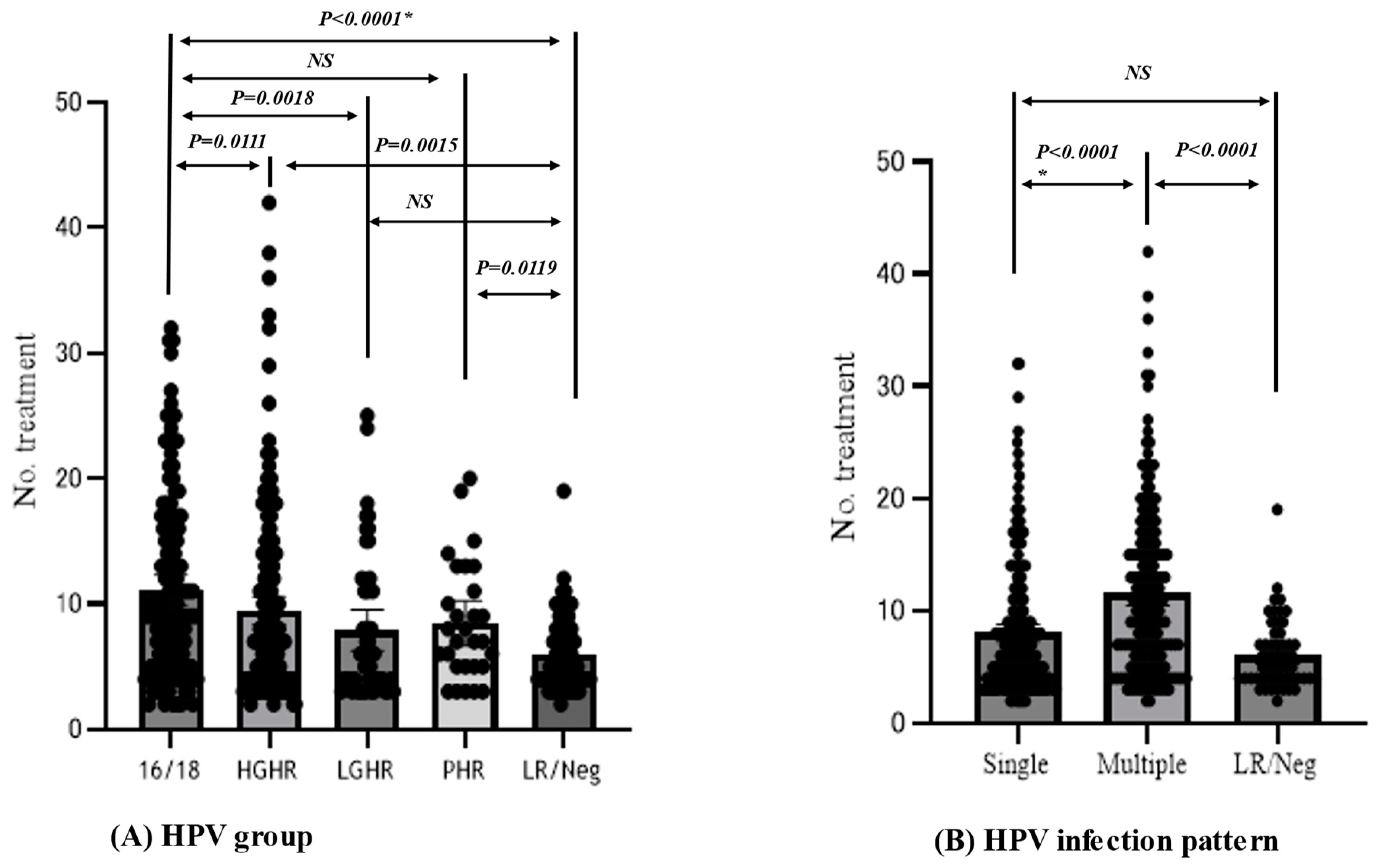
3.4. Factors Conferring Resistance to Phenol Therapy
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Ervik, M.; Lam, F.; Colombet, M.; Mery, L.; Piñeros, M. Global Cancer Observatory: Cancer Today; International Agency for Research on Cancer: Lyon, France, 2020; Available online: https://gco.iarc.fr/today (accessed on 26 September 2020).
- Cancer Statistics. Cancer Information Service, National Cancer Center, Japan (Vital Statistics of Japan, Ministry of Health, Labour and Welfare) for Incidence and Mortality. 2019. Available online: https://ganjoho.jp/public/qa_links/report/statistics/pdf/cancer_statistics_2019.pdf (accessed on 26 September 2023).
