A Scoping Review on Progression Towards Freedom from Peste des Petits Ruminants (PPR) and the Role of the PPR Monitoring and Assessment Tool (PMAT)
Abstract
:1. Introduction
2. Methodology
2.1. Search Strategy
2.2. Study Eligibility
2.3. Record Screening and Data Extraction
2.4. Data Synthesis
2.5. Bias Assessment
3. Results
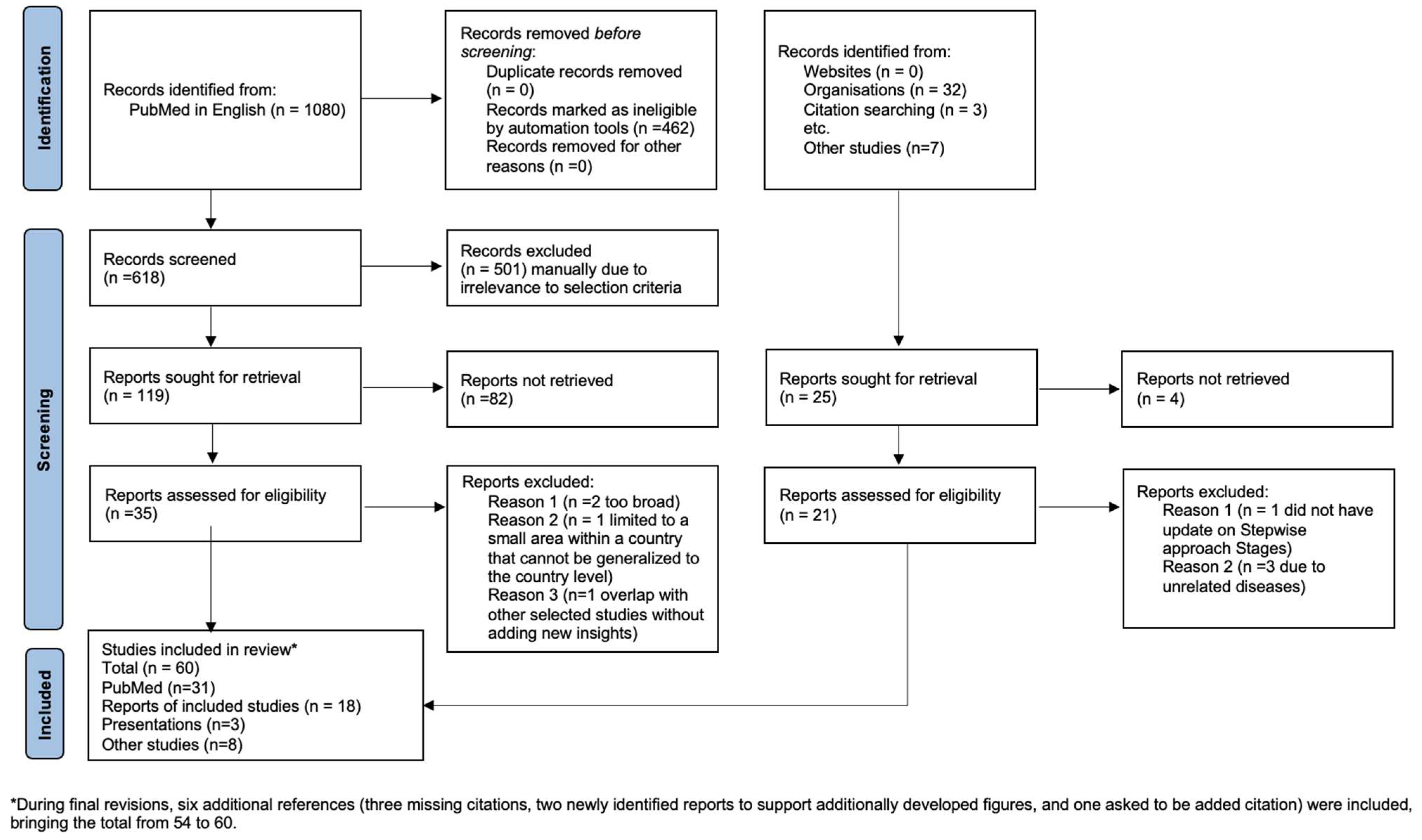
3.1. Screening Results
3.2. Characteristics of Included Studies
3.3. The Global Control and Eradication Strategy (GCES) and PPR Monitoring and Assessment Tool (PMAT)
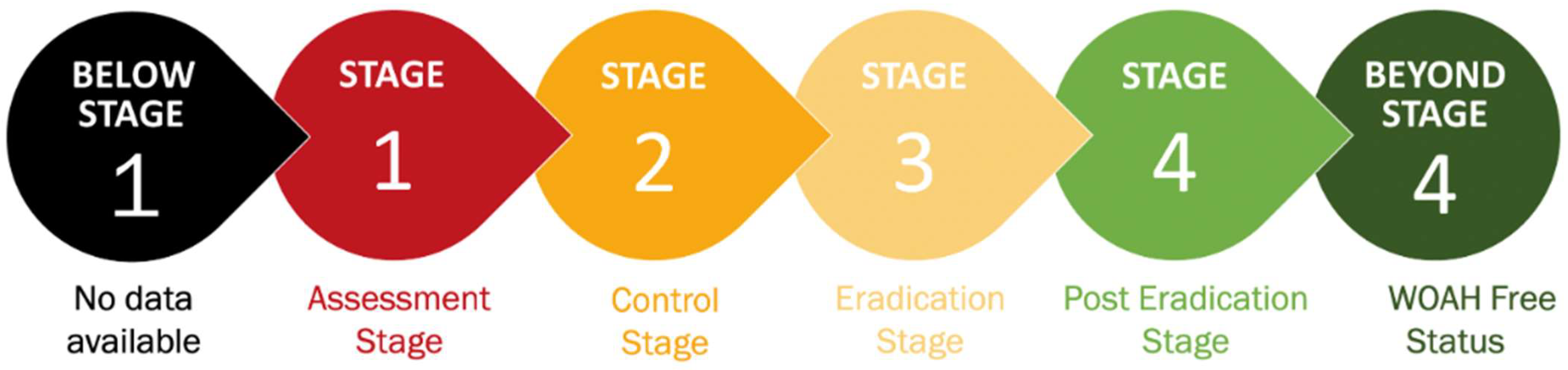
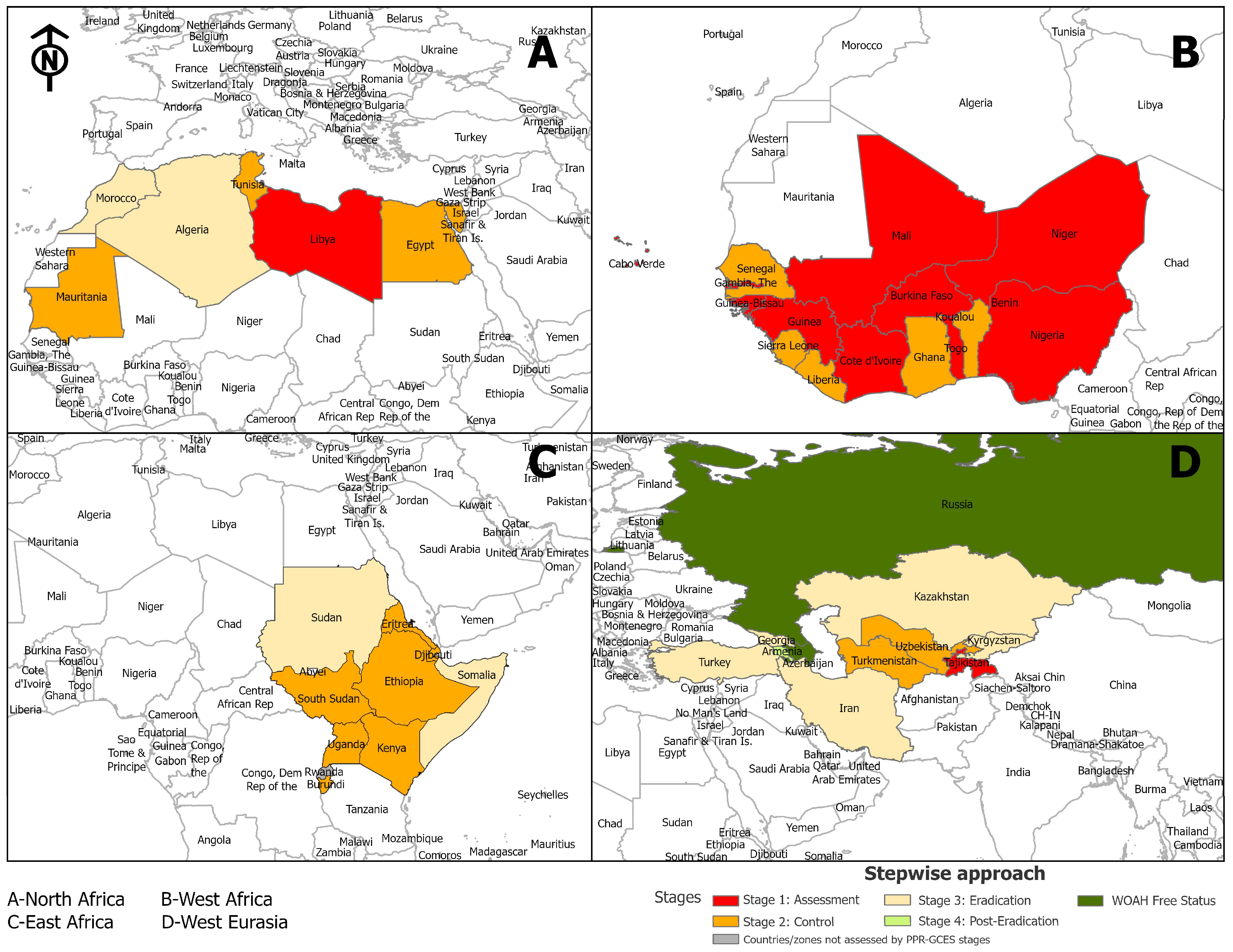
- Below Stage 1 (black): no data available
- Stage 1 (red): assessment—countries evaluate the current PPR situation and identify high-risk areas.
- Stage 2 (orange): control—implement targeted vaccination and movement control measures.
- Stage 3 (yellow): eradication—intensified vaccination and surveillance efforts focus on reducing PPR incidence.
- Stage 4 (green): post-eradication—countries monitor for re-emergence and ensure PPR-free status, certified by WOAH.
- Beyond Stage 4 (dark green): WOAH-free status.
3.4. Regional Findings
3.4.1. Introduction to Regional Findings
3.4.2. Eastern Africa
| Country | 2017 | 2018 | 2019 | 2020 | 2021 | 2022 | 2023 | 2024 | 2025 | Last WAHIS 1 Outbreak Reported |
|---|---|---|---|---|---|---|---|---|---|---|
| Ethiopia | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | No reports |
| Kenya | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | No reports since 2007 |
| Uganda | 1 | 1 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | No reports since 2007 |
| Sudan | 2 | 2 | 2 | 2 | 2 | 3 | 3 | 3 | 3 | No reports |
| Somalia | 2 | 2 | 2 | 2 | 2 | 3 | 3 | 3 | 3 | No reports |
| Burundi | 1 | 1 | 1 * | 1 | 1 | 2 | 2 | 2 | 2 | May 2019 |
| Djibouti | 1 | 1 | 1 | 1 | 1 | 2 | 2 | 2 | 2 | No reports |
| Eritrea | 1 | 1 | 1 | 1 | 1 | 2 | 2 | 2 | 2 | No reports |
| Rwanda | May 2024 | |||||||||
| South Sudan | 1 | 1 | 1 | 1 | 1 | 2 | 2 | 2 | 2 | No reports |

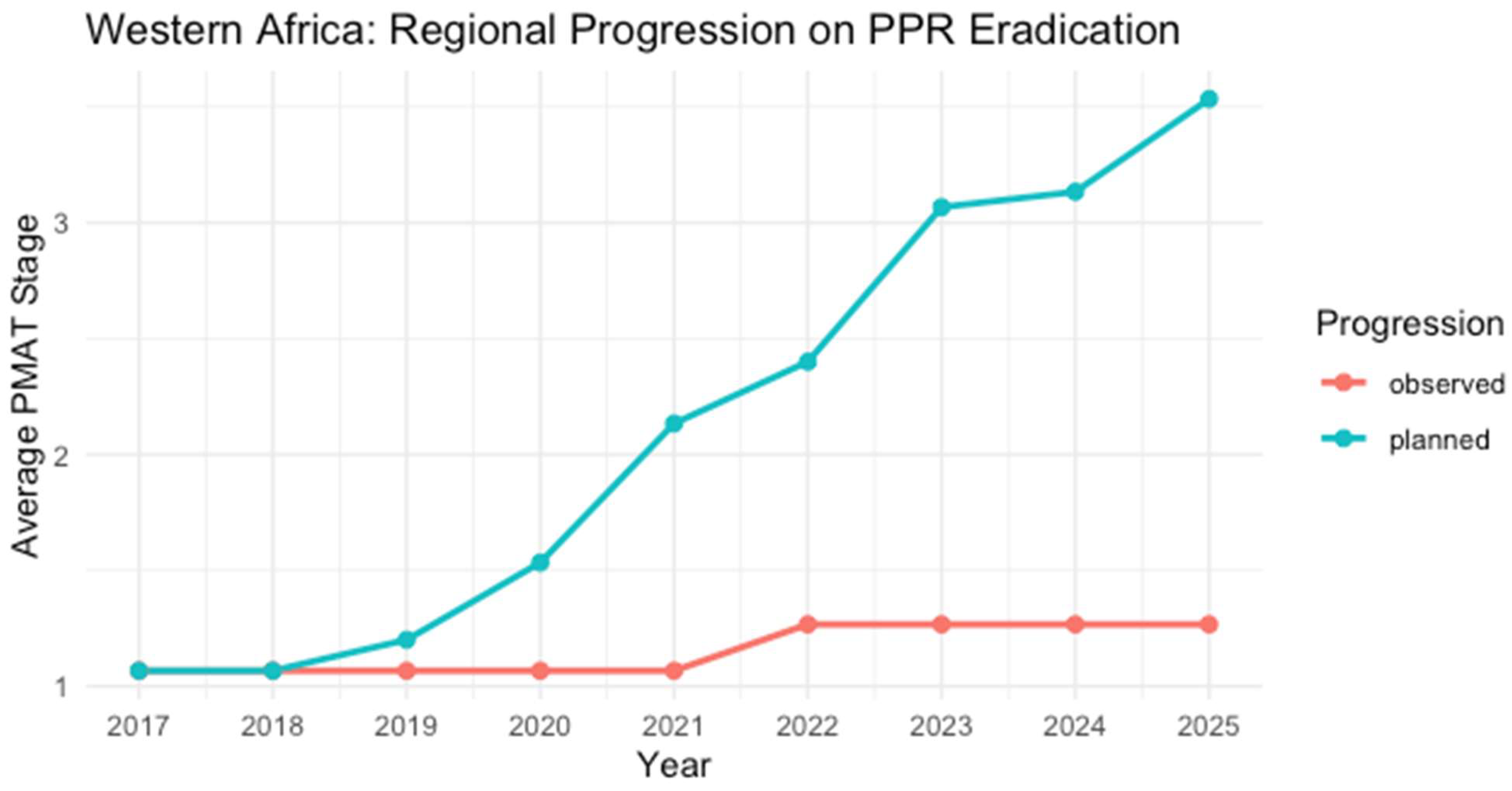
3.4.3. Western Africa
| Country | 2017 | 2018 | 2019 | 2020 | 2021 | 2022 | 2023 | 2024 | 2025 | Last Outbreak Reported |
|---|---|---|---|---|---|---|---|---|---|---|
| Benin | 1 | 1 | 1 | 1 | 1 | 2 | 2 | 2 | 2 | No reports |
| Burkina Faso | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | No reports |
| Cabo Verde | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | No reports |
| Cô d’Ivoire | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | No reports |
| Gambia | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | No reports |
| Ghana | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | No reports |
| Guinea | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | No reports |
| Guinea-Bissau | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | No reports |
| Liberia | 1 * | 1 | 1 | 1 | 1 | 2 | 2 | 2 | 2 | * December 2017 |
| Mali | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | Last report in May 2005 |
| Nigeria | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | No reports |
| Niger | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | No reports |
| Senegal | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | No reports |
| Sierra Leone | 1 | 1 * | 1 | 1 | 1 | 2 | 2 | 2 | 2 | September 2018 |
| Togo | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | No reports |
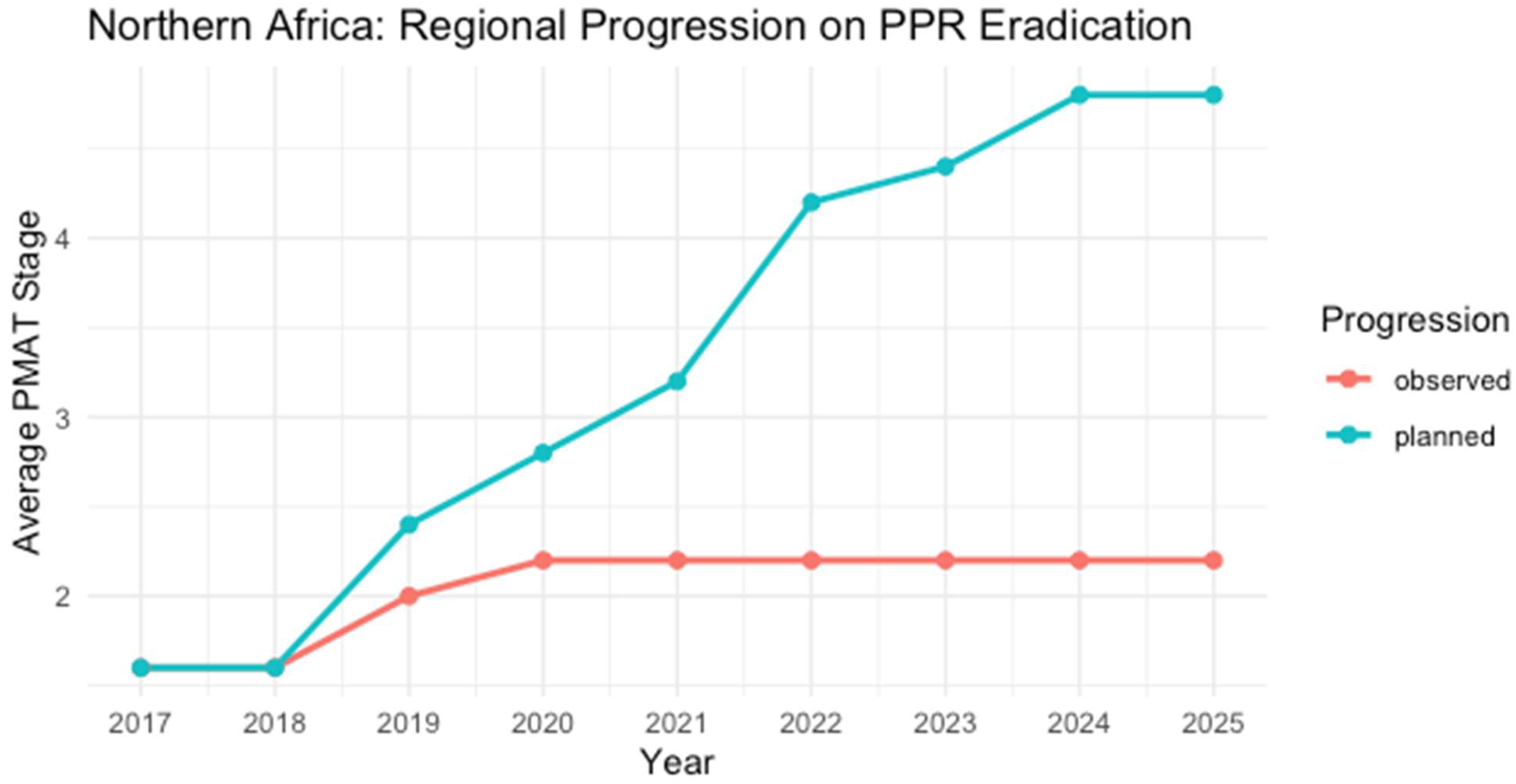
3.4.4. Northern Africa
| Country | 2017 | 2018 | 2019 | 2020 | 2021 | 2022 | 2023 | 2024 | 2025 | Last Outbreak Reported |
|---|---|---|---|---|---|---|---|---|---|---|
| Algeria | 2 | 2 | 3 | 3 | 3 | 3 * | 3 | 3 | 3 | December 2022 |
| Libya | 1 | 1 | 1 * | 1 * | 1 | 1 | 1 | 1 | 1 | May 2019, October 2020 |
| Morocco | 2 | 2 | 3 | 3 | 3 | 3 * | 3 | 3 | 3 | October 2023 |
| Mauritania | 1 | 1 | 1 | 2 | 2 | 2 | 2 | 2 | 2 | No WAHIS reports |
| Tunisia | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | * September 2016 |
| Egypt | 1 * | 1 | 1 | 1 | 1 | 2 | 2 | 2 | 2 | No outbreaks have been reported since May 2013 in WAHIS 1. FAO 2 documented no cases since 2017 |
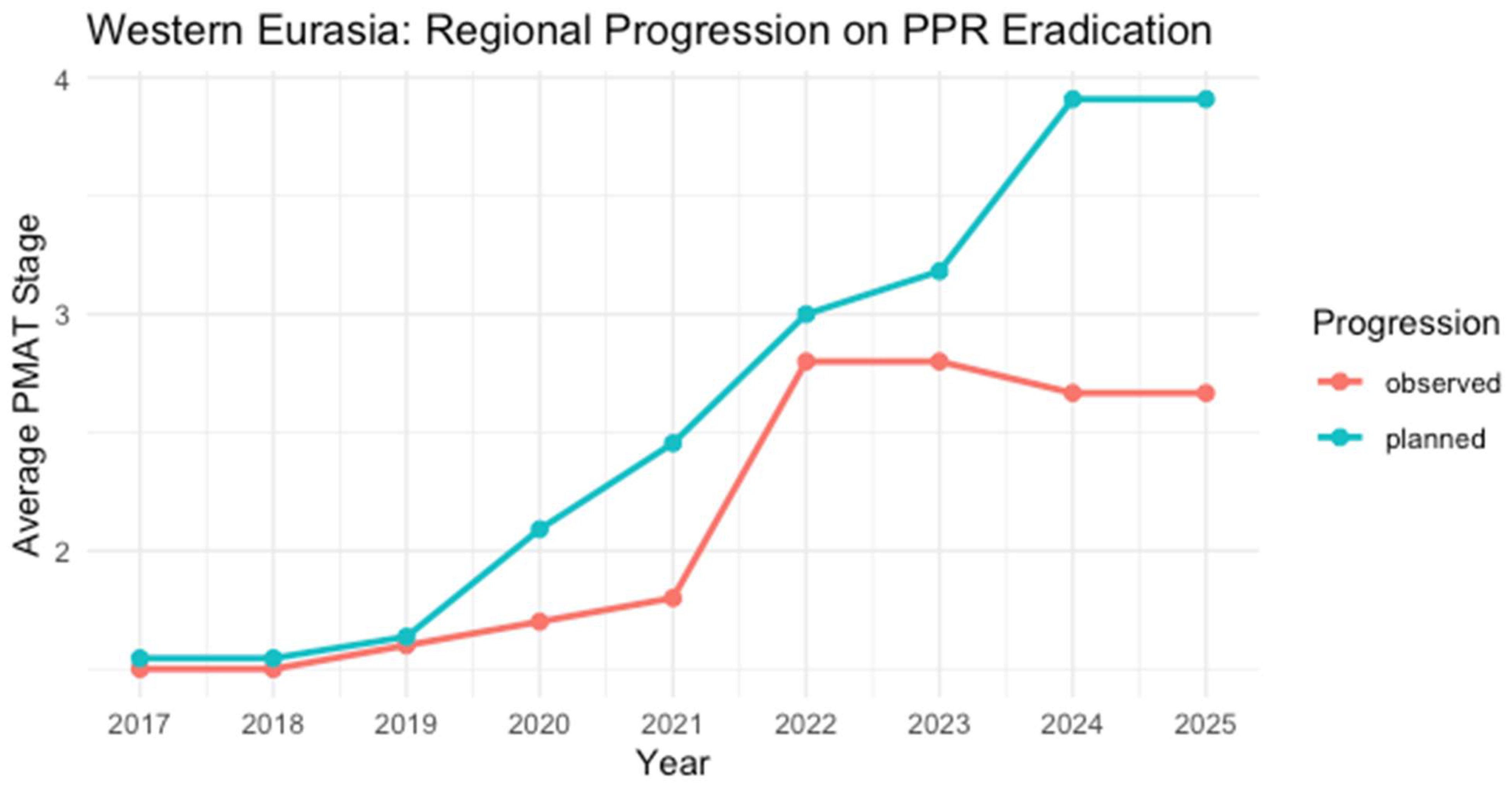
3.4.5. Western Eurasia
| Country | 2017 | 2018 | 2019 | 2020 | 2021 | 2022 | 2023 | 2024 | 2025 | Last Outbreak Reported |
|---|---|---|---|---|---|---|---|---|---|---|
| Armenia | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | Never reported |
| Azerbaijan | 1 | 1 | 1 | 1 | 1 | 4 | 4 | Free | Free | Never reported |
| Georgia | 1 | 1 | 2 | 2 | 3 | 3 | 3 * | 3 | 3 | May 2024 |
| Iran | 1 | 1 | 1 | 2 | 2 | 3 | 3 * | 3 | 3 | No reports |
| Kazakhstan | 2 | 2 | 2 | 2 | 2 | 3 | 3 * | 3 | 3 | No reports |
| Kyrgyzstan | 1 * | 1 | 1 | 1 | 1 | 3 | 3 * | 3 | 3 | No reports |
| Russian Federation | Free | Free | Free | Free | Free | Free | No reports | |||
| Tajikistan | 1 | 1 | 1 * | 1 * | 1 | 1 | 1 | 1 | 1 | September 2015 |
| Türkiye | 2 | 2 | 2 | 2 | 2 | 3 | 3 * | 3 | 3 | February 2024 |
| Turkmenistan | 1 | 1 | 1 | 1 | 1 | 2 | 2 | 2 | 2 | No reports |
| Uzbekistan | 1 | 1 | 1 | 1 | 1 | 2 | 2 | 2 | 2 | No reports |
4. Discussion
4.1. Animal Movement and Trade Impact
4.2. Veterinary Infrastructure and Disease Control Challenges
4.3. Vaccination Challenges
4.4. Regional Differences in PPR Control Progress
5. Key Findings
6. Limitations
6.1. Data Availability
6.2. Methodological Challenges
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhao, H.; Njeumi, F.; Parida, S.; Benfield, C.T.O. Progress towards Eradication of Peste Petits Ruminants through Vaccination. Viruses 2021, 13, 59. [Google Scholar] [CrossRef] [PubMed]
- Cameron, A.R. Strategies for the Global Eradication of Peste Petits Ruminants: An Argument for the Use of Guerrilla Rather Than Trench Warfare. Front. Vet. Sci. 2019, 6, 331. [Google Scholar] [CrossRef] [PubMed]
- Torres-Velez, F.; Havas, K.A.; Spiegel, K.; Brown, C. Transboundary Animal Diseases as Re-Emerging Threats—Impact on One Health. Semin. Diagn. Pathol. 2019, 36, 193–196. [Google Scholar] [CrossRef]
- World Organisation for Animal Health (OIE); Food and Agriculture Organization of the United Nations (FAO). The Global Strategy for the Control and Eradication of PPR; OIE: Paris, France; FAO: Rome, Italy, 2015. [Google Scholar]
- Global Framework for the Control of Transboundary Animal Diseases. Available online: https://www.gf-tads.org/ (accessed on 17 December 2024).
- Torsson, E.; Kgotlele, T.; Misinzo, G.; Johansson Wensman, J.; Berg, M.; Karlsson Lindsjö, O. Field-Adapted Full Genome Sequencing of Peste-Petits-Ruminants Virus Using Nanopore Sequencing. Front. Vet. Sci. 2020, 7, 542724. [Google Scholar] [CrossRef]
- World Organisation for Animal Health (OIE); Food and Agriculture Organization of the United Nations (FAO). Guidelines for the Control and Prevention of Peste Petits Ruminants (PPR) in Wildlife Populations. In Peste Petits Ruminants Global Eradication Programme; OIE: Paris, France; FAO: Rome, Italy, 2021. [Google Scholar]
