Inactivated Viral Vaccine BBV87 Protects Against Chikungunya Virus Challenge in a Non-Human Primate Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Challenge Agent Chikungunya Virus LR2006 OPY1
2.2. Challenge Model
2.3. Surgical Implants
2.4. Vaccine
2.5. Experimental Outline (See Figure 1)
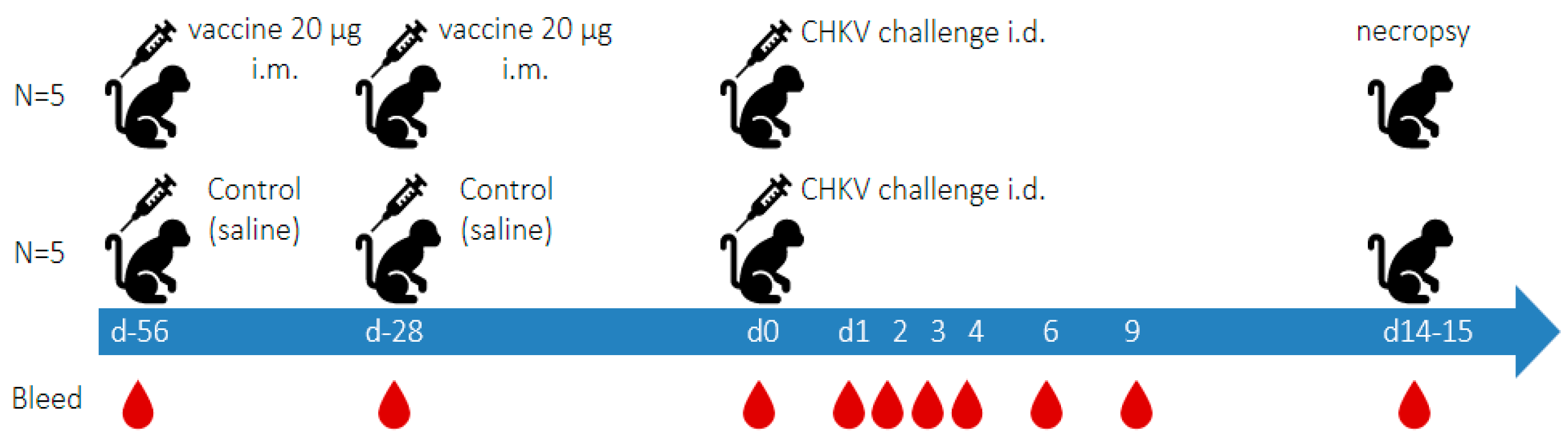
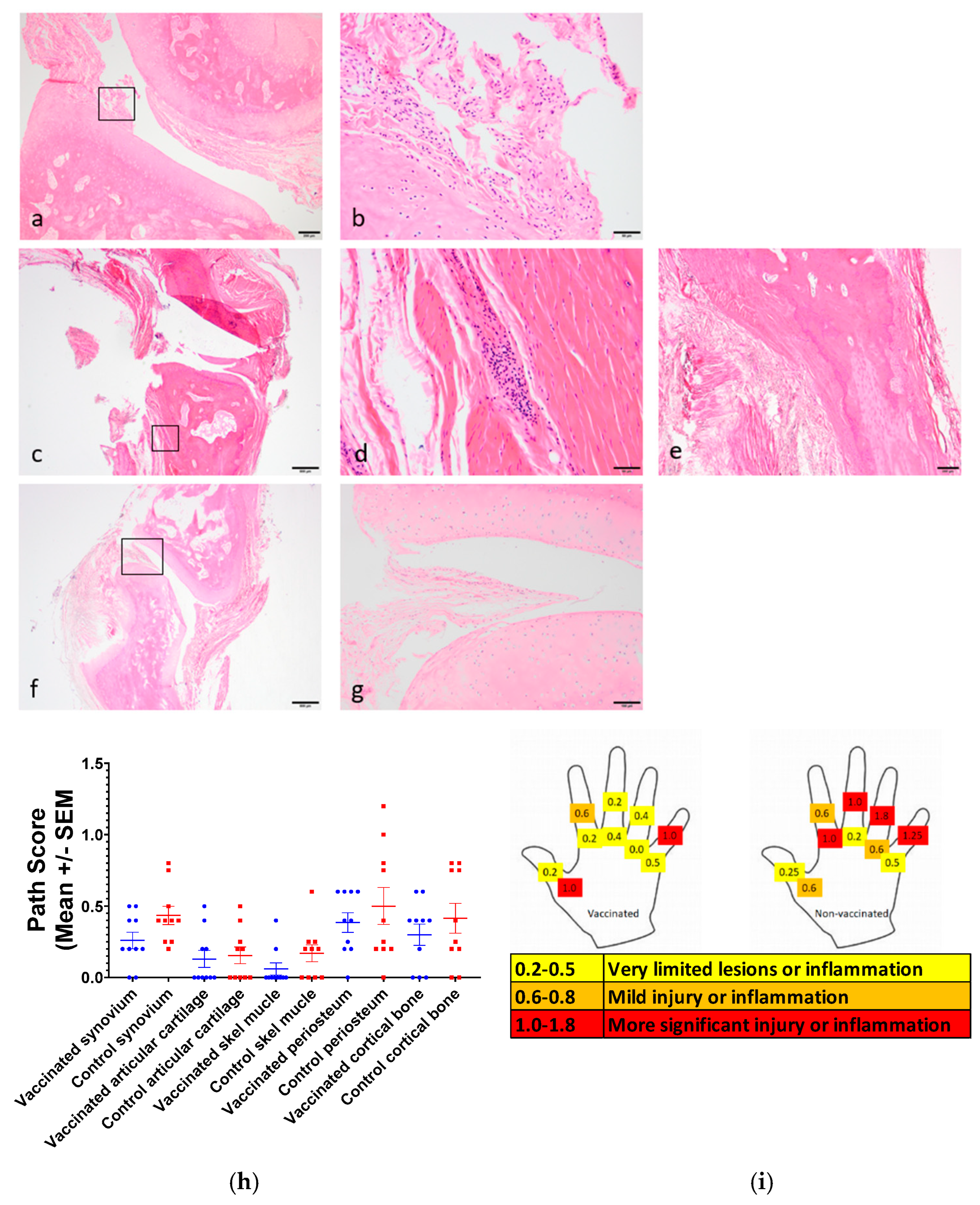
2.6. Histopathology
2.7. Plaque Assay for Determination of Viral Load in Blood and Selected Tissues
2.8. Quantitative CHIKV RT-qPCR Determinations in Plasma and Tissues
2.9. Binding Antibody ELISA
2.10. CHIKV Plaque Reduction Neutralisation Test
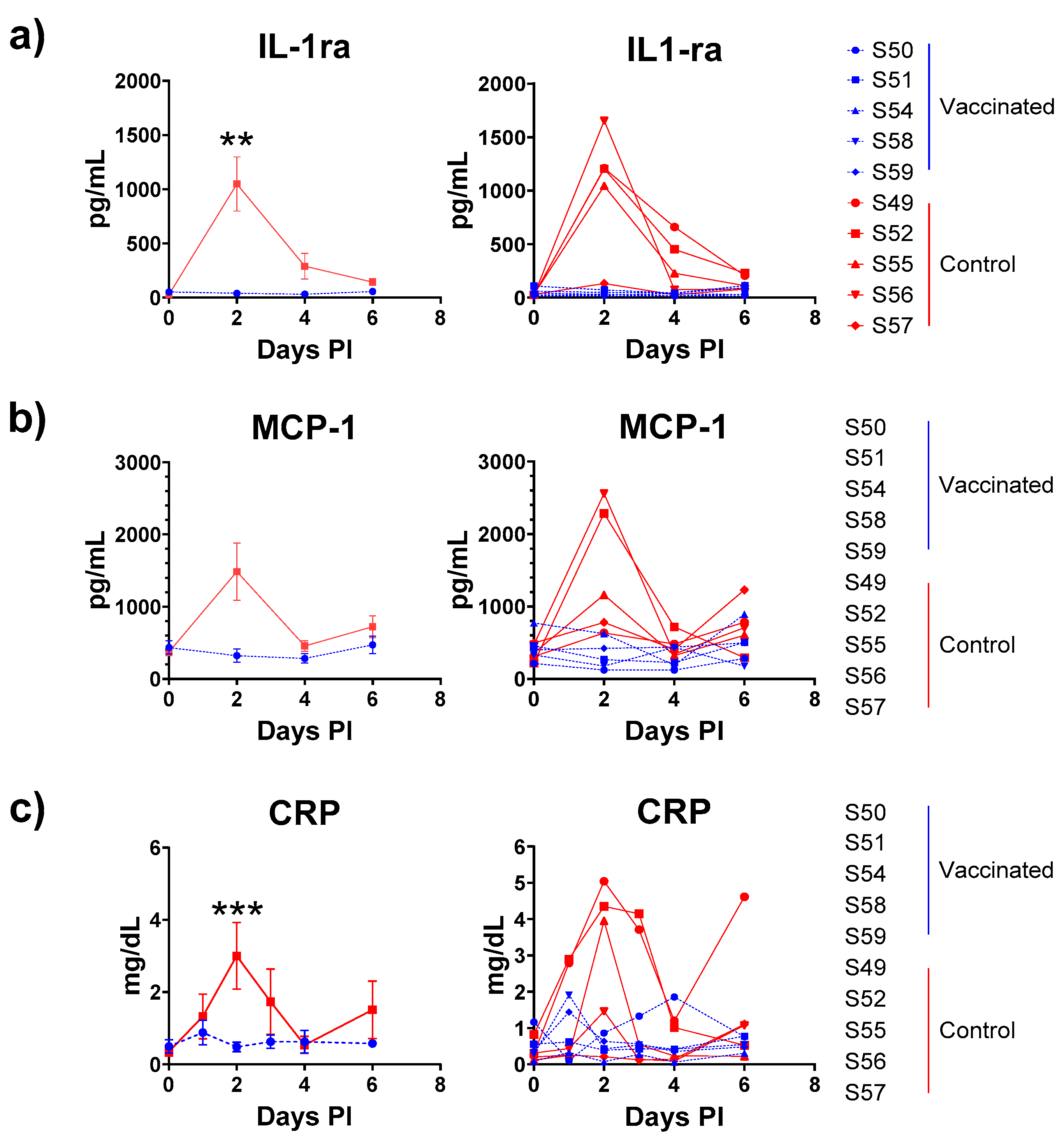
2.11. Multiplex Cytokine Assay
3. Results
3.1. Virus Sequence of the Challenge Stock
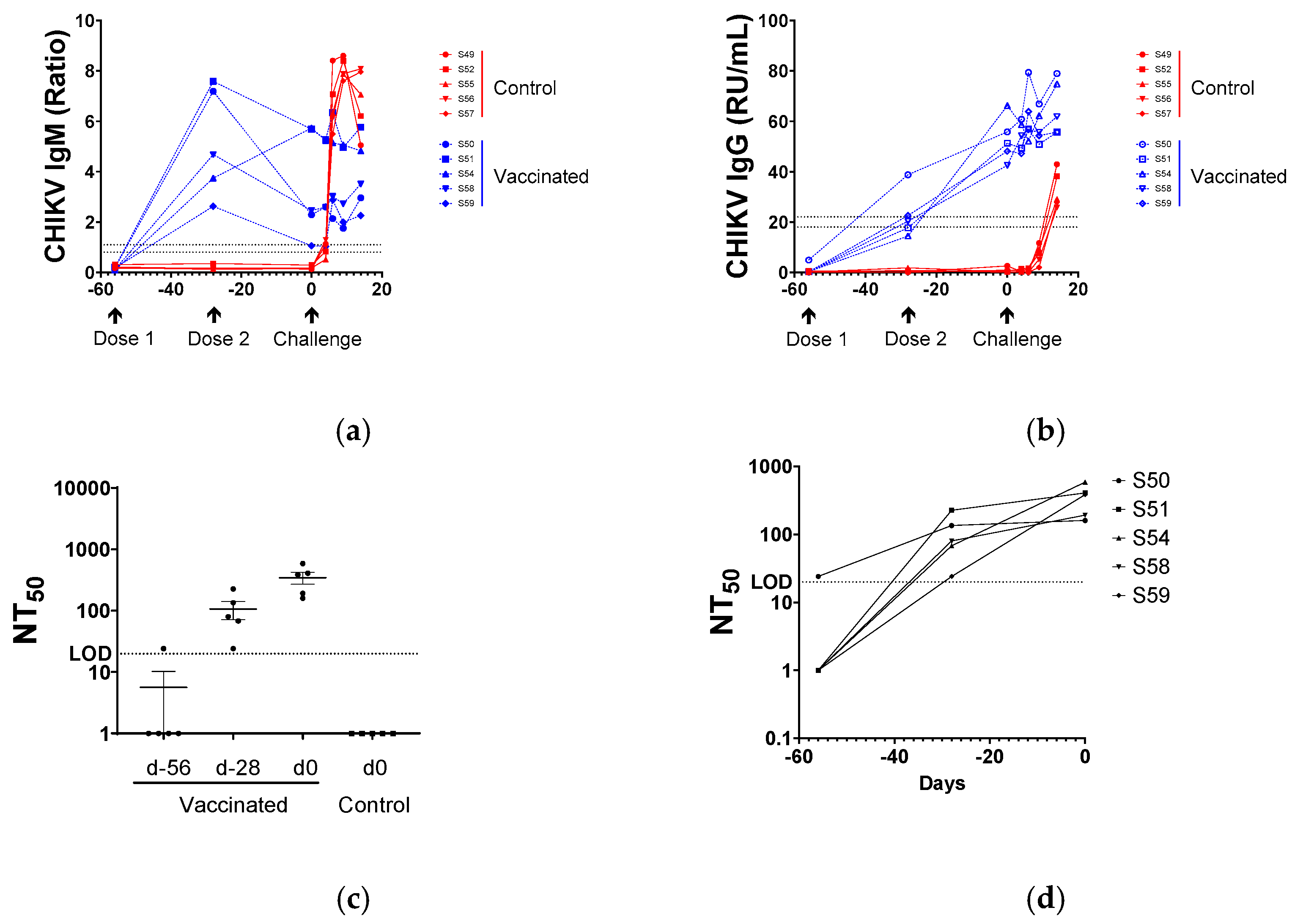
3.2. Serological Responses to BBV87 Vaccination and CHIKV Infection
3.3. Virus Neutralisation
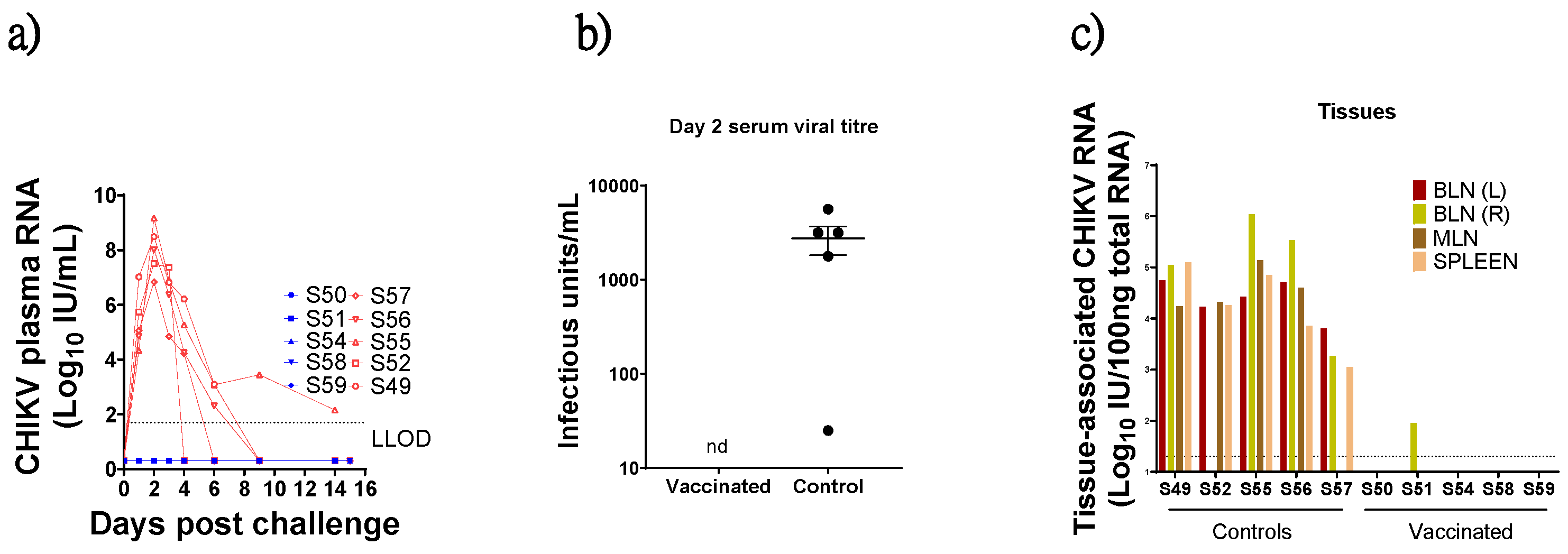
3.4. Virus Detection in Blood Post CHIKV Challenge
3.5. Virus Detection in Tissues
3.6. Temperature Monitoring
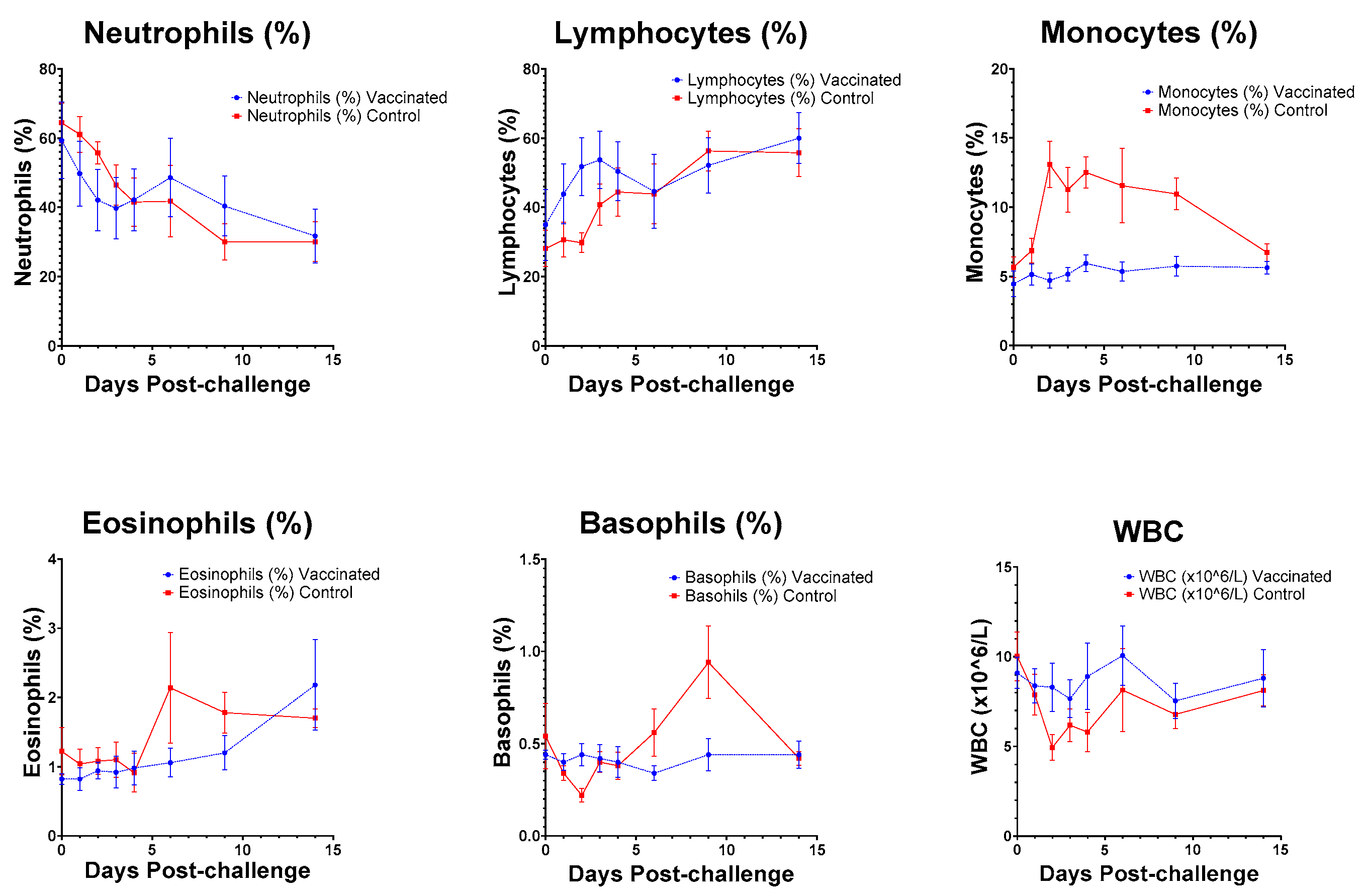
3.7. Haematology
3.8. Joint Pathology
3.9. Cytokine Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Puntasecca, C.J.; King, C.H.; LaBeaud, A.D. Measuring the global burden of chikungunya and Zika viruses: A systematic review. PLoS Neglected Trop. Dis. 2021, 15, e0009055. [Google Scholar] [CrossRef] [PubMed]
- Schneider, A.B.; Ochsenreiter, R.; Hostager, R.; Hofacker, I.L.; Janies, D.; Wolfinger, M.T. Updated Phylogeny of Chikungunya Virus Suggests Lineage-Specific RNA Architecture. Viruses 2019, 11, 798. [Google Scholar] [CrossRef]
- Sumathy, K.; Ella, K.M. Genetic diversity of Chikungunya virus, India 2006–2010: Evolutionary dynamics and serotype analyses. J. Med. Virol. 2012, 84, 462–470. [Google Scholar] [CrossRef] [PubMed]
