Nanotechnology-Driven Strategy Against SARS-CoV-2: Pluronic F127-Based Nanomicelles with or Without Atazanavir Reduce Viral Replication in Calu-3 Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Poloxamer 407 Characterization and CMC Determination Methodology
2.2. Development of Nanomicelles
2.3. Size, Polydispersity Index Analysis (PDI), Zeta Potential (mV), and TEM (Transmission Electron Microscopy)
2.4. In Vitro Release Study of ATV
2.5. Cell and Virus Culture
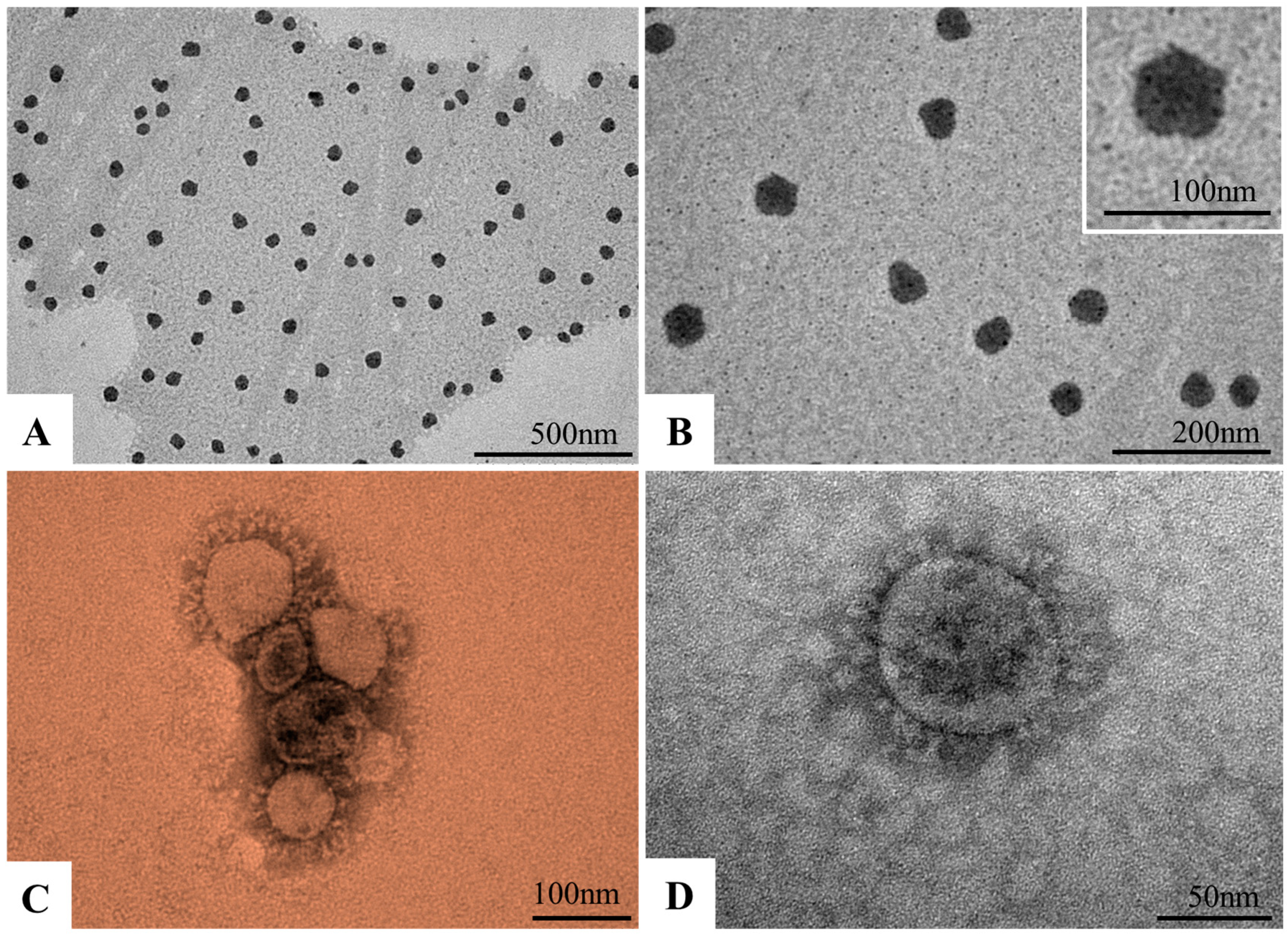
2.6. Ultrastructural Analysis of Micelles and SARS-CoV-2 Particles by Negative Staining Technique
2.7. Cytotoxicity Test
2.8. Evaluation of the Micelles’ Activity on SARS-CoV-2 Replication and Cytokine Production
2.9. Evaluation of the Virucidal Activity of Micelles on SARS-CoV-2
2.10. Evaluation of the Micelle’s Activity on the SARS-CoV-2 Adsorption
2.11. Pre-Treatment Test with Micelles on SARS-CoV-2 Replication
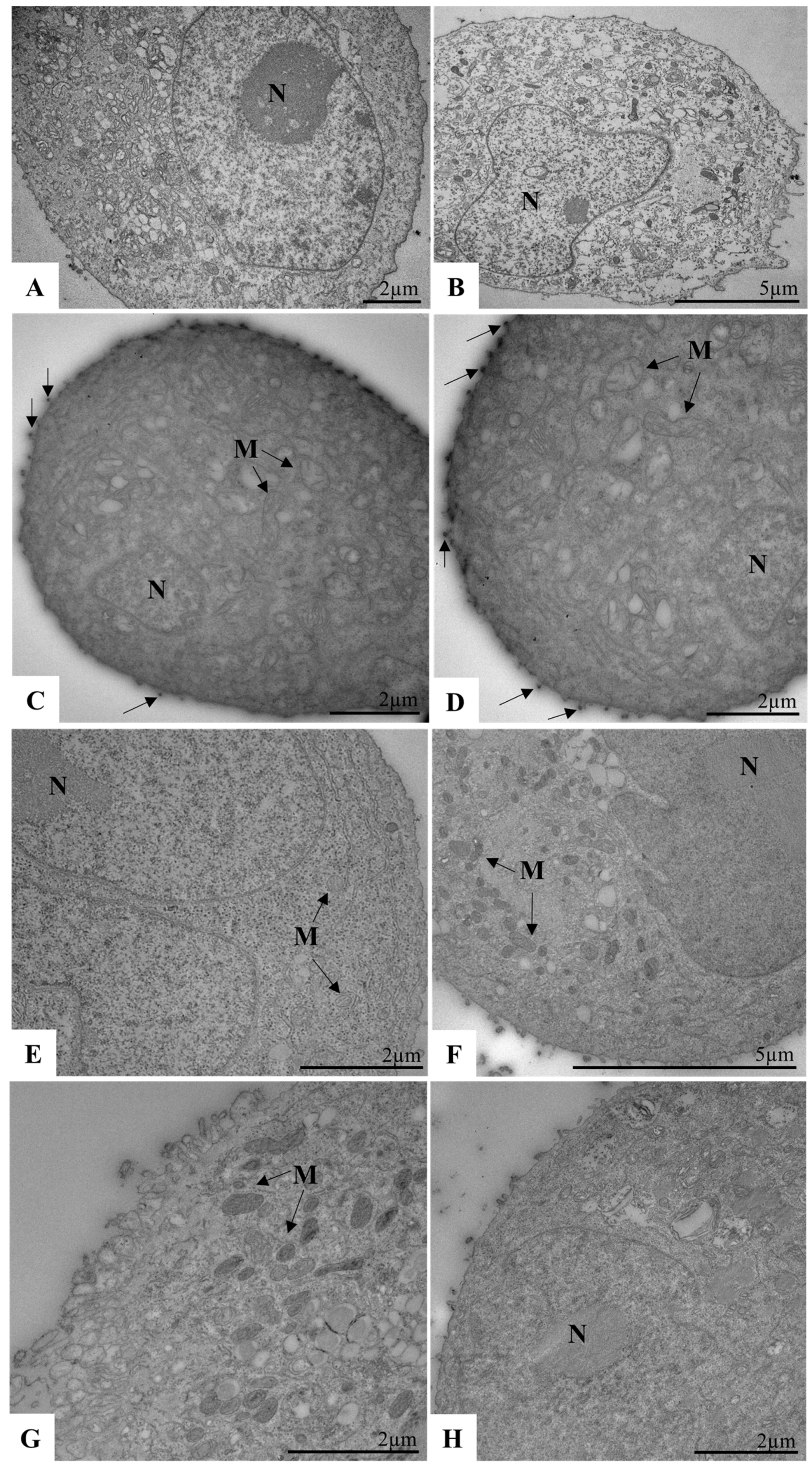
2.12. Biological Assays to Evaluate SARS-CoV-2 Replication in Calu-3 Cells by TEM
2.13. Processing of Sample Cells for Ultrastructural Analysis by TEM
2.14. Statistical Analysis
3. Results and Discussion
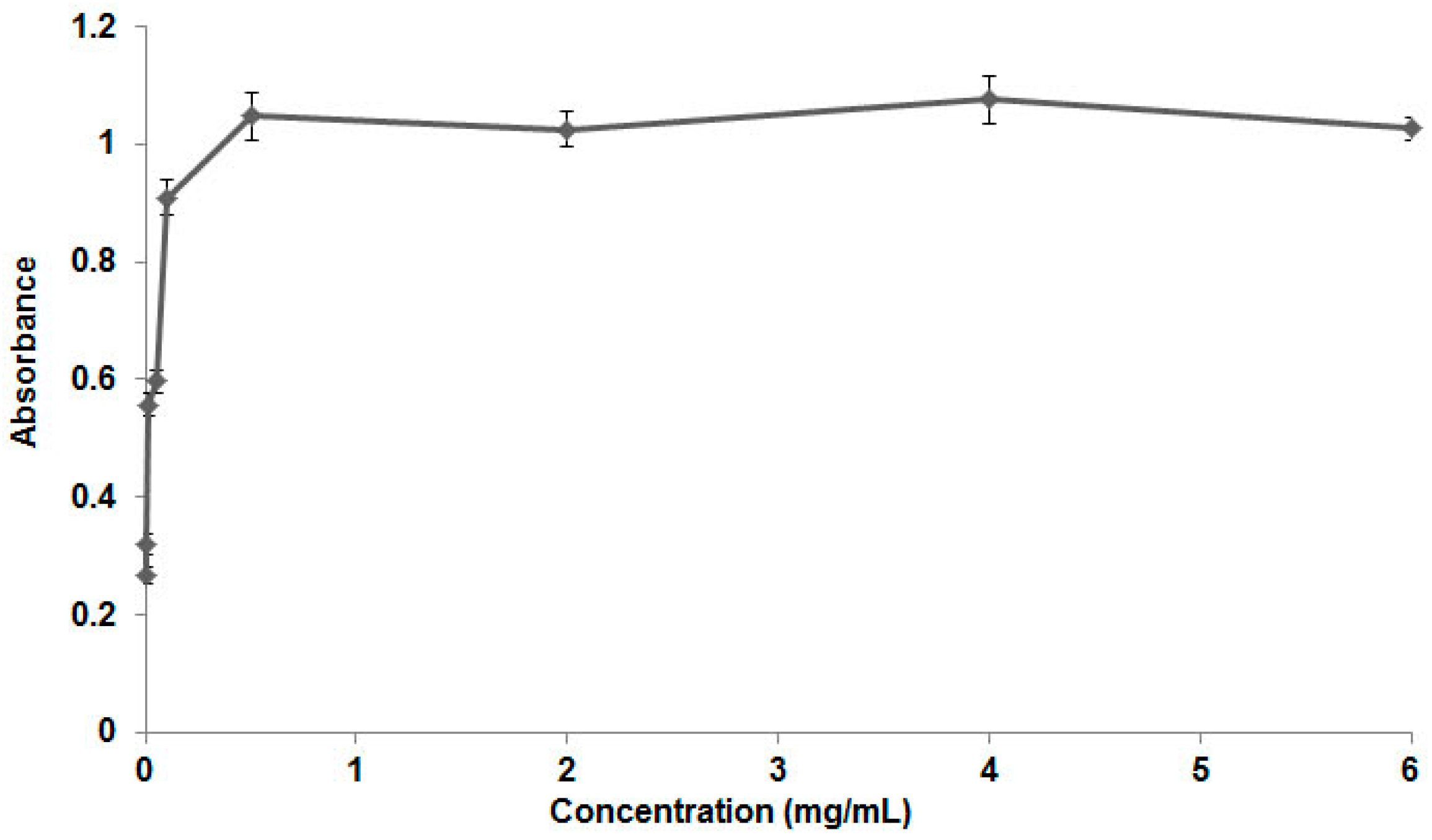
3.1. Poloxamer 407 Characterization and CMC Determination
3.2. Development of the Nanomicelles, Size, Polydispersity Index, and Zeta Potential
3.3. Morphological Characterization of Nanomicelles and SARS-CoV-2 Particles by TEM
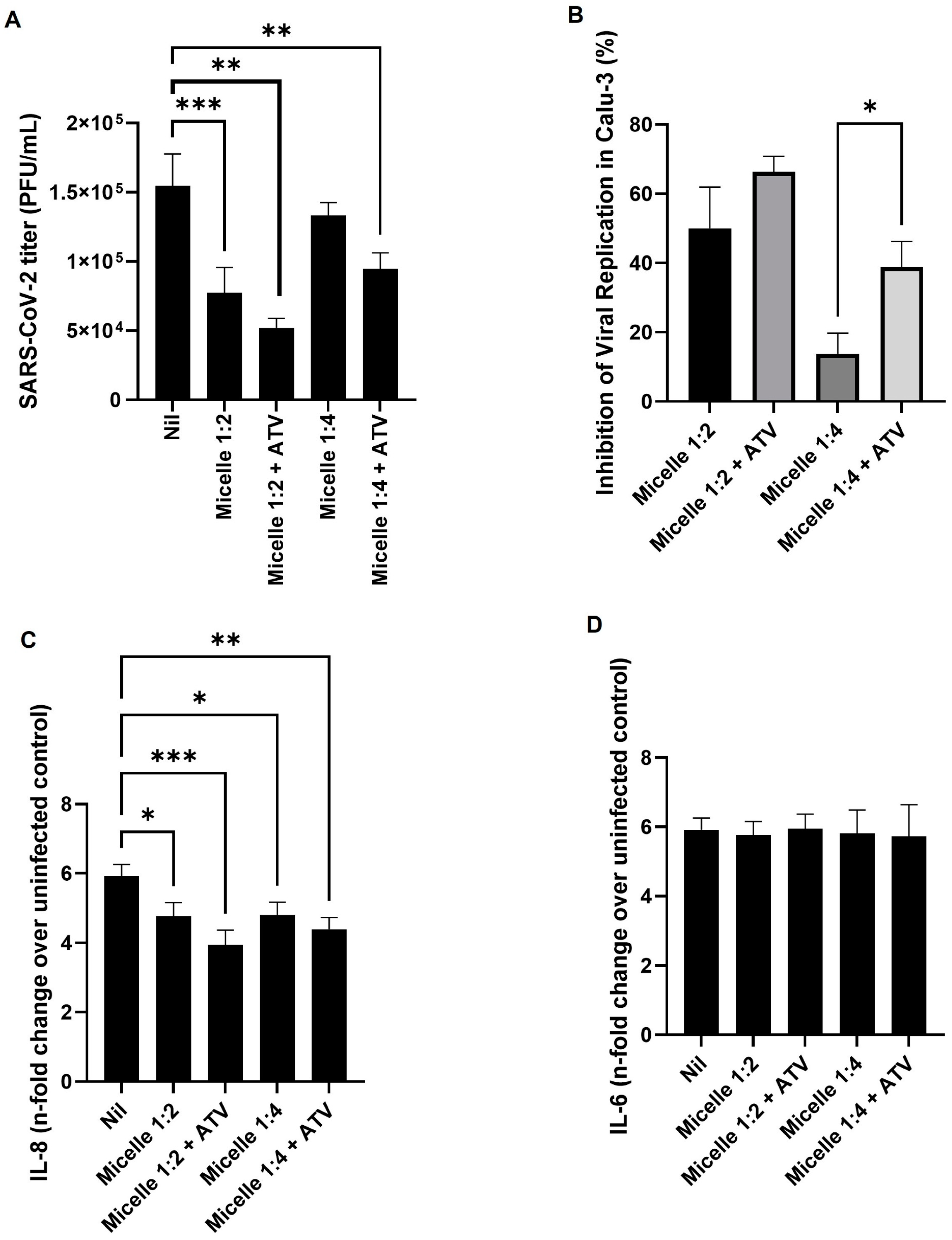
3.4. The Micelles Were Not Toxic to the Main Cell Model Studied and Were Able to Inhibit the Replication of SARS-CoV-2
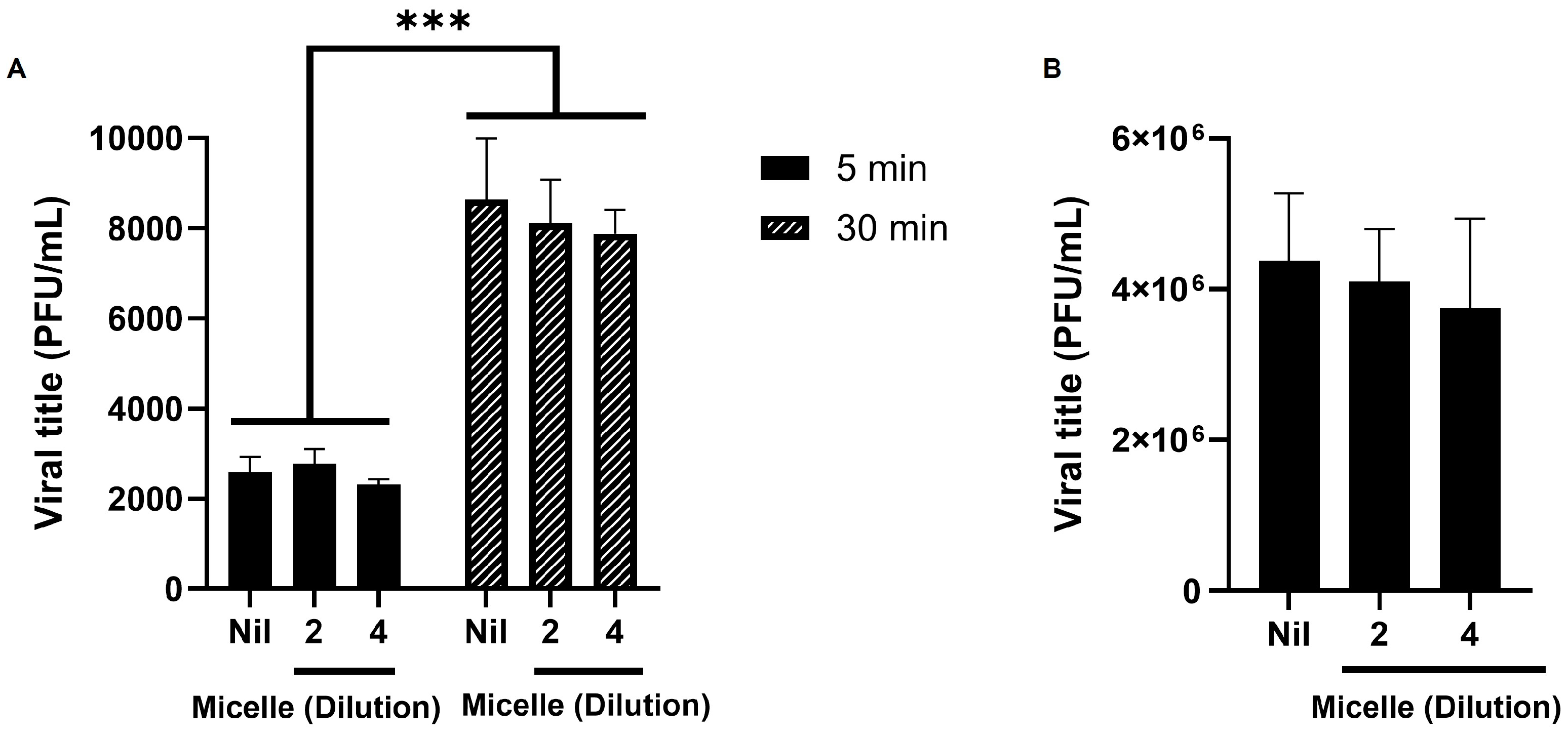
3.5. The Micelles Do Not Seem to Have Virucidal nor Anti-Adsorption Activity Against SARS-CoV-2
3.6. Pre-Treatment with Micelles Inhibits Viral Replication
3.7. Unveiling the Antiviral and Immunomodulatory Properties of Pluronic Micelles in SARS-CoV-2 Infection
3.8. Spectrophotometric Validation, Release Studies, and Kinetic Modeling of Nanomicelles with ATV
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mostafavi, M.; Shabani, M. Leveraging Nanotechnology for Addressing COVID-19: Revealing Antiviral Approaches and Hurdles. World J. Nano Sci. Eng. 2024, 14, 1–14. [Google Scholar] [CrossRef]
- Adekoya, O.C.; Adekoya, G.J.; Liu, W.; Sadiku, E.R.; Hamam, Y. Pegylated Graphene Oxide For 4′-Fluorouridine Delivery: An Ab Initio Approach to Antiviral Therapy. Adv. Theory Simul. 2024, 20, 2401145. [Google Scholar] [CrossRef]
- Roy, K.; Saikia, B.K.; Konwar, R. Exploring the role of carbon quantum dots as countermeasure for SARS-CoV-2 virus. Virology 2025, 603, 110339. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Zhi, X.; Ye, T.; Wang, X.; Li, K.; Li, Y.; Zhang, Q.; Jiang, L.; Ding, X. Boosting humoral and cellular immunity with enhanced STING activation by hierarchical mesoporous metal-organic framework adjuvants. J. Control. Release 2024, 370, 691–706. [Google Scholar] [CrossRef]
- Yazdanian, M.; Memarnejadian, A.; Mahdavi, M.; Sadat, S.M.; Motevali, F.; Vahabpour, R.; Khanahmad, H.; Siadat, S.D.; Aghasadeghi, M.R.; Roohvand, F. Immunization of Mice by BCG Formulated HCV Core Protein Elicited Higher Th1-Oriented Responses Compared to Pluronic-F127 Copolymer. Hepat. Mon. 2013, 13, e14178. [Google Scholar] [CrossRef]