- Parida, S.; Muniraju, M.; Mahapatra, M.; Muthuchelvan, D.; Buczkowski, H.; Banyard, A.C. Peste Petits Ruminants. Vet. Microbiol. 2015, 181, 90–106. [Google Scholar] [CrossRef]
- Baron, M.D.; Diallo, A.; Lancelot, R.; Libeau, G. Peste Petits Ruminants Virus. Adv. Virus Res. 2016, 95, 1–42. [Google Scholar] [CrossRef] [PubMed]
- Legnardi, M.; Raizman, E.; Beltran-Alcrudo, D.; Cinardi, G.; Robinson, T.; Falzon, L.C.; Djomgang, H.K.; Okori, E.; Parida, S.; Njeumi, F.; et al. Peste Petits Ruminants in Central and Eastern Asia/West Eurasia: Epidemiological Situation and Status of Control and Eradication Activities after the First Phase of the PPR Global Eradication Programme (2017–2021). Animals 2022, 12, 2030. [Google Scholar] [CrossRef]
- Couacy-Hymann, E.; Berete, K.; Odoom, T.; Zerbo, L.H.; Mathurin, K.Y.; Kouakou, V.K.; Doumbouya, M.I.; Balde, A.; Ababio, P.T.; Ouoba, L.B.; et al. The Spread of Peste Petits Ruminants Virus Lineage IV in West Africa. Animals 2023, 13, 1268. [Google Scholar] [CrossRef]
- Mariner, J.C.; Jones, B.A.; Rich, K.M.; Thevasagayam, S.; Anderson, J.; Jeggo, M.; Cai, Y.; Peters, A.R.; Roeder, P.L. The Opportunity To Eradicate Peste Petits Ruminants. J. Immunol. 2016, 196, 3499–3506. [Google Scholar] [CrossRef]
- Terrestrial Animal Health Code. Available online: https://www.woah.org/en/what-we-do/standards/codes-and-manuals/terrestrial-code-online-access/ (accessed on 23 September 2024).
- Taylor, W. The Global Eradication of Peste Petits Ruminants (PPR) within 15 Years—Is This a Pipe Dream? Trop. Anim. Health Prod. 2016, 48, 559–567. [Google Scholar] [CrossRef]
- Parida, S.; Yusuf, J.; Njeumi, F. Update on Peste Petits Ruminants in Europe. Vet. Rec. 2024, 195, 211. [Google Scholar] [CrossRef] [PubMed]
- PPR Free Members. Available online: https://www.woah.org/en/disease/peste-des-petits-ruminants/#ui-id-5 (accessed on 14 March 2025).
- World Organization for Animal Health. First Detection of Peste Petits Ruminants (PPR) in Greece and Romania. Available online: https://www.woah.org/en/first-detection-of-peste-des-petits-ruminants-ppr-in-greece-and-romania/ (accessed on 8 August 2024).
- Britton, A.; Caron, A.; Bedane, B. Progress to Control and Eradication of Peste Petits Ruminants in the Southern African Development Community Region. Front. Vet. Sci. 2019, 6, 343. [Google Scholar] [CrossRef] [PubMed]
- Jones, B.A.; Rich, K.M.; Mariner, J.C.; Anderson, J.; Jeggo, M.; Thevasagayam, S.; Cai, Y.; Peters, A.R.; Roeder, P. The Economic Impact of Eradicating Peste Petits Ruminants: A Benefit-Cost Analysis. PLoS ONE 2016, 11, e0149982. [Google Scholar] [CrossRef] [PubMed]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Leboucq, N.; Ferrari, G.; Domenech, J. PPR Monitoring and Evaluation Tool: A Companion Tool of the Global Strategy for the Control and Eradication of PPR; Annex, 3.3; OIE: Paris, France; FAO: Rome, Italy, 2015. [Google Scholar]
- Food and Agriculture Organization of the United Nations. Peste Petits Ruminants: Programme Approach. Available online: https://www.fao.org/ppr/global-programme/stepwise-approach/en/ (accessed on 16 March 2025).
- Munir, M. Role of Wild Small Ruminants in the Epidemiology of Peste Petits Ruminants. Transbound. Emerg. Dis. 2014, 61, 411–424. [Google Scholar] [CrossRef]
- WOAH. Sara Lysholm Update of Peste Petits Ruminants Global Control and Eradication Strategy (PPR GCES). In Proceedings of the Presented at the WOAH Regional Workshop on PPR in Asia and the Pacific, Qingdao, China, 18–20 June 2024. [Google Scholar]
- FAO; WOAH. Overview of The Plan of Action Peste Petits Ruminants Global Eradication Programme II & III Together for Peste Petits Ruminants Global Eradication by 2030 Blueprint 2022; FAO: Rome, Italy; WOAH: Paris, France, 2022. [Google Scholar]
- Viola Chemis PPR Monitoring and Evaluation Tool. Presented at the FMD/PPR Consultative meeting for East Mediterranean Countries, 2022. Available online: https://rr-middleeast.woah.org/app/uploads/2022/09/2-pmat-presentation-v-chemis.pdf (accessed on 11 April 2025).
- Dundon, W.G.; Diallo, A.; Cattoli, G. Peste Petits Ruminants in Africa: A Review of Currently Available Molecular Epidemiological Data, 2020. Arch. Virol. 2020, 165, 2147–2163. [Google Scholar] [CrossRef]
- AU-IBAR; FAO. 7th Regional PPR Control And Eradication Coordination Committee (PPR-CECC) Meeting: The Communiqué; AU-IBAR: Nairobi, Kenya, 2019. [Google Scholar]
- WOAH. Burundi:Burundi—Peste Petits Ruminants Virus (Inf. with)—Follow up Report 8 [FINAL]; WOAH: Paris, France, 2019. [Google Scholar]
- Wendimu, T.G.; Dinbiso, T.D.; Lobago, D.S.; Marami, L.M. Seroprevalence and Associated Risk Factors of Peste Petits Ruminants in Sheep and Goats in Three Districts of the Central Oromia Region, Ethiopia. Front. Vet. Sci. 2024, 11, 1402342. [Google Scholar] [CrossRef]
- Fournié, G.; Waret-Szkuta, A.; Camacho, A.; Yigezu, L.M.; Pfeiffer, D.U.; Roger, F. A Dynamic Model of Transmission and Elimination of Peste Petits Ruminants in Ethiopia. Proc. Natl. Acad. Sci. USA 2018, 115, 8454–8459. [Google Scholar] [CrossRef]
- Kumbe, A.; Negussie, H.; Getachew, Y.; Alemu, B.; Alemayehu, G.; Girma, S.; Sibhatu, D.; Emiyu, K.; Waktole, H.; Leta, S. Epidemiology of Peste Petits Ruminants in Selected Districts of Borena Zone, Ethiopia. BMC Vet. Res. 2024, 20, 451. [Google Scholar] [CrossRef]
- Nkamwesiga, J.; Coffin-Schmitt, J.; Ochwo, S.; Mwiine, F.N.; Palopoli, A.; Ndekezi, C.; Isingoma, E.; Nantima, N.; Nsamba, P.; Adiba, R.; et al. Identification of Peste Petits Ruminants Transmission Hotspots in the Karamoja Subregion of Uganda for Targeting of Eradication Interventions. Front. Vet. Sci. 2019, 6, 221. [Google Scholar] [CrossRef] [PubMed]
- Nkamwesiga, J.; Lumu, P.; Nalumenya, D.P.; Korennoy, F.; Roesel, K.; Wieland, B.; Perez, A.; Kiara, H.; Muhanguzi, D. Seroprevalence and Risk Factors of Peste Petits Ruminants in Different Production Systems in Uganda. Prev. Vet. Med. 2023, 221, 106051. [Google Scholar] [CrossRef] [PubMed]
- Ayebazibwe, C.; Akwongo, C.J.; Waiswa, J.; Ssemakula, O.; Akandinda, A.; Barasa, M.; Nkamwesiga, J.; Mabirizi, A.; Lule, P.M.; Roesel, K.; et al. Peste Petits Ruminants (PPR) in Uganda Assessment of Animal Health Systems and Coordination Mechanisms; ILRI: Nairobi, Kenya, 2022; Available online: https://cgspace.cgiar.org/server/api/core/bitstreams/104bbca0-67f9-4460-9046-28183b9523ac/content (accessed on 17 March 2025).
- Tounkara, K. Western Africa Regional PPR Strategy and Roadmap. In Presented at the OIE Procedures for the Endorsement of National Official Control Programmes with Regard to FMD and PPR; OIE: Kigali, Rwanda, 2017. [Google Scholar]
- Adeoye, F.; Kolapo, A.; Ogunmolawa, O.; Aluko, A.; Meseko, C.; Oluwayelu, D. Molecular Detection of Peste Petits Ruminants Virus (PPRV) in Goats and in Sheep in Ibadan, Oyo State, Nigeria. Vet. Ital. 2022, 58. [Google Scholar] [CrossRef]
- Tounkara, K.; Bataille, A.; Adombi, C.M.; Maikano, I.; Djibo, G.; Settypalli, T.B.K.; Loitsch, A.; Diallo, A.; Libeau, G. First Genetic Characterization of Peste Petits Ruminants from Niger: On the Advancing Front of the Asian Virus Lineage. Transbound. Emerg. Dis. 2018, 65, 1145–1151. [Google Scholar] [CrossRef] [PubMed]
- FAO; WOAH. In Proceedings of the Atelier de Revue de la Feuille de Route Pour le Contrôle et L’éradication de la PPR dans les Pays de l’Union du Maghreb Arabe (UMA) une Approche Régionale Coordonnée, Tunis, Tunisia, 2–3 April 2019.
- Intisar, K.S.; Ali, Y.H.; Haj, M.A.; Sahar, M.A.T.; Shaza, M.M.; Baraa, A.M.; Ishag, O.M.; Nouri, Y.M.; Taha, K.M.; Nada, E.M.; et al. Peste Petits Ruminants Infection in Domestic Ruminants in Sudan. Trop. Anim. Health Prod. 2017, 49, 747–754. [Google Scholar] [CrossRef]
- Ali, S.E.M.; Ahmed, Y.A.M.; Osman, A.A.; Gamal Eldin, O.A.; Osman, N.A. Prevalence of Peste Petits Ruminants Virus Antibodies in Sheep and Goats Sera from Central-Western Sudan. Onderstepoort J. Vet. Res. 2023, 90, e1–e8. [Google Scholar] [CrossRef]
- Ali, W.H.; Osman, N.A.; Asil, R.M.; Mohamed, B.A.; Abdelgadir, S.O.; Mutwakil, S.M.; Mohamed, N.E.B. Serological Investigations of Peste Petits Ruminants among Cattle in the Sudan. Trop. Anim. Health Prod. 2019, 51, 655–659. [Google Scholar] [CrossRef]
- Soltan, M.A.; Abd-Eldaim, M.M. Emergence of Peste Petits Ruminants Virus Lineage IV in Ismailia Province, Egypt. Infect. Genet. Evol. J. Mol. Epidemiol. Evol. Genet. Infect. Dis. 2014, 28, 44–47. [Google Scholar] [CrossRef]
- WOAH. Algeria—Peste Petits Ruminants Virus (Inf. with)—Follow up Report 3 [FINAL]; WOAH: Paris, France, 2022. [Google Scholar]
- Tunisia—Peste Petits Ruminants—Follow up Report 2. 2004. Available online: https://wahis.woah.org/#/in-review/2004 (accessed on 11 April 2025).
- WAHIS. Report: Morocco. Available online: https://wahis.woah.org/#/in-review/3991 (accessed on 13 March 2025).
- Peste Petits Ruminants: Egypt. Available online: https://www.fao.org/ppr/current-situation/country-detail/en/?country_iso3=EGY (accessed on 16 February 2025).
- WAHIS. Report: Libya. Available online: https://wahis.woah.org/#/in-event/3321/dashboard (accessed on 11 April 2025).
- Meetings of the West Eurasia PPR Roadmap (GF-TADs). Available online: https://rr-europe.woah.org/en/our-missions/animal-diseases/peste-des-petits-ruminants-ppr/ppr-west-eurasia-roadmap-meetings/#:~:text=2nd%20PPR%20Roadmap%20meeting,and%20vaccination%20in%20the%20region (accessed on 17 March 2025).
- World Organization for Animal Health. Five Countries Receive Official Recognition of Animal Health Status from WOAH. Available online: https://www.woah.org/en/five-countries-receive-official-recognition-of-animal-health-status-from-woah/#:~:text=In%202024%2C%20five%20countries%20obtained,African%20horse%20sickness%20(AHS)%3B (accessed on 4 October 2024).
- Chenais, E.; Wennström, P.; Kartskhia, N.; Fischer, K.; Risatti, G.; Chaligava, T.; Enukidze, T.; Ståhl, K.; Vepkhvadze, N.G. Perceptions of Pastoralist Problems: A Participatory Study on Animal Management, Disease Spectrum and Animal Health Priorities of Small Ruminant Pastoralists in Georgia. Prev. Vet. Med. 2021, 193, 105412. [Google Scholar] [CrossRef]
- FAO; WOAH. In Proceedings of the Peste de Petits Ruminants Regional Meeting and Blueprint Consultation for Economic Cooperation Organization (ECO) Countries, Baku, Azerbaijan, 25–27 April 2023.
- Arede, M.; Beltrán-Alcrudo, D.; Aliyev, J.; Chaligava, T.; Keskin, I.; Markosyan, T.; Morozov, D.; Oste, S.; Pavlenko, A.; Ponea, M.; et al. Corrigendum: Examination of Critical Factors Influencing Ruminant Disease Dynamics in the Black Sea Basin. Front. Vet. Sci. 2024, 11, 1376956. [Google Scholar] [CrossRef]
- Kock, R.A.; Orynbayev, M.B.; Sultankulova, K.T.; Strochkov, V.M.; Omarova, Z.D.; Shalgynbayev, E.K.; Rametov, N.M.; Sansyzbay, A.R.; Parida, S. Detection and Genetic Characterization of Lineage IV Peste Petits Ruminant Virus in Kazakhstan. Transbound. Emerg. Dis. 2015, 62, 470–479. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Xu, G.; Zeng, Z.; Lv, J.; Huang, L.; Wang, H.; Wang, X. Transboundary Spread of Peste Petits Ruminants Virus in Western China: A Prediction Model. PLoS ONE 2021, 16, e0257898. [Google Scholar] [CrossRef] [PubMed]
- Abdrakhmanov, S.K.; Mukhanbetkaliyev, Y.Y.; Sultanov, A.A.; Yessembekova, G.N.; Borovikov, S.N.; Namet, A.; Abishov, A.A.; Perez, A.M.; Korennoy, F.I. Mapping the Risks of the Spread of Peste Petits Ruminants in the Republic of Kazakhstan. Transbound. Emerg. Dis. 2022, 69, 2296–2305. [Google Scholar] [CrossRef] [PubMed]
- Pruvot, M.; Fine, A.E.; Hollinger, C.; Strindberg, S.; Damdinjav, B.; Buuveibaatar, B.; Chimeddorj, B.; Bayandonoi, G.; Khishgee, B.; Sandag, B.; et al. Outbreak of Peste Petits Ruminants among Critically Endangered Mongolian Saiga and Other Wild Ungulates, Mongolia, 2016–2017. Emerg. Infect. Dis. 2020, 26, 51–62. [Google Scholar] [CrossRef]
- Benfield, C.T.O.; Hill, S.; Shatar, M.; Shiilegdamba, E.; Damdinjav, B.; Fine, A.; Willett, B.; Kock, R.; Bataille, A. Molecular Epidemiology of Peste Petits Ruminants Virus Emergence in Critically Endangered Mongolian Saiga Antelope and Other Wild Ungulates. Virus Evol. 2021, 7, veab062. [Google Scholar] [CrossRef]
- Tounkara, K.; Kwiatek, O.; Niang, M.; Abou Kounta Sidibe, C.; Sery, A.; Dakouo, M.; Salami, H.; Lo, M.M.; Ba, A.; Diop, M.; et al. Genetic Evidence for Transboundary Circulation of Peste Petits Ruminants Across West Africa. Front. Vet. Sci. 2019, 6, 275. [Google Scholar] [CrossRef]
| Concept | Keywords |
|---|---|
| Peste des petits ruminants | “Peste des petits ruminants” OR “PPRV” OR “PPR eradication” OR “PPR control” OR “PPR outbreaks” OR “PPR vaccination” |
| Regions/countries | “Western Africa” “Benin” OR “Burkina Faso” OR “Cape Verde” OR “Côte d’Ivoire” OR “Gambia” OR “Ghana” OR “Guinea” OR “Guinea-Bissau” OR “Liberia” OR “Mali” OR “Niger” OR “Nigeria” OR “Senegal” OR “Sierra Leone” OR “Togo” “Northern Africa” “Algeria” OR “Egypt” OR “Libya” OR “Mauritania” OR “Morocco” OR “Sudan” OR “Tunisia” “Eastern Africa” “Burundi” OR “Djibouti” OR “Eritrea” OR “Ethiopia” OR “Kenya” OR “Rwanda” OR “Somalia” OR “South Sudan” OR “Uganda” OR “Kazakhstan” OR “Kyrgyzstan” OR “Tajikistan” OR “Turkmenistan” OR “Uzbekistan” OR “Central Asia” OR “Africa” OR “Eastern Europe” OR “Russia” OR “Greece” OR “Romania” OR “West Eurasia” OR “Armenia” OR “Azerbaijan” OR “Georgia” OR “Turkey” OR “Iran” OR “Europe” |
| GCES | “diagnostics” OR “surveillance” OR “prevention” OR “vaccination” OR “legal framework” OR “stakeholder involvement” OR “veterinary capacities” OR “PMAT” |
| Author (Year) | Study Region | Study Focus | Key Findings | Relevance to PPR Control |
|---|---|---|---|---|
| Global Level | ||||
| [1] Zhao et al. (2021) | Global | Progress toward PPR eradication through vaccination | Emphasized vaccination campaigns achieving up to 70% efficacy in high-risk zones | Highlights vaccination as a critical component of the PPR eradication strategy under GCES |
| [3] Torres-Velez et al. (2019) | Global | Review of TADs as re-emerging threats with an emphasis on their One Health impact | Socio-economic impact of TADs, including PPR, highlighting the need for diagnostics, vaccination, and regional cooperation | Underscores PPR’s role as a significant TAD affecting small ruminants, emphasizing the importance of surveillance, vaccination, and control strategies to mitigate its impact |
| [4] FAO/WOAH (2015) | Global | Launch and framework of the PPR Global Control and Eradication Strategy (GCES) | A stepwise approach for PPR eradication, emphasizing vaccination, surveillance, and legal frameworks, is outlined | Provides the foundation for global coordination and guidance toward PPR eradication by 2030, including monitoring tools like PMAT |
| [22] Leboucq et al. (2015) | Global | PMAT for evaluating PPR control progress | A structured, evidence-based framework for assessing country progress in PPR control | Supports risk-based decision-making, regional coordination, and harmonized interventions |
| [2] Cameron (2019) | Global | Guerrilla strategy for PPR eradication | Proposed a short-duration, high-coverage vaccination strategy integrated with movement management and real-time surveillance | Advocates for sustainable, efficient eradication methods over long-term campaigns, emphasizing rapid response and targeted interventions |
| [5] FAO/WOAH (2024) | Global | Initiation of the Global Framework for the Progressive Management of Transboundary Animal Diseases (GF-TADs) | The Joint FAO-WOAH initiative aims to stop, identify, and manage transboundary animal diseases (TADs) by fostering regional partnership | Establishes a fundamental framework for international collaboration and local strengthening in the fight against TADs, such as PPR, through the alignment of resources and strategies at various levels |
| [6] Torsson et al. (2020) | Global, field applications | Field-adapted complete genome sequencing of PPRV using Nanopore technology | Demonstrated successful field-adapted protocol for full genome sequencing of PPRV, achieving >90% genome coverage in resource-constrained settings with portable devices | Enables rapid diagnostics and surveillance for PPR in remote areas, supporting FAO/WOAH eradication efforts by 2030 |
| [7] FAO/WOAH (2021) | Global | Guidelines for PPR Control in Wildlife Populations | Approaches to incorporating wildlife aspects into PPR control initiatives encompass monitoring, risk evaluation, and managing outbreaks in wildlife populations | Emphasizes the importance of a multisectoral approach and tailored strategies to manage PPR at the wildlife–livestock interface for successful eradication |
| [9] Baron et al. (2016) | Global | Genetic characterization and molecular biology of PPRV | Described four distinct PPRV lineages (I–IV), which can be discriminated based on nucleotide variations in the N gene | Provides molecular tools for epidemiological surveillance, lineage tracking, and targeted control strategies for specific regions |
| [13] World Organisation for Animal Health (WOAH). Terrestrial Code Online Access (2024) | Global | Terrestrial Animal Health Code | Section 5 of the Terrestrial Code focuses on trade measures, import/export procedures, and veterinary certification to manage TAD outbreaks, mitigate trade restrictions, and protect territories | Provides guidelines for implementing disease control strategies aligned with international standards to minimize PPR spread and economic burdens while enabling trade continuity |
| [14] Taylor (2016) | Global | Eradication strategies for PPR, building on lessons from rinderpest eradication | Highlights the critical role of international cooperation, vaccination campaigns, and sanitary livestock movement to reduce virus spread and achieve control | Provides a strategic framework for PPR eradication efforts globally based on proven methods |
| [19] Jones et al. (2016) | Global | Benefit-cost analysis for the eradication of PPR | The analysis highlights strong economic returns from eradication through reduced mortality, increased production, and avoided control costs | Emphasizing the importance of targeted vaccination and international cooperation for resource allocation |
| [20] Tricco et al. (2018) | Global | Development of the PRISMA Extension for Scoping Reviews (PRISMA-ScR) Checklist | Offers detailed explanations and examples for each checklist item of scoping review | Ensures the methodological transparency and consistency of scoping reviews, applicable for structuring and reporting reviews in PPR-related research |
| [21] Page et al. (2021) | Global | Updates to PRISMA guidelines for systematic reviews | A 27-item checklist for transparent and comprehensive reporting of systematic reviews | Systematic reviews could enhance the quality and reliability of PPR-related research synthesis, aiding in evidence-based policy and control strategies |
| [23] Food and Agriculture Organization (FAO) (2016) | Global | Stepwise Approach for PPR Eradication | Progressive Control Pathway (PCP) as a framework for countries to achieve PPR eradication by 2030; identified stages and criteria for monitoring progress | A structured and systematic approach to guide countries in assessing their PPR control capabilities, identifying gaps, and implementing targeted measures for eradication |
| [24] Munir (2014) | Global | Role of small wild ruminants in PPR epidemiology | Phylogenetic analysis confirmed all PPRV isolates from wild ungulates belong to lineage IV | Highlights the uncertain role of wildlife in PPR epidemiology |
| [25] Lysholm (2024) | Global | WOAH guidelines for PPR control and disease status recognition | Outlined key provisions of the WOAH Terrestrial Animal Health Code related to PPR | Provides regulatory guidance for PPR control and eradication |
| [26] FAO & WOAH (2022) | Global | PPR Global Eradication Programme (GEP) II & III Blueprint | Provides a strategic roadmap for the global eradication of PPR by 2030 | Outlines a structured, stepwise strategy for achieving global PPR eradication |
| [23] FAO (2015) | Global | Stepwise approach to PPR control and eradication | The PPR Global Eradication Programme (PPR GEP) outlines a four-stage stepwise approach | Provides a structured framework for countries’ progressive control and eradication of PPR |
| [27] Chemis, V. (2022) | Middle East, global | Implementation of a PPR Monitoring and Assessment Tool | Insights into PMAT’s role in evaluating national and regional efforts in PPR eradication | Demonstrates PMAT’s value in providing a structured framework for countries to assess their progress in PPR control, identify gaps, and implement corrective actions |
| [8] Parida et al. (2015) | Africa, Asia | Overview of PPR epidemiology and its impact | Detailed the socio-economic impact of PPR and the role of vaccination and diagnostics in eradication efforts | Supports prioritization of vaccination campaigns and diagnostic improvements in endemic regions |
| [12] Mariner et al. (2016) | Africa, Asia, Middle East | Challenges and Opportunities in PPR Eradication | Political instability, resource constraints, and weak veterinary systems hinder PPR control; emphasized the critical role of surveillance, vaccination, and stakeholder collaboration | Provides insights into aligning eradication strategies with socio-economic and political realities, ensuring feasibility and sustainability in resource-limited regions |
| [28] Dundon et al. (2020) | Africa | Review of molecular epidemiological data on PPR in Africa | Consolidated data from multiple African countries highlighting the shift toward lineage IV as the dominant strain | Provides a comprehensive molecular epidemiological perspective on PPRV circulation in Africa |
| Eastern Africa | ||||
| [29] AU-IBAR (2019) | Eastern Africa | Regional coordination and progress review on PPR control and eradication | Reviewed regional and national PPR control progress | Strengthens the case for regional collaboration, structured funding approaches, and cross-border coordination to advance PPR eradication |
| [30] WOAH (2019) | World Animal Health Information System (WAHIS) report | PPR outbreak follow-up report | Outbreak that started in 2018 was resolved in 2019 | Burundi continues experiencing outbreaks |
| [31] Wendimu et al. (2024) | Central Oromia, Ethiopia | Seroprevalence associated risk factors | High seroprevalence was found in nomadic areas, necessitating tailored vaccination strategies for pastoral systems | Indicates risk factors that hinder vaccination coverage in regions with mobile livestock populations |
| [32] Fournié et al. (2018) | Ethiopia | Mathematical modeling of PPRV transmission | Used a dynamic transmission model to estimate PPRV spread and the required vaccination coverage for elimination in Ethiopia | Provides evidence-based vaccination strategies for PPR eradication |
| [33] Kumbe et al. (2024) | Ethiopia (Borena Zone) | Seroprevalence and risk factors of PPRV | A cross-sectional study found a PPR seroprevalence of 32.1% in non-vaccinated animals, 45.5% in animals with unknown vaccination history, and 68.8% in vaccinated animals | Highlights critical gaps in Ethiopia’s PPR vaccination coverage and eradication strategy |
| [34] Nkamwesiga et al. (2019) | Karamoja, Uganda | Identifying PPR transmission hotspots for targeting eradication interventions | Identified hotspots using phylogenetic and movement data to tailor vaccination campaigns | Demonstrates the importance of hotspot mapping for efficient use of resources |
| [35] Nkamwesiga et al. (2023) | Uganda | Seroprevalence and risk factors of PPRV in different production systems | Found a true seroprevalence of 27.3%, with the highest rates in pastoral (44.1%) and agropastoral (31.7%) systems | Emphasizes the need for targeted PPR control strategies, particularly in pastoralist communities |
| [36] Ayebazibwe et al. (2022) | Uganda | Assessment of animal health systems and coordination mechanisms for PPR control | PPR was first detected in Karamoja in 2007 and has since spread to over 50 districts; the study found that Uganda remains at Stage 2 of the PPR GCES | Highlights the need for improved veterinary governance, strengthened surveillance, and better vaccination logistics |
| [18] Britton et al. (2019) | Southern African Development Community (SADC) region | Progress to control and eradication of PPR, gaps in veterinary services, and surveillance in the SADC region | Gaps in vaccination, diagnostics, and surveillance systems are described | The need for regional cooperation, risk-based surveillance, and cross-border livestock trade control are emphasized |
| Western Africa | ||||
| [37] Tounkara (2017) | Western Africa | Regional PPR Strategy and Progression | Countries indicated planned progression along the Progressive Stepwise Approach | The event sought to synchronize national efforts and boost the collective ability to combat PPR |
| [11] Couacy-Hymann et al. (2023) | Burkina Faso, Côte d’Ivoire, Ghana, Guinea | Molecular characterization of PPRV strains | Identified genetic heterogeneity within Lineage IV, subdivided into distinct subclades, and highlighted transboundary animal movements as a key driver of virus spread | Reinforces the importance of cross-border collaboration and lineage-specific diagnostics for effective disease control |
| [38] Adeoye et al. (2022) | Nigeria | Molecular detection of PPRV in goats and sheep in Ibadan, Oyo State | Confirmed a 7.4% prevalence of PPRV in field samples; confirmed ongoing circulation of PPRV in Ibadan despite vaccination programs | Highlights the need for continuous PPR surveillance and more effective vaccination strategies |
| [39] Tounkara et al. (2018) | Niger and Western Africa | Genetic characterization of PPR in Niger | Demonstrates transboundary spread of the Asian virus lineage in West Africa | Provides evidence for the need for regional coordination in genetic surveillance and vaccination |
| Northern Africa | ||||
| [40] FAO & WOAH (2019) | Northern Africa | The PPR roadmap meeting in the Maghreb region (Union du Maghreb Arabe, UMA) | Countries indicated planned progression along the Progressive Stepwise Approach | The event sought to synchronize national efforts and boost the collective ability to combat PPR |
| [41] Intisar et al. (2017) | Sudan (Northern Africa) | Seroprevalence and molecular detection of PPRV in domestic ruminants and camels | Investigated the prevalence of PPR in sheep, goats, cattle, and camels in Sudan from 2008 to 2012 | Provides strong evidence of widespread PPRV circulation in Sudan |
| [42] Ali et al. (2023) | Central–Western Sudan | PPRV antibodies prevalence in livestock populations | High antibody prevalence indicates ongoing virus circulation | Reinforces the role of serological studies in monitoring and targeting eradication strategies |
| [43] Ali et al. (2019) | Sudan | Serological investigations of PPR in cattle populations | A serosurveillance study conducted from 2015 to 2016 found a 42.0% prevalence of PPRV antibodies among 1000 cattle in five Sudanese states | Reinforces the importance of cross-species monitoring for effective PPR eradication |
| [44] Soltan et al. (2014) | Egypt | Molecular detection and genetic characterization of PPRV in Ismailia province | Confirmed the emergence of PPRV in Egypt despite the country previously declared PPR-free | Demonstrates the importance of continuous surveillance to detect emerging PPR outbreaks |
| [45] WOAH (2022) | Algeria | PPR outbreak follow-up report | Recurrence of PPR | Insights into the current PPR situation in Algeria |
| [46] WOAH (2016) | Tunisia | PPR outbreak follow-up report | The report provides detailed information on the latest PPR outbreaks in Tunisia | Highlights Tunisia’s efforts to control PPR |
| [47] WOAH (2023) | Morocco | PPR outbreak follow-up report | The report provides detailed information on the latest PPR outbreaks and its resolution in 2023 | Highlights ongoing outbreaks registration |
| [48] FAO (2024) | Egypt | Overview of PPR epidemiological situation and control measures in Egypt | PPRV was confirmed in a sheep farm in 2012; between 2013 and 2017, at least 35 outbreaks were reported annually | Overview of Egypt’s PPR status and control strategies |
| [49] WOAH (2021) | Libya | PPR outbreak follow-up report | The report provides detailed information on the latest PPR outbreaks and its resolution in 2021 | Highlights ongoing outbreaks registration |
| Western Eurasia | ||||
| [50] WOAH (2025) | Western Eurasia | Third PPR roadmap meeting | Planned progression of countries along the Progressive Stepwise Approach | Majority of the countries were planning to be free or at Stage 4 by 2025 |
| [15] Parida et al. (2024) | Europe (Greece, Romania) | Outbreaks and control measures for PPR reintroduction to the European Union | Linked to migratory livestock movement and trade, with control measures application | Emphasizes the importance of rapid response, strict movement controls, and enhanced surveillance |
| [16] WOAH (2025) | Europe (Hungary) | Suspension on PPR-free status in Hungary due to outbreak | Update on Resolution No. 25 adopted in May 2024 by the World Assembly of Delegates listing Hungary as a “PPR free country”: Hungary’s status had been suspended with effect on 23 January 2025 | Emphasizes the importance of rapid response, strict movement controls, and enhanced surveillance |
| [17] WOAH (2024) | Europe (Greece, Romania) | First detection of PPR in Greece and Romania | In July 2024, PPR was detected for the first time in Greece and Romania, both previously free of the disease | Highlights the importance of rapid detection and implementation of control measures in regions previously free from PPR |
| [51] WOAH (2024) | Azerbaijan | Official recognition of PPR-free status | In 2024, WOAH declared Azerbaijan free of PPR | Provides a model for other countries aiming to achieve PPR-free status |
| [52] Chenais et al. (2021) | Georgia | Participatory epidemiology study on small ruminant health and PPR perception | PPR was not identified as a priority disease by the participants, and no historical unreported outbreaks were detected | Suggests that PPR interventions should integrate broader livestock health management approaches |
| [53] FAO & WOAH (2023) | Economic Cooperation Organization (ECO) countries | Regional meeting and PPR Blueprint consultation | Evaluated national PPR eradication progress, identified challenges, and aligned country strategies with the PPR Global Eradication Program | Reinforces the need for harmonized policies, surveillance strategies, and funding mechanisms |
| [54] Arede et al. (2024) | Black Sea Basin | Examination of factors influencing ruminant disease dynamics, including transboundary animal diseases | Surveillance gaps, trade movements, and disease reporting inconsistencies in the Black Sea Basin | Importance of regional cooperation, robust surveillance, and policy frameworks |
| [10] Legnardi et al. (2022) | Western Eurasia | Epidemiological situation and control activities after Phase 1 of the PPR Global Eradication Programme | Documented the success of phased vaccination and surveillance efforts, reducing virus transmission | Highlights strategic implementation of GCES steps in higher-capacity regions. |
| [55] Kock et al. (2015) | Kazakhstan | Detection of PPR Lineage IV | Identifies genetic lineage IV in Kazakhstan, showing the regional spread | Highlights the importance of gene surveillance for targeted vaccination strategies |
| [56] Gao et al. (2021) | Western China and neighboring countries | Predictive modeling for PPR spread | Develops a predictive model showing high-risk areas for PPR spread in Western China | Provides tools for risk-based control and prioritization of resources |
| [57] Abdrakhmanov et al. (2022) | Kazakhstan | Mapping PPR risks | Highlights high-risk areas for PPR spread due to livestock movement and trade | Guides targeted vaccination and surveillance efforts. |
| [58] Pruvot et al. (2020) | Mongolia | PPRV outbreak in wild ungulates and its impact on conservation efforts | Confirmed spillover of PPRV from livestock to wild species (Mongolian saiga, ibex, goitered gazelle), causing mass mortality | Highlights the importance of including wildlife in PPR eradication efforts. |
| [59] Benfield et al. (2021) | Mongolia | Molecular epidemiology of PPRV in Mongolian wildlife | Phylogenetic analysis confirmed that PPRV in Mongolian wildlife likely originated from livestock transmission | Demonstrates the importance of early surveillance at the livestock–wildlife interface |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Imanbayeva, D.; Pérez Aguirreburualde, M.S.; Knauer, W.; Tegzhanov, A.; Yustyniuk, V.; Arzt, J.; Perez, A.; Njeumi, F.; Parida, S. A Scoping Review on Progression Towards Freedom from Peste des Petits Ruminants (PPR) and the Role of the PPR Monitoring and Assessment Tool (PMAT). Viruses 2025, 17, 563. https://doi.org/10.3390/v17040563
Imanbayeva D, Pérez Aguirreburualde MS, Knauer W, Tegzhanov A, Yustyniuk V, Arzt J, Perez A, Njeumi F, Parida S. A Scoping Review on Progression Towards Freedom from Peste des Petits Ruminants (PPR) and the Role of the PPR Monitoring and Assessment Tool (PMAT). Viruses. 2025; 17(4):563. https://doi.org/10.3390/v17040563
Chicago/Turabian StyleImanbayeva, Dinara, Maria Sol Pérez Aguirreburualde, Whitney Knauer, Azimkhan Tegzhanov, Valeriia Yustyniuk, Jonathan Arzt, Andres Perez, Felix Njeumi, and Satya Parida. 2025. "A Scoping Review on Progression Towards Freedom from Peste des Petits Ruminants (PPR) and the Role of the PPR Monitoring and Assessment Tool (PMAT)" Viruses 17, no. 4: 563. https://doi.org/10.3390/v17040563
APA StyleImanbayeva, D., Pérez Aguirreburualde, M. S., Knauer, W., Tegzhanov, A., Yustyniuk, V., Arzt, J., Perez, A., Njeumi, F., & Parida, S. (2025). A Scoping Review on Progression Towards Freedom from Peste des Petits Ruminants (PPR) and the Role of the PPR Monitoring and Assessment Tool (PMAT). Viruses, 17(4), 563. https://doi.org/10.3390/v17040563