- Ng, L.C.; Hapuarachchi, H.C. Tracing the path of Chikungunya virus-evolution and adaptation. Infect. Genet. Evol. 2010, 10, 876–885. [Google Scholar] [CrossRef]
- Arankalle, V.A.; Shrivastava, S.; Cherian, S.; Gunjikar, R.S.; Walimbe, A.M.; Jadhav, S.M.; Sudeep, A.B.; Mishra, A.C. Genetic divergence of Chikungunya viruses in India (1963–2006) with special reference to the 2005–2006 explosive epidemic. J. Gen. Virol. 2007, 88 Pt 7, 1967–1976. [Google Scholar] [CrossRef] [PubMed]
- Phadungsombat, J.; Imad, H.A.; Nakayama, E.E.; Leaungwutiwong, P.; Ramasoota, P.; Nguitragool, W.; Matsee, W.; Piyaphanee, W.; Shioda, T. Spread of a Novel Indian Ocean Lineage Carrying E1-K211E/E2-V264A of Chikungunya Virus East/Central/South African Genotype across the Indian Subcontinent, Southeast Asia, and Eastern Africa. Microorganisms 2022, 10, 354. [Google Scholar] [CrossRef]
- Goupil, B.A.; Mores, C.N. A Review of Chikungunya Virus-induced Arthralgia: Clinical Manifestations, Therapeutics, and Pathogenesis. Open Rheumatol. J. 2016, 10, 129–140. [Google Scholar] [CrossRef]
- Broeckel, R.; Haese, N.; Messaoudi, I.; Streblow, D.N. Nonhuman Primate Models of Chikungunya Virus Infection and Disease (CHIKV NHP Model). Pathogens 2015, 4, 662–681. [Google Scholar] [CrossRef]
- Rezza, G. Do we need a vaccine against chikungunya? Pathog. Glob. Health 2015, 109, 170–173. [Google Scholar] [CrossRef]
- Akahata, W.; Yang, Z.Y.; Andersen, H.; Sun, S.; Holdaway, H.A.; Kong, W.P.; Lewis, M.G.; Higgs, S.; Rossmann, M.G.; Rao, S.; et al. A virus-like particle vaccine for epidemic Chikungunya virus protects nonhuman primates against infection. Nat. Med. 2010, 16, 334–338. [Google Scholar] [CrossRef]
