Laboratory Characterization of Co-Infections in Individuals Infected with HHV-8
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Collection
2.2. Statistical Analysis
3. Results
3.1. Demographics and Clinical Presentation
3.2. Diagnostic Testing Utilized for Patients with HHV-8 Infection
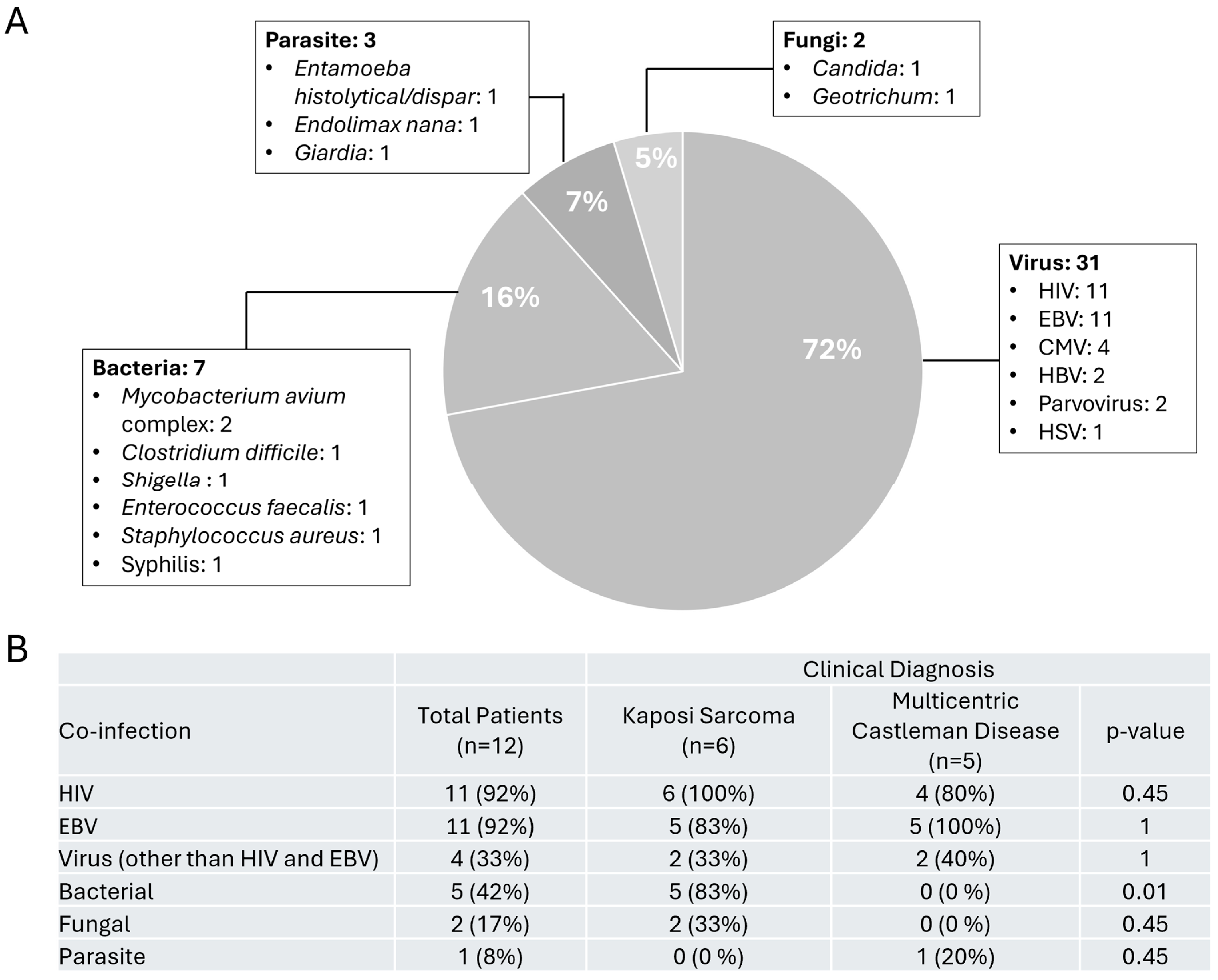
3.3. Co-Infections Detected in Our Patient with HHV-8 Infection
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Whitby, D.; Howard, M.R.; Tenant-Flowers, M.; Brink, N.S.; Copas, A.; Boshoff, C.; Hatzioannou, T.; Suggett, F.E.; Aldam, D.M.; Denton, A.S.; et al. Detection of Kaposi sarcoma associated herpesvirus in peripheral blood of HIV-infected individuals and progression to Kaposi’s sarcoma. Lancet 1995, 346, 799–802. [Google Scholar] [CrossRef] [PubMed]
- Ganem, D. KSHV infection and the pathogenesis of Kaposi’s sarcoma. Annu. Rev. Pathol. 2006, 1, 273–296. [Google Scholar] [CrossRef] [PubMed]
- Cesarman, E.; Chang, Y.; Moore, P.S.; Said, J.W.; Knowles, D.M. Kaposi’s sarcoma-associated herpesvirus-like DNA sequences in AIDS-related body-cavity-based lymphomas. N. Engl. J. Med. 1995, 332, 1186–1191. [Google Scholar] [CrossRef] [PubMed]
- Soulier, J.; Grollet, L.; Oksenhendler, E.; Cacoub, P.; Cazals-Hatem, D.; Babinet, P.; d’Agay, M.F.; Clauvel, J.P.; Raphael, M.; Degos, L.; et al. Kaposi’s sarcoma-associated herpesvirus-like DNA sequences in multicentric Castleman’s disease. Blood 1995, 86, 1276–1280. [Google Scholar]
- Engels, E.A.; Atkinson, J.O.; Graubard, B.I.; McQuillan, G.M.; Gamache, C.; Mbisa, G.; Cohn, S.; Whitby, D.; Goedert, J.J. Risk factors for human herpesvirus 8 infection among adults in the United States and evidence for sexual transmission. J. Infect. Dis. 2007, 196, 199–207. [Google Scholar] [CrossRef]
- Rohner, E.; Wyss, N.; Heg, Z.; Faralli, Z.; Mbulaiteye, S.M.; Novak, U.; Zwahlen, M.; Egger, M.; Bohlius, J. HIV and human herpesvirus 8 co-infection across the globe: Systematic review and meta-analysis. Int. J. Cancer 2016, 138, 45–54. [Google Scholar] [CrossRef]
- Bhutani, M.; Polizzotto, M.N.; Uldrick, T.S.; Yarchoan, R. Kaposi sarcoma-associated herpesvirus-associated malignancies: Epidemiology, pathogenesis, and advances in treatment. Semin. Oncol. 2015, 42, 223–246. [Google Scholar] [CrossRef]
- Ground, M.; Veenendaal, T.; Chiluzi, D.R.; Nkhonjera, G.; Glas, A.C.; Glas-van Dijk, L. HHV8-Associated Multicentric Castleman Disease: A Case Report on a Rare Complication of HIV in a Low-Income Setting. Res. Rep. Trop. Med. 2024, 15, 91–97. [Google Scholar] [CrossRef]
- Dierickx, D.; Keane, C.; Natkunam, Y. Genetic and immunological features of immune deficiency and dysregulation-associated lymphoproliferations and lymphomas as a basis for classification. Histopathology 2025, 86, 106–118. [Google Scholar] [CrossRef]