- Jino Affrald, R.; Narayan, S. Intranasal Drug Delivery of Antiviral Agents—A Revisit and Way Forward. Curr. Drug Ther. 2024, 19, 130–150. [Google Scholar] [CrossRef]
- Kim, N.W.; Kim, S.Y.; Lee, J.E.; Yin, Y.; Lee, J.H.; Lim, S.Y.; Kim, E.S.; Duong, H.T.T.; Kim, H.K.; Kim, S.; et al. Enhanced Cancer Vaccination by In Situ Nanomicelle-Generating Dissolving Microneedles. ACS Nano 2018, 12, 9702–9713. [Google Scholar] [CrossRef]
- Khaliq, N.U.; Lee, J.; Kim, S.; Sung, D.; Kim, H. Pluronic F-68 and F-127 Based Nanomedicines for Advancing Combination Cancer Therapy. Pharmaceutics 2023, 15, 2102. [Google Scholar] [CrossRef]
- Wu, S.J.; Ewing, D.; Sundaram, A.K.; Chen, H.W.; Liang, Z.; Cheng, Y.; Jani, V.; Sun, P.; Gromowski, G.D.; De La Barrera, R.A.; et al. Enhanced Immunogenicity of Inactivated Dengue Vaccines by Novel Polysaccharide-Based Adjuvants in Mice. Microorganisms 2022, 10, 1034. [Google Scholar] [CrossRef]
- Singla, P.; Garg, S.; McClements, J.; Jamieson, O.; Peeters, M.; Mahajan, R.K. Advances in the therapeutic delivery and applications of functionalized Pluronics: A critical review. Adv. Colloid. Interface Sci. 2022, 299, 102563. [Google Scholar] [CrossRef]
- Kumar, G.; Virmani, T.; Misra, S.K.; Sharma, A.; Pathak, K. Exploration of chitosan and its modified derivatives as vaccine adjuvant: A review. Carbohydr. Polym. Technol. Appl. 2024, 8, 100537. [Google Scholar] [CrossRef]
- Toscanini, M.A.; Limeres, M.J.; Garrido, A.V.; Cagel, M.; Bernabeu, E.; Moretton, M.A.; Chiappetta, D.A.; Cuestas, M.L. Polymeric micelles and nanomedicines: Shaping the future of next generation therapeutic strategies for infectious diseases. J. Drug Deliv. Sci. Technol. 2021, 66, 102927. [Google Scholar] [CrossRef]
- Fintelman-Rodrigues, N.; Sacramento, C.Q.; Ribeiro Lima, C.; Souza da Silva, F.; Ferreira, A.C.; Mattos, M.; de Freitas, C.S.; Cardoso Soares, V.; da Silva Gomes Dias, S.; Temerozo, J.R.; et al. Atazanavir, Alone or in Combination with Ritonavir, Inhibits SARS-CoV-2 Replication and Proinflammatory Cytokine Production. Antimicrob. Agents Chemother. 2020, 64, e00825-20. [Google Scholar] [CrossRef]
- Chaves, O.A.; Sacramento, C.Q.; Ferreira, A.C.; Mattos, M.; Fintelman-Rodrigues, N.; Temerozo, J.R.; Vazquez, L.; Pinto, D.P.; da Silveira, G.P.E.; da Fonseca, L.B.; et al. Atazanavir Is a Competitive Inhibitor of SARS-CoV-2 M(pro), Impairing Variants Replication In Vitro and In Vivo. Pharmaceuticals 2021, 15, 21. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, Q.; Guo, D. Emerging coronaviruses: Genome structure, replication, and pathogenesis. J. Med. Virol. 2020, 92, 2249. [Google Scholar] [CrossRef]
- Jackson, C.B.; Farzan, M.; Chen, B.; Choe, H. Mechanisms of SARS-CoV-2 entry into cells. Nat. Rev. Mol. Cell Biol. 2022, 23, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Dong, M.; Galvan Achi, J.M.; Du, R.; Rong, L.; Cui, Q. Development of SARS-CoV-2 entry antivirals. Cell Insight 2024, 3, 100144. [Google Scholar] [CrossRef] [PubMed]
- Trougakos, I.P.; Stamatelopoulos, K.; Terpos, E.; Tsitsilonis, O.E.; Aivalioti, E.; Paraskevis, D.; Kastritis, E.; Pavlakis, G.N.; Dimopoulos, M.A. Insights to SARS-CoV-2 life cycle, pathophysiology, and rationalized treatments that target COVID-19 clinical complications. J. Biomed. Sci. 2021, 28, 9. [Google Scholar] [CrossRef]
- Song, P.; Li, W.; Xie, J.; Hou, Y.; You, C. Cytokine storm induced by SARS-CoV-2. Clin. Chim. Acta 2020, 509, 280–287. [Google Scholar] [CrossRef]
- Tsai, C.; Wilson, S.E.; Rubinos, C. SARS-CoV-2 infection and seizures: The perfect storm. J. Integr. Neurosci. 2022, 21, 115. [Google Scholar] [CrossRef]
- Samith, V.D.; Miño, G.; Ramos-Moore, E.; Arancibia-Miranda, N. Effects of pluronic F68 micellization on the viability of neuronal cells in culture. J. Appl. Polym. Sci. 2013, 130, 2159–2164. [Google Scholar] [CrossRef]
- Feitosa, V.A.; Almeida, V.C.; Malheiros, B.; Castro, R.D.; Barbosa, L.R.S.; Cerize, N.N.P.; Rangel-Yagui, C.O. Polymeric micelles of pluronic F127 reduce hemolytic potential of amphiphilic drugs. Colloids Surf. B Biointerfaces 2019, 180, 177–185. [Google Scholar] [CrossRef]
- Sotoudegan, F.; Amini, M.; Faizi, M.; Aboofazeli, R. Nimodipine-Loaded Pluronic((R)) Block Copolymer Micelles: Preparation, Characterization, In-vitro and In-vivo Studies. Iran. J. Pharm. Res. 2016, 15, 641–661. [Google Scholar]
- Yang, L.; Wu, X.; Liu, F.; Duan, Y.; Li, S. Novel biodegradable polylactide/poly(ethylene glycol) micelles prepared by direct dissolution method for controlled delivery of anticancer drugs. Pharm. Res. 2009, 26, 2332–2342. [Google Scholar] [CrossRef] [PubMed]
- Bruschi, M.L. 5-Mathematical models of drug release. In Strategies to Modify the Drug Release from Pharmaceutical Systems; Bruschi, M.L., Ed.; Woodhead Publishing: Cambridge, UK, 2015; pp. 63–86. [Google Scholar]
- Peppas, N.A.; Narasimhan, B. Mathematical models in drug delivery: How modeling has shaped the way we design new drug delivery systems. J. Control. Release 2014, 190, 75–81. [Google Scholar] [CrossRef]
- Ghante, M.R.; Shelar, R.S.; Sawant, S.D.; Kadam, M.M. Development and Validation of Uv Spectrophotometric Method for Estimation of Darunavir Ethanolate in Bulk and Tablet Dosage Form. Int. J. Pharm. Pharm. Sci. 2014, 6, 240–242. [Google Scholar]
- WHO. Laboratory Biosafety Guidance Related to Coronavirus Disease 2019 (COVID-19); WHO: Geneva, Switzerland, 2020. [Google Scholar]
- Barreto-Vieira, D.F.; Barth, O.M. Negative and positive staining in transmission electron microscopy for virus diagnosis. In Microbiology in Agriculture and Human Health; Intechopen: London, UK, 2015; pp. 45–56. [Google Scholar]
- Caldas, G.C.; Barth, O.M.; Barreto-Vieira, D.F. Transmission Electron Microscopy Studies of Dengue Viruses. Methods Mol. Biol. 2022, 2409, 11–30. [Google Scholar] [CrossRef]
- Caleffi, G.S.; Rosa, A.S.; de Souza, L.G.; Avelar, J.L.S.; Nascimento, S.M.R.; de Almeida, V.M.; Tucci, A.R.; Ferreira, V.N.; da Silva, A.J.M.; Santos-Filho, O.A.; et al. Aurones: A Promising Scaffold to Inhibit SARS-CoV-2 Replication. J. Nat. Prod. 2023, 86, 1536–1549. [Google Scholar] [CrossRef]
- Wildner, G.; Tucci, A.R.; Prestes, A.d.S.; Muller, T.; Rosa, A.d.S.; Borba, N.R.R.; Ferreira, V.N.; Rocha, J.B.T.; Miranda, M.D.; Barbosa, N.V. Ebselen and Diphenyl Diselenide Inhibit SARS-CoV-2 Replication at Non-Toxic Concentrations to Human Cell Lines. Vaccines 2023, 11, 1222. [Google Scholar] [CrossRef]
- Tucci, A.R.; da Rosa, R.M.; Rosa, A.S.; Augusto Chaves, O.; Ferreira, V.N.S.; Oliveira, T.K.F.; Coutinho Souza, D.D.; Borba, N.R.R.; Dornelles, L.; Rocha, N.S.; et al. Antiviral Effect of 5′-Arylchalcogeno-3-aminothymidine Derivatives in SARS-CoV-2 Infection. Molecules 2023, 28, 6696. [Google Scholar] [CrossRef]
- Barth, O.M.; Silva, M.A.; Barreto-Vieira, D.F. Low impact to fixed cell processing aiming transmission electron microscopy. Mem. Inst. Oswaldo Cruz 2016, 111, 411–413. [Google Scholar] [CrossRef] [PubMed]
- Escobar-Chavez, J.J.; Lopez-Cervantes, M.; Naik, A.; Kalia, Y.N.; Quintanar-Guerrero, D.; Ganem-Quintanar, A. Applications of thermo-reversible pluronic F-127 gels in pharmaceutical formulations. J. Pharm. Pharm. Sci. 2006, 9, 339–358. [Google Scholar] [PubMed]
- Croy, S.R.; Kwon, G.S. Polymeric micelles for drug delivery. Curr. Pharm. Des. 2006, 12, 4669–4684. [Google Scholar] [CrossRef]
- Bodratti, A.M.; Alexandridis, P. Formulation of Poloxamers for Drug Delivery. J. Funct. Biomater. 2018, 9, 11. [Google Scholar] [CrossRef]
- Chen, W.N.; Shaikh, M.F.; Bhuvanendran, S.; Date, A.; Ansari, M.T.; Radhakrishnan, A.K.; Othman, I. Poloxamer 188 (P188), A Potential Polymeric Protective Agent for Central Nervous System Disorders: A Systematic Review. Curr. Neuropharmacol. 2022, 20, 799–808. [Google Scholar] [CrossRef]
- Alexandridis, P.; Holzwarth, J.F.; Hatton, T.A. Micellization of Poly(ethylene oxide)-Poly(propylene oxide)-Poly(ethylene oxide) Triblock Copolymers in Aqueous Solutions: Thermodynamics of Copolymer Association. Macromolecules 1994, 27, 2414–2425. [Google Scholar] [CrossRef]
- Tehrani-Bagha, A.R.; Holmberg, K. Solubilization of Hydrophobic Dyes in Surfactant Solutions. Materials 2013, 6, 580–608. [Google Scholar] [CrossRef] [PubMed]
- Attwood, D.; Booth, C. Solubilization of a Poorly Soluble Aromatic Drug by Micellar Solutions of Amphiphilic Block Copoly(oxyalkylene)s. In Colloid Stability; Intechopen: London, UK, 2010; pp. 61–78. [Google Scholar]
- Sezgin, Z.; Yuksel, N.; Baykara, T. Preparation and characterization of polymeric micelles for solubilization of poorly soluble anticancer drugs. Eur. J. Pharm. Biopharm. 2006, 64, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Ghezzi, M.; Pescina, S.; Padula, C.; Santi, P.; Del Favero, E.; Cantu, L.; Nicoli, S. Polymeric micelles in drug delivery: An insight of the techniques for their characterization and assessment in biorelevant conditions. J. Control. Release 2021, 332, 312–336. [Google Scholar] [CrossRef]
- Makhmalzade, B.S.; Chavoshy, F. Polymeric micelles as cutaneous drug delivery system in normal skin and dermatological disorders. J. Adv. Pharm. Technol. Res. 2018, 9, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Manjappa, A.S.; Kumbhar, P.S.; Disouza, J.I.; Patravale, V.B. Polymeric Mixed Micelles: Improving the Anticancer Efficacy of Single-Copolymer Micelles. Crit. Rev. Ther. Drug Carrier Syst. 2019, 36, 1–58. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Lin, Y.J.; Cheng, L.T.; Lin, C.H.; Ke, G.M. Poloxamer-188 Adjuvant Efficiently Maintains Adaptive Immunity of SARS-CoV-2 RBD Subunit Vaccination through Repressing p38MAPK Signaling. Vaccines 2022, 10, 715. [Google Scholar] [CrossRef]
- Dumortier, G.; Grossiord, J.L.; Agnely, F.; Chaumeil, J.C. A review of poloxamer 407 pharmaceutical and pharmacological characteristics. Pharm. Res. 2006, 23, 2709–2728. [Google Scholar] [CrossRef] [PubMed]
- Giuliano, E.; Paolino, D.; Fresta, M.; Cosco, D. Drug-Loaded Biocompatible Nanocarriers Embedded in Poloxamer 407 Hydrogels as Therapeutic Formulations. Medicines 2018, 6, 7. [Google Scholar] [CrossRef]
- Klang, V.; Matsko, N.B.; Valenta, C.; Hofer, F. Electron microscopy of nanoemulsions: An essential tool for characterisation and stability assessment. Micron 2012, 43, 85–103. [Google Scholar] [CrossRef]
- Manaia, E.B.; Abucafy, M.P.; Chiari-Andreo, B.G.; Silva, B.L.; Oshiro Junior, J.A.; Chiavacci, L.A. Physicochemical characterization of drug nanocarriers. Int. J. Nanomed. 2017, 12, 4991–5011. [Google Scholar] [CrossRef]
- Jain, A.K.; Thareja, S. In vitro and in vivo characterization of pharmaceutical nanocarriers used for drug delivery. Artif. Cells Nanomed. Biotechnol. 2019, 47, 524–539. [Google Scholar] [CrossRef]
- Chen, L.; Liang, J. An overview of functional nanoparticles as novel emerging antiviral therapeutic agents. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 112, 110924. [Google Scholar] [CrossRef]
- De Jong, W.H.; Borm, P.J. Drug delivery and nanoparticles: Applications and hazards. Int. J. Nanomed. 2008, 3, 133–149. [Google Scholar] [CrossRef]
- Hwang, D.; Ramsey, J.D.; Kabanov, A.V. Polymeric micelles for the delivery of poorly soluble drugs: From nanoformulation to clinical approval. Adv. Drug Deliv. Rev. 2020, 156, 80–118. [Google Scholar] [CrossRef]
- Ghosh, B.; Biswas, S. Polymeric micelles in cancer therapy: State of the art. J. Control. Release 2021, 332, 127–147. [Google Scholar] [CrossRef]
- Andrés, F.O.; Héctor, C.; Luis, E.; Betsabe, A. Solubilization of P-Alkylphenols in Pluronics F-68 and F-127 Micelles: Partition Coefficients and Effect of Solute on the Aggregate Structure. J. Chil. Chem. Soc. 2014, 59, 2451–2454. [Google Scholar] [CrossRef]
- Teja, P.K.; Mithiya, J.; Kate, A.S.; Bairwa, K.; Chauthe, S.K. Herbal nanomedicines: Recent advancements, challenges, opportunities and regulatory overview. Phytomedicine 2022, 96, 153890. [Google Scholar] [CrossRef] [PubMed]
- Domingo, M.; Guzman, H.V.; Kanduc, M.; Faraudo, J. Electrostatic Interaction between SARS-CoV-2 and Charged Surfaces: Spike Protein Evolution Changed the Game. J. Chem. Inf. Model. 2025, 65, 240–251. [Google Scholar] [CrossRef]
- Mahmoud, D.B.; Bakr, M.M.; Al-Karmalawy, A.A.; Moatasim, Y.; El Taweel, A.; Mostafa, A. Scrutinizing the Feasibility of Nonionic Surfactants to Form Isotropic Bicelles of Curcumin: A Potential Antiviral Candidate Against COVID-19. AAPS PharmSciTech 2021, 23, 44. [Google Scholar] [CrossRef] [PubMed]
- Tavares, G.S.V.; Mendonca, D.V.C.; Pereira, I.A.G.; Oliveira-da-Silva, J.A.; Ramos, F.F.; Lage, D.P.; Machado, A.S.; Carvalho, L.M.; Reis, T.A.R.; Perin, L.; et al. A clioquinol-containing Pluronic® F127 polymeric micelle system is effective in the treatment of visceral leishmaniasis in a murine model. Parasite 2020, 27, 29. [Google Scholar] [CrossRef]
- Ruiz-Hitzky, E.; Darder, M.; Wicklein, B.; Ruiz-Garcia, C.; Martin-Sampedro, R.; Del Real, G.; Aranda, P. Nanotechnology Responses to COVID-19. Adv. Healthc. Mater. 2020, 9, e2000979. [Google Scholar] [CrossRef]
- Brant, A.C.; Tian, W.; Majerciak, V.; Yang, W.; Zheng, Z.M. SARS-CoV-2: From its discovery to genome structure, transcription, and replication. Cell Biosci. 2021, 11, 136. [Google Scholar] [CrossRef]
- Houghton, M.J.; Balland, E.; Gartner, M.J.; Thomas, B.J.; Subbarao, K.; Williamson, G. The flavonoid quercetin decreases ACE2 and TMPRSS2 expression but not SARS-CoV-2 infection in cultured human lung cells. Biofactors 2024, 50, 1268–1286. [Google Scholar] [CrossRef]
- Zhu, Y.; Chidekel, A.; Shaffer, T.H. Cultured human airway epithelial cells (calu-3): A model of human respiratory function, structure, and inflammatory responses. Crit. Care Res. Pract. 2010, 2010, 394578. [Google Scholar] [CrossRef]
- Sousa, F.; Castro, P. 3.4-Cell-based in vitro models for nasal permeability studies. In Concepts and Models for Drug Permeability Studies; Sarmento, B., Ed.; Woodhead Publishing: Cambridge, UK, 2016; pp. 83–100. [Google Scholar]
- Ladel, S.; Schindowski, K. Chapter 3.4-Cell-based in vitro models for nasal permeability studies. In Concepts and Models for Drug Permeability Studies, 2nd ed.; Sarmento, B., Leite Pereira, C., Neves, J.D., Eds.; Woodhead Publishing: Cambridge, UK, 2024; pp. 109–135. [Google Scholar]
- Simon, M.; Veit, M.; Osterrieder, K.; Gradzielski, M. Surfactants-Compounds for inactivation of SARS-CoV-2 and other enveloped viruses. Curr. Opin. Colloid. Interface Sci. 2021, 55, 101479. [Google Scholar] [CrossRef] [PubMed]
- Matlin, K.S.; Reggio, H.; Helenius, A.; Simons, K. Infectious entry pathway of influenza virus in a canine kidney cell line. J. Cell Biol. 1981, 91, 601–613. [Google Scholar] [CrossRef] [PubMed]
- Marsh, M.; Helenius, A. Virus entry into animal cells. Adv. Virus Res. 1989, 36, 107–151. [Google Scholar] [CrossRef] [PubMed]
- Klasse, P.J.; Bron, R.; Marsh, M. Mechanisms of enveloped virus entry into animal cells. Adv. Drug Deliv. Rev. 1998, 34, 65–91. [Google Scholar] [CrossRef]
- Kausar, S.; Said Khan, F.; Ishaq Mujeeb Ur Rehman, M.; Akram, M.; Riaz, M.; Rasool, G.; Hamid Khan, A.; Saleem, I.; Shamim, S.; Malik, A. A review: Mechanism of action of antiviral drugs. Int. J. Immunopathol. Pharmacol. 2021, 35, 20587384211002621. [Google Scholar] [CrossRef]
- Bayati, A.; Kumar, R.; Francis, V.; McPherson, P.S. SARS-CoV-2 infects cells after viral entry via clathrin-mediated endocytosis. J. Biol. Chem. 2021, 296, 100306. [Google Scholar] [CrossRef]
- Gerl, M.J.; Sampaio, J.L.; Urban, S.; Kalvodova, L.; Verbavatz, J.M.; Binnington, B.; Lindemann, D.; Lingwood, C.A.; Shevchenko, A.; Schroeder, C.; et al. Quantitative analysis of the lipidomes of the influenza virus envelope and MDCK cell apical membrane. J. Cell Biol. 2012, 196, 213–221. [Google Scholar] [CrossRef]
- Simons, K.; Gerl, M.J. Revitalizing membrane rafts: New tools and insights. Nat. Rev. Mol. Cell Biol. 2010, 11, 688–699. [Google Scholar] [CrossRef]
- Glende, J.; Schwegmann-Wessels, C.; Al-Falah, M.; Pfefferle, S.; Qu, X.; Deng, H.; Drosten, C.; Naim, H.Y.; Herrler, G. Importance of cholesterol-rich membrane microdomains in the interaction of the S protein of SARS-coronavirus with the cellular receptor angiotensin-converting enzyme 2. Virology 2008, 381, 215–221. [Google Scholar] [CrossRef]
- Lingwood, D.; Simons, K. Lipid rafts as a membrane-organizing principle. Science 2010, 327, 46–50. [Google Scholar] [CrossRef]
- Staring, J.; Raaben, M.; Brummelkamp, T.R. Viral escape from endosomes and host detection at a glance. J. Cell Sci. 2018, 131, jcs216259. [Google Scholar] [CrossRef] [PubMed]
- Meher, G.; Bhattacharjya, S.; Chakraborty, H. Membrane Cholesterol Modulates Oligomeric Status and Peptide-Membrane Interaction of Severe Acute Respiratory Syndrome Coronavirus Fusion Peptide. J. Phys. Chem. B 2019, 123, 10654–10662. [Google Scholar] [CrossRef]
