Sulfatide Binds to Influenza B Virus and Enhances Viral Replication
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines
2.2. Viruses
2.3. Antibody
2.4. Lipids
2.5. Reagents
2.6. Plasmids
2.7. 50% Tissue Culture Infectious Dose (TCID50) Assay
2.8. Measurement of IBV Replication
2.9. Focus-Forming Assays
2.10. Enzyme-Linked Immunosorbent Assay (ELISA) for Sulfatide Binding to IBV
2.11. Thin-Layer-Chromatography (TLC) Overlay Assay
2.12. Expression and Purification of IBV sHA
2.13. Hemagglutination Assays
2.14. ELISA Evaluation of HA-Glycolipid Binding
2.15. Fluorescence Microscopic Imaging of IBV Infectivity Under Inhibited Sulfatide Synthesis
2.16. IBV Replication When Sulfatide Synthesis Was Inhibited
2.17. Fluorescence Microscopy Imaging of Nuclear Export of the vRNP Complex
2.18. Statistical Analyses
3. Results
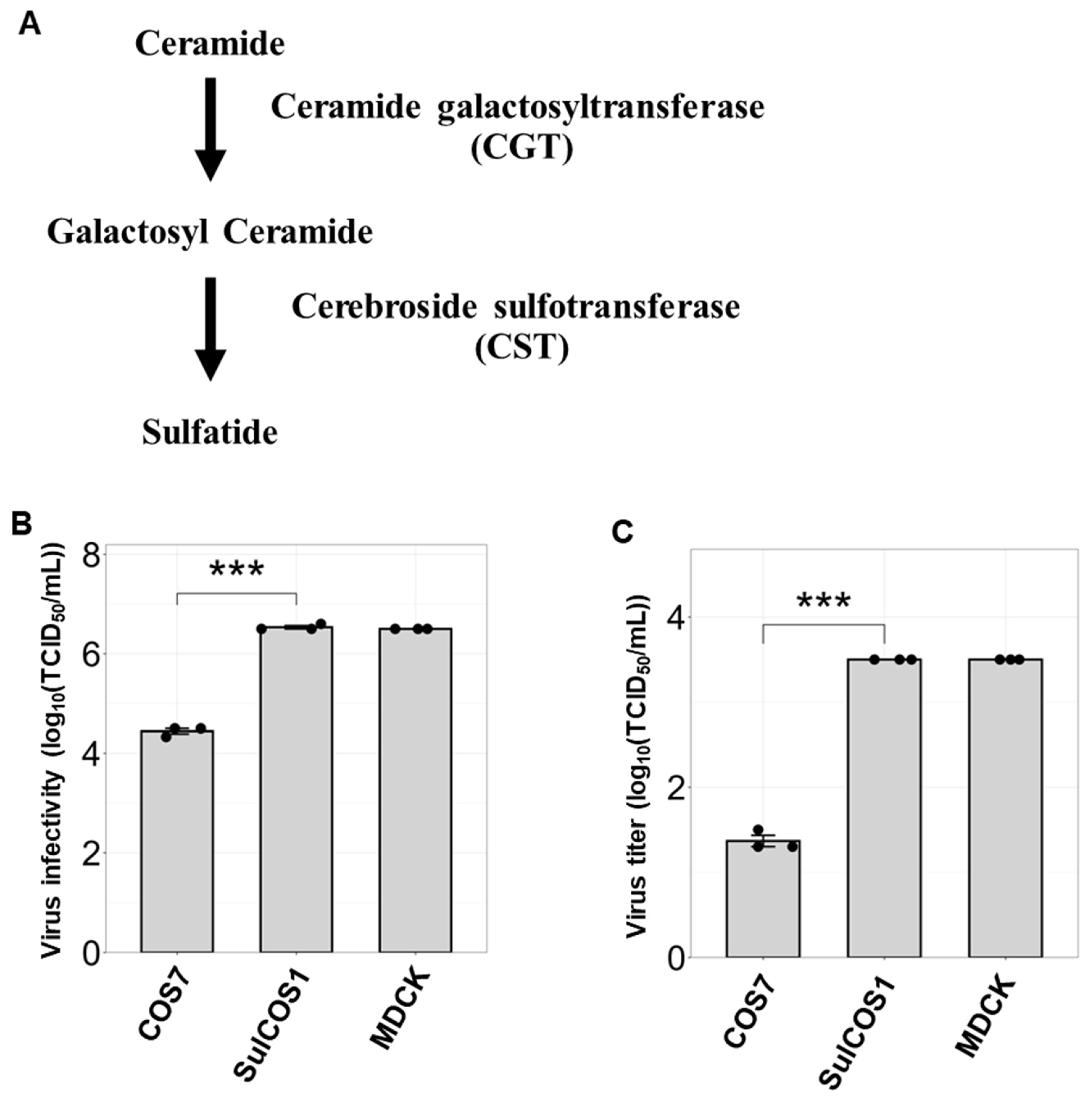
3.1. Sulfatide Promotes Viral Entry and Replication
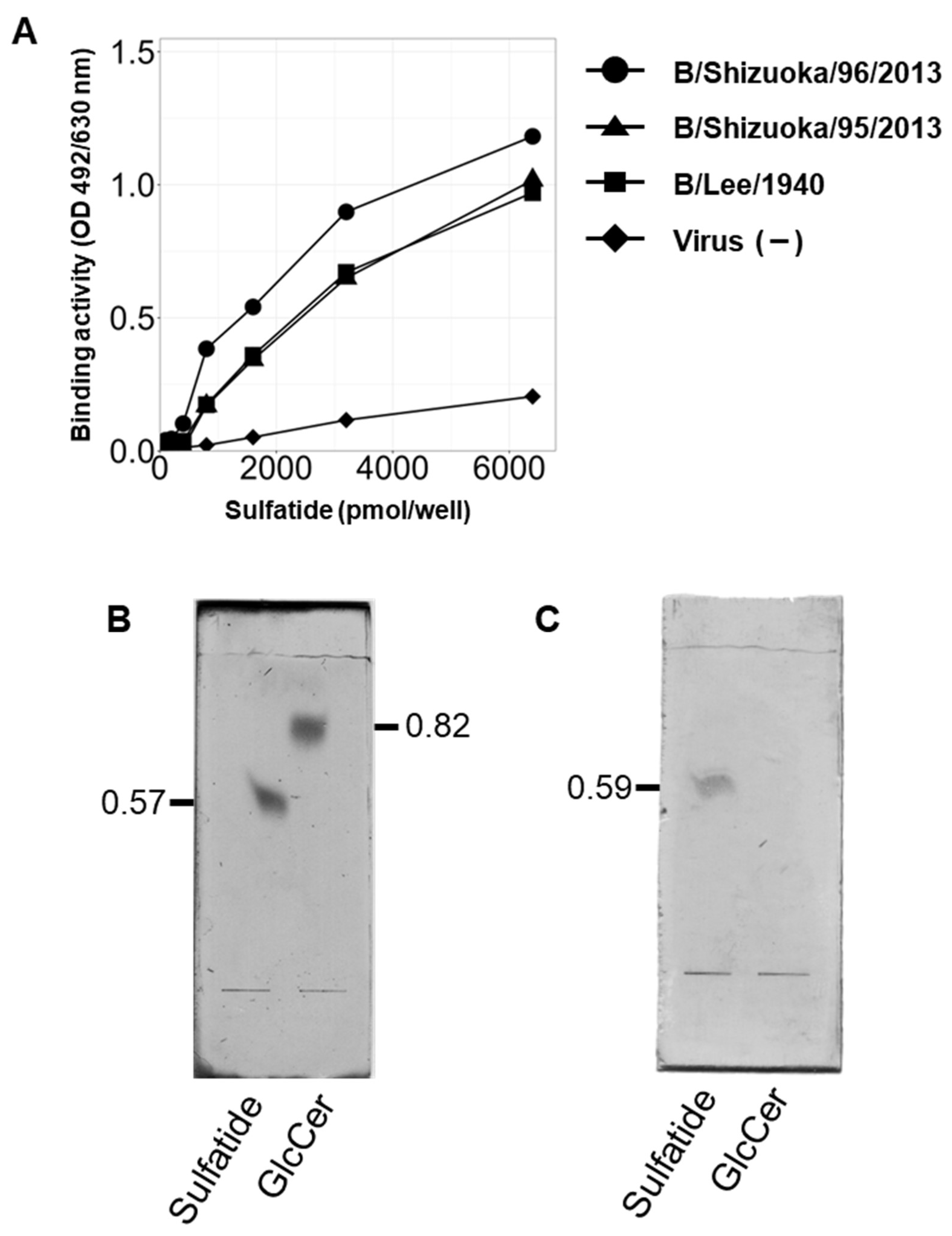
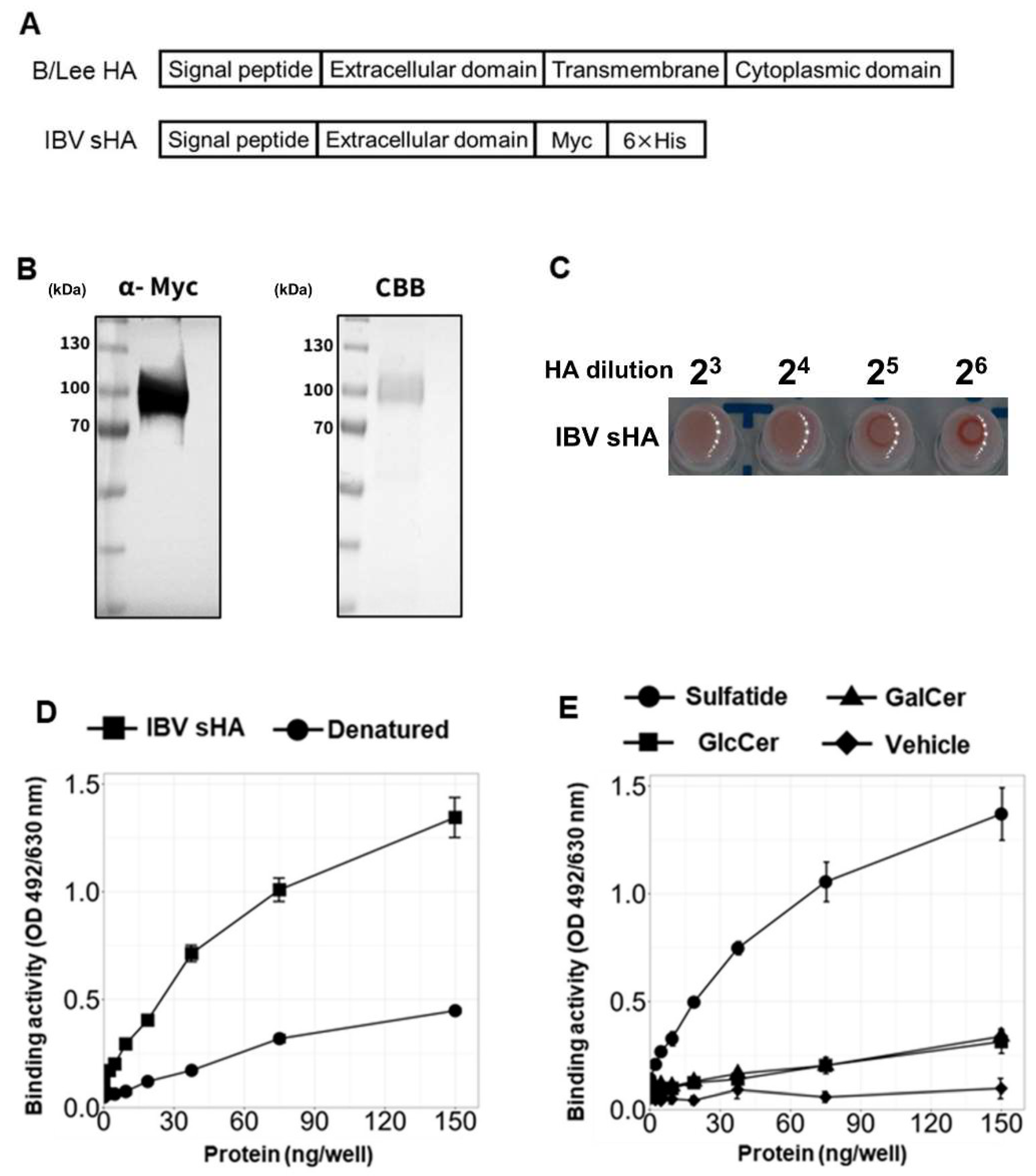
3.2. IBV Hemagglutinin Specifically Binds to Sulfatide
3.3. Sulfatide Inhibition via Anti-Sulfatide Antibody or Sulfatase Suppresses IBV Replication
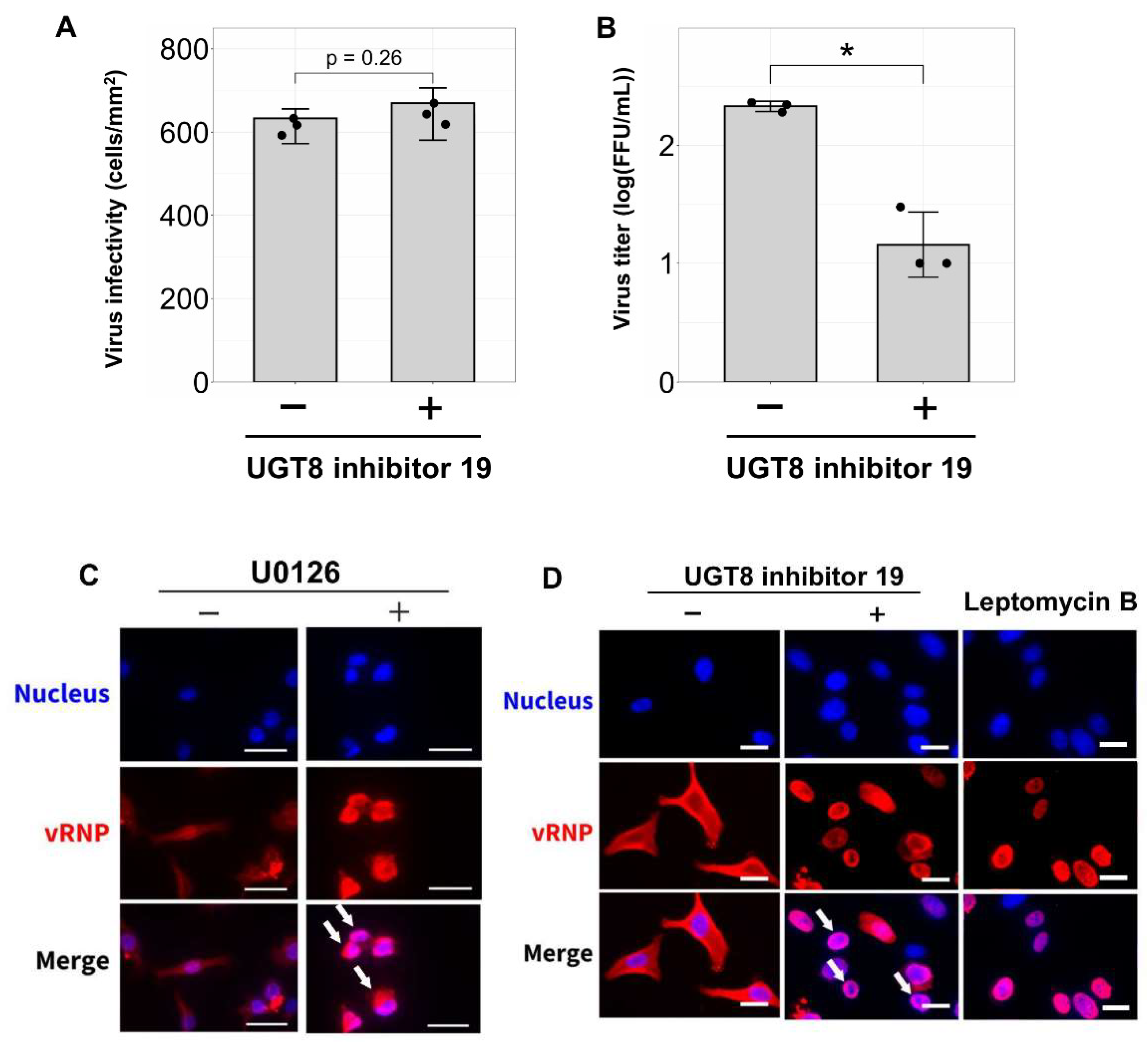
3.4. Inhibition of Sulfatide Synthesis Suppresses IBV Replication and Viral Ribonucleoprotein Translocation in Human Lung Derived-Cells
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Collin, E.A.; Sheng, Z.; Lang, Y.; Ma, W.; Hause, B.M.; Li, F. Cocirculation of two distinct genetic and antigenic lineages of proposed influenza D virus in cattle. J. Virol. 2015, 89, 1036–1042. [Google Scholar] [CrossRef] [PubMed]
- Taubenberger, J.K.; Kash, J.C. Influenza virus evolution, host adaptation, and pandemic formation. Cell Host Microbe 2010, 7, 440–451. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.P.; New, A.E.; Taylor, J.F.; Chiang, H.S. Influenza virus isolations from dogs during a human epidemic in Taiwan. Int. J. Zoonoses 1976, 3, 61–64. [Google Scholar] [PubMed]
- Osterhaus, A.D.; Rimmelzwaan, G.F.; Martina, B.E.; Bestebroer, T.M.; Fouchier, R.A. Influenza B virus in seals. Science 2000, 288, 1051–1053. [Google Scholar] [CrossRef]
- Ren, H.; Zhou, P. Epitope-focused vaccine design against influenza A and B viruses. Curr. Opin. Immunol. 2016, 42, 83–90. [Google Scholar] [CrossRef]
- Li, Y.; Huo, S.; Yin, Z.; Tian, Z.; Huang, F.; Liu, P.; Liu, Y.; Yu, F. Retracted and republished from: “The current state of research on influenza antiviral drug development: Drugs in clinical trial and licensed drugs”. mBio 2023, 14, e0127323. [Google Scholar] [CrossRef]
- Kumar, G.; Sakharam, K.A. Tackling Influenza A virus by M2 ion channel blockers: Latest progress and limitations. Eur. J. Med. Chem. 2024, 267, 116172. [Google Scholar] [CrossRef]
- Fodor, E.; Te Velthuis, A.J.W. Structure and Function of the Influenza Virus Transcription and Replication Machinery. Cold Spring Harb. Perspect. Med. 2020, 10, a038398. [Google Scholar] [CrossRef]
- Ashraf, M.A.; Raza, M.A.; Amjad, M.N.; Ud Din, G.; Yue, L.; Shen, B.; Chen, L.; Dong, W.; Xu, H.; Hu, Y. A comprehensive review of influenza B virus, its biological and clinical aspects. Front. Microbiol. 2024, 15, 1467029. [Google Scholar] [CrossRef]
- Suzuki, Y.; Nakao, T.; Ito, T.; Watanabe, N.; Toda, Y.; Xu, G.; Suzuki, T.; Kobayashi, T.; Kimura, Y.; Yamada, A.; et al. Structural determination of gangliosides that bind to influenza A, B, and C viruses by an improved binding assay: Strain-specific receptor epitopes in sialo-sugar chains. Virology 1992, 189, 121–131. [Google Scholar] [CrossRef]