- Trivedi, P.; Takazawa, K.; Zompetta, C.; Cuomo, L.; Anastasiadou, E.; Carbone, A.; Uccini, S.; Belardelli, F.; Takada, K.; Frati, L.; et al. Infection of HHV-8+ primary effusion lymphoma cells with a recombinant Epstein-Barr virus leads to restricted EBV latency, altered phenotype, and increased tumorigenicity without affecting TCL1 expression. Blood 2004, 103, 313–316. [Google Scholar] [CrossRef][Green Version]
- Wells, R.; Stensland, L.; Vieira, J. The human cytomegalovirus UL112-113 locus can activate the full Kaposi’s sarcoma-associated herpesvirus lytic replication cycle. J. Virol. 2009, 83, 4695–4699. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Thakker, S.; Verma, S.C. Co-infections and Pathogenesis of KSHV-Associated Malignancies. Front. Microbiol. 2016, 7, 151. [Google Scholar] [CrossRef]
- Dai, L.; DeFee, M.R.; Cao, Y.; Wen, J.; Wen, X.; Noverr, M.C.; Qin, Z. Lipoteichoic acid (LTA) and lipopolysaccharides (LPS) from periodontal pathogenic bacteria facilitate oncogenic herpesvirus infection within primary oral cells. PLoS ONE 2014, 9, e101326. [Google Scholar] [CrossRef]
- Williams, S.C.; Sweeney, J.; Parameswaran, L. Diagnostic and management considerations in the modern patient with AIDS: A case of concurrent disseminated Kaposi sarcoma and colesional Cryptococcus neoformans. BMJ Case Rep. 2020, 13, e233860. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, M.C. Malaria may influence the transmission of Kaposi sarcoma associated herpesvirus in endemic areas. J. Acquir. Immune Defic. Syndr. 2014, 67, e41–e43. [Google Scholar] [CrossRef]
- Coluzzi, M.; Manno, D.; Guzzinati, S.; Tognazzo, S.; Zambon, P.; Arcà, B.; Costantini, C.; Ascoli, V. The bloodsucking arthropod bite as possible cofactor in the transmission of human herpesvirus-8 infection and in the expression of Kaposi’s sarcoma disease. Parassitologia 2002, 44, 123–129. [Google Scholar]
- Coluzzi, M.; Calabrò, M.L.; Manno, D.; Chieco-Bianchi, L.; Schulz, T.F.; Ascoli, V. HHV-8 transmission via saliva to soothe blood-sucking arthropod bites. Br. J. Cancer 2004, 91, 998–999. [Google Scholar] [CrossRef]
- Oksenhendler, E.; Carcelain, G.; Aoki, Y.; Boulanger, E.; Maillard, A.; Clauvel, J.P.; Agbalika, F. High levels of human herpesvirus 8 viral load, human interleukin-6, interleukin-10, and C reactive protein correlate with exacerbation of multicentric castleman disease in HIV-infected patients. Blood 2000, 96, 2069–2073. [Google Scholar]
- Begré, L.; Rohner, E.; Mbulaiteye, S.M.; Egger, M.; Bohlius, J. Is human herpesvirus 8 infection more common in men than in women? Systematic review and meta-analysis. Int. J. Cancer 2016, 139, 776–783. [Google Scholar] [CrossRef]