- Torchilin, V.P. Micellar nanocarriers: Pharmaceutical perspectives. Pharm. Res. 2007, 24, 1–16. [Google Scholar] [CrossRef]
- Yu, X.; Trase, I.; Ren, M.; Duval, K.; Guo, X.; Chen, Z. Design of Nanoparticle-Based Carriers for Targeted Drug Delivery. J. Nanomater. 2016, 2016, 1087250. [Google Scholar] [CrossRef]
- Wan, Z.; Zheng, R.; Moharil, P.; Liu, Y.; Chen, J.; Sun, R.; Song, X.; Ao, Q. Polymeric Micelles in Cancer Immunotherapy. Molecules 2021, 26, 1220. [Google Scholar] [CrossRef]
- Abdessalem, M.A.; Adham, S.A. Research advancements in nanoparticles and cell-based drug delivery systems for the targeted killing of cancer cells. Oncol. Res. 2025, 33, 27–44. [Google Scholar] [CrossRef]
- Wong, H.L.; Chattopadhyay, N.; Wu, X.Y.; Bendayan, R. Nanotechnology applications for improved delivery of antiretroviral drugs to the brain. Adv. Drug Deliv. Rev. 2010, 62, 503–517. [Google Scholar] [CrossRef] [PubMed]
- Karahmet Sher, E.; Alebic, M.; Markovic Boras, M.; Boskailo, E.; Karahmet Farhat, E.; Karahmet, A.; Pavlovic, B.; Sher, F.; Lekic, L. Nanotechnology in medicine revolutionizing drug delivery for cancer and viral infection treatments. Int. J. Pharm. 2024, 660, 124345. [Google Scholar] [CrossRef] [PubMed]
- Shende, P.; Khair, R.; Gaud, R.S. Nanostructured cochleates: A multi-layered platform for cellular transportation of therapeutics. Drug Dev. Ind. Pharm. 2019, 45, 869–881. [Google Scholar] [CrossRef]
- Pradhan, D.; Biswasroy, P.; Goyal, A.; Ghosh, G.; Rath, G. Recent Advancement in Nanotechnology-Based Drug Delivery System Against Viral Infections. AAPS PharmSciTech 2021, 22, 47. [Google Scholar] [CrossRef]
- Chakravarty, M.; Vora, A. Nanotechnology-based antiviral therapeutics. Drug Deliv. Transl. Res. 2021, 11, 748–787. [Google Scholar] [CrossRef] [PubMed]
- Jindal, N.; Mehta, S.K. Nevirapine loaded Poloxamer 407/Pluronic P123 mixed micelles: Optimization of formulation and in vitro evaluation. Colloids Surf. B Biointerfaces 2015, 129, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Wahid, B.; Rani, N.; Idrees, M. Cytokine storm syndrome in SARS-CoV-2: A review. Z. Naturforschung C J. Biosci. 2022, 77, 65–69. [Google Scholar] [CrossRef]
- Sanduzzi Zamparelli, S.; Sanduzzi Zamparelli, A.; Bocchino, M. Immune-Boosting and Antiviral Effects of Antioxidants in COVID-19 Pneumonia: A Therapeutic Perspective. Life 2025, 15, 113. [Google Scholar] [CrossRef]
- Li, L.; Li, J.; Gao, M.; Fan, H.; Wang, Y.; Xu, X.; Chen, C.; Liu, J.; Kim, J.; Aliyari, R.; et al. Interleukin-8 as a Biomarker for Disease Prognosis of Coronavirus Disease-2019 Patients. Front. Immunol. 2020, 11, 602395. [Google Scholar] [CrossRef]
- Gubernatorova, E.O.; Gorshkova, E.A.; Polinova, A.I.; Drutskaya, M.S. IL-6: Relevance for immunopathology of SARS-CoV-2. Cytokine Growth Factor. Rev. 2020, 53, 13–24. [Google Scholar] [CrossRef]
- Anton, D.B.; de Lima, J.C.; Dahmer, B.R.; Camini, A.M.; Goettert, M.I.; Timmers, L. Taming the storm: Potential anti-inflammatory compounds targeting SARS-CoV-2 MPro. Inflammopharmacology 2024, 32, 3007–3035. [Google Scholar] [CrossRef]
- Sampath, A.; Banerjee, A.; Atal, S.; Jhaj, R. Use of baricitinib in treatment of COVID-19: A systematic review. Med. Res. Rev. 2023, 43, 1322–1345. [Google Scholar] [CrossRef]