- Pekarek, M.J.; Weaver, E.A. Existing Evidence for Influenza B Virus Adaptations to Drive Replication in Humans as the Primary Host. Viruses 2023, 15, 2032. [Google Scholar] [CrossRef] [PubMed]
- Bui, C.H.; Chan, R.W.; Ng, M.M.; Cheung, M.-C.; Ng, K.-C.; Chan, M.P.; Chan, L.L.; Fong, J.H.; Nicholls, J.; Peiris, J.M.; et al. Tropism of influenza B viruses in human respiratory tract explants and airway organoids. Eur. Respir. J. 2019, 54, 1900008. [Google Scholar] [CrossRef] [PubMed]
- Fields, B.N.; Knipe, D.M.; Howley, P.M. Fields Virology; Wolters Kluwer Health/Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2013. [Google Scholar]
- Dumm, R.E.; Heaton, N.S. The Development and Use of Reporter Influenza B Viruses. Viruses 2019, 11, 736. [Google Scholar] [CrossRef] [PubMed]
- To, J.; Torres, J. Viroporins in the Influenza Virus. Cells 2019, 8, 654. [Google Scholar] [CrossRef]
- Din, G.U.; Hasham, K.; Amjad, M.N.; Hu, Y. Natural History of Influenza B Virus—Current Knowledge on Treatment, Resistance and Therapeutic Options. Curr. Issues Mol. Biol. 2024, 46, 183–199. [Google Scholar] [CrossRef]
- Barman, S.; Nayak, D.P. Analysis of the transmembrane domain of influenza virus neuraminidase, a type II transmembrane glycoprotein, for apical sorting and raft association. J. Virol. 2000, 74, 6538–6545. [Google Scholar] [CrossRef]
- Maeda, T.; Kawasaki, K.; Ohnishi, S. Interaction of influenza virus hemagglutinin with target membrane lipids is a key step in virus-induced hemolysis and fusion at pH 5.2. Proc. Natl. Acad. Sci. USA 1981, 78, 4133–4137. [Google Scholar] [CrossRef]