- Wang, Y.; Dong, C.; Han, Y.; Gu, Z.; Sun, C. Immunosenescence, aging and successful aging. Front. Immunol. 2022, 13, 942796. [Google Scholar] [CrossRef]
- Rossi, G.; Cozzi, I.; Della Starza, I.; De Novi, L.A.; De Propris, M.S.; Gaeta, A.; Petrucci, L.; Pulsoni, A.; Pulvirenti, F.; Ascoli, V. Human herpesvirus-8-positive primary effusion lymphoma in HIV-negative patients: Single institution case series with a multidisciplinary characterization. Cancer Cytopathol. 2021, 129, 62–74. [Google Scholar] [CrossRef]
- Ross, S.A.; Novak, Z.; Pati, S.; Boppana, S.B. Overview of the diagnosis of cytomegalovirus infection. Infect. Disord. Drug Targets 2011, 11, 466–474. [Google Scholar] [CrossRef] [PubMed]
- Nowalk, A.; Green, M. Epstein-Barr Virus. Microbiol. Spectr. 2016, 4, 10-1128. [Google Scholar] [CrossRef]
- Halstead, D.C.; Sautter, R.L. A Literature Review on How We Can Address Medical Laboratory Scientist Staffing Shortages. Lab. Med. 2023, 54, e31–e36. [Google Scholar] [CrossRef] [PubMed]
- Lewinski, M.A.; Alby, K.; Babady, N.E.; Butler-Wu, S.M.; Bard, J.D.; Greninger, A.L.; Hanson, K.; Naccache, S.N.; Newton, D.; Temple-Smolkin, R.L.; et al. Exploring the Utility of Multiplex Infectious Disease Panel Testing for Diagnosis of Infection in Different Body Sites: A Joint Report of the Association for Molecular Pathology, American Society for Microbiology, Infectious Diseases Society of America, and Pan American Society for Clinical Virology. J. Mol. Diagn. 2023, 25, 857–875. [Google Scholar] [CrossRef]

| Characteristic | No. (%) |
|---|---|
| Male | 11 (92%) |
| Age (average) | 56 years |
| Clinical Symptoms | |
| Fever | 9 (75%) |
| Night Sweats | 7 (58%) |
| Shortness of breath | 7 (58%) |
| Fatigue | 7 (58%) |
| Weight Loss | 5 (42%) |
| Headache | 3 (25%) |
| Emesis/Nausea | 3 (25%) |
| Anemia | 3 (25%) |
| Cough | 1 (8%) |
| Radiology | |
| Lymphadenopathy | 10 (83%) |
| Splenomegaly | 4 (33%) |
| Pulmonary Nodules or Opacities | 4 (33%) |
| Clinical Diagnosis | |
| Multicentric Castleman Disease | 5 (42%) |
| Kaposi’s Sarcoma and Multicentric Castleman Disease | 4 (33%) |
| Kaposi’s Sarcoma and Primary Effusion Lymphoma | 2 (17%) |
| Primary Effusion Lymphoma | 1 (8%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jett, A.; Tariq, Z.; Yee, R. Laboratory Characterization of Co-Infections in Individuals Infected with HHV-8. Viruses 2025, 17, 460. https://doi.org/10.3390/v17040460
Jett A, Tariq Z, Yee R. Laboratory Characterization of Co-Infections in Individuals Infected with HHV-8. Viruses. 2025; 17(4):460. https://doi.org/10.3390/v17040460
Chicago/Turabian StyleJett, Alex, Zoon Tariq, and Rebