Abstract
Orf virus (ORFV) is the type species of Parapoxvirus of the Poxviridae family that induces cutaneous pustular skin lesions in sheep and goats, and causes zoonotic infections in humans. Pattern recognition receptors (PRRs) sense pathogen-associated molecular patterns (PAMPs), leading to the triggering of the innate immune response through multiple signalling pathways involving type I interferons (IFNs). The major PAMPs generated during viral infection are nucleic acids, which are the most important molecules that are recognized by the host. The induction of type l IFNs leads to activation of the Janus kinase (JAK)-signal transducer activator of transcription (STAT) pathway, which results in the induction of hundreds of interferon-stimulated genes (ISGs), many of which encode proteins that have antiviral roles in eliminating virus infection and create an antiviral state. Genetic and functional analyses have revealed that ORFV, as found for other poxviruses, has evolved multiple immunomodulatory genes and strategies that manipulate the innate immune sensing response.
Keywords:
Poxvirus; parapoxvirus; orf virus; innate immunity; innate sensing; interferon; immune evasion 1. Introduction
The Parapoxvirus genus (family Poxviridae, subfamily Chordopoxvirus) includes Orf virus (ORFV), Pseudocowpoxvirus (PCPV), Bovine popular stomatitis virus (BPSV) and Parapoxvirus of red deer in New Zealand (PVNZ). ORFV is the type species of the Parapoxvirus genus and the causative agent of Orf disease, a contagious debilitating skin condition that induces pustular skin lesions in sheep and goats and causes zoonotic infection in humans [1,2]. The symptoms of Orf disease in humans start as erythema, vesicles, pustules, and finally scabs. The lesions typically resolve within 6 to 12 weeks. In addition, ORFV is known to cause severe disease in those that are immunocompromised [1,2]. In these cases, the virus causes large vascularized tumour-like lesions of the skin [3,4].
Poxviruses have large linear dsDNA genomes and the basic genetic structure of a poxvirus was first shown for vaccinia virus (VACV), which has a genome of approximately 190 kbp [5]. VACV is a member of the Orthopoxvirus genus, the prototype of the poxvirus family, and the most extensively studied of the poxviruses. Poxviruses encode almost all factors required for replication [6,7]. All other poxviruses are essentially similar, but the genetic differences mainly lie within the termini of the genome. The genomes of several ORFV strains have been fully sequenced [8,9]. It was predicted that the ORFV genome contains 132 genes and the distribution of these genes is typical of poxviruses, with the central region containing genes essential for its life cycle, and the terminal regions containing host range restriction or virulence genes that are non-essential for growth in cell culture [1,2,10,11].
ORFV infects skin-resident keratinocytes and replicates exclusively in the cytoplasm, causing a localized infection that persists for weeks [12,13,14]. Keratinocytes reside in the epidermal layer of skin, constituting 90% of epidermal cells. They act as immune sentinels and have the capability to activate the innate immune response [15,16,17,18,19,20]. Innate immunity is a first line of defence and an immediate non-specific response against viral infection. Although an immune response is generated against ORFV infection, the virus has the ability to repeatedly infect its host [21,22]. This strongly indicates that ORFV expresses factors that antagonize this response. In this review, our current knowledge of strategies employed by ORFV to antagonize the innate immune sensing response will be reviewed.
2. Innate Immune Sensing
Host cells adopt multiple defence mechanisms to respond to invading viruses. Innate immunity is a first line of defence and an immediate response against viral infection [23]. A key step in this response is the detection of virus infection. Sensing pathogens by innate immunity is mediated by host pattern recognition receptors (PRRs) that recognize pathogen-associated molecular patterns (PAMPs). The major PAMPs generated during viral infection are nucleic acids and are the most important molecules that are recognized by the host [24].
There are many receptors responsible for triggering the innate immune response and can be classified based on their subcellular localization and the distinct virus-derived molecules they recognize. C-type lectin receptors (CLRs) and Toll-like receptors (TLRs) are endosomal or membrane-bound receptors, whereas nucleotide-binding oligomerization domain (NOD)-like receptors (NLRs), AIM2-like receptors (ALRs), and retinoic acid inducible gene (RIG-I)-like receptors (RLRs) are cytosolic (Figure 1). Each class of these receptors activates specific signalling cascades, which, in turn, activate specific transcription factors to induce the expression of target genes, namely, type I IFNs and proinflammatory cytokines.
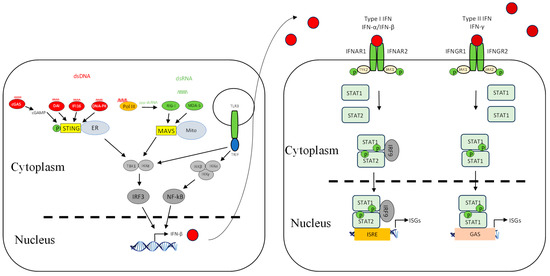
Figure 1.
Interferon induction and signalling pathways. Shown are DNA- and RNA-sensing mechanisms that lead to IFN signalling. The DNA sensors cGAS, DAI, IFI16, DNA-PK, and Pol III are localized in the cytosol and utilize the adaptor STING residing on the endoplasmic reticulum. They detect DNA in the cytosol and trigger STING-dependent signalling, leading to the activation of TBK1/IKKε and IRF3 that results in IFN-β expression. cGAS synthesizes the production of cGAMP dinucleotides that bind STING. RIG-like receptors (RLRs) are localized in the cytosol and utilize the adaptor MAVS. The RIG-I receptor senses short dsRNA and also ppp-dsRNA derived from polymerase III-transcribed poly (dA:dT), whereas MDA5 senses long dsRNA. Upon ligand binding, RLRs engage the adaptor MAVS located on the outer membrane of mitochondria, which leads to downstream signalling via TBK1/IKKε and thus phosphorylation of IRF3 followed by IFN expression. TLR3 is localized in the endosome and utilizes the adaptor TRIF. Upon recognizing dsRNA, it can lead to IFN-β expression through TBK1/IKKβ-IRF3 and the IKKα/IKKβ/IKKγ-NF-κB axis. Upon IFN induction, a second line of signalling is initiated in which the induced IFNs interact with IFN receptors, in an autocrine or paracrine manner, leading to transcription of a diverse set of genes called IFN-stimulated genes via the Janus kinase (JAK)-signal transducer activator of transcription (STAT) pathway. Red circle is type I IFN, darker red circle is type II IFN, dashed line is nuclear membrane.
Among those receptors, the RIG-I-like receptor family is the main cytosolic RNA sensor. It consists of three groups of molecules: RIG-I, melanoma differentiation associated gene 5 (MDA5), and laboratory of genetic and physiology 2 (LGP2) [25]. In addition, there are other molecules that act as sensors for foreign dsRNAs: protein kinase R (PKR) and 2′-5′-Oligoadenylate Synthetase (OAS)/RNaseL. They are present in most cell types at basal levels and involve the inhibition of protein synthesis and RNA degradation, respectively [26,27,28].
In addition, host cells employ a number of DNA sensors that can detect DNA in the cytosol (Figure 1). These include DNA-dependent activator of interferon-regulatory factor (DAI) [29], RNA Polymerase III/RIG-I (RNA Pol III) [30,31,32,33], PYHIN family protein (IFI16/p204) [34,35], DExD/H-Box Helicases (DDX) protein [36], DNA-dependent protein kinase (DNA-PK) [37], Cyclic GMP-AMP (2′3′-cGAMP) [38], and Cyclic GMP-AMP Synthase (cGAS) [39,40,41].
Once the sensors are activated, RIG-I and MDA5 interact with mitochondrial antiviral signalling protein (MAVS) [42,43,44], whereas cytosolic DNA sensors interact with stimulator of interferon genes (STINGs) [45,46] (Figure 1). The cytosolic PRR-mediated signalling pathways converge at the IKK-related serine/threonine kinases TBK1/IKKε, which then phosphorylate and activate IRF3 and IRF7 [47,48,49] (Figure 1). TLR9 recruits MyD88 to transmit cellular signalling, leading to the activation of IRF7 and NF-κB [24,50], whereas TLR3 recruits TRIF to mediate signalling, leading to IRF3 and NF-κB activation [51,52,53]. The complex of TBK1/IKKε and associated subunits is important for the activation of IRF3 and IRF7 [54], whereas the complex of IKKα/IKKβ/IKKγ is important for the activation of NF-κB. Upon IRF3/IRF7 and NF-κB activation, they translocate to the nucleus and bind to their respective regulatory elements, culminating in the induction of type I IFNs and inflammatory cytokines, respectively [55]. The activated factors translocate to the nucleus, where they bind to the promoter regions of IFNs and proinflammatory cytokines for activation.
Upon IFN induction, a second line of signalling is initiated in which the induced IFNs interact with IFN receptors (IFNRs), in an autocrine or paracrine manner, leading to the transcription of a diverse set of genes called IFN-stimulated genes (ISGs) via the Janus kinase (JAK)-signal transducer activator of transcription (STAT) pathway (Figure 1). These ISGs are involved in eliminating viral infection from infected cells and conferring resistance to neighbouring cells. The established antiviral state will inhibit viral replication at various stages [27,56]. Some ISGs are induced directly by viral infection, but less effectively than the IFN response itself; however, their induction is amplified significantly by IFNs [57,58,59,60].
Among all of the TLRs characterized to date, only TLR-3, -7, and -8 are known to detect viral RNA [50,61,62]. TLR-7 and TLR-8 recruit Myeloid differentiation primary-response gene 88 (MyD88) to transmit cellular signalling, leading to the activation of transcription factors including IFN regulatory factors (IRFs), nuclear factor kappa B (NF-κB), and activating transcription factor 2 (ATF2)/c-Jun [63], whereas TLR3 recruits TIR-domain-containing adapter-inducing interferon-β (TRIF) to mediate signalling, leading to the activation of two transcription factors, IRF3 and NF-κB, resulting in the induction of type I IFNs and proinflammatory cytokines [51,64,65]. TLR7 is mostly found in plasmacytoid dendritic cells, and TLR8 is mainly expressed in myeloid dendritic cells and monocytes. However, TLR3 is ubiquitously expressed and detects viral dsRNA generated during virus replication and its synthetic analogue Poly (I:C) [66]. TLRs are not important in other cell types for the production of type I IFNs in response to viral infection; instead, cytosolic RNA sensors are essential [67].