- World Health Organization (WHO). Global Strategy to Accelerate the Elimination of Cervical Cancer as a Public Health Problem; World Health Organization (WHO): Geneva, Switzerland, 2000. [Google Scholar]
- Kyrgiou, M.; Athanasiou, A.; Kalliala, I.E.J.; Paraskevaidi, M.; Mitra, A.; Martin-Hirsch, P.P.L.; Arbyn, M.; Bennett, P.; Paraskevaidis, E. Cochrane Gynaecological, Neuro-oncology and Orphan Cancer Group. Obstetric outcomes after conservative treatment for cervical intraepithelial lesions and early invasive disease. Cochrane Database Syst. Rev. 2017, 2017, CD012847. [Google Scholar] [CrossRef]
- Santesso, N.; Mustafa, R.A.; Wiercioch, W.; Kehar, R.; Gandhi, S.; Chen, Y.; Cheung, A.; Hopkins, J.; Khatib, R.; Ma, B.; et al. Systematic reviews and meta-analyses of benefits and harms of cryotherapy, LEEP, and cold knife conization to treat cervical intraepithelial neoplasia. Int. J. Gynecol. Obs. 2016, 132, 266–271. [Google Scholar] [CrossRef]
- Bosch, F.X.; Lorincz, A.; Munoz, N.; Meijer, C.J.L.M.; Shah, K.V. The causal relation between human papillomavirus and cervical cancer. J. Clin. Pathol. 2002, 55, 244–265. [Google Scholar] [CrossRef] [PubMed]
- Ho, G.Y.; Bierman, R.; Beardsley, L.; Chang, C.J.; Burk, R.D. Natural history of cervicovaginal papillomavirus infection in young women. N. Engl. J. Med. 1998, 338, 423–428. [Google Scholar] [CrossRef] [PubMed]
- Doorbar, J.; Quint, W.; Banks, L.; Bravo, I.G.; Stoler, M.; Broker, T.R.; Stanley, M.A. The biology and life-cycle of human papillomaviruses. Vaccine 2012, 30 (Suppl. 5), F55–F70. [Google Scholar] [CrossRef] [PubMed]
- Sasagawa, T.; Takagi, H.; Makinoda, S. Immune responses against human papillomavirus (HPV) infection and evasion of host defense in cervical cancer. J. Infect. Chemother. 2012, 18, 807–815. [Google Scholar] [CrossRef]
- Tang, J.; Li, M.; Zhao, C.; Shen, D.; Liu, L.; Zhang, X.; Wei, L. Therapeutic DNA Vaccines against HPV-Related Malignancies: Promising Leads from Clinical Trials. Viruses 2022, 14, 239. [Google Scholar] [CrossRef] [PubMed]
- Tindle, R.W. Immune evasion in human papillomavirus-associated cervical cancer. Nat. Rev. Cancer 2002, 2, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Truchuelo, M.; Cerdá, P.; Fernández, L.F. Chemical Peeling: A Useful Tool in the Office. Actas Dermosifiliogr. 2017, 108, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Banihashemi, M.; Pezeshkpoor, F.; Yazdanpanah, M.J.; Family, S. Efficacy of 80% phenol solution incomparison with cryotherapy in thetreatment of common warts of hands. Singapore Med. J. 2008, 49, 1035–1037. [Google Scholar]
- Geisler, S.; Speiser, S.; Speiser, L.; Heinze, G.; Rosenthal, A.; Speiser, P. Short-Term Efficacy of Trichloroacetic Acid in the Treatment of Cervical Intraepithelial Neoplasia. Obs. Gynecol. 2016, 127, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Sasagawa, T.; Maehama, T.; Ideta, K.; Irie, T.; Itoh, F.; J-HERS Study Group, Multicenter Study. Population-based study for human papillomavirus (HPV) infection in young women in Japan: A multicenter study by the Japanese human papillomavirus disease education research survey group (J-HERS). J. Med. Virol. 2016, 88, 324–335. [Google Scholar] [CrossRef] [PubMed]
- Okodo, M.; Okayama, K.; Teruya, K.; Sasagawa, T. Uniplex E6/E7 PCR method detecting E6 or E7 genes in 39 human papillomavirus types. J. Med. Virol. 2018, 90, 981–988. [Google Scholar] [CrossRef]
- Arbyn, M.; Redman, C.W.E.; Verdoodt, F.; Kyrgiou, M.; Tzafetas, M.; Ghaem-Maghami, S.; Petry, K.U.; Leeson, S.; Bergeron, C.; Nieminen, P.; et al. Incomplete excision of cervical precancer as a predictor of treatment failure: A systematic review and meta-analysis. Lancet Oncol. 2017, 18, 1665–1679. [Google Scholar] [CrossRef]