- Fujioka, Y.; Nishide, S.; Ose, T.; Suzuki, T.; Kato, I.; Fukuhara, H.; Fujioka, M.; Horiuchi, K.; Satoh, A.O.; Nepal, P.; et al. A Sialylated Voltage-Dependent Ca(2+) Channel Binds Hemagglutinin and Mediates Influenza A Virus Entry into Mammalian Cells. Cell Host Microbe 2018, 23, 809–818.e805. [Google Scholar] [CrossRef]
- Gaur, P.; Ranjan, P.; Sharma, S.; Patel, J.R.; Bowzard, J.B.; Rahman, S.K.; Kumari, R.; Gangappa, S.; Katz, J.M.; Cox, N.J.; et al. Influenza A virus neuraminidase protein enhances cell survival through interaction with carcinoembryonic antigen-related cell adhesion molecule 6 (CEACAM6) protein. J. Biol. Chem. 2012, 287, 15109–15117. [Google Scholar] [CrossRef]
- Goren, M.B.; D’Arcy Hart, P.; Young, M.R.; Armstrong, J.A. Prevention of phagosome-lysosome fusion in cultured macrophages by sulfatides of Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. USA 1976, 73, 2510–2514. [Google Scholar] [CrossRef]
- Benjamins, J.A.; Hadden, T.; Skoff, R.P. Cerebroside sulfotransferase in Golgi-enriched fractions from rat brain. J. Neurochem. 1982, 38, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, K.; Schaeren-Wiemers, N.; Klein, D.; Sandhoff, R.; Schwarz, A.; Yaghootfam, A.; Gieselmann, V.; Franken, S. Specific downregulation and mistargeting of the lipid raft-associated protein MAL in a glycolipid storage disorder. Neurobiol. Dis. 2004, 16, 396–406. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Ito, K.; Fukushima, K.; Takaguchi, M.; Hayakawa, T.; Suzuki, Y.; Suzuki, T. Sulfatide negatively regulates the fusion process of human parainfluenza virus type 3. J. Biochem. 