3. Detection of Parapoxviruses by Innate Immune Sensing Receptors
Poxviruses replicate in the cytoplasm of infected cells, making their nucleic acids targets of cytosolic PRRs. After the virion core is released into the cytoplasm and before complete uncoating, early transcribed mRNAs are extruded from the core into the cytoplasm to be translated [7,68]. Early gene expression then ceases when the core is completely uncoated, and the viral genome is released into the cytoplasm, in which intermediate gene transcription and replication of the genome is initiated. Both the exposed viral RNA and DNA become targets of cellular receptors. Little is known about the specific detection of parapoxviruses by innate immune sensing at this time; however, based on the findings for other poxviruses in particular VACV and DNA viruses, it is more than likely that the same cytosolic RNA receptors and DNA receptors will have a role.
3.1. Intracellular Detection of Viral RNA
During replication, poxviruses produce appreciable amounts of dsRNA in the cytoplasm as a result of convergent transcription [69,70,71,72]. In addition, the generation of dsRNA can be produced from dsDNA by cellular RNA polymerase III (Pol III) [30,32]. The role of Pol III on DNA virus sensing was shown to mediate an antiviral response against VACV [32]. Not surprisingly, dsRNA generated from poxvirus replication can be a target for cytosolic RNA sensors. VACV dsRNA was shown to be detected by RIG-I and MDA5, thus activating an antiviral response [73,74]. Furthermore, MYXV elicited an IFN response via RIG-I-dependent sensing [75]. Protein kinase receptor (PKR) is an intracellular sensor of stress manifested during viral infection. Virally produced dsRNA activates PKR, which arrests protein synthesis by phosphorylating the alpha subunit of the translation initiation factor elF2. In addition, PKR is a key component of the IFN antiviral response against poxviruses. It was shown that PKR induced MDA5-mediated IFN-β induction during VACVΔE3L infection [76]. Interestingly, a homolog of VACV-E3L is found in ORFV, ORF020, that has been shown to bind dsRNA and inhibit the activation of PKR [77,78]. In addition, OAS and RNase L are dsRNA sensors that have antiviral roles [79] that have been illustrated by the finding that RNase L knock-out mice were susceptible to VACV infection [80,81]. It is likely that the range of RNA sensors that detect orthopoxviruses will be important for parapoxvirus detection.
3.2. Intracellular Detection of Viral DNA
Several studies have shown that viral genomic DNA can elicit an IFN response, and cytosolic PRRs are more important in detecting DNA viruses in the cytoplasm [82,83,84]. Although a number of cytosolic DNA sensors have been shown to have a role in a DNA-dependent IFN response, only a few have been implicated in poxvirus recognition.
Of all the DNA receptors identified at this time, cGAS appears to be critical for poxvirus detection. cGAS is an essential STING-dependent cytosolic DNA receptor, as proven by the induction of type I IFNs being severely impaired in several cell types (fibroblasts, macrophages, and DCs) lacking cGAS [40]. cGAS is required to detect DNA viruses such as HSV-1, KSHV, ECTV, and VACV [40,41,46,85,86]. It was shown to be the most potent inducer of IFN-β expression in comparison with other cytosolic DNA receptors such as DAI, IFI16, and DDX41 [39,40,41]. As eluded to above, RNA Pol III is also considered to be a DNA sensor for some DNA viruses including HSV-1, EBV, and VACV [32,33]. IFI16 is a DNA sensor that shuttles from the nucleus to viral factories for viral DNA detection. HSV-1 and VACV infection, but not their transfected DNA, can activate IFI16 that leads to IFN-β induction via IRF3 [35,87]. DNA-PK, besides its DNA repair activity, can sense cytosolic DNA and initiate an immune response to VACV [37].
4. Innate Immune Evasion Strategies by ORFV
4.1. Evasion of RNA-Dependent Sensing
We have recently shown that ORFV employs a strategy that antagonizes the RNA-dependent signalling pathway of IFN induction. The virus potently inhibits the induction of IFN-β upon stimulation with dsRNA via RIG-I-dependent signalling [88]. The exact mechanism underlying this observation is yet to be elucidated, and whether the virus targets upstream at the sensing level or downstream within the signalling pathway. One of the ORFV factors that can be attributed to this dsRNA-dependent signalling inhibition is ORF020. As noted above, it is a homolog of the VACV interferon resistance gene E3L that binds dsRNA to prevent the activation of PKR and OAS (Figure 2) [77,78]. Its inhibitory effect on the dsRNA-mediated induction of type I IFN was demonstrated from ectopic vector expression [88,89]. Despite the fact that the exact mechanism of this inhibition is not known, it is likely to be similar to that of VACV E3L [90,91]. VACV E3L sequesters RNA and thus inhibits the dsRNA-mediated induction of type I IFNs (Figure 3) [33,92]. VACV K3L is a PKR inhibitor that has homology to the n-terminal region of eIF2α. It is believed that K3L acts as a pseudosubstrate for PKR in lieu of eIF2α (Figure 3) [93,94,95]. Interestingly ORFV does not have a homolog of this gene.
Poxviruses have co-evolved mechanisms to counteract the TLR-dependent response. VACV encodes two proteins known to counteract TLR-mediated signalling, A46R and A52R [96,97], neither of which are encoded by parapoxvirues. They have distinct modes of action and target different cellular proteins involved in TLR signalling (Figure 3). A46R is a TIR domain-containing protein that can associate with several TIR domain-containing adaptors, such as MyD88, TIRAP, TRIF, and TRAM, the cytoplasmic domains of TLRs, leading to the inhibition of TLR-induced NF-κB and IRF3 activation (Figure 3) [98]. A52R has the ability to inhibit TLR-induced NF-κB activation by interacting with IRAK2 and TRAF6 (Figure 3) [99].
Other strategies that poxviruses utilize to avoid RNA sensing is by modifying the structure of their RNA. They can cap the end of their newly synthesized mRNA to mimic cellular RNAs, or even decap them to prevent their accumulation and an antiviral response, as shown in VACV [100,101,102,103].
4.2. Evasion of DNA-Dependent Sensing
Primary keratinocytes, in which ORFV replicates, express a number of cytosolic DNA sensors such as ZBP1 (DAI), IFI16, cGAS, STING, and AIM2, in addition to the endosomal TLR9 [15,104,105,106]. Our recent studies have shown that ORFV has evolved a strategy to evade cytosolic DNA-dependent signalling [31]. These results strongly suggest that ORFV encodes factors that interfere with the DNA-dependent signalling pathway of IFN-β expression; however, the underlying mechanism is yet to be determined. Several poxvirus antagonists have been identified that directly interfere with the cytosolic DNA sensing signalling pathways, and VACV in particular devotes a considerable number of factors that target this pathway. VACV E3L contains a Z-DNA-binding domain that can prevent the interaction of DAI with DNA, resulting in the inhibition of DNA-induced IFN-β (Figure 3) [107]. DNA-PK was identified to be targeted by the VACV C16 and C4 proteins in which it binds to the Ku subunit and prevents it from binding to DNA (Figure 3) [108,109]. DNA-PK is a heterotrimeric complex consisting of heterodimer Ku70 and Ku80 and acts as a DNA sensor [37]. Two other factors expressed by VACV were recently discovered to counteract DNA detection. VACV proteins E5 and Poxin counteract cGAS and cGAMP, respectively, by promoting their degradation, which results in STING inhibition (Figure 3) [110,111,112]. From our studies, ORFV appears to target cytosolic DNA sensing similarly to VACV, although homologs of the above VACV genes have not been discovered in ORFV and may suggest that some of the unknown genes in ORFV are involved.
4.3. Inhibition of Signalling Molecules
We have recently shown that ORFV, in common with a number of other poxviruses, potently inhibits dsDNA-mediated IFN induction via a STING-dependent pathway, although the underlying mechanism is not known for ORFV (Figure 2) [31]. Georgana, Sumner [113] found that VACV, CPXV, and ECTV, but not MVA, interfere with DNA-induced STING signalling. A number of factors were discovered in VACV that inhibit this pathway. VACV C6 was described as an inhibitor of IFN-β expression by preventing activation of IRF3 and IRF7 at the level of TBK1/IKKε (Figure 3). C6 interacts with subunits of TBK1/IKKε: NAK-associated protein 1 (NAP1), TRAF family member-associated NF-κB activator (TANK), and TBK1 adaptor (SINTBAD), to inhibit IRF3 and IRF7 activation [114]. VACV N1L is another viral protein that has been shown to interfere with DNA sensing by inhibiting the activation of TBK1 (Figure 3). N1L, expressed from MVA, caused a reduction in IFN-β expression, possibly through STING [115]. Furthermore, the induction of IFN-β is inhibited by VACV K7R by binding with DEAD-box protein 3 (DDX3), resulting in the inhibition of TBK1/IKKε-mediated IRF3 activation [116] (Figure 3). VACV B14R interacts with IKKβ and inhibits its phosphorylation, whereas KSHC K13 interacts with the IKKα/IKKβ complex and both result in the inhibition of NF-κB activation (Figure 3) [117,118]. Homologs of the above VACV genes have not been found in ORFV, suggesting that ORFV may have evolved a unique set of genes to inhibit STING-dependent signalling.
The transcription factors that drive the expression of antiviral genes are also key molecules that viruses target. VACV N2 acts downstream of IRF3 phosphorylation and inhibits the activation of IRF3 after translocation into the nucleus through an unknown mechanism (Figure 3) [119]. In addition, VACV E3L has multiple functions including the inhibition of IRF3 and IRF7 activation (Figure 3) [120,121,122].