- Murakami, I.; Ohno, A.; Ikeda, M.; Yamashita, H.; Mikami, M.; Kobayashi, Y.; Nagase, S.; Yokoyama, M.; Enomoto, T.; Katabuchi, H. Analysis of pathological and clinical characteristics of cervical conization according to age group in Japan. Heliyon 2020, 6, e05193. [Google Scholar] [CrossRef]
- Kocken, M.; Helmerhorst, T.J.; Berkhof, J.; Louwers, J.A.; Nobbenhuis, M.A.; Bais, A.G.; Hogewoning, C.J.; Zaal, A.; Verheijen, R.H.; Snijders, P.J.; et al. Risk of recurrent high-grade cervical intraepithelial neoplasia after successful treatment: A long-term multi-cohort study. Lancet Oncol. 2011, 12, 441–450. [Google Scholar] [CrossRef]
- Kesic, V.; Carcopino, X.; Preti, M.; Vieira-Baptista, P.; Bevilacqua, F.; Bornstein, J.; Chargari, C.; Cruickshank, M.; Erzeneoglu, E.; Gallio, N.; et al. The European Society of Gynaecological Oncology (ESGO), the International Society for the Study of Vulvovaginal Disease (ISSVD), the European College for the Study of Vulval Disease (ECSVD), and the European Federation for Colposcopy (EFC) Consensus Statement on the Management of Vaginal Intraepithelial Neoplasia. J. Low. Genit. Tract. Dis. 2023, 27, 131–145. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Health, Labour and Welfare, Japan. Changes in Marriage Age in Japan. Available online: https://www.mhlw.go.jp/stf/wp/hakusyo/kousei/19/backdata/01-01-01-09.html (accessed on 26 September 2020).
- Kim, M.; Park, N.J.-Y.; Jeong, J.Y.; Park, J.Y. Multiple Human Papilloma Virus (HPV) Infections Are Associated with HSIL and Persistent HPV Infection Status in Korean Patients. Viruses 2021, 13, 1342. [Google Scholar] [CrossRef]
- Dickson, E.L.; Vogel, R.I.; Geller, M.A.; Downs, L.S., Jr. Cervical cytology and multiple type HPV infection: A study of 8182 women ages 31–65. Gynecol. Oncol. 2014, 133, 405–408. [Google Scholar] [CrossRef]
- Bruno, M.T.; Scalia, G.; Cassaro, N.; Boemi, S. Multiple HPV 16 infection with two strains: A possible marker of neoplastic progression. BMC Cancer 2020, 20, 444. [Google Scholar] [CrossRef]
- Castle, P.E.; Einstein, M.H.; Sahasrabuddhe, V.V. Cervical cancer prevention and control in women living with human immunodeficiency virus. CA Cancer J. Clin. 2021, 71, 505–526. [Google Scholar] [CrossRef] [PubMed]
- Clifford, G.M.; Tully, S.; Franceschi, S. Carcinogenicity of Human Papillomavirus (HPV) Types in HIV-Positive Women: A Meta-Analysis from HPV Infection to Cervical Cancer. Clin. Infect. Dis. 2017, 64, 1228–1235. [Google Scholar] [CrossRef] [PubMed]
- Gillison, M.L. Oropharyngeal cancer: A potential consequence of concomitant HPV and HIV infection. Curr. Opin. Oncol. 2009, 21, 439–444. [Google Scholar] [CrossRef]
- de Sanjosé, S.; Brotons, M.; Pavón, M.A. The natural history of human papillomavirus infection. Best Pract. Res. Clin. Obs. Gynaecol. 2018, 47, 2–13. [Google Scholar] [CrossRef]
- Su, B.; Qin, W.; Xue, F.; Wei, X.; Guan, Q.; Jiang, W.; Wang, S.; Xu, M.; Yu, S. The relation of passive smoking with cervical cancer: A systematic review and meta-analysis. Medicine 2018, 97, e13061. [Google Scholar] [CrossRef] [PubMed]
- Wen, Q.; Wang, X.; Lv, J.; Guo, Y.; Pei, P.; Yang, L.; Chen, Y.; Du, H.; Burgess, S.; China Kadoorie Biobank Collaborative Group; et al. Association between involuntary smoking and risk of cervical cancer in Chinese female never smokers: A prospective cohort study. Environ. Res. 2022, 212(Pt. C), 113371. [Google Scholar] [CrossRef]
- Chellappan, S. Smoking Cessation after Cancer Diagnosis and Enhanced Therapy Response: Mechanisms and Significance. Curr. Oncol. 2022, 29, 9956–9969. [Google Scholar] [CrossRef]
- Qiu, F.; Liang, C.-L.; Liu, H.; Zeng, Y.-Q.; Hou, S.; Huang, S.; Lai, X.; Da, Z. Impacts of cigarette smoking on immune responsiveness: Up and down or upside down? Oncotarget 2017, 8, 268–284. [Google Scholar] [CrossRef]
- Mitra, A.; MacIntyre, D.A.; Ntritsos, G.; Smith, A.; Tsilidis, K.K.; Marchesi, J.R.; Bennett, P.R.; Moscicki, A.B.; Kyrgiou, M. The vaginal microbiota associates with the regression of untreated cervical intraepithelial neoplasia 2 lesions. Nat. Commun. 2020, 11, 1999. [Google Scholar] [CrossRef]
- Saito, M.; Rajesh, A.; Innes, C.; van der Griend, R.; Fitzgerald, P.; Simcock, B.; Sykes, P.; Hibma, M. Blimp-1 is a prognostic indicator for progression of cervical intraepithelial neoplasia grade 2. J. Cancer Res. Clin. Oncol. 2022, 148, 1991–2002. [Google Scholar] [CrossRef]

| Monovariate Analysis | Multivariate Analysis | |||||
|---|---|---|---|---|---|---|
| Variable Factors | ORs | 95%CI | Probability | ORs | 95%CI | Probability |
| Age group | NS | p = 0.0344 (Wald test) | ||||
| 35–39 yrs vs. 16–29 yrs | 1.0 | 0.54–1.91 | NS | 3.6 | 1.37–9.26 | p = 0.0090 |
| 35–39 yrs vs. 30–34 yrs | 1.7 | 0.89–3.36 | NS | 4.5 | 165–12.4 | p = 0.0034 |
| 35–39 yrs vs. 40–44 yrs | 1.6 | 0.82–3.06 | NS | 3.4 | 1.29–9.02 | p = 0.0134 |
| 35–39 yrs vs. over 45 yrs | 1.4 | 0.71–2.77 | NS | 2.8 | 1.04–7.79 | p = 0.0415 |
| Grade of lesion | p < 0.0001(Wald test) | |||||
| CIN2 or/andVAIN1/2 vs. CIN1 or/and VAIN1 | 3.5 | 1.92–6.62 | p < 0.0001 | 3.4 | 1.58–7.31 | p = 0.0017 |
| CIN3 or/and VAIN1/2 vs. CIN1 or/and VAIN1 | 5.3 | 2.86–10.1 | p < 0.0001 | 7.8 | 3.29–18.6 | p < 0.0001 |
| CIN3 or/and VAIN1/2 vs. CIN2 or/andVAIN1/2 | 1.5 | 0.93–2.46 | NS | 2.3 | 1.14–4.64 | p = 0.0198 |
| HPV group (a) | NS (Wald test) | |||||
| HGHR/PHR vs. LGHR/ LR/ Neg | 4.6 | 2.44–9.81 | p < 0.0001 | 1.6 | 0.64–3.77 | NS |
| Multiple or Single type HPV infection | p = 0.0008 (Wald test) | |||||
| Multiple vs. Negative | 3.6 | 2.31–5.63 | p < 0.0001 | 3.1 | 1.59–5.85 | |
| Lesion area size (b) | NS (Wald test) | |||||
| Over 4 sections vs. 1–3 sections of Cx/Vag | 2.1 | 1.14–3.94 | p = 0.0162 | 1.3 | 0.63–2.78 | NS |
| Active/passive smoking | NS | |||||
| Direct/passive smoking vs. no smoking | 1.9 | 1.19–2.92 | p = 0.0063 | 1.4 | 0.76–2.62 | NS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maehama, T.; Shimada, S.; Sakamoto, J.; Shibata, T.; Fujita, S.; Takakura, M.; Takagi, H.; Sasagawa, T. Chemical Peeling Therapy Using Phenol for the Cervico-Vaginal Intraepithelial Neoplasia. Viruses 2023, 15, 2219. https://doi.org/10.3390/v15112219
Maehama T, Shimada S, Sakamoto J, Shibata T, Fujita S, Takakura M, Takagi H, Sasagawa T. Chemical Peeling Therapy Using Phenol for the Cervico-Vaginal Intraepithelial Neoplasia. Viruses. 2023; 15(11):2219. https://doi.org/10.3390/v15112219
Chicago/Turabian StyleMaehama, Toshiyuki, Sumire Shimada, Jinichi Sakamoto, Takeo Shibata, Satoko Fujita, Masahiro Takakura, Hiroaki Takagi, and Toshiyuki Sasagawa. 2023. "Chemical Peeling Therapy Using Phenol for the Cervico-Vaginal Intraepithelial Neoplasia" Viruses 15, no. 11: 2219. https://doi.org/10.3390/v15112219
APA StyleMaehama, T., Shimada, S., Sakamoto, J., Shibata, T., Fujita, S., Takakura, M., Takagi, H., & Sasagawa, T. (2023). Chemical Peeling Therapy Using Phenol for the Cervico-Vaginal Intraepithelial Neoplasia. Viruses, 15(11), 2219. https://doi.org/10.3390/v15112219