- Mallilankaraman, K.; Shedlock, D.J.; Bao, H.; Kawalekar, O.U.; Fagone, P.; Ramanathan, A.A.; Ferraro, B.; Stabenow, J.; Vijayachari, P.; Sundaram, S.G.; et al. A DNA vaccine against chikungunya virus is protective in mice and induces neutralizing antibodies in mice and nonhuman primates. PLoS Neglected Trop. Dis. 2011, 5, e928. [Google Scholar] [CrossRef] [PubMed]
- Roques, P.; Ljungberg, K.; Kummerer, B.M.; Gosse, L.; Dereuddre-Bosquet, N.; Tchitchek, N.; Hallengärd, D.; García-Arriaza, J.; Meinke, A.; Esteban, M.; et al. Attenuated and vectored vaccines protect nonhuman primates against Chikungunya virus. JCI Insight 2017, 2, e83527. [Google Scholar] [CrossRef] [PubMed]
- Cherian, N.; Bettis, A.; Deol, A.; Kumar, A.; Di Fabio, J.L.; Chaudhari, A.; Yimer, S.; Fahim, R.; Endy, T. Strategic considerations on developing a CHIKV vaccine and ensuring equitable access for countries in need. NPJ Vaccines 2023, 8, 123. [Google Scholar] [CrossRef] [PubMed]
- Simon, F.; Parola, P.; Grandadam, M.; Fourcade, S.; Oliver, M.; Brouqui, P.; Hance, P.; Kraemer, P.; Mohamed, A.A.; de Lamballerie, X.; et al. Chikungunya Infection: An Emerging Rheumatism Among Travelers Returned From Indian Ocean Islands. Report of 47 Cases. Medicine 2007, 86, 123–137. [Google Scholar] [CrossRef]
- Parola, P.; de Lamballerie, X.; Jourdan, J.; Rovery, C.; Vaillant, V.; Minodier, P.; Brouqui, P.; Flahault, A.; Raoult, D.; Charrel, R. Novel chikungunya virus variant in travelers returning from Indian Ocean islands. Emerg. Infect. Dis. 2006, 12, 1493–1499. [Google Scholar] [CrossRef]
- Labadie, K.; Larcher, T.; Joubert, C.; Mannioui, A.; Delache, B.; Brochard, P.; Guigand, L.; Dubreil, L.; Lebon, P.; Verrier, B.; et al. Chikungunya disease in nonhuman primates involves long-term viral persistence in macrophages. J. Clin. Investig. 2010, 120, 894–906. [Google Scholar] [CrossRef] [PubMed]