It is well established that NF-κB drives the expression of proinflammatory cytokines once activated; however, it is also a critical player in regulating the cellular response to IFNs [123]. Accordingly, ORFV and VACV encode a number of proteins that interfere with NF-κB activation. They are early genes and act at different stages in the signalling pathway to inhibit NF-κB activation. ORFV encodes ORF121, ORF002, and ORF024, which inhibit NF-κB-regulated cytokines: IL-1α, IL-6, IL-8, CCL20, CXCL1, CXCL2, CXCL3, ICAM-1, and PTGS2 (Figure 2) [12,124,125]. Bioinformatics analysis has shown that the above ORFV ORFs have no homologs in VACV [9]. VACV encodes A46, A49, A52, B14, C4, E3, K1, K7, M2, and N1 that inhibit NF-κB (Figure 3) [126,127]. It is likely that there are even more NF-κB inhibitors encoded by VACV, as the virus was still able to inhibit NF-κB activation even when all known inhibitors were deleted from the genome [128].
Molluscum contagiosum virus (MCV), a poxvirus that infects humans only and causes a localized skin infection, has developed strategies to evade the innate immune response. MCV expresses multiple proteins that target the signalling pathways, and, interestingly, although MCV is also keratinocyte-tropic, none of these proteins have homologs in ORFV. MC005, MC008, MC132, MC160, and MC159 inhibit NF-κB by targeting different molecules that lead to its activation [129,130,131,132,133]. Moreover, the virus MC159 and MC089 can also target IRF3 and prevent its activation [133,134].
4.4. Inhibition of IFN-Induced Signalling
Apart from other biological properties of type I IFNs, i.e., regulating cellular differentiation and proliferation and immunomodulation, they can activate the JAK/STAT signalling pathway [135]. The interaction between IFN-α/β and its receptor (IFNAR1/2) recruits JAK1 and TYK2, leading to activation of the receptor-associated JAK1 and the phosphorylation of STAT 1 and 2. Upon phosphorylation, STAT1 and STAT2 form a heterotrimeric transcription factor complex, IFN-stimulated gene factor 3 (ISGF3) with interferon regulatory factor (IRF-9), which translocates to the nucleus. This trimeric complex binds to the interferon-stimulated response element (ISRE) in the promoters of interferon-stimulated genes (ISGs) to induce their expression (Figure 1). On the other hand, the interaction between IFN-γ and its receptor (IFNGR1/2) recruits JAK1 and JAK2, leading to the activation of STAT1. Upon its phosphorylation, they form a homodimeric complex that translocates to the nucleus. This complex then binds gamma-activated sequence (GAS) of ISGs to drive their expression (Figure 1).
The activity of IFNs is targeted by poxviruses by producing factors that block the IFN-signalling cascade. ORFV modulates the JAK/STAT pathway by dephosphorylating STAT1 at Tyr701 immediately upon infection via a structural protein, ORF057 [136] (Figure 2). ORF057 has 41% amino acid identity with VACV VH1 [8,9,137], also known to dephosphorylate STAT1 (Figure 3) [136,138,139]. In the case of VACV, it was thought that this dephosphorylation activity was due solely to VH1, until the discovery of ORF018 (Figure 3). This factor was shown to bind directly to the SH2 domain of STAT1 and prevent its association with the receptor and, consequently, its phosphorylation [140].
4.5. Inhibition of Interferon-Stimulated Genes
As described above, IFNs induce hundreds of antiviral effectors [56,141,142], and poxviruses in particular encode factors that interfere with their actions [127,143].
Recently, a functional analysis of ORF116 encoded by ORFV was investigated in which the transcriptome of HeLa cells infected with either OV-NZ2 wild type or OV-NZ2Δ116 knockout was analyzed by a gene-expression microarray. The microarray data revealed that the expression level of a number of ISGs had been upregulated in the mutant virus-infected cells to higher levels than in wild type-infected cells. These ISGs include IFI44, RIG-I, IFIT2, MDA5, OAS1, OASL, DDX60, ISG20, and IFIT1. The data show that ORF116 has a role in manipulating the antiviral effectors expressed in HeLa cells; however, its direct effect and mechanism are yet to be determined (Figure 2) [144].
Guanylate-binding protein 1 is a large GTPase (GBP1) of the dynamin superfamily and is involved in the regulation of membrane cytoskeleton and cell cycle progression [145]. In many cell types, GBP1 is strongly induced by IFN-γ and acts to reduce cellular proliferation during inflammation [145,146]. Harvey, McCaughan [136] showed that GBP1 is strongly inhibited in ORFV-infected HeLa cells stimulated with IFN-γ. Furthermore, human myxovirus resistance protein A (MxA) is an IFN type l-induced dynamin-like GTPase that protects cells from viral pathogens and is part of the innate immune response and is strongly inhibited in ORFV-infected Hela cells stimulated by IFN-α [136].
VACV KIL and C7L were shown to have a role in inhibiting the IFN effector response by targeting SAMD9 (Figure 3), an ISG that plays a critical antiviral role in viral infection, and the deletion of these two viral genes makes the virus sensitive to IFNs [147,148,149]. ORFV lacks these two genes; however, despite this, ORFV can grow in HeLa cells in which a VACV C7L/K1L deletion mutant does not replicate. Furthermore, ORFV can partially restore the growth of the VACV C7L/K1L deletion mutant in HeLa cells [150], indicating that ORFV encodes factors that are functionally similar to K1L and C7L that subvert the effects of SAMD9.
As described above, a further ORFV factor known to disrupt the activity of ISGs is ORF020, which binds dsRNA to inhibit the activation of PKR [77,78]. More recently, an ORFV factor (ORF129) was discovered that has an inhibitory effect on innate immunity by inhibiting C1QBP, a cellular protein that regulates the immune response [151].
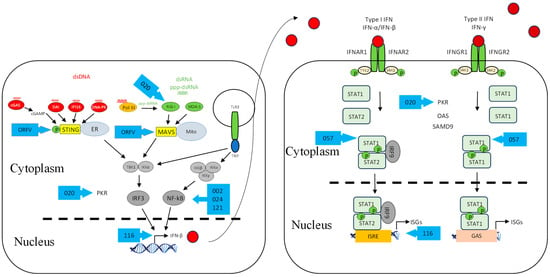
Figure 2.
ORFV inhibitors of the interferon induction and signalling pathways. As in Figure 1, shown are DNA- and RNA-sensing mechanisms that lead to IFN signalling. ORFV inhibitors are shown as blue arrows. Further details of the inhibitors are shown in Table 1. Red circle is type I IFN, darker red circle is type II IFN, dashed line is nuclear membrane.
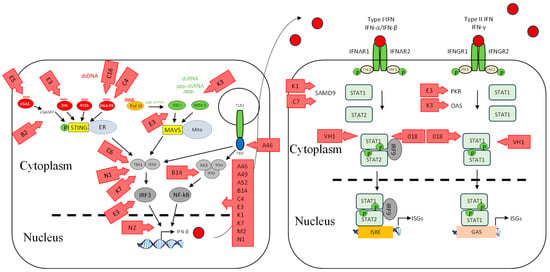
Figure 3.
VACV inhibitors of the interferon induction and signalling pathways. As for Figure 1, shown are DNA- and RNA-sensing mechanisms that lead to IFN signalling. VACV inhibitors are shown as red arrows. Further details of the inhibitors are shown in Table 1. Red circle is type I IFN, darker red circle is type II IFN, dashed line is nuclear membrane.

Table 1.
VACV and ORFV inhibitors.
Table 1.
VACV and ORFV inhibitors.
| Signalling Pathway | Protein | References |
|---|---|---|
| RNA Sensing | K3 | [95,152] |
| E3 | [33] | |
| ORF020 * | [77,78] | |
| DNA Sensing | E3 | [107] |
| C16 | [108,109] | |
| C4 | [109] | |
| E5 | [112] | |
| B2 | [110,111] | |
| Signalling Molecules | C6 | [114] |
| N1 | [115,153] | |
| K7 | [116] | |
| N2 | [119] | |
| E3 | [120,121,122] | |
| ORF002 * | [12,154] | |
| ORF024 * | [124] | |
| ORF121 * | [125] | |
| A46 | [97,98] | |
| A49 | [155] | |
| A52 | [97] | |
| B14 | [117,156] | |
| C4 | [157] | |
| E3 | [158] | |
| K1 | [159] | |
| K7 | [160] | |
| M2 | [161] | |
| N1 | [162,163] | |
| IFN-Induced Signalling | ORF057 * | [136] |
| VH1 | [137,138] | |
| 018 | [140] | |
| ISGs | ORF116 * | [144] |
| ORF020 * | [77,78] | |
| K1 | [147,148,149] | |
| C7 | [147,148,149] |
* indicates protein from ORFV.
5. Conclusions and Future Perspectives
Studies in recent years have revealed that ORFV has evolved strategies in common with other poxviruses that counteract immune detection and disrupt the innate response, in particular, the effects of type I IFNs. Although a number of these immune evasion molecules are similar to other poxvirus proteins, many appear to be unique with no homology to other poxvirus genes. We predict that the unexplained inhibitory effects on the viral sensing and modulation of type I IFN signalling pathways observed is due to the unique genes that ORFV encodes. Although the functionality of these genes appears to be conserved, a major shift in their structural evolution could have occurred or the virus could be targeting other factors within signalling pathways. It is also apparent that ORFV encodes far fewer factors than the more virulent poxviruses to subvert the immune response, and this may due to the life cycle of the virus in that it only infects keratinocytes in the infected host and generally causes benign lesions. Further, studies will elucidate the mechanisms that ORFV employs to subvert the host immune response and whether some of the unique genes ORFV encodes have a role.
Author Contributions
Conceptualization: B.A.A. and S.B.F. Writing: B.A.A. and S.B.F. Graphic design and figure drawing: B.A.A. and S.B.F. Editing the final draft: B.A.A. and S.B.F. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported in part by funding from the Health Research Council of New Zealand HRC Programme Grant 13/774 Professor A A Mercer and Dr S B Fleming.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
We thank Andrew Mercer for proofreading the manuscript.
Conflicts of Interest
The authors declare that there are no conflicts of interest.