2012, 152, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Krivan, H.C.; Olson, L.D.; Barile, M.F.; Ginsburg, V.; Roberts, D.D. Adhesion of Mycoplasma pneumoniae to sulfated glycolipids and inhibition by dextran sulfate. J. Biol. Chem. 1989, 264, 9283–9288. [Google Scholar] [CrossRef]
- Marcus, J.; Honigbaum, S.; Shroff, S.; Honke, K.; Rosenbluth, J.; Dupree, J.L. Sulfatide is essential for the maintenance of CNS myelin and axon structure. Glia 2006, 53, 372–381. [Google Scholar] [CrossRef]
- Yaghootfam, A.; Sorkalla, T.; Häberlein, H.; Gieselmann, V.; Kappler, J.; Eckhardt, M. Cerebroside sulfotransferase forms homodimers in living cells. Biochemistry 2007, 46, 9260–9269. [Google Scholar] [CrossRef]
- Takahashi, T.; Suzuki, T. Role of sulfatide in normal and pathological cells and tissues. J. Lipid Res. 2012, 53, 1437–1450. [Google Scholar] [CrossRef]
- Fantini, J.; Hammache, D.; Delézay, O.; Piéroni, G.; Tamalet, C.; Yahi, N. Sulfatide inhibits HIV-1 entry into CD4-/CXCR4+ cells. Virology 1998, 246, 211–220. [Google Scholar] [CrossRef]
- Sundell, I.B.; Cortado, R.V.; Koka, P.S. Sulfatide—A new candidate for ART treatment in HIV-1 infection. J. Stem Cells 2012, 7, 61–72. [Google Scholar]
- Tsukamoto, B.; Kurebayashi, Y.; Takahashi, T.; Abe, Y.; Ota, R.; Wakabayashi, Y.; Nishiie, A.; Minami, A.; Suzuki, T.; Takeuchi, H. VP1 of human and murine noroviruses recognizes glycolipid sulfatide via the P domain. J. Biochem. 2024, 176, 299–312. [Google Scholar] [CrossRef]
- Takahashi, T.; Murakami, K.; Nagakura, M.; Kishita, H.; Watanabe, S.; Honke, K.; Ogura, K.; Tai, T.; Kawasaki, K.; Miyamoto, D.; et al. Sulfatide is required for efficient replication of influenza A virus. J. Virol. 2008, 82, 5940–5950. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Suzuki, T.; Takahashi, T.; Nishinaka, D.; Murakami, M.; Fujii, S.; Hidari, K.I.; Miyamoto, D.; Li, Y.T.; Suzuki, Y. Inhibition of influenza A virus sialidase activity by sulfatide. FEBS Lett. 2003, 553, 355–359. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Sometani, A.; Yamazaki, Y.; Horiike, G.; Mizutani, Y.; Masuda, H.; Yamada, M.; Tahara, H.; Xu, G.; Miyamoto, D.; et al. Sulphatide binds to human and animal influenza A viruses, and inhibits the viral infection. Biochem. J. 1996, 318 Pt. 2, 389–393. [Google Scholar] [CrossRef]
- Terajima, M.; Babon, J.A.; Co, M.D.; Ennis, F.A. Cross-reactive human B cell and T cell epitopes between influenza A and B viruses. Virol. J. 2013, 10, 244. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Nishi, H.; Hidari, K.; Hirabayashi, Y.; Matsumoto, M.; Kobayashi, T.; Watarai, S.; Yasuda, T.; Nakayama, J.; Maeda, H.; et al. A new monoclonal antibody directed to sialyl alpha 2-3lactoneotetraosylceramide and its application for detection of human gastrointestinal neoplasms. J. Biochem. 1991, 109, 354–360. [Google Scholar]
- Shikata, K.; Suzuki, Y.; Wada, J.; Hirata, K.; Matsuda, M.; Kawashima, H.; Suzuki, T.; Iizuka, M.; Makino, H.; Miyasaka, M. L-selectin and its ligands mediate infiltration of mononuclear cells into kidney interstitium after ureteric obstruction. J. Pathol. 1999, 188, 93–99. [Google Scholar] [CrossRef]
- Ding, Z.; Kawashima, H.; Suzuki, Y.; Suzuki, T.; Ward, P.A.; Miyasaka, M. A sulfatide receptor distinct from L-selectin is involved in lymphocyte activation. FEBS Lett. 1997, 418, 310–314. [Google Scholar] [CrossRef]
- Nakamura, T.; Tanaka, R.; Ashida, H. Possible evidence of contamination by catechins in deconjugation enzymes from Helix pomatia and Abalone entrails. Biosci. Biotechnol. Biochem. 2011, 75, 1506–1510. [Google Scholar] [CrossRef]
- Thurairatnam, S.; Lim, S.; Barker, R.H., Jr.; Choi-Sledeski, Y.M.; Hirth, B.H.; Jiang, J.; Macor, J.E.; Makino, E.; Maniar, S.; Musick, K.; et al. Brain Penetrable Inhibitors of Ceramide Galactosyltransferase for the Treatment of Lysosomal Storage Disorders. ACS Med. Chem. Lett. 2020, 11, 2010–2016. [Google Scholar] [CrossRef]
- Kärber, G. Beitrag zur kollektiven Behandlung pharmakologischer Reihenversuche. Naunyn-Schmiedebergs Arch. Für Exp. Pathol. Und Pharmakologie 1931, 162, 480–483. [Google Scholar] [CrossRef]
- Ohgane, K.; Yoshioka, H. Quantification of Gel Bands by an Image J Macro, Band/Peak Quantification Tool. protocols.io 2019. [Google Scholar] [CrossRef]
- Yamada, S.; Yasuhara, A.; Kawaoka, Y. Soluble Recombinant Hemagglutinin Protein of H1N1pdm09 Influenza Virus Elicits Cross-Protection Against a Lethal H5N1 Challenge in Mice. Front. Microbiol. 2019, 10, 2031. [Google Scholar] [CrossRef]
- Rossman, J.S.; Lamb, R.A. Influenza virus assembly and budding. Virology 2011, 411, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Kawagishi, S.; Masuda, M.; Suzuki, T. Binding kinetics of sulfatide with influenza A virus hemagglutinin. Glycoconj. J. 2013, 30, 709–716. [Google Scholar] [CrossRef]
- Schreiber, A.; Boff, L.; Anhlan, D.; Krischuns, T.; Brunotte, L.; Schuberth, C.; Wedlich-Söldner, R.; Drexler, H.; Ludwig, S. Dissecting the mechanism of signaling-triggered nuclear export of newly synthesized influenza virus ribonucleoprotein complexes. Proc. Natl. Acad. Sci. USA 2020, 117, 16557–16566. [Google Scholar] [CrossRef]