- Mohamed Ali, S.; Amroun, A.; de Lamballerie, X.; Nougairede, A. Evolution of Chikungunya virus in mosquito cells. Sci. Rep. 2018, 8, 16175. [Google Scholar] [CrossRef]
- Verbeke, G.; Molenberghs, G. Linear Mixed Models for Longitudinal Data; Springer: New York, NY, USA, 2000. [Google Scholar]
- Hernandez, L.M.; Sumathy, K.; Sahastrabuddhe, S.; Excler, J.L.; Kochhar, S.; Smith, E.R.; Gurwith, M.; Chen, R.T. A Brighton Collaboration standardized template with key considerations for a benefit/risk assessment for an inactivated viral vaccine against Chikungunya virus. Vaccine 2022, 40, 5263–5274. [Google Scholar] [CrossRef]
- Reed, L.J.; Muench, H. A Simple Method of Estimating Fifty Per Cent Endpoints. Am. J. Epidemiol. 1938, 27, 493–497. [Google Scholar] [CrossRef]
- Kress, J.A.; Hanschmann, K.-M.; Chudy, M.; Collaborative Study Group. Collaborative Study to Evaluate a Candidate World Health Organization International Standard for Chikungunya Virus for Nucleic Acid Amplification Technique (NAT)-Based Assays; WHO: Geneva, Switzerland, 2017. [Google Scholar]
- Jones, J.E.; Long, K.M.; Whitmore, A.C.; Sanders, W.; Thurlow, L.R.; Brown, J.A.; Morrison, C.R.; Vincent, H.; Peck, K.M.; Browning, C.; et al. Disruption of the Opal Stop Codon Attenuates Chikungunya Virus-Induced Arthritis and Pathology. mBio 2017, 8. [Google Scholar] [CrossRef]
- Mounce, B.C.; Cesaro, T.; Vlajnic, L.; Vidina, A.; Vallet, T.; Weger-Lucarelli, J.; Passoni, G.; Stapleford, K.A.; Levraud, J.-P.; Vignuzzi, M. Chikungunya Virus Overcomes Polyamine Depletion by Mutation of nsP1 and the Opal Stop Codon To Confer Enhanced Replication and Fitness. J. Virol. 2017, 91, e00344-17. [Google Scholar] [CrossRef] [PubMed]