- Abassi, Z.; Higazi, A.A.R.; Kinaneh, S.; Armaly, Z.; Skorecki, K.; Heyman, S.N. ACE2, COVID-19 Infection, Inflammation, and Coagulopathy: Missing Pieces in the Puzzle. Front. Physiol. 2020, 11, 574753. [Google Scholar] [CrossRef]
- Raiteri, A.; Piscaglia, F.; Granito, A.; Tovoli, F. Tocilizumab: From Rheumatic Diseases to COVID-19. Curr. Pharm. Des. 2021, 27, 1597–1607. [Google Scholar] [CrossRef]
- Alosaimi, B.; Mubarak, A.; Hamed, M.E.; Almutairi, A.Z.; Alrashed, A.A.; AlJuryyan, A.; Enani, M.; Alenzi, F.Q.; Alturaiki, W. Complement Anaphylatoxins and Inflammatory Cytokines as Prognostic Markers for COVID-19 Severity and In-Hospital Mortality. Front. Immunol. 2021, 12, 668725. [Google Scholar] [CrossRef]
- Zarrintaj, P.; Ramsey, J.D.; Samadi, A.; Atoufi, Z.; Yazdi, M.K.; Ganjali, M.R.; Amirabad, L.M.; Zangene, E.; Farokhi, M.; Formela, K.; et al. Poloxamer: A versatile tri-block copolymer for biomedical applications. Acta Biomater. 2020, 110, 37–67. [Google Scholar] [CrossRef] [PubMed]
- Van Zee, N.J.; Peroutka, A.S.; Hillmyer, M.A.; Lodge, T.P. Effect of Poloxamer Binding on the Elasticity and Toughness of Model Lipid Bilayers. Langmuir 2023, 39, 7258–7267. [Google Scholar] [CrossRef]
- Fouad, S.A.; Malaak, F.A.; Teaima, M.H.; Omar, S.; Kutkat, O.; Elhabal, S.F.; El-Nabarawi, M. Novel inhalable nano-based/microparticles for enhanced sustained pulmonary delivery of remdesivir—A patient malleable treatment for coronaviruses infection: In vitro aerosolization, cytotoxicity assays and antiviral activity studies. J. Drug Deliv. Sci. Technol. 2024, 101, 106196. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ricci-Junior, E.; Rosa, A.S.; do Nascimento, T.; Santos-Oliveira, R.; da Silva, M.A.N.; Barreto-Vieira, D.F.; Batista, L.T.; da Conceição, G.B.; Quintão, T.A.N.; Ferreira, V.N.S.; et al. Nanotechnology-Driven Strategy Against SARS-CoV-2: Pluronic F127-Based Nanomicelles with or Without Atazanavir Reduce Viral Replication in Calu-3 Cells. Viruses 2025, 17, 518. https://doi.org/10.3390/v17040518
Ricci-Junior E, Rosa AS, do Nascimento T, Santos-Oliveira R, da Silva MAN, Barreto-Vieira DF, Batista LT, da Conceição GB, Quintão TAN, Ferreira VNS, et al. Nanotechnology-Driven Strategy Against SARS-CoV-2: Pluronic F127-Based Nanomicelles with or Without Atazanavir Reduce Viral Replication in Calu-3 Cells. Viruses. 2025; 17(4):518. https://doi.org/10.3390/v17040518
Chicago/Turabian StyleRicci-Junior, Eduardo, Alice Santos Rosa, Tatielle do Nascimento, Ralph Santos-Oliveira, Marcos Alexandre Nunes da Silva, Debora Ferreira Barreto-Vieira, Luísa Tozatto Batista, Giovanna Barbosa da Conceição, Tayane Alvites Nunes Quintão, Vivian Neuza Santos Ferreira, and et al. 2025. "Nanotechnology-Driven Strategy Against SARS-CoV-2: Pluronic F127-Based Nanomicelles with or Without Atazanavir Reduce Viral Replication in Calu-3 Cells" Viruses 17, no. 4: 518. https://doi.org/10.3390/v17040518
APA StyleRicci-Junior, E., Rosa, A. S., do Nascimento, T., Santos-Oliveira, R., da Silva, M. A. N., Barreto-Vieira, D. F., Batista, L. T., da Conceição, G. B., Quintão, T. A. N., Ferreira, V. N. S., & Miranda, M. D. (2025). Nanotechnology-Driven Strategy Against SARS-CoV-2: Pluronic F127-Based Nanomicelles with or Without Atazanavir Reduce Viral Replication in Calu-3 Cells. Viruses, 17(4), 518. https://doi.org/10.3390/v17040518