- Marjuki, H.; Alam, M.I.; Ehrhardt, C.; Wagner, R.; Planz, O.; Klenk, H.D.; Ludwig, S.; Pleschka, S. Membrane accumulation of influenza A virus hemagglutinin triggers nuclear export of the viral genome via protein kinase Calpha-mediated activation of ERK signaling. J. Biol. Chem. 2006, 281, 16707–16715. [Google Scholar] [CrossRef]
- Roy, A.; Patra, S.K. Lipid Raft Facilitated Receptor Organization and Signaling: A Functional Rheostat in Embryonic Development, Stem Cell Biology and Cancer. Stem Cell Rev. Rep. 2023, 19, 2–25. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurebayashi, Y.; Wakabayashi, Y.; Takahashi, T.; Sakakibara, K.; Takahashi, S.; Minami, A.; Suzuki, T.; Takeuchi, H. Sulfatide Binds to Influenza B Virus and Enhances Viral Replication. Viruses 2025, 17, 530. https://doi.org/10.3390/v17040530
Kurebayashi Y, Wakabayashi Y, Takahashi T, Sakakibara K, Takahashi S, Minami A, Suzuki T, Takeuchi H. Sulfatide Binds to Influenza B Virus and Enhances Viral Replication. Viruses. 2025; 17(4):530. https://doi.org/10.3390/v17040530
Chicago/Turabian StyleKurebayashi, Yuuki, Yoshiki Wakabayashi, Tadanobu Takahashi, Keiko Sakakibara, Shunsaku Takahashi, Akira Minami, Takashi Suzuki, and Hideyuki Takeuchi. 2025. "Sulfatide Binds to Influenza B Virus and Enhances Viral Replication" Viruses 17, no. 4: 530. https://doi.org/10.3390/v17040530
APA StyleKurebayashi, Y., Wakabayashi, Y., Takahashi, T., Sakakibara, K., Takahashi, S., Minami, A., Suzuki, T., & Takeuchi, H. (2025). Sulfatide Binds to Influenza B Virus and Enhances Viral Replication. Viruses, 17(4), 530. https://doi.org/10.3390/v17040530