- Ozden, S.; Lucas-Hourani, M.; Ceccaldi, P.E.; Basak, A.; Valentine, M.; Benjannet, S.; Hamelin, J.; Jacob, Y.; Mamchaoui, K.; Mouly, V.; et al. Inhibition of Chikungunya virus infection in cultured human muscle cells by furin inhibitors: Impairment of the maturation of the E2 surface glycoprotein. J. Biol. Chem. 2008, 283, 21899–21908. [Google Scholar] [CrossRef] [PubMed]
- Ashbrook, A.W.; Burrack, K.S.; Silva, L.A.; Montgomery, S.A.; Heise, M.T.; Morrison, T.E.; Dermody, T.S. Residue 82 of the Chikungunya virus E2 attachment protein modulates viral dissemination and arthritis in mice. J. Virol. 2014, 88, 12180–12192. [Google Scholar] [CrossRef]
- Wressnigg, N.; Hochreiter, R.; Zoihsl, O.; Fritzer, A.; Bezay, N.; Klingler, A.; Lingnau, K.; Schneider, M.; Lundberg, U.; Meinke, A.; et al. Single-shot live-attenuated chikungunya vaccine in healthy adults: A phase 1, randomised controlled trial. Lancet Infect. Dis. 2020, 20, 1193–1203. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.C.; Kam, Y.W.; Lin, R.T.; Ng, M.M.; Ng, L.F.; Chu, J.J. Comparative analysis of the genome sequences and replication profiles of chikungunya virus isolates within the East, Central and South African (ECSA) lineage. Virol. J. 2013, 10, 169. [Google Scholar] [CrossRef]
- Strauss, J.H.; Strauss, E.G. The alphaviruses: Gene expression, replication, and evolution. Microbiol. Rev. 1994, 58, 491–562. [Google Scholar] [CrossRef]
- Lentscher, A.J.; McAllister, N.; Griswold, K.A.; Martin, J.L.; Welsh, O.L.; Sutherland, D.M.; Silva, L.A.; Dermody, T.S. Chikungunya Virus Vaccine Candidate Incorporating Synergistic Mutations Is Attenuated and Protects Against Virulent Virus Challenge. J. Infect. Dis. 2023, 227, 457–465. [Google Scholar] [CrossRef]
- Chen, C.I.; Clark, D.C.; Pesavento, P.; Lerche, N.W.; Luciw, P.A.; Reisen, W.K.; Brault, A.C. Comparative pathogenesis of epidemic and enzootic Chikungunya viruses in a pregnant Rhesus macaque model. Am. J. Trop. Med. Hyg. 2010, 83, 1249–1258. [Google Scholar] [CrossRef]
- Roques, P.; Fritzer, A.; Dereuddre-Bosquet, N.; Wressnigg, N.; Hochreiter, R.; Bossevot, L.; Pascal, Q.; Guehenneux, F.; Bitzer, A.; Ramljak, I.C.; et al. Effectiveness of CHIKV vaccine VLA1553 demonstrated by passive transfer of human sera. JCI Insight 2022, 7, e160173. [Google Scholar] [CrossRef]
- Rossi, S.L.; Comer, J.E.; Wang, E.; Azar, S.R.; Lawrence, W.S.; Plante, J.A.; Ramsauer, K.; Schrauf, S.; Weaver, S.C. Immunogenicity and Efficacy of a Measles Virus-Vectored Chikungunya Vaccine in Nonhuman Primates. J. Infect. Dis. 2019, 220, 735–742. [Google Scholar] [CrossRef]
- Cirimotich, C.M.; Vela, E.M.; Garver, J.; Barnewall, R.E.; Miller, B.D.; Meister, G.T.; Rogers, J.V. Chikungunya virus infection in Cynomolgus macaques following Intradermal and aerosol exposure. Virol. J. 2017, 14, 135. [Google Scholar] [CrossRef] [PubMed]
- t’Hart, B.A.; Vervoordeldonk, M.; Heeney, J.L.; Tak, P.P. Gene therapy in nonhuman primate models of human autoimmune disease. Gene Ther. 2003, 10, 890–901. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Arriaza, J.; Cepeda, V.; Hallengard, D.; Sorzano, C.O.; Kummerer, B.M.; Liljestrom, P.; Esteban, M. A novel poxvirus-based vaccine, MVA-CHIKV, is highly immunogenic and protects mice against chikungunya infection. J. Virol. 2014, 88, 3527–3547. [Google Scholar] [CrossRef] [PubMed]
- Partidos, C.D.; Weger, J.; Brewoo, J.; Seymour, R.; Borland, E.M.; Ledermann, J.P.; Powers, A.M.; Weaver, S.C.; Stinchcomb, D.T.; Osorio, J.E. Probing the attenuation and protective efficacy of a candidate chikungunya virus vaccine in mice with compromised interferon (IFN) signaling. Vaccine 2011, 29, 3067–3073. [Google Scholar] [CrossRef]
- Yoon, I.K.; Alera, M.T.; Lago, C.B.; Tac-An, I.A.; Villa, D.; Fernandez, S.; Thaisomboonsuk, B.; Klungthong, C.; Levy, J.W.; Velasco, J.M.; et al. High rate of subclinical chikungunya virus infection and association of neutralizing antibody with protection in a prospective cohort in the Philippines. PLoS Neglected Trop. Dis. 2015, 9, e0003764. [Google Scholar] [CrossRef]
- Chu, H.; Das, S.C.; Fuchs, J.F.; Suresh, M.; Weaver, S.C.; Stinchcomb, D.T.; Partidos, C.D.; Osorio, J.E. Deciphering the protective role of adaptive immunity to CHIKV/IRES a novel candidate vaccine against Chikungunya in the A129 mouse model. Vaccine 2013, 31, 3353–3360. [Google Scholar] [CrossRef]

| Position (nt). | Ref | Alt | Proportion (% Alt, Ivar) | Total Reads | Coding | Location | Comments |
|---|---|---|---|---|---|---|---|
| 20 | G | A | 6 | 2602 | - | 5′ UTR | n/a |
| 20 | G | C | 22 | 3181 | - | 5′ UTR | n/a |
| 22 | A | G | 11 | 3654 | - | 5′ UTR | n/a |
| 24 | C | T | 21 | 3977 | - | 5′ UTR | n/a |
| 995 | CAC | TAC | 76 | 12,524 | H to Y | non-structural polyprotein | n/a |
| 1052 | R (A or G)TG | GTG | 100 | 12,186 | - | non-structural polyprotein | no change, already a possible nt in complete genome sequence |
| 1445 | TCG | CCG | 8 | 11,478 | S to P | non-structural polyprotein | n/a |
| 4167 | GRC (A or G) | GAC | 98 | 23,828 | - | non-structural polyprotein | no change, already a possible nt in complete genome sequence |
| 5049 | AKA (G or T) | AGA | 96 | 20,962 | - | non-structural polyprotein | no change, already a possible nt in complete genome sequence |
| 5645 | TGA | CGA | 6 | 24,158 | Stop to R | non-structural polyprotein | opal stop mutation [22,23] |
| 6505 | GAA | GAT | 100 | 2926 | E to D | non-structural polyprotein | n/a |
