Landscape of H5 Infections in ASEAN Region: Past Insights, Present Realities, & Future Strategies
Abstract
1. Introduction
2. Epidemiology, Transmission, and Zoonotic Potential of H5 Infections in the ASEAN Region
2.1. H5N1 Subtype
2.2. H5N2 Subtype
2.3. H5N6 Subtype
2.4. H5N8 Subtype
3. Socioeconomic Impacts
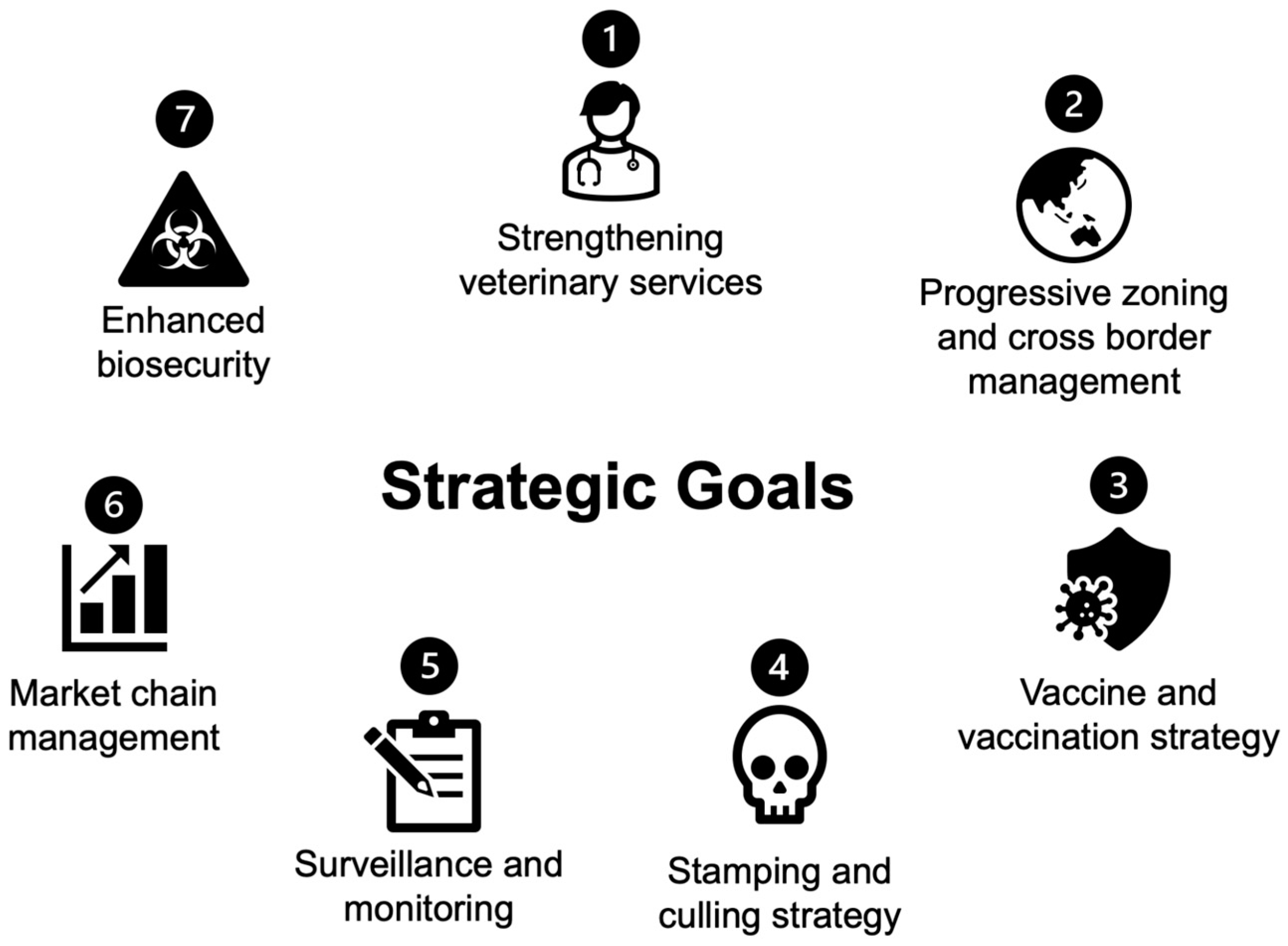
4. ASEAN Regional Responses and Collaborative Efforts
4.1. Strengthening Veterinary Services
4.2. Progressive Zoning and Cross-Border Management
4.3. Vaccine and Vaccination Strategy
4.4. Stamping Out and Culling
4.5. Surveillance and Monitoring
4.6. Market Chain Management
4.7. Enhanced Biosecurity
5. Lesson Learned from Past Outbreaks
6. One Health Approaches
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chan, P.K.S. Outbreak of Avian Influenza A (H5N1) Virus Infection in Hong Kong in 1997. Clin. Infect. Dis. 2002, 34, S58–S64. [Google Scholar] [CrossRef] [PubMed]
- Coker, R.J.; Hunter, B.M.; Rudge, J.W.; Liverani, M.; Hanvoravongchai, P. Emerging Infectious Diseases in Southeast Asia: Regional Challenges to Control. Lancet 2011, 377, 599–609. [Google Scholar] [CrossRef]
- Gutiérrez, R.A.; Naughtin, M.J.; Horm, S.V.; San, S.; Buchy, P. A(H5N1) Virus Evolution in South East Asia. Viruses 2009, 1, 335–361. [Google Scholar] [CrossRef]
- AbuBakar, U.; Amrani, L.; Kamarulzaman, F.A.; Karsani, S.A.; Hassandarvish, P.; Khairat, J.E. Avian Influenza Virus Tropism in Humans. Viruses 2023, 15, 833. [Google Scholar] [CrossRef] [PubMed]
- Caserta, L.C.; Frye, E.A.; Butt, S.L.; Laverack, M.; Nooruzzaman, M.; Covaleda, L.M.; Thompson, A.C.; Koscielny, M.P.; Cronk, B.; Johnson, A.; et al. Spillover of Highly Pathogenic Avian Influenza H5N1 Virus to Dairy Cattle. Nature 2024, 634, 669–676. [Google Scholar] [CrossRef] [PubMed]
- Sreenivasan, C.C.; Li, F.; Wang, D. Emerging Threats of Highly Pathogenic Avian Influenza A (H5N1) in US Dairy Cattle: Understanding Cross-Species Transmission Dynamics in Mammalian Hosts. Viruses 2024, 16, 1703. [Google Scholar] [CrossRef]
- Du, R.; Cui, Q.; Chen, Z.; Zhao, X.; Lin, X.; Rong, L. Revisiting Influenza A Virus Life Cycle from a Perspective of Genome Balance. Virol. Sin. 2023, 38, 1–8. [Google Scholar] [CrossRef]
- Dou, D.; Revol, R.; Östbye, H.; Wang, H.; Daniels, R. Influenza A Virus Cell Entry, Replication, Virion Assembly and Movement. Front. Immunol. 2018, 9, 1581. [Google Scholar] [CrossRef]
- Kosik, I.; Yewdell, J.W. Influenza Hemagglutinin and Neuraminidase: Yin–Yang Proteins Coevolving to Thwart Immunity. Viruses 2019, 11, 346. [Google Scholar] [CrossRef]
- Wang, Y.; Song, T.; Li, K.; Jin, Y.; Yue, J.; Ren, H.; Liang, L. Different Subtypes of Influenza Viruses Target Different Human Proteins and Pathways Leading to Different Pathogenic Phenotypes. Biomed Res. Int. 2019, 2019, 4794910. [Google Scholar] [CrossRef]
- U.S. Center for Disease Control and Prevention. Avian Influenza (Bird Flu). Available online: https://www.cdc.gov/bird-flu/index.html (accessed on 23 October 2024).
- The Association of Southeast Asian Nations. Post-2020 Avian Influenza Control Framework in ASEAN. Available online: https://asean.org/book/post-2020-avian-influenza-control-framework-in-asean/ (accessed on 1 September 2024).
- Ammali, N.; Kara, R.; Guetarni, D.; Chebloune, Y. Highly Pathogenic Avian Influenza H5N8 and H5N1 Outbreaks in Algerian Avian Livestock Production. Comp. Immunol. Microbiol. Infect. Dis. 2024, 111, 102202. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Li, X.; Dong, J.; Bo, H.; Liu, J.; Yang, J.; Zhang, Y.; Wei, H.; Huang, W.; Zhao, X.; et al. Epidemiologic, Clinical, and Genetic Characteristics of Human Infections with Influenza A(H5N6) Viruses, China. Emerg. Infect. Dis. 2022, 28, 1332–1344. [Google Scholar] [CrossRef]
- Burggraaf, S.; Karpala, A.J.; Bingham, J.; Lowther, S.; Selleck, P.; Kimpton, W.; Bean, A.G.D. H5N1 Infection Causes Rapid Mortality and High Cytokine Levels in Chickens Compared to Ducks. Virus Res. 2014, 185, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Poovorawan, Y.; Pyungporn, S.; Prachayangprecha, S.; Makkoch, J. Global Alert to Avian Influenza Virus Infection: From H5N1 to H7N9. Pathog. Glob. Health 2013, 107, 217–233. [Google Scholar] [CrossRef] [PubMed]
- Kintz, E.; Trzaska, W.J.; Pegg, E.; Perry, W.; Tucker, A.W.; Kyriakides, A.; Antic, D.; Callaghan, K.; Wilson, A.J. The Risk of Acquiring Avian Influenza from Commercial Poultry Products and Hen Eggs: A Qualitative Assessment. Microb. Risk Anal. 2024, 27–28, 27–28. [Google Scholar] [CrossRef]
- James, J.; Warren, C.J.; De Silva, D.; Lewis, T.; Grace, K.; Reid, S.M.; Falchieri, M.; Brown, I.H.; Banyard, A.C. The Role of Airborne Particles in the Epidemiology of Clade 2.3.4.4b H5N1 High Pathogenicity Avian Influenza Virus in Commercial Poultry Production Units. Viruses 2023, 15, 1002. [Google Scholar] [CrossRef]
- Gutiérrez, R.A.; Buchy, P. Contaminated Soil and Transmission of Influenza Virus (H5N1). Emerg. Infect. Dis. 2012, 18, 1530–1531. [Google Scholar] [CrossRef]
- Xu, X.; Subbarao, K.; Cox, N.J.; Guo, Y. Genetic Characterization of the Pathogenic Influenza A/Goose/Guangdong/1/96 (H5N1) Virus: Similarity of Its Hemagglutinin Gene to Those of H5N1 Viruses from the 1997 Outbreaks in Hong Kong. Virology 1999, 261, 15–19. [Google Scholar] [CrossRef]
- Yee, K.S.; Carpenter, T.E.; Cardona, C.J. Epidemiology of H5N1 Avian Influenza. Comp. Immunol. Microbiol. Infect. Dis. 2009, 32, 325–340. [Google Scholar] [CrossRef]
- Charostad, J.; Rukerd, M.R.Z.; Mahmoudvand, S.; Bashash, D.; Hashemi, S.M.A.; Nakhaie, M.; Zandi, K. A Comprehensive Review of Highly Pathogenic Avian Influenza (HPAI) H5N1: An Imminent Threat at Doorstep. Travel Med. Infect. Dis. 2023, 55, 102638. [Google Scholar] [CrossRef]
- Chen, H.; Smith, G.J.D.; Li, K.S.; Wang, J.; Fan, X.H.; Rayner, J.M.; Vijaykrishna, D.; Zhang, J.X.; Zhang, L.J.; Guo, C.T.; et al. Establishment of Multiple Sublineages of H5N1 Influenza Virus in Asia: Implications for Pandemic Control. Proc. Natl. Acad. Sci. USA 2006, 103, 2845–2850. [Google Scholar] [CrossRef] [PubMed]
- Harvey, J.A.; Mullinax, J.M.; Runge, M.C.; Prosser, D.J. The Changing Dynamics of Highly Pathogenic Avian Influenza H5N1: Next Steps for Management & Science in North America. Biol. Conserv. 2023, 282, 110041. [Google Scholar] [CrossRef]
- Nidra, F.Y.; Monir, M.B.; Dewan, S.M.R. Avian Influenza A (H5N1) Outbreak 2024 in Cambodia: Worries Over the Possible Spread of the Virus to Other Asian Nations and the Strategic Outlook for Its Control. Environ. Health Insights 2024, 18, 11786302241246453. [Google Scholar] [CrossRef] [PubMed]
- Peiris, J.S.M.; De Jong, M.D.; Guan, Y. Avian Influenza Virus (H5N1): A Threat to Human Health. Clin. Microbiol. Rev. 2007, 20, 243–267. [Google Scholar] [CrossRef]
- Mok, C.K.P.; Guan, W.D.; Liu, X.Q.; Lamers, M.M.; Li, X.B.; Wang, M.; Zhang, T.J.S.; Zhang, Q.L.; Li, Z.T.; Huang, J.C.; et al. Genetic Characterization of Highly Pathogenic Avian Influenza A(H5N6) Virus, Guangdong, China. Emerg. Infect. Dis. 2015, 21, 2268–2271. [Google Scholar] [CrossRef]
- Yang, Y.; Halloran, M.E.; Sugimoto, J.D.; Longini, I.M. Detecting Human-to-Human Transmission of Avian Influenza A (H5N1). Emerg. Infect. Dis. 2007, 13, 1348. [Google Scholar] [CrossRef]
- Alexander, D.J.; Brown, I.H. History of Highly Pathogenic Avian Influenza. Sci. Tech. Rev. 2009, 28, 19–38. [Google Scholar] [CrossRef]
- Lee, C.-C.D.; Zhu, H.; Huang, P.-Y.; Peng, L.; Chang, Y.-C.; Yip, C.-H.; Li, Y.-T.; Cheung, C.-L.; Compans, R.; Yang, C.; et al. Emergence and Evolution of Avian H5N2 Influenza Viruses in Chickens in Taiwan. J. Virol. 2014, 88, 5677–5686. [Google Scholar] [CrossRef]
- Cheng, M.C.; Soda, K.; Lee, M.S.; Lee, S.H.; Sakoda, Y.; Kida, H.; Wang, C.H. Isolation and Characterization of Potentially Pathogenic H5N2 Influenza Virus from a Chicken in Taiwan in 2008. Avian Dis. 2010, 54, 885–893. [Google Scholar] [CrossRef]
- Okamatsu, M.; Saito, T.; Yamamoto, Y.; Mase, M.; Tsuduku, S.; Nakamura, K.; Tsukamoto, K.; Yamaguchi, S. Low Pathogenicity H5N2 Avian Influenza Outbreak in Japan during the 2005-2006. Vet. Microbiol. 2007, 124, 35–46. [Google Scholar] [CrossRef]
- Yamazaki, Y.; Doy, M.; Okabe, N.; Yasui, Y.; Nakashima, K.; Fujieda, T.; Yamato, S.I.; Kawata, Y.; Ogata, T. Serological Survey of Avian H5N2-Subtype Influenza Virus Infections in Human Populations. Arch. Virol. 2009, 154, 421–427. [Google Scholar] [CrossRef]
- Mahase, E. Bird Flu: First Person with Confirmed H5N2 Infection Dies. BMJ 2024, 385, q1260. [Google Scholar] [CrossRef] [PubMed]
- Adibah, N.M.; Zailina, H.; Arshad, S.S. Avian Influenza Outbreaks in Malaysia, 1980-2017. Asia Pac. Environ. Occup. Health J. 2017, 3, 1–14. [Google Scholar]
- Lee, J.H.; Pascua, P.N.Q.; Song, M.-S.; Baek, Y.H.; Kim, C.-J.; Choi, H.-W.; Sung, M.-H.; Webby, R.J.; Webster, R.G.; Poo, H.; et al. Isolation and Genetic Characterization of H5N2 Influenza Viruses from Pigs in Korea. J. Virol. 2009, 83, 4205–4215. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Gu, X.; Lu, X.; Pan, J.; Duan, Z.; Zhao, K.; Gu, M.; Liu, Q.; He, L.; Chen, J.; et al. Novel Reassortant Highly Pathogenic H5N2 Avian Influenza Viruses in Poultry in China. PLoS ONE 2012, 7, e46183. [Google Scholar] [CrossRef]
- Baek, Y.H.; Pascua, P.N.Q.; Song, M.S.; Park, K.J.; Kwon, H.I.; Lee, J.H.; Kim, S.Y.; Moon, H.J.; Kim, C.J.; Choi, Y.K. Surveillance and Characterization of Low Pathogenic H5 Avian Influenza Viruses Isolated from Wild Migratory Birds in Korea. Virus Res. 2010, 150, 119–128. [Google Scholar] [CrossRef]
- Sultan, S.; Bui, V.N.; Hill, N.J.; Hussein, I.T.M.; Trinh, D.Q.; Inage, K.; Hashizume, T.; Runstadler, J.A.; Ogawa, H.; Imai, K. Genetic Characterization of H5N2 Influenza Viruses Isolated from Wild Birds in Japan Suggests Multiple Reassortment. Arch. Virol. 2016, 161, 3309–3322. [Google Scholar] [CrossRef]
- Guang-jian, Z.; Zong-shuai, L.; Yan-li, Z.; Shi-jin, J.; Zhi-jing, X. Genetic Characterization of a Novel Influenza A Virus H5N2 Isolated from a Dog in China. Vet. Microbiol. 2012, 155, 409–416. [Google Scholar] [CrossRef]
- Belot, G.; Claes, F.; Von Dobschuetz, S.; Kamata, A.; Newman, S.; Chanthavisouk, C.; Phommachanh, P.; Wongsathapornchai, K.; Fusheng, G.; Edwards, J.; et al. Avian Influenza A(H5N6): The Latest Addition to Emerging Zoonotic Avian Influenza Threats in East and Southeast Asia. Empress Watch. 2014, 30, 1–6. [Google Scholar]
- Kang, Y.; Liu, L.; Feng, M.; Yuan, R.; Huang, C.; Tan, Y.; Gao, P.; Xiang, D.; Zhao, X.; Li, Y.; et al. Highly Pathogenic H5N6 Influenza A Viruses Recovered from Wild Birds in Guangdong, Southern China, 2014-2015. Sci. Rep. 2017, 7, 44410. [Google Scholar] [CrossRef]
- He, Z.; Wang, X.; Lin, Y.; Feng, S.; Huang, X.; Zhao, L.; Zhang, J.; Ding, Y.; Li, W.; Yuan, R.; et al. Genetic Characteristics of Waterfowl-Origin H5N6 Highly Pathogenic Avian Influenza Viruses and Their Pathogenesis in Ducks and Chickens. Front. Microbiol. 2023, 14, 1211355. [Google Scholar] [CrossRef] [PubMed]
- Bi, Y.; Mei, K.; Shi, W.; Liu, D.; Yu, X.; Gao, Z.; Zhao, L.; Gao, G.F.; Chen, J.; Chen, Q. Two Novel Reassortants of Avian Influenza A (H5N6) Virus in China. J. Gen. Virol. 2015, 96, 975–981. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.; Gao, R.; Lv, Q.; Huang, S.; Zhou, Z.; Yang, L.; Li, X.; Zhao, X.; Zou, X.; Tong, W.; et al. Human Infection with a Novel, Highly Pathogenic Avian Influenza A (H5N6) Virus: Virological and Clinical Findings. J. Infect. 2016, 72, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chen, Y.; Shan, N.; Wang, X.; Lin, S.; Ma, K.; Li, B.; Li, H.; Liao, M.; Qi, W. Genetic Diversity, Phylogeography, and Evolutionary Dynamics of Highly Pathogenic Avian Influenza A (H5N6) Viruses. Virus Evol. 2020, 6, veaa079. [Google Scholar] [CrossRef]
- Bean, A.G.D.; Baker, M.L.; Stewart, C.R.; Cowled, C.; Deffrasnes, C.; Wang, L.F.; Lowenthal, J.W. Studying Immunity to Zoonotic Diseases in the Natural Host—Keeping It Real. Nat. Rev. Immunol. 2013, 13, 851–861. [Google Scholar] [CrossRef]
- Wang, B.; Su, Q.; Luo, J.; Li, M.; Wu, Q.; Chang, H.; Du, J.; Huang, C.; Ma, J.; Han, S.; et al. Differences in Highly Pathogenic H5N6 Avian Influenza Viral Pathogenicity and Inflammatory Response in Chickens and Ducks. Front. Microbiol. 2021, 12, 593202. [Google Scholar] [CrossRef]
- World Health Organization. Surveillance in Emergencies. Available online: https://www.who.int/westernpacific/wpro-emergencies/surveillance (accessed on 12 November 2024).
- Sun, Y.; Hu, Z.; Zhang, X.; Chen, M.; Wang, Z.; Xu, G.; Bi, Y.; Tong, Q.; Wang, M.; Sun, H.; et al. An R195K Mutation in the PA-X Protein Increases the Virulence and Transmission of Influenza A Virus in Mammalian Hosts. J. Virol. 2020, 94, 20. [Google Scholar] [CrossRef]
- Herfst, S.; Mok, C.K.P.; van den Brand, J.M.A.; van der Vliet, S.; Rosu, M.E.; Spronken, M.I.; Yang, Z.; de Meulder, D.; Lexmond, P.; Bestebroer, T.M.; et al. Human Clade 2.3.4.4 A/H5N6 Influenza Virus Lacks Mammalian Adaptation Markers and Does Not Transmit via the Airborne Route between Ferrets. mSphere 2018, 3, e00405-17. [Google Scholar] [CrossRef]
- McParland, P.J.; Allan, G.M.; McCracken, R.M.; McNulty, M.S. Isolation of a Highly Pathogenic Influenza Virus from Turkeys. Avian Pathol. 1985, 14, 173–176. [Google Scholar] [CrossRef]
- Yamaguchi, E.; Hayama, Y.; Murato, Y.; Sawai, K.; Kondo, S.; Yamamoto, T. A Case-Control Study of the Infection Risk of H5N8 Highly Pathogenic Avian Influenza in Japan during the Winter of 2020–2021. Res. Vet. Sci. 2024, 168, 105149. [Google Scholar] [CrossRef]
- Nagarajan, S.; Kumar, M.; Murugkar, H.V.; Tripathi, S.; Shukla, S.; Agarwal, S.; Dubey, G.; Nagi, R.S.; Singh, V.P.; Tosh, C. Novel Reassortant Highly Pathogenic Avian Influenza (H5N8) Virus in Zoos, India. Emerg. Infect. Dis. 2017, 23, 717–719. [Google Scholar] [CrossRef] [PubMed]
- Si, Y.J.; Jang, S.-G.; Kim, Y.-I.; Casel, M.A.B.; Kim, D.-J.; Ji, H.Y.; Choi, J.H.; Gil, J.R.; Rollon, R.; Jang, H.; et al. Evolutional Dynamics of Highly Pathogenic Avian Influenza H5N8 Genotypes in Wintering Bird Habitats: Insights from South Korea’s 2020–2021 Season. One Health 2024, 18, 100719. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.S.; Chen, L.H.; Chen, Y.P.; Liu, Y.P.; Li, W.C.; Lin, Y.L.; Lee, F. Highly Pathogenic Avian Influenza Viruses H5N2, H5N3, and H5N8 in Taiwan in 2015. Vet. Microbiol. 2016, 187, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Ku, K.B.; Park, E.H.; Yum, J.; Kim, J.A.; Oh, S.K.; Seo, S.H. Highly Pathogenic Avian Influenza A(H5N8) Virus from Waterfowl, South Korea, 2014. Emerg. Infect. Dis. 2014, 20, 1587–1588. [Google Scholar] [CrossRef]
- Li, M.; Liu, H.; Bi, Y.; Sun, J.; Wong, G.; Liu, D.; Li, L.; Liu, J.; Chen, Q.; Wang, H.; et al. Highly Pathogenic Avian Influenza A(H5N8) Virus in Wild Migratory Birds, Qinghai Lake, China. Emerg. Infect. Dis. 2017, 23, 637–641. [Google Scholar] [CrossRef]
- Caliendo, V.; Leijten, L.; Begeman, L.; Poen, M.J.; Fouchier, R.A.M.; Beerens, N.; Kuiken, T. Enterotropism of Highly Pathogenic Avian Influenza Virus H5N8 from the 2016/2017 Epidemic in Some Wild Bird Species. Vet. Res. 2020, 51, 117. [Google Scholar] [CrossRef]
- Floyd, T.; Banyard, A.C.; Lean, F.Z.; Byrne, A.M.; Fullick, E.; Whittard, E.; Mollett, B.C.; Bexton, S.; Swinson, V.; Macrelli, M.; et al. Encephalitis and Death in Wild Mammals at a Rehabilitation Center after Infection with Highly Pathogenic Avian Influenza A(H5N8) Virus, United Kingdom. Emerg. Infect. Dis. 2021, 27, 2856–2863. [Google Scholar] [CrossRef]
- Shin, D.L.; Siebert, U.; Lakemeyer, J.; Grilo, M.; Pawliczka, I.; Wu, N.H.; Valentin-Weigand, P.; Haas, L.; Herrler, G. Highly Pathogenic Avian Influenza A(H5N8) Virus in Gray Seals, Baltic Sea. Emerg. Infect. Dis. 2019, 25, 2295–2298. [Google Scholar] [CrossRef]
- Pyankova, O.G.; Susloparov, I.M.; Moiseeva, A.A.; Kolosova, N.P.; Onkhonova, G.S.; Danilenko, A.V.; Vakalova, E.V.; Shendo, G.L.; Nekeshina, N.N.; Noskova, L.N.; et al. Isolation of Clade 2.3.4.4b A(H5N8), a Highly Pathogenic Avian Influenza Virus, from a Worker during an Outbreak on a Poultry Farm, Russia, December 2020. Eurosurveillance 2021, 26, 2100439. [Google Scholar] [CrossRef]
- Musa, E.; Nia, Z.M.; Bragazzi, N.L.; Leung, D.; Lee, N.; Kong, J.D. Avian Influenza: Lessons from Past Outbreaks and an Inventory of Data Sources, Mathematical and AI Models, and Early Warning Systems for Forecasting and Hotspot Detection to Tackle Ongoing Outbreaks. Healthcare 2024, 12, 1959. [Google Scholar] [CrossRef]
- Subedi, D.; Farhan, M.H.R.; Niraula, A.; Shrestha, P.; Chandran, D.; Acharya, K.P.; Ahmad, M. Avian Influenza in Low and Middle-Income Countries (LMICs): Outbreaks, Vaccination Challenges and Economic Impact. Pak. Vet. J. 2024, 44, 9–17. [Google Scholar] [CrossRef]
- McLeod, A.; Morgan, N.; Prakash, A.; Hinrichs, J. Economic and Social Impacts of Avian Influenza. Available online: https://openknowledge.fao.org/items/7f8e1b13-f130-474f-9ea7-65ea1449ecf4 (accessed on 12 November 2024).
- The World Bank. Countering Global Shocks. Available online: https://documents.worldbank.org/en/publication/documents-reports/documentdetail/220041468245427647/countering-global-shocks (accessed on 12 November 2024).
- Pramuwidyatama, M.G.; Indrawan, D.; Boeters, M.; Poetri, O.N.; Saatkamp, H.W.; Hogeveen, H. Economic Impact of Highly Pathogenic Avian Influenza Outbreaks in Western Java Smallholder Broiler Farms. Prev. Vet. Med. 2023, 212, 105833. [Google Scholar] [CrossRef] [PubMed]
- Elçi, C. The Impact of HPAI of the H5N1 Strain on Economies of Affected Countries. In International Conference on Human and Economic Resources; Izmir University of Economics: Izmir, Turkey, 2006; pp. 101–115. [Google Scholar]
- Hinrichs, J.; Sims, L.; Mcleod, A. Some Direct Costs of Control for Avian Influenza. In 11th International Symposium on Veterinary Epidemiology and Economics; International Symposia on Veterinary Epidemiology and Economics: Cairns, Australia, 2006; p. 811. [Google Scholar]
- Simmerman, J.M.; Lertiendumrong, J.; Dowell, S.F.; Uyeki, T.; Olsen, S.J.; Chittaganpitch, M.; Chunsutthiwat, S.; Tangcharoensathien, V. The Cost of Influenza in Thailand. Vaccine 2006, 24, 4417–4426. [Google Scholar] [CrossRef]
- Malik, Y.A. Impact of Influenza in South-East Asia. Int. J. Infect. Dis. 2023, 130, S40–S41. [Google Scholar] [CrossRef]
- Burgos, S.; Burgos, S.A. Avian Influenza Outbreaks in Southeast Asia Affects Prices, Markets and Trade: A Short Case Study. Int. J. Poult. Sci. 2007, 6, 1006–1009. [Google Scholar] [CrossRef][Green Version]
- Buaprommee, N.; Polyorat, K. The Antecedents of Purchase Intention of Meat with Traceability in Thai Consumers. Asia Pac. Manag. Rev. 2016, 21, 161–169. [Google Scholar] [CrossRef]
- Vapnek, J. Regulatory Measures Against Outbreaks of Highly Pathogenic Avian Influenza. Available online: https://www.fao.org/fileadmin/user_upload/legal/docs/lpo82.pdf (accessed on 13 November 2024).
- Azhar, M.; Lubis, A.S.; Siregar, E.S.; Alders, R.G.; Brum, E.; McGrane, J.; Morgan, I.; Roeder, P. Participatory Disease Surveillance and Response in Indonesia: Strengthening Veterinary Services and Empowering Communities to Prevent and Control Highly Pathogenic Avian Influenza. Avian Dis. 2010, 54, 749–753. [Google Scholar] [CrossRef]
- Leong, H.K.; Goh, C.S.; Chew, S.T.; Lim, C.W.; Lin, Y.N.; Chang, S.F.; Yap, H.H.; Chua, S.B. Prevention and Control of Avian Influenza in Singapore. Ann. Acad. Med. Singap. 2008, 37, 504–509. [Google Scholar] [CrossRef]
- Pribadi, E.S.; Hanun, H.; Haryanto, A.P.; Sutarman, D.C.; Utami, S.S.; Harahap, R.H.; Safika, S.; Kompudu, A.J.M.; Schoonman, L.; Utomo, G.B.; et al. Correlation Strength Assessment of Animal Husbandry Components to the Implementation of ASEAN Good Animal Husbandry Practices: A Case Study in Layer Farming. J. Ilmu-Ilmu Peternak. 2023, 33, 116–124. [Google Scholar] [CrossRef]
- Nielsen, S.S.; Alvarez, J.; Bicout, D.J.; Calistri, P.; Canali, E.; Drewe, J.A.; Garin-Bastuji, B.; Rojas, J.L.G.; Gortázar, C.; Herskin, M.; et al. Vaccination of Poultry against Highly Pathogenic Avian Influenza—Part 1. Available Vaccines and Vaccination Strategies. EFSA J. 2023, 21, e08271. [Google Scholar] [CrossRef]
- Zeng, X.Y.; He, X.W.; Meng, F.; Ma, Q.; Wang, Y.; Bao, H.M.; Liu, Y.J.; Deng, G.H.; Shi, J.Z.; Li, Y.B.; et al. Protective Efficacy of an H5/H7 Trivalent Inactivated Vaccine (H5-Re13, H5-Re14, and H7-Re4 Strains) in Chickens, Ducks, and Geese against Newly Detected H5N1, H5N6, H5N8, and H7N9 Viruses. J. Integr. Agric. 2022, 21, 2086–2094. [Google Scholar] [CrossRef]
- Bertran, K.; Moresco, K.; Swayne, D.E. Impact of Vaccination on Infection with Vietnam H5N1 High Pathogenicity Avian Influenza Virus in Hens and the Eggs They Lay. Vaccine 2015, 33, 1324–1330. [Google Scholar] [CrossRef]
- Mighell, E.; Ward, M.P. African Swine Fever Spread across Asia, 2018–2019. Transbound. Emerg. Dis. 2021, 68, 2722–2732. [Google Scholar] [CrossRef] [PubMed]
- Tibenderana, J.R. Stamping out Marburg Virus Disease in Tanzania: Cutting-Edge Interventions and Recommendations for a Healthier Future. IJS Glob. Health 2023, 6, e0203. [Google Scholar] [CrossRef]
- Looi, L.M.; Chua, K.B. Lessons from the Nipah Virus Outbreak in Malaysia. Malays. J. Pathol. 2007, 29, 63–67. [Google Scholar] [PubMed]
- Norulhuda, W.; Makmal, T.J.; Kawasan, V.; Bharu, K.; Kerian, J.K. An Overview of Highly Pathogenic Avian Influenza (H5N1) Outbreak Cases in Kelantan, West Malaysia in Year 2017. Malays. J. Vet. Res. 2018, 9, 102–108. [Google Scholar]
- Magalhães, R.J.S.; Pfeiffer, D.U.; Otte, J. Evaluating the Control of HPAIV H5N1 in Vietnam: Virus Transmission within Infected Flocks Reported before and after Vaccination. BMC Vet. Res. 2010, 6, 31. [Google Scholar] [CrossRef]
- Tiensin, T.; Chaitaweesub, P.; Songserm, T.; Chaisingh, A.; Hoonsuwan, W.; Buranathai, C.; Parakamawongsa, T.; Premashthira, S.; Amonsin, A.; Gilbert, M.; et al. Highly Pathogenic Avian Influenza H5N1, Thailand, 2004. Emerg. Infect. Dis. 2005, 11, 1664–1672. [Google Scholar] [CrossRef]
- Ziegler, T.; Moen, A.; Zhang, W.; Cox, N.J. Global Influenza Surveillance and Response System: 70 Years of Responding to the Expected and Preparing for the Unexpected. Lancet 2022, 400, 981–982. [Google Scholar] [CrossRef]
- Mon, H.H.; Hadrill, D.; Brioudes, A.; Mon, C.C.S.; Sims, L.; Win, H.H.; Thein, W.Z.; Mok, W.S.; Kyin, M.M.; Maw, M.T.; et al. Longitudinal Analysis of Influenza A(H5) Sero-Surveillance in Myanmar Ducks, 2006–2019. Microorganisms 2021, 9, 2114. [Google Scholar] [CrossRef]
- Norrulashikin, M.A.; Yusof, F.; Hanafiah, N.H.M.; Norrulashikin, S.M. Modelling Monthly Influenza Cases in Malaysia. PLoS ONE 2021, 16, e0254137. [Google Scholar] [CrossRef] [PubMed]
- Fournié, G.; Guitian, J.; Desvaux, S.; Cuong, V.C.; Dung, D.H.; Pfeiffer, D.U.; Mangtani, P.; Ghani, A.C. Interventions for Avian Influenza A (H5N1) Risk Management in Live Bird Market Networks. Proc. Natl. Acad. Sci. USA 2013, 110, 9177–9182. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Wang, Y.; Liu, H.; Guo, F.; Doi, S.A.; Smith, C.; Clements, A.C.A.; Edwards, J.; Huang, B.; Magalhães, R.J.S. Effectiveness of Market-Level Biosecurity at Reducing Exposure of Poultry and Humans to Avian Influenza: A Systematic Review and Meta-Analysis. J. Infect. Dis. 2018, 218, 1861–1875. [Google Scholar] [CrossRef]
- Van Kerkhove, M.D.; Vong, S.; Guitian, J.; Holl, D.; Mangtani, P.; San, S.; Ghani, A.C. Poultry Movement Networks in Cambodia: Implications for Surveillance and Control of Highly Pathogenic Avian Influenza (HPAI/H5N1). Vaccine 2009, 27, 6345–6352. [Google Scholar] [CrossRef]
- The Association of Southeast Asian Nations. Asean Biosecurity Management Manual for Commercial Poultry Farming. Available online: https://asean.org/wp-content/uploads/2021/09/AMAF-33-Biosecurity-Manual.pdf (accessed on 13 November 2024).
- Osman, N. The Legal Regulation of Biosafety Risk: A Comparative Legal Study with Singapore Biosafety Law. Ph.D. Thesis, Nottingham Trent University, Nottingham, UK, 2018. [Google Scholar] [CrossRef]
- Shafie, N.F.; Osman, N.D. Overview of Biosecurity Legislation in Malaysia. Perdana: Int. J. Acad. Res. 2024, 19, 1–12. [Google Scholar]
- Soisangwan, P. Biosafety and Biosecurity Law in Thailand: From Legislation to Practice. J. Biosaf. Biosecurity 2021, 3, 91–98. [Google Scholar] [CrossRef]
- The Association of Southeast Asian Nations. Prevention, Control, and Eradication of Avian Influenza in ASEAN: Strategies and Success Stories. Available online: https://asean.org/wp-content/uploads/2012/07/HPAI-Strategies.pdf (accessed on 11 October 2024).
- Asia-Pacific Alliances for the Control of Influenza. Pandemic Preparedness Plans for the Asia-Pacific Region. Available online: https://apaci.asia/influenza/pandemic-preparedness/pandemic-preparedness-plans-for-the-asia-pacific-region/ (accessed on 20 November 2024).
- Ministry of Health Malaysia. MYCDCGP—National Influenza Preparedness Plan. Available online: https://books.google.com.my/books?id=ncpcDwAAQBAJ (accessed on 31 October 2024).
- Department of Veterinary Services Malaysia. Manual for the Control of Highly Pathogenic Avian Influenza (HPAI). Available online: https://www.woah.org/fileadmin/database/ASIA/Malaysia/Manual_for_the_control_of_Highly_Pathogenic_Avian_Influenza_(HPAI)_Malaysia.pdf (accessed on 31 October 2024).
- Ministry of Health Union of Myanmar. National Strategic Plan for Prevention and Control of Avian Influenza and Human Influenza Pandemic Preparedness and Response. Available online: https://www.mohs.gov.mm/ckfinder/connector?command=Proxy&lang=en&type=Main¤tFolder=/Publications/CEU/CEU_May+2018/&hash=a6a1c319429b7abc0a8e21dc137ab33930842cf5&fileName=Pandemic+plan+(+latest)27-6-06+Eng.pdf (accessed on 2 November 2024).
- Department of Agriculture-Bureau of Animal Industry Philippines. Avian Influenza Protection Programme Manuals of Procedure. Available online: https://drive.google.com/file/d/1YGacdiinAgXOOz0ZzHbxYygVws0Ys70S/view?pli=1 (accessed on 2 November 2024).
- Ministry of Health Vietnam. Vietnam Integrated National Operational Program on Avian Influenza, Pandemic Preparedness and Emerging Infectious Diseases (AIPED) 2011–2015. Available online: https://onehealth.org.vn/upload/upload/AIPED+2011-2015+-+Final.pdf (accessed on 2 November 2024).
- World Health Organization. Avian Influenza A (H5N1)-Cambodia. Available online: https://www.who.int/emergencies/disease-outbreak-news/item/2024-DON501 (accessed on 10 December 2024).
- Institute of Medicine. The Threat of Pandemic Influenza: Are We Ready. In Workshop Summary; Knobler, S.L., Mack, A., Mahmoud, A., Lemon, S.M., Eds.; The National Academies Press: Washington, DC, USA, 2005. [Google Scholar] [CrossRef]
- Hassan Nizam, Q.N. Self-Declaration on the Recovery of Freedom from Highly Pathogenic Avian Influenza by Malaysia. Available online: http://www.dvs.gov.my/index.php/pages/view/538 (accessed on 6 September 2024).
- Sengkeopraseuth, B.; Co, K.C.; Leuangvilay, P.; Mott, J.A.; Khomgsamphanh, B.; Somoulay, V.; Tsuyuoka, R.; Chiew, M.; Ketmayoon, P.; Jones, J.; et al. First Human Infection of Avian Influenza A(H5N6) Virus Reported in Lao People’s Democratic Republic, February–March 2021. Influenza Other Respir. Viruses 2022, 16, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Walsh, D.P.; Ma, T.F.; Ip, H.S.; Zhu, J. Artificial Intelligence and Avian Influenza: Using Machine Learning to Enhance Active Surveillance for Avian Influenza Viruses. Transbound. Emerg. Dis. 2019, 66, 2537–2545. [Google Scholar] [CrossRef]
- Blagodatski, A.; Trutneva, K.; Glazova, O.; Mityaeva, O.; Shevkova, L.; Kegeles, E.; Onyanov, N.; Fede, K.; Maznina, A.; Khavina, E.; et al. Avian Influenza in Wild Birds and Poultry: Dissemination Pathways, Monitoring Methods, and Virus Ecology. Pathogens 2021, 10, 630. [Google Scholar] [CrossRef]
- Fair, J.M.; Al-Hmoud, N.; Alrwashdeh, M.; Bartlow, A.W.; Balkhamishvili, S.; Daraselia, I.; Elshoff, A.; Fakhouri, L.; Javakhishvili, Z.; Khoury, F.; et al. Transboundary Determinants of Avian Zoonotic Infectious Diseases: Challenges for Strengthening Research Capacity and Connecting Surveillance Networks. Front. Microbiol. 2024, 15, 1341842. [Google Scholar] [CrossRef]
- Horefti, E. The Importance of the One Health Concept in Combating Zoonoses. Pathogens 2023, 12, 977. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, M.; Chaitaweesub, P.; Parakamawongsa, T.; Premashthira, S.; Tiensin, T.; Kalpravidh, W.; Wagner, H.; Slingenbergh, J. Free-Grazing Ducks and Highly Pathogenic Avian Influenza, Thailand. Emerg. Infect. Dis. 2006, 12, 227–234. [Google Scholar] [CrossRef]
- Nguyen-Viet, H.; Lam, S.; Nguyen-Mai, H.; Trang, D.T.; Phuong, V.T.; Tuan, N.D.A.; Tan, D.Q.; Thuy, N.T.; Linh, D.T.; Pham-Duc, P. Decades of Emerging Infectious Disease, Food Safety, and Antimicrobial Resistance Response in Vietnam: The Role of One Health. One Health 2022, 14, 100361. [Google Scholar] [CrossRef]
- Creanga, A.; Nguyen, D.T.; Gerloff, N.; Do, H.T.; Balish, A.; Nguyen, H.D.; Jang, Y.; Dam, V.T.; Thor, S.; Jones, J.; et al. Emergence of Multiple Clade 2.3.2.1 Influenza A (H5N1) Virus Subgroups in Vietnam and Detection of Novel Reassortants. Virology 2013, 444, 12–20. [Google Scholar] [CrossRef]
- Dao, D.T.; Coleman, K.K.; Bui, V.N.; Bui, A.N.; Tran, L.H.; Nguyen, Q.D.; Than, S.; Pulscher, L.A.; Marushchak, L.V.; Robie, E.R.; et al. High Prevalence of Highly Pathogenic Avian Influenza: A Virus in Vietnam’s Live Bird Markets. Open Forum Infect. Dis. 2024, 11, ofae355. [Google Scholar] [CrossRef] [PubMed]
- The Association of Southeast Asian Nations. ASEAN Statistical Yearbook 2023. Available online: https://asean.org/wp-content/uploads/2023/12/ASEAN-Statistical-Yearbook-2023.pdf (accessed on 3 December 2024).
- Lee, C.-Y. Exploring Potential Intermediates in the Cross-Species Transmission of Influenza A Virus to Humans. Viruses 2024, 16, 1129. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y. Pathogenicity and Virulence of Influenza. Virulence 2023, 14, 2223057. [Google Scholar] [CrossRef]
- Huang, P.; Sun, L.; Li, J.; Wu, Q.; Rezaei, N.; Jiang, S.; Pan, C. Potential Cross-Species Transmission of Highly Pathogenic Avian Influenza H5 Subtype (HPAI H5) Viruses to Humans Calls for the Development of H5-Specific and Universal Influenza Vaccines. Cell Discov. 2023, 9, 1–13. [Google Scholar] [CrossRef]
- Sellwood, C.; Asgari-Jirhandeh, N.; Salimee, S. Bird Flu: If or When? Planning for the next Pandemic. Postgrad. Med. J. 2007, 83, 445–450. [Google Scholar] [CrossRef]
- Seri, N.A.; Rahman, A.A. Impact of Climate Change on Migratory Birds in Asia. Pertanika J. Sci. Technol. 2021, 29, 2937–2965. [Google Scholar] [CrossRef]
| Country | Local Mitigation/Preparedness Plans(s) | Year Published/Revised | References |
|---|---|---|---|
| Brunei | Influenza Pandemic Preparedness—Recommendations for Workplaces and Business Continuity Plan | 2008 | - * |
| Cambodia | Cambodia National Comprehensive Avian and Human Influenza Plan | 2007 | [98] |
| Indonesia | National Strategic Plan for Avian Influenza Control and Pandemic Influenza Preparedness | 2006 | [98] |
| Laos | National Avian Influenza Control and Pandemic Preparedness Plan | 2006 | [98] |
| Malaysia | National Influenza Pandemic Preparedness Plan | 2008 | [99] |
| Manual for the Control of Highly Pathogenic Avian Influenza | 2005 | [100] | |
| Myanmar | National Strategic Plan for Prevention and Control of Avian Influenza and Human Influenza Pandemic Preparedness and Response | 2006 | [101] |
| Philippines | Avian Influenza Protection Program Manuals of Procedure (2020) | 2020 | [102] |
| Singapore | MOH Pandemic Readiness and Response Plan for Influenza and Other Acute Respiratory Diseases | 2014 | - * |
| Thailand | Thailand National Strategic Plan for Emerging Infectious Disease Preparedness, Prevention, and Response | 2013 | [98] |
| Vietnam | Vietnam Integrated Program on Avian Influenza, Pandemic Preparedness, and Emerging Infectious Diseases 2011–2015 | 2011 | [103] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hatta, M.N.A.; Nga, Y.X.; Amirnuddin, E.N.; Muzafar, S.N.; Khairat, J.E. Landscape of H5 Infections in ASEAN Region: Past Insights, Present Realities, & Future Strategies. Viruses 2025, 17, 535. https://doi.org/10.3390/v17040535
Hatta MNA, Nga YX, Amirnuddin EN, Muzafar SN, Khairat JE. Landscape of H5 Infections in ASEAN Region: Past Insights, Present Realities, & Future Strategies. Viruses. 2025; 17(4):535. https://doi.org/10.3390/v17040535
Chicago/Turabian StyleHatta, Muhammad Nur Adam, Yi Xin Nga, Ezryn Najwa Amirnuddin, Siti Nuraisyah Muzafar, and Jasmine Elanie Khairat. 2025. "Landscape of H5 Infections in ASEAN Region: Past Insights, Present Realities, & Future Strategies" Viruses 17, no. 4: 535. https://doi.org/10.3390/v17040535
APA StyleHatta, M. N. A., Nga, Y. X., Amirnuddin, E. N., Muzafar, S. N., & Khairat, J. E. (2025). Landscape of H5 Infections in ASEAN Region: Past Insights, Present Realities, & Future Strategies. Viruses, 17(4), 535. https://doi.org/10.3390/v17040535