References
- Fleming, S.B.; Mercer, A.A. Genus Parapoxvirus. In Poxviruses; Mercer, A.A., Schmidt, A., Weber, O., Eds.; Birkhäuser: Basel, Switzerland, 2007; pp. 127–165. [Google Scholar]
- Mercer, A.; Fleming, S. Parapoxvirus. In The Springer Index of Viruses; Tidona, C., Darai, G., Eds.; Springer: New York, NY, USA, 2011; pp. 1495–1504. [Google Scholar]
- Savage, J.; Black, M.M. ‘Giant’ orf of finger in a patient with a lymphoma. Proc. R. Soc. Med. 1972, 65, 766–768. [Google Scholar] [CrossRef]
- Tan, S.T.; Blake, G.B.; Chambers, S. Recurrent orf in an immunocompromised host. Br. J. Plast. Surg. 1991, 44, 465–467. [Google Scholar] [CrossRef] [PubMed]
- Paoletti, E.; Grady, L.J. Transcriptional complexity of vaccinia virus in vivo and in vitro. J. Virol. 1977, 23, 608–615. [Google Scholar] [CrossRef]
- Buller, R.M.; Palumbo, G.J. Poxvirus pathogenesis. Microbiol. Rev. 1991, 55, 80–122. [Google Scholar] [CrossRef]
- Moss, B. Poxviridae: The Virus and Their Replication. In Fields Virology, 5th ed.; Knipe, D.M., Lamb, R.A., Straus, S.E., Howley, P.M., Martin, M.A., Roizman, B., Eds.; Wolters Kluwer-Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2007; Volume 2, pp. 2905–2946. [Google Scholar]
- Delhon, G.; Tulman, E.R.; Afonso, C.L.; Lu, Z.; De La Concha-Bermejillo, A.; Lehmkuhl, H.D.; Piccone, M.E.; Kutish, G.F.; Rock, D.L. Genomes of the Parapoxviruses Orf Virus and Bovine Papular Stomatitis Virus. J. Virol. 2004, 78, 168–177. [Google Scholar] [CrossRef]
- Mercer, A.A.; Ueda, N.; Friederichs, S.-M.; Hofmann, K.; Fraser, K.M.; Bateman, T.; Fleming, S.B. Comparative analysis of genome sequences of three isolates of Orf virus reveals unexpected sequence variation. Virus Res. 2006, 116, 146–158. [Google Scholar] [CrossRef] [PubMed]
- Fleming, S.B.; Blok, J.; Fraser, K.M.; Mercer, A.A.; Robinson, A.J. Conservation of Gene Structure and Arrangement between Vaccinia Virus and Orf Virus. Virology 1993, 195, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Mercer, A.A.; Lyttle, D.J.; Whelan, E.M.; Fleming, S.B.; Sullivan, J.T. The Establishment of a Genetic Map of Orf Virus Reveals a Pattern of Genomic Organization That Is Highly Conserved among Divergent Poxviruses. Virology 1995, 212, 698–704. [Google Scholar] [CrossRef]
- Diel, D.G.; Luo, S.; Delhon, G.; Peng, Y.; Flores, E.F.; Rock, D.L. A nuclear inhibitor of NF-kappaB encoded by a poxvirus. J. Virol. 2011, 85, 264–275. [Google Scholar] [CrossRef]
- Jenkinson, D.M.; Mc, E.P.; Moss, V.A.; Elder, H.Y.; Reid, H.W. Location and Spread of Orf Virus Antigen in Infected Ovine Skin. Vet. Dermatol. 1990, 1, 189–195. [Google Scholar] [CrossRef]
- McKeever, D.J.; McEwan Jenkinson, D.; Hutchison, G.; Reid, H.W. Studies of the pathogenesis of orf virus infection in sheep. J. Comp. Pathol. 1988, 99, 317–328. [Google Scholar] [CrossRef]
- Almine, J.F.; O’Hare, C.A.J.; Dunphy, G.; Haga, I.R.; Naik, R.J.; Atrih, A.; Connolly, D.J.; Taylor, J.; Kelsall, I.R.; Bowie, A.G.; et al. IFI16 and cGAS cooperate in the activation of STING during DNA sensing in human keratinocytes. Nat. Commun. 2017, 8, 14392. [Google Scholar] [CrossRef]
- Fleming, S.B.; Wise, L.W.; Mercer, A.A. Molecular genetic analysis of orf virus: A poxvirus that has adapted to skin. Viruses 2015, 7, 1505–1539. [Google Scholar] [CrossRef] [PubMed]
- Fujisawa, H.; Kondo, S.; Wang, B.; Shivji, G.M.; Sauder, D.N. The Expression and Modulation of IFN-α and IFN-β in Human Keratinocytes. J. Interferon Cytokine Res. 1997, 17, 721–725. [Google Scholar] [CrossRef]
- Nestle, F.O.; Di Meglio, P.; Qin, J.-Z.; Nickoloff, B.J. Skin immune sentinels in health and disease. Nat. Rev. Immunol. 2009, 9, 679–691. [Google Scholar] [CrossRef] [PubMed]
- Schnipper, L.E.; Levin, M.; Crumpacker, C.S.; Gilchrest, B.A. Virus replication and induction of interferon in human epidermal keratinocytes following infection with herpes simplex virus. J. Investig. Dermatol. 1984, 82, 94–96. [Google Scholar] [CrossRef] [PubMed]
- Torseth, J.W.; Nickoloff, B.J.; Basham, T.Y.; Merigan, T.C. β Interferon Produced by Keratinocytes in Human Cutaneous Infection with Herpes Simplex Virus. J. Infect. Dis. 1987, 155, 641–648. [Google Scholar] [CrossRef]
- Haig, D.M.; McInnes, C.J. Immunity and counter-immunity during infection with the parapoxvirus orf virus. Virus Res. 2002, 88, 3–16. [Google Scholar] [CrossRef]
- Haig, D.M.; Thomson, J.; McInnes, C.; McCaughan, C.; Imlach, W.; Mercer, A.; Fleming, S. Orf virus immuno-modulation and the host immune response. Vet. Immunol. Immunopathol. 2002, 87, 395–399. [Google Scholar] [CrossRef]
- McNab, F.; Mayer-Barber, K.; Sher, A.; Wack, A.; O’garra, A. Type I interferons in infectious disease. Nat. Rev. Immunol. 2015, 15, 87–103. [Google Scholar] [CrossRef]
- Yoneyama, M.; Fujita, T. Recognition of viral nucleic acids in innate immunity. Rev. Med. Virol. 2010, 20, 4–22. [Google Scholar] [CrossRef] [PubMed]
- Rehwinkel, J.; Gack, M.U. RIG-I-like receptors: Their regulation and roles in RNA sensing. Nat. Rev. Immunol. 2020, 20, 537–551. [Google Scholar] [CrossRef]
- Der, S.D.; Lau, A.S. Involvement of the double-stranded-RNA-dependent kinase PKR in interferon expression and interferon-mediated antiviral activity. Proc. Natl. Acad. Sci. USA 1995, 92, 8841–8845. [Google Scholar] [CrossRef]
- Samuel, C.E. Antiviral Actions of Interferons. Clin. Microbiol. Rev. 2001, 14, 778–809. [Google Scholar] [CrossRef]
- McAllister, C.S.; Taghavi, N.; Samuel, C.E. Protein Kinase PKR Amplification of Interferon β Induction Occurs through Initiation Factor eIF-2α-mediated Translational Control. J. Biol. Chem. 2012, 287, 36384–36392. [Google Scholar] [CrossRef] [PubMed]
- Takaoka, A.; Wang, Z.; Choi, M.K.; Yanai, H.; Negishi, H.; Ban, T.; Lu, Y.; Miyagishi, M.; Kodama, T.; Honda, K.; et al. DAI (DLM-1/ZBP1) is a cytosolic DNA sensor and an activator of innate immune response. Nature 2007, 448, 501–505. [Google Scholar] [CrossRef]
- Ablasser, A.; Bauernfeind, F.; Hartmann, G.; Latz, E.; Fitzgerald, K.A.; Hornung, V. RIG-I-dependent sensing of poly(dA:dT) through the induction of an RNA polymerase III–transcribed RNA intermediate. Nat. Immunol. 2009, 10, 1065–1072. [Google Scholar] [CrossRef]
- AlDaif, B.A.; Mercer, A.A.; Fleming, S.B. The parapoxvirus Orf virus inhibits dsDNA-mediated type I IFN expression via STING-dependent and STING-independent signalling pathways. J. Gen. Virol. 2023, 104, 001912. [Google Scholar] [CrossRef] [PubMed]
- Chiu, Y.-H.; MacMillan, J.B.; Chen, Z.J. RNA Polymerase III Detects Cytosolic DNA and Induces Type I Interferons through the RIG-I Pathway. Cell 2009, 138, 576–591. [Google Scholar] [CrossRef]
- Valentine, R.; Smith, G.L. Inhibition of the RNA polymerase III-mediated dsDNA-sensing pathway of innate immunity by vaccinia virus protein E3. J. Gen. Virol. 2010, 91, 2221–2229. [Google Scholar] [CrossRef]
- Jin, T.; Perry, A.; Jiang, J.; Smith, P.; Curry, J.A.; Unterholzner, L.; Jiang, Z.; Horvath, G.; Rathinam, V.A.; Johnstone, R.W.; et al. Structures of the HIN Domain:DNA Complexes Reveal Ligand Binding and Activation Mechanisms of the AIM2 Inflammasome and IFI16 Receptor. Immunity 2012, 36, 561–571. [Google Scholar] [CrossRef] [PubMed]
- Unterholzner, L.; Keating, S.E.; Baran, M.; Horan, K.A.; Jensen, S.B.; Sharma, S.; Sirois, C.M.; Jin, T.; Latz, E.; Xiao, T.S.; et al. IFI16 is an innate immune sensor for intracellular DNA. Nat. Immunol. 2010, 11, 997–1004. [Google Scholar] [CrossRef]
- Zhang, Z.; Yuan, B.; Bao, M.; Lu, N.; Kim, T.; Liu, Y.-J. The helicase DDX41 senses intracellular DNA mediated by the adaptor STING in dendritic cells. Nat. Immunol. 2011, 12, 959–965. [Google Scholar] [CrossRef]
- Ferguson, B.J.; Mansur, D.S.; Peters, N.E.; Ren, H.; Smith, G.L. DNA-PK is a DNA sensor for IRF-3-dependent innate immunity. elife 2012, 1, e00047. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Sun, L.; Chen, X.; Du, F.; Shi, H.; Chen, C.; Chen, Z.J. Cyclic GMP-AMP Is an Endogenous Second Messenger in Innate Immune Signaling by Cytosolic DNA. Science 2013, 339, 826–830. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Wu, J.; Du, F.; Chen, X.; Chen, Z.J. Cyclic GMP-AMP Synthase Is a Cytosolic DNA Sensor That Activates the Type I Interferon Pathway. Science 2013, 339, 786–791. [Google Scholar] [CrossRef]
- Li, X.-D.; Wu, J.; Gao, D.; Wang, H.; Sun, L.; Chen, Z.J. Pivotal Roles of cGAS-cGAMP Signaling in Antiviral Defense and Immune Adjuvant Effects. Science 2013, 341, 1390–1394. [Google Scholar] [CrossRef]
- Schoggins, J.W.; MacDuff, D.A.; Imanaka, N.; Gainey, M.D.; Shrestha, B.; Eitson, J.L.; Mar, K.B.; Richardson, R.B.; Ratushny, A.V.; Litvak, V.; et al. Pan-viral specificity of IFN-induced genes reveals new roles for cGAS in innate immunity. Nature 2014, 505, 691–695. [Google Scholar] [CrossRef]
- Kawai, T.; Takahashi, K.; Sato, S.; Coban, C.; Kumar, H.; Kato, H.; Ishii, K.J.; Takeuchi, O.; Akira, S. IPS-1, an adaptor triggering RIG-I- and Mda5-mediated type I interferon induction. Nat. Immunol. 2005, 6, 981–988. [Google Scholar] [CrossRef]
- Xu, L.-G.; Wang, Y.-Y.; Han, K.-J.; Li, L.-Y.; Zhai, Z.; Shu, H.-B. VISA Is an Adapter Protein Required for Virus-Triggered IFN-β Signaling. Mol. Cell 2005, 19, 727–740. [Google Scholar] [CrossRef]
- Zhong, B.; Yang, Y.; Li, S.; Wang, Y.-Y.; Li, Y.; Diao, F.; Lei, C.; He, X.; Zhang, L.; Tien, P.; et al. The Adaptor Protein MITA Links Virus-Sensing Receptors to IRF3 Transcription Factor Activation. Immunity 2008, 29, 538–550. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, H.; Barber, G.N. STING is an endoplasmic reticulum adaptor that facilitates innate immune signalling. Nature 2008, 455, 674–678. [Google Scholar] [CrossRef]
- Ishikawa, H.; Ma, Z.; Barber, G.N. STING regulates intracellular DNA-mediated, type I interferon-dependent innate immunity. Nature 2009, 461, 788–792. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, K.A.; McWhirter, S.M.; Faia, K.L.; Rowe, D.C.; Latz, E.; Golenbock, D.T.; Coyle, A.J.; Liao, S.-M.; Maniatis, T. IKKε and TBK1 are essential components of the IRF3 signaling pathway. Nat. Immunol. 2003, 4, 491–496. [Google Scholar] [CrossRef]
- Ishii, K.J.; Kawagoe, T.; Koyama, S.; Matsui, K.; Kumar, H.; Kawai, T.; Uematsu, S.; Takeuchi, O.; Takeshita, F.; Coban, C.; et al. TANK-binding kinase-1 delineates innate and adaptive immune responses to DNA vaccines. Nature 2008, 451, 725–729. [Google Scholar] [CrossRef] [PubMed]
- Miyahira, A.K.; Shahangian, A.; Hwang, S.; Sun, R.; Cheng, G. TANK-Binding Kinase-1 Plays an Important Role during In Vitro and In Vivo Type I IFN Responses to DNA Virus Infections1. J. Immunol. 2009, 182, 2248–2257. [Google Scholar] [CrossRef]
- Kawai, T.; Akira, S. The role of pattern-recognition receptors in innate immunity: Update on Toll-like receptors. Nat. Immunol. 2010, 11, 373–384. [Google Scholar] [CrossRef]
- Oshiumi, H.; Matsumoto, M.; Funami, K.; Akazawa, T.; Seya, T. TICAM-1, an adaptor molecule that participates in Toll-like receptor 3–mediated interferon-β induction. Nat. Immunol. 2003, 4, 161–167. [Google Scholar] [CrossRef]
- Yamamoto, M.; Sato, S.; Mori, K.; Hoshino, K.; Takeuchi, O.; Takeda, K.; Akira, S. Cutting Edge: A Novel Toll/IL-1 Receptor Domain-Containing Adapter That Preferentially Activates the IFN-β Promoter in the Toll-Like Receptor Signaling. J. Immunol. 2002, 169, 6668–6672. [Google Scholar] [CrossRef]
- Yamamoto, M.; Sato, S.; Hemmi, H.; Hoshino, K.; Kaisho, T.; Sanjo, H.; Takeuchi, O.; Sugiyama, M.; Okabe, M.; Takeda, K.; et al. Role of Adaptor TRIF in the MyD88-Independent Toll-Like Receptor Signaling Pathway. Science 2003, 301, 640–643. [Google Scholar] [CrossRef]
- Tanaka, Y.; Chen, Z.J. STING Specifies IRF3 Phosphorylation by TBK1 in the Cytosolic DNA Signaling Pathway. Sci. Signal. 2012, 5, ra20. [Google Scholar] [CrossRef] [PubMed]
- Iwanaszko, M.; Kimmel, M. NF-κB and IRF pathways: Cross-regulation on target genes promoter level. BMC Genom. 2015, 16, 307. [Google Scholar] [CrossRef] [PubMed]
- Schoggins, J.W. Interferon-Stimulated Genes: What Do They All Do? Annu. Rev. Virol. 2019, 6, 567–584. [Google Scholar] [CrossRef] [PubMed]
- Andersen, J.; VanScoy, S.; Cheng, T.F.; Gomez, D.; Reich, N.C. IRF-3-dependent and augmented target genes during viral infection. Genes Immun. 2008, 9, 168–175. [Google Scholar] [CrossRef]
- Grandvaux, N.; Servant, M.J.; tenOever, B.; Sen, G.C.; Balachandran, S.; Barber, G.N.; Rongtuan, L.; Hiscott, J. Transcriptional Profiling of Interferon Regulatory Factor 3 Target Genes: Direct Involvement in the Regulation of Interferon-Stimulated Genes. J. Virol. 2002, 76, 5532–5539. [Google Scholar] [CrossRef]
- Sen, G.C.; Peters, G.A. Viral Stress-Inducible Genes. In Advances in Virus Research; Academic Press: Cambridge, MA, USA, 2007; Volume 70, pp. 233–263. [Google Scholar]
- Wathelet, M.G.; Berr, P.M.; Huez, G.A. Regulation of gene expression by cytokines and virus in human cells lacking the type-I interferon locus. Eur. J. Biochem. 1992, 206, 901–910. [Google Scholar] [CrossRef]
- Bowie, A.G.; Haga, I.R. The role of Toll-like receptors in the host response to viruses. Mol. Immunol. 2005, 42, 859–867. [Google Scholar] [CrossRef]
- Vaidya, S.A.; Cheng, G. Toll-like receptors and innate antiviral responses. Curr. Opin. Immunol. 2003, 15, 402–407. [Google Scholar] [CrossRef]
- Gilliet, M.; Cao, W.; Liu, Y.-J. Plasmacytoid dendritic cells: Sensing nucleic acids in viral infection and autoimmune diseases. Nat. Rev. Immunol. 2008, 8, 594–606. [Google Scholar] [CrossRef]
- Jiang, Z.; Mak, T.W.; Sen, G.; Li, X. Toll-like receptor 3-mediated activation of NF-κB and IRF3 diverges at Toll-IL-1 receptor domain-containing adapter inducing IFN-β. Proc. Natl. Acad. Sci. USA 2004, 101, 3533–3538. [Google Scholar] [CrossRef]
- O’Neill, L.A.J.; Bowie, A.G. The family of five: TIR-domain-containing adaptors in Toll-like receptor signalling. Nat. Rev. Immunol. 2007, 7, 353–364. [Google Scholar] [CrossRef] [PubMed]
- Schröder, M.; Bowie, A.G. TLR3 in antiviral immunity: Key player or bystander? Trends Immunol. 2005, 26, 462–468. [Google Scholar] [CrossRef] [PubMed]
- Kato, H.; Sato, S.; Yoneyama, M.; Yamamoto, M.; Uematsu, S.; Matsui, K.; Tsujimura, T.; Takeda, K.; Fujita, T.; Takeuchi, O.; et al. Cell Type-Specific Involvement of RIG-I in Antiviral Response. Immunity 2005, 23, 19–28. [Google Scholar] [CrossRef]
- Boyle, K.; Traktman, P. Poxviruses. In Viral Genome Replication; Raney, K.D., Gotte, M., Cameron, C.E., Eds.; Springer: Boston, MA, USA, 2009; pp. 225–247. [Google Scholar]
- Boone, R.F.; Parr, R.P.; Moss, B. Intermolecular duplexes formed from polyadenylylated vaccinia virus RNA. J. Virol. 1979, 30, 365–374. [Google Scholar] [CrossRef] [PubMed]
- Colby, C.; Duesberg, P.H. Double-stranded RNA in Vaccinia Virus Infected Cells. Nature 1969, 222, 940–944. [Google Scholar] [CrossRef]
- Colby, C.; Jurale, C.; Kates, J.R. Mechanism of Synthesis of Vaccinia Virus Double-Stranded Ribonucleic Acid In Vivo and In Vitro. J. Virol. 1971, 7, 71–76. [Google Scholar] [CrossRef]