| 8552 | GAC | GGC | 11 | 21,761 | D to G | structural polyprotein | downstream of furin cleavage site between E3 and E2 [24] |
| 8785 | GGG | CGG | 17 | 14,143 | G to R | structural polyprotein | G82R mutation in E2 glycoprotein, recognised as attenuating [23,25] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kempster, S.L.; Ferguson, D.; Ham, C.; Hall, J.; Jenkins, A.; Giles, E.; Priestnall, S.L.; Suarez-Bonnet, A.; Roques, P.; Le Grand, R.; et al. Inactivated Viral Vaccine BBV87 Protects Against Chikungunya Virus Challenge in a Non-Human Primate Model. Viruses 2025, 17, 550. https://doi.org/10.3390/v17040550
Kempster SL, Ferguson D, Ham C, Hall J, Jenkins A, Giles E, Priestnall SL, Suarez-Bonnet A, Roques P, Le Grand R, et al. Inactivated Viral Vaccine BBV87 Protects Against Chikungunya Virus Challenge in a Non-Human Primate Model. Viruses. 2025; 17(4):550. https://doi.org/10.3390/v17040550
Chicago/Turabian StyleKempster, Sarah L., Deborah Ferguson, Claire Ham, Joanna Hall, Adrian Jenkins, Elaine Giles, Simon L. Priestnall, Alejandro Suarez-Bonnet, Pierre Roques, Roger Le Grand, and et al. 2025. "Inactivated Viral Vaccine BBV87 Protects Against Chikungunya Virus Challenge in a Non-Human Primate Model" Viruses 17, no. 4: 550. https://doi.org/10.3390/v17040550
APA StyleKempster, S. L., Ferguson, D., Ham, C., Hall, J., Jenkins, A., Giles, E., Priestnall, S. L., Suarez-Bonnet, A., Roques, P., Le Grand, R., Kandaswamy, S., Sahastrabuddhe, S., Hernandez, L. M., Chuasuwan, S., Ahn, H. S., Kim, D. R., Wartel, A., Zellweger, R. M., Berry, N., & Almond, N. (2025). Inactivated Viral Vaccine BBV87 Protects Against Chikungunya Virus Challenge in a Non-Human Primate Model. Viruses, 17(4), 550. https://doi.org/10.3390/v17040550