- Willis, K.L.; Langland, J.O.; Shisler, J.L. Viral Double-stranded RNAs from Vaccinia Virus Early or Intermediate Gene Transcripts Possess PKR Activating Function, Resulting in NF-κB Activation, When the K1 Protein Is Absent or Mutated. J. Biol. Chem. 2011, 286, 7765–7778. [Google Scholar] [CrossRef]
- Myskiw, C.; Arsenio, J.; Booy, E.P.; Hammett, C.; Deschambault, Y.; Gibson, S.B.; Cao, J. RNA species generated in vaccinia virus infected cells activate cell type-specific MDA5 or RIG-I dependent interferon gene transcription and PKR dependent apoptosis. Virology 2011, 413, 183–193. [Google Scholar] [CrossRef]
- Pichlmair, A.; Schulz, O.; Tan, C.-P.; Rehwinkel, J.; Kato, H.; Takeuchi, O.; Akira, S.; Way, M.; Schiavo, G.; Reis e Sousa, C. Activation of MDA5 Requires Higher-Order RNA Structures Generated during Virus Infection. J. Virol. 2009, 83, 10761–10769. [Google Scholar] [CrossRef]
- Wang, F.; Gao, X.; Barrett, J.W.; Shao, Q.; Bartee, E.; Mohamed, M.R.; Rahman, M.; Werden, S.; Irvine, T.; Cao, J.; et al. RIG-I mediates the co-induction of tumor necrosis factor and type I interferon elicited by myxoma virus in primary human macrophages. PLoS Pathog. 2008, 4, e1000099. [Google Scholar] [CrossRef]
- Pham, A.M.; Santa Maria, F.G.; Lahiri, T.; Friedman, E.; Marié, I.J.; Levy, D.E. PKR Transduces MDA5-Dependent Signals for Type I IFN Induction. PLoS Pathog. 2016, 12, e1005489. [Google Scholar] [CrossRef] [PubMed]
- Haig, D.M.; McInnes, C.J.; Thomson, J.; Wood, A.; Bunyan, K.; Mercer, A. The orf virus OV20.0L gene product is involved in interferon resistance and inhibits an interferon-inducible, double-stranded RNA-dependent kinase. Immunology 1998, 93, 335–340. [Google Scholar] [CrossRef] [PubMed]
- McInnes, C.J.; Wood, A.R.; Mercer, A.A. Orf Virus Encodes a Homolog of the Vaccinia Virus Interferon-Resistance Gene E3L. Virus Genes 1998, 17, 107–115. [Google Scholar] [CrossRef]
- Hovanessian, A.G. On the discovery of interferon-inducible, double-stranded RNA activated enzymes: The 2′–5′oligoadenylate synthetases and the protein kinase PKR. Cytokine Growth Factor Rev. 2007, 18, 351–361. [Google Scholar] [CrossRef] [PubMed]
- DÍAz-Guerra, M.; Rivas, C.; Esteban, M. Inducible Expression of the 2-5A Synthetase/RNase L System Results in Inhibition of Vaccinia Virus Replication. Virology 1997, 227, 220–228. [Google Scholar] [CrossRef]
- Rice, A.D.; Turner, P.C.; Embury, J.E.; Moldawer, L.L.; Baker, H.V.; Moyer, R.W. Roles of Vaccinia Virus Genes E3L and K3L and Host Genes PKR and RNase L during Intratracheal Infection of C57BL/6 Mice. J. Virol. 2011, 85, 550–567. [Google Scholar] [CrossRef]
- Hochrein, H.; Schlatter, B.; O’Keeffe, M.; Wagner, C.; Schmitz, F.; Schiemann, M.; Bauer, S.; Suter, M.; Wagner, H. Herpes simplex virus type-1 induces IFN-α production via Toll-like receptor 9-dependent and -independent pathways. Proc. Natl. Acad. Sci. USA 2004, 101, 11416–11421. [Google Scholar] [CrossRef]
- Horan, K.A.; Hansen, K.; Jakobsen, M.R.; Holm, C.K.; Søby, S.; Unterholzner, L.; Thompson, M.; West, J.A.; Iversen, M.B.; Rasmussen, S.B.; et al. Proteasomal Degradation of Herpes Simplex Virus Capsids in Macrophages Releases DNA to the Cytosol for Recognition by DNA Sensors. J. Immunol. 2013, 190, 2311–2319. [Google Scholar] [CrossRef]
- Ishii, K.J.; Coban, C.; Kato, H.; Takahashi, K.; Torii, Y.; Takeshita, F.; Ludwig, H.; Sutter, G.; Suzuki, K.; Hemmi, H.; et al. A Toll-like receptor–independent antiviral response induced by double-stranded B-form DNA. Nat. Immunol. 2006, 7, 40–48. [Google Scholar] [CrossRef]
- Cheng, W.-Y.; He, X.-B.; Jia, H.-J.; Chen, G.-H.; Jin, Q.-W.; Long, Z.-L.; Jing, Z.-Z. The cGas–Sting Signaling Pathway Is Required for the Innate Immune Response Against Ectromelia Virus. Front. Immunol. 2018, 9, 1297. [Google Scholar] [CrossRef]
- Ma, Z.; Jacobs, S.R.; West, J.A.; Stopford, C.; Zhang, Z.; Davis, Z.; Barber, G.N.; Glaunsinger, B.A.; Dittmer, D.P.; Damania, B. Modulation of the cGAS-STING DNA sensing pathway by gammaherpesviruses. Proc. Natl. Acad. Sci. USA 2015, 112, E4306–E4315. [Google Scholar] [CrossRef]
- Orzalli, M.H.; DeLuca, N.A.; Knipe, D.M. Nuclear IFI16 induction of IRF-3 signaling during herpesviral infection and degradation of IFI16 by the viral ICP0 protein. Proc. Natl. Acad. Sci. USA 2012, 109, E3008–E3017. [Google Scholar] [CrossRef] [PubMed]
- AlDaif, B.A.; Mercer, A.A.; Fleming, S.B. The parapoxvirus Orf virus inhibits IFN-β expression induced by dsRNA. Virus Res. 2022, 307, 198619. [Google Scholar] [CrossRef]
- Tseng, Y.-Y.; Lin, F.-Y.; Cheng, S.-F.; Tscharke, D.; Chulakasian, S.; Chou, C.-C.; Liu, Y.-F.; Chang, W.-S.; Wong, M.-L.; Hsu, W.-L. Functional Analysis of the Short Isoform of Orf Virus Protein OV20.0. J. Virol. 2015, 89, 4966–4979. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chang, H.W.; Watson, J.C.; Jacobs, B.L. The E3L gene of vaccinia virus encodes an inhibitor of the interferon-induced, double-stranded RNA-dependent protein kinase. Proc. Natl. Acad. Sci. USA 1992, 89, 4825–4829. [Google Scholar] [CrossRef] [PubMed]
- Perdiguero, B.; Esteban, M. The Interferon System and Vaccinia Virus Evasion Mechanisms. J. Interferon Cytokine Res. 2009, 29, 581–598. [Google Scholar] [CrossRef]
- Marq, J.-B.; Hausmann, S.; Luban, J.; Kolakofsky, D.; Garcin, D. The Double-stranded RNA Binding Domain of the Vaccinia Virus E3L Protein Inhibits Both RNA- and DNA-induced Activation of Interferon β. J. Biol. Chem. 2009, 284, 25471–25478. [Google Scholar] [CrossRef]
- Beattie, E.; Paoletti, E.; Tartaglia, J. Distinct Patterns of IFN Sensitivity Observed in Cells Infected with Vaccinia K3L- and E3L- Mutant Viruses. Virology 1995, 210, 254–263. [Google Scholar] [CrossRef]
- Beattie, E.; Tartaglia, J.; Paoletti, E. Vaccinia virus-encoded elF-2α homolog abrogates the antiviral effect of interferon. Virology 1991, 183, 419–422. [Google Scholar] [CrossRef]
- Langland, J.O.; Jacobs, B.L. The Role of the PKR-Inhibitory Genes, E3L and K3L, in Determining Vaccinia Virus Host Range. Virology 2002, 299, 133–141. [Google Scholar] [CrossRef]
- Hurst, T.; Bowie, A.G. Innate Immune Signaling Pathways: Lessons from Vaccinia Virus. Future Virol. 2008, 3, 147–156. [Google Scholar] [CrossRef]
- Bowie, A.; Kiss-Toth, E.; Symons, J.A.; Smith, G.L.; Dower, S.K.; O’Neill, L.A.J. A46R and A52R from vaccinia virus are antagonists of host IL-1 and toll-like receptor signaling. Proc. Natl. Acad. Sci. USA 2000, 97, 10162–10167. [Google Scholar] [CrossRef] [PubMed]
- Stack, J.; Haga, I.R.; Schröder, M.; Bartlett, N.W.; Maloney, G.; Reading, P.C.; Fitzgerald, K.A.; Smith, G.L.; Bowie, A.G. Vaccinia virus protein A46R targets multiple Toll-like–interleukin-1 receptor adaptors and contributes to virulence. J. Exp. Med. 2005, 201, 1007–1018. [Google Scholar] [CrossRef]
- Harte, M.T.; Haga, I.R.; Maloney, G.; Gray, P.; Reading, P.C.; Bartlett, N.W.; Smith, G.L.; Bowie, A.; O’Neill, L.-J. The Poxvirus Protein A52R Targets Toll-like Receptor Signaling Complexes to Suppress Host Defense. J. Exp. Med. 2003, 197, 343–351. [Google Scholar] [CrossRef]
- Barbosa, E.; Moss, B. mRNA(nucleoside-2’-)-methyltransferase from vaccinia virus. Characteristics and substrate specificity. J. Biol. Chem. 1978, 253, 7698–7702. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.-W.; Katsafanas, G.C.; Liu, R.; Wyatt, L.S.; Moss, B. Poxvirus Decapping Enzymes Enhance Virulence by Preventing the Accumulation of dsRNA and the Induction of Innate Antiviral Responses. Cell Host Microbe 2015, 17, 320–331. [Google Scholar] [CrossRef]
- Martin, S.A.; Paoletti, E.; Moss, B. Purification of mRNA guanylyltransferase and mRNA (guanine-7-) methyltransferase from vaccinia virions. J. Biol. Chem. 1975, 250, 9322–9329. [Google Scholar] [CrossRef]
- Wei, C.M.; Moss, B. Methylated nucleotides block 5’-terminus of vaccinia virus messenger RNA. Proc. Natl. Acad. Sci. USA 1975, 72, 318–322. [Google Scholar] [CrossRef]
- Devos, M.; Tanghe, G.; Gilbert, B.; Dierick, E.; Verheirstraeten, M.; Nemegeer, J.; de Reuver, R.; Lefebvre, S.; De Munck, J.; Rehwinkel, J.; et al. Sensing of endogenous nucleic acids by ZBP1 induces keratinocyte necroptosis and skin inflammation. J. Exp. Med. 2020, 217, e20191913. [Google Scholar] [CrossRef]
- Kopfnagel, V.; Wittmann, M.; Werfel, T. Human keratinocytes express AIM2 and respond to dsDNA with IL-1β secretion. Exp. Dermatol. 2011, 20, 1027–1029. [Google Scholar] [CrossRef]
- Lebre, M.C.; van der Aar, A.M.G.; van Baarsen, L.; van Capel, T.M.M.; Schuitemaker, J.H.N.; Kapsenberg, M.L.; de Jong, E.C. Human Keratinocytes Express Functional Toll-Like Receptor 3, 4, 5, and 9. J. Investig. Dermatol. 2007, 127, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Choi, M.K.; Ban, T.; Yanai, H.; Negishi, H.; Lu, Y.; Tamura, T.; Takaoka, A.; Nishikura, K.; Taniguchi, T. Regulation of innate immune responses by DAI (DLM-1/ZBP1) and other DNA-sensing molecules. Proc. Natl. Acad. Sci. USA 2008, 105, 5477–5482. [Google Scholar] [CrossRef]
- Peters, N.E.; Ferguson, B.J.; Mazzon, M.; Fahy, A.S.; Krysztofinska, E.; Arribas-Bosacoma, R.; Pearl, L.H.; Ren, H.; Smith, G.L. A Mechanism for the Inhibition of DNA-PK-Mediated DNA Sensing by a Virus. PLoS Pathog. 2013, 9, e1003649. [Google Scholar] [CrossRef] [PubMed]
- Scutts, S.R.; Ember, S.W.; Ren, H.; Ye, C.; Lovejoy, C.A.; Mazzon, M.; Veyer, D.L.; Sumner, R.P.; Smith, G.L. DNA-PK Is Targeted by Multiple Vaccinia Virus Proteins to Inhibit DNA Sensing. Cell Rep. 2018, 25, 1953–1965.e4. [Google Scholar] [CrossRef]
- Eaglesham, J.B.; Pan, Y.; Kupper, T.S.; Kranzusch, P.J. Viral and metazoan poxins are cGAMP-specific nucleases that restrict cGAS-STING signalling. Nature 2019, 566, 259–263. [Google Scholar] [CrossRef]
- Hernáez, B.; Alonso, G.; Georgana, I.; El-Jesr, M.; Martín, R.; Shair, K.H.Y.; Fischer, C.; Sauer, S.; Maluquer de Motes, C.; Alcamí, A. Viral cGAMP nuclease reveals the essential role of DNA sensing in protection against acute lethal virus infection. Sci. Adv. 2020, 6, eabb4565. [Google Scholar] [CrossRef]
- Yang, N.; Wang, Y.; Dai, P.; Li, T.; Zierhut, C.; Tan, A.; Zhang, T.; Xiang, J.Z.; Ordureau, A.; Funabiki, H.; et al. Vaccinia E5 is a major inhibitor of the DNA sensor cGAS. Nat. Commun. 2023, 14, 2898. [Google Scholar] [CrossRef] [PubMed]
- Georgana, I.; Sumner, R.P.; Towers, G.J.; de Motes, C.M. Virulent Poxviruses Inhibit DNA Sensing by Preventing STING Activation. J. Virol. 2018, 92, e02145-17. [Google Scholar] [CrossRef]
- Unterholzner, L.; Sumner, R.P.; Baran, M.; Ren, H.; Mansur, D.S.; Bourke, N.M.; Randow, F.; Smith, G.L.; Bowie, A.G. Vaccinia Virus Protein C6 Is a Virulence Factor that Binds TBK-1 Adaptor Proteins and Inhibits Activation of IRF3 and IRF7. PLoS Pathog. 2011, 7, e1002247. [Google Scholar] [CrossRef]
- DiPerna, G.; Stack, J.; Bowie, A.G.; Boyd, A.; Kotwal, G.; Zhang, Z.; Arvikar, S.; Latz, E.; Fitzgerald, K.A.; Marshall, W.L. Poxvirus Protein N1L Targets the I-κB Kinase Complex, Inhibits Signaling to NF-κB by the Tumor Necrosis Factor Superfamily of Receptors, and Inhibits NF-κB and IRF3 Signaling by Toll-like Receptors. J. Biol. Chem. 2004, 279, 36570–36578. [Google Scholar] [CrossRef]
- Schröder, M.; Baran, M.; Bowie, A.G. Viral targeting of DEAD box protein 3 reveals its role in TBK1/IKKε-mediated IRF activation. EMBO J. 2008, 27, 2147–2157. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.A.J.; Ryzhakov, G.; Cooray, S.; Randow, F.; Smith, G.L. Inhibition of IκB Kinase by Vaccinia Virus Virulence Factor B14. PLoS Pathog. 2008, 4, e22. [Google Scholar] [CrossRef] [PubMed]
- Matta, H.; Mazzacurati, L.; Schamus, S.; Yang, T.; Sun, Q.; Chaudhary, P.M. Kaposi′s Sarcoma-associated Herpesvirus (KSHV) Oncoprotein K13 Bypasses TRAFs and Directly Interacts with the IκB Kinase Complex to Selectively Activate NF-κB without JNK Activation. J. Biol. Chem. 2007, 282, 24858–24865. [Google Scholar] [CrossRef]
- Ferguson, B.J.; Benfield, C.T.O.; Ren, H.; Lee, V.H.; Frazer, G.L.; Strnadova, P.; Sumner, R.P.; Smith, G.L. Vaccinia virus protein N2 is a nuclear IRF3 inhibitor that promotes virulence. J. Gen. Virol. 2013, 94, 2070–2081. [Google Scholar] [CrossRef]
- Smith, E.J.; Marié, I.; Prakash, A.; García-Sastre, A.; Levy, D.E. IRF3 and IRF7 Phosphorylation in Virus-infected Cells Does Not Require Double-stranded RNA-dependent Protein Kinase R or IκB Kinase but Is Blocked by Vaccinia Virus E3L Protein. J. Biol. Chem. 2001, 276, 8951–8957. [Google Scholar] [CrossRef]
- Zhang, P.; Samuel, C.E. Induction of Protein Kinase PKR-dependent Activation of Interferon Regulatory Factor 3 by Vaccinia Virus Occurs through Adapter IPS-1 Signaling. J. Biol. Chem. 2008, 283, 34580–34587. [Google Scholar] [CrossRef]
- Xiang, Y.; Condit, R.C.; Vijaysri, S.; Jacobs, B.; Williams, B.R.G.; Silverman, R.H. Blockade of Interferon Induction and Action by the E3L Double-Stranded RNA Binding Proteins of Vaccinia Virus. J. Virol. 2002, 76, 5251–5259. [Google Scholar] [CrossRef] [PubMed]
- Pfeffer, L.M. The Role of Nuclear Factor κB in the Interferon Response. J. Interferon Cytokine Res. 2011, 31, 553–559. [Google Scholar] [CrossRef]
- Diel, D.G.; Delhon, G.; Luo, S.; Flores, E.F.; Rock, D.L. A novel inhibitor of the NF-κB signaling pathway encoded by the parapoxvirus orf virus. J. Virol. 2010, 84, 3962–3973. [Google Scholar] [CrossRef]
- Diel, D.G.; Luo, S.; Delhon, G.; Peng, Y.; Flores, E.F.; Rock, D.L. Orf virus ORFV121 encodes a novel inhibitor of NF-kappaB that contributes to virus virulence. J. Virol. 2011, 85, 2037–2049. [Google Scholar] [CrossRef]
- Smith, G.L.; Benfield, C.T.O.; Maluquer de Motes, C.; Mazzon, M.; Ember, S.W.J.; Ferguson, B.J.; Sumner, R.P. Vaccinia virus immune evasion: Mechanisms, virulence and immunogenicity. J. Gen. Virol. 2013, 94, 2367–2392. [Google Scholar] [CrossRef]
- Smith, G.L.; Symons, J.A.; Alcamí, A. Poxviruses: Interfering with Interferon. Semin. Virol. 1998, 8, 409–418. [Google Scholar] [CrossRef]
- Sumner, R.P.; de Motes, C.M.; Veyer, D.L.; Smith, G.L. Vaccinia Virus Inhibits NF-κB-Dependent Gene Expression Downstream of p65 Translocation. J. Virol. 2014, 88, 3092–3102. [Google Scholar] [CrossRef] [PubMed]
- Brady, G.; Haas, D.A.; Farrell, P.J.; Pichlmair, A.; Bowie, A.G. Poxvirus Protein MC132 from Molluscum Contagiosum Virus Inhibits NF-B Activation by Targeting p65 for Degradation. J. Virol. 2015, 89, 8406–8415. [Google Scholar] [CrossRef] [PubMed]
- Brady, G.; Haas, D.A.; Farrell, P.J.; Pichlmair, A.; Bowie, A.G. Molluscum Contagiosum Virus Protein MC005 Inhibits NF-κB Activation by Targeting NEMO-Regulated IκB Kinase Activation. J. Virol. 2017, 91, e00545-17. [Google Scholar] [CrossRef]
- Nichols, D.B.; Shisler, J.L. Poxvirus MC160 Protein Utilizes Multiple Mechanisms To Inhibit NF-κB Activation Mediated via Components of the Tumor Necrosis Factor Receptor 1 Signal Transduction Pathway. J. Virol. 2009, 83, 3162–3174. [Google Scholar] [CrossRef]
- Phelan, T.; Lawler, C.; Pichlmair, A.; Little, M.A.; Bowie, A.G.; Brady, G. Molluscum Contagiosum Virus Protein MC008 Targets NF-κB Activation by Inhibiting Ubiquitination of NEMO. J. Virol. 2023, 97, e0010823. [Google Scholar] [CrossRef]
- Randall, C.M.; Jokela, J.A.; Shisler, J.L. The MC159 protein from the molluscum contagiosum poxvirus inhibits NF-κB activation by interacting with the IκB kinase complex. J. Immunol. 2012, 188, 2371–2379. [Google Scholar] [CrossRef]
- Al Hamrashdi, M.; Sanchez Perez, C.; Haas, D.A.; Vishwakarma, J.; Pichlmair, A.; Bowie, A.G.; Brady, G. Molluscum contagiosum virus protein MC089 inhibits interferon regulatory factor 3 activation. J. Gen. Virol. 2024, 105, 002015. [Google Scholar] [CrossRef]
- Shuai, K.; Liu, B. Regulation of JAK–STAT signalling in the immune system. Nat. Rev. Immunol. 2003, 3, 900–911. [Google Scholar] [CrossRef]
- Harvey, R.; McCaughan, C.; Wise, L.M.; Mercer, A.A.; Fleming, S.B. Orf virus inhibits interferon stimulated gene expression and modulates the JAK/STAT signalling pathway. Virus Res. 2015, 208, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Sun, J.; Zhang, Z. An Overview of the Protein Tyrosine Phosphatase Superfamily. Curr. Top. Med. Chem. 2003, 3, 739–748. [Google Scholar] [CrossRef] [PubMed]
- Najarro, P.; Traktman, P.; Lewis, J.A. Vaccinia virus blocks gamma interferon signal transduction: Viral VH1 phosphatase reverses Stat1 activation. J. Virol. 2001, 75, 3185–3196. [Google Scholar] [CrossRef]
- Schmidt, F.I.; Bleck, C.-E.; Reh, L.; Novy, K.; Wollscheid, B.; Helenius, A.; Stahlberg, H.; Mercer, J. Vaccinia Virus Entry Is Followed by Core Activation and Proteasome-Mediated Release of the Immunomodulatory Effector VH1 from Lateral Bodies. Cell Rep. 2013, 4, 464–476. [Google Scholar] [CrossRef]
- Talbot-Cooper, C.; Pantelejevs, T.; Shannon, J.P.; Cherry, C.R.; Au, M.T.; Hyvönen, M.; Hickman, H.D.; Smith, G.L. Poxviruses and paramyxoviruses use a conserved mechanism of STAT1 antagonism to inhibit interferon signaling. Cell Host Microbe 2022, 30, 357–372.e11. [Google Scholar] [CrossRef] [PubMed]
- Katze, M.G.; He, Y.; Gale, M. Viruses and interferon: A fight for supremacy. Nat. Rev. Immunol. 2002, 2, 675–687. [Google Scholar] [CrossRef]
- Schoggins, J.W.; Rice, C.M. Interferon-stimulated genes and their antiviral effector functions. Curr. Opin. Virol. 2011, 1, 519–525. [Google Scholar] [CrossRef]
- Smith, G.L.; Talbot-Cooper, C.; Lu, Y. Chapter Fourteen—How Does Vaccinia Virus Interfere With Interferon? In Advances in Virus Research; Kielian, M., Mettenleiter, T.C., Roossinck, M.J., Eds.; Academic Press: Cambridge, MA, USA, 2018; Volume 100, pp. 355–378. [Google Scholar]
- AlDaif, B.A.; Mercer, A.A.; Fleming, S.B. The parapoxvirus Orf virus ORF116 gene encodes an antagonist of the interferon response. J. Gen. Virol. 2021, 102, 001695. [Google Scholar] [CrossRef]
- Ostler, N.; Britzen-Laurent, N.; Liebl, A.; Naschberger, E.; Lochnit, G.; Ostler, M.; Forster, F.; Kunzelmann, P.; Ince, S.; Supper, V.; et al. Gamma Interferon-Induced Guanylate Binding Protein 1 Is a Novel Actin Cytoskeleton Remodeling Factor. Mol. Cell. Biol. 2014, 34, 196–209. [Google Scholar] [CrossRef]
- Praefcke, G.J.K. Regulation of innate immune functions by guanylate-binding proteins. Int. J. Med. Microbiol. 2018, 308, 237–245. [Google Scholar] [CrossRef]
- Meng, X.; Jiang, C.; Arsenio, J.; Dick, K.; Cao, J.; Xiang, Y. Vaccinia Virus K1L and C7L Inhibit Antiviral Activities Induced by Type I Interferons. J. Virol. 2009, 83, 10627–10636. [Google Scholar] [CrossRef]
- Meng, X.; Schoggins, J.; Rose, L.; Cao, J.; Ploss, A.; Rice, C.M.; Xiang, Y. C7L Family of Poxvirus Host Range Genes Inhibits Antiviral Activities Induced by Type I Interferons and Interferon Regulatory Factor 1. J. Virol. 2012, 86, 4538–4547. [Google Scholar] [CrossRef] [PubMed]
- Lemos de Matos, A.; Liu, J.; McFadden, G.; Esteves, P.J. Evolution and divergence of the mammalian SAMD9/SAMD9L gene family. BMC Evol. Biol. 2013, 13, 121. [Google Scholar] [CrossRef] [PubMed]
- Riad, S.; Xiang, Y.; AlDaif, B.; Mercer, A.A.; Fleming, S.B. Rescue of a Vaccinia Virus Mutant Lacking IFN Resistance Genes K1L and C7L by the Parapoxvirus Orf Virus. Front. Microbiol. 2020, 11, 1797. [Google Scholar] [CrossRef]
- Dan, Y.; Yang, L.; Zhang, H.; Ren, Y.; He, H.; Yang, F.; Zhu, J.; Xiang, H. The orf virus 129 protein can inhibit immune responses by interacting with host complement C1q binding protein in goat turbinate bone cells. Vet. Microbiol. 2023, 283, 109782. [Google Scholar] [CrossRef] [PubMed]
- Beattie, E.; Denzler, K.L.; Tartaglia, J.; Perkus, M.E.; Paoletti, E.; Jacobs, B.L. Reversal of the interferon-sensitive phenotype of a vaccinia virus lacking E3L by expression of the reovirus S4 gene. J. Virol. 1995, 69, 499–505. [Google Scholar] [CrossRef]
- Cooray, S.; Bahar, M.W.; Abrescia, N.G.A.; McVey, C.E.; Bartlett, N.W.; Chen, R.A.; Stuart, D.I.; Grimes, J.M.; Smith, G.L. Functional and structural studies of the vaccinia virus virulence factor N1 reveal a Bcl-2-like anti-apoptotic protein. J. Gen. Virol. 2007, 88 Pt 6, 1656–1666. [Google Scholar] [CrossRef]
- Chen, Q.; Sun, L.; Chen, Z.J. Regulation and function of the cGAS–STING pathway of cytosolic DNA sensing. Nat. Immunol. 2016, 17, 1142–1149. [Google Scholar] [CrossRef]
- Mansur, D.S.; Maluquer de Motes, C.; Unterholzner, L.; Sumner, R.P.; Ferguson, B.J.; Ren, H.; Strnadova, P.; Bowie, A.G.; Smith, G.L. Poxvirus Targeting of E3 Ligase β-TrCP by Molecular Mimicry: A Mechanism to Inhibit NF-κB Activation and Promote Immune Evasion and Virulence. PLoS Pathog. 2013, 9, e1003183. [Google Scholar] [CrossRef]
- Chen, R.A.; Jacobs, N.; Smith, G.L. Vaccinia virus strain Western Reserve protein B14 is an intracellular virulence factor. J. Gen. Virol. 2006, 87 Pt 6, 1451–1458. [Google Scholar] [CrossRef]
- Ember, S.W.J.; Ren, H.; Ferguson, B.J.; Smith, G.L. Vaccinia virus protein C4 inhibits NF-κB activation and promotes virus virulence. J. Gen. Virol. 2012, 93 Pt 10, 2098–2108. [Google Scholar] [CrossRef] [PubMed]
- Myskiw, C.; Arsenio, J.; van Bruggen, R.; Deschambault, Y.; Cao, J. Vaccinia virus E3 suppresses expression of diverse cytokines through inhibition of the PKR, NF-kappaB, and IRF3 pathways. J. Virol. 2009, 83, 6757–6768. [Google Scholar] [CrossRef] [PubMed]
- Shisler, J.L.; Jin, X.L. The vaccinia virus K1L gene product inhibits host NF-kappaB activation by preventing IkappaBalpha degradation. J. Virol. 2004, 78, 3553–3560. [Google Scholar] [CrossRef]
- Benfield, C.T.O.; Ren, H.; Lucas, S.J.; Bahsoun, B.; Smith, G.L. Vaccinia virus protein K7 is a virulence factor that alters the acute immune response to infection. J. Gen. Virol. 2013, 94 Pt 7, 1647–1657. [Google Scholar] [CrossRef]
- Gedey, R.; Jin, X.L.; Hinthong, O.; Shisler, J.L. Poxviral regulation of the host NF-kappaB response: The vaccinia virus M2L protein inhibits induction of NF-kappaB activation via an ERK2 pathway in virus-infected human embryonic kidney cells. J. Virol. 2006, 80, 8676–8685. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, N.; Symons, J.A.; Tscharke, D.C.; Smith, G.L. The vaccinia virus N1L protein is an intracellular homodimer that promotes virulence. J. Gen. Virol. 2002, 83 Pt 8, 1965–1976. [Google Scholar] [CrossRef]
- Kotwal, G.J.; Hügin, A.W.; Moss, B. Mapping and insertional mutagenesis of a vaccinia virus gene encoding a 13,800-Da secreted protein. Virology 1989, 171, 579–587. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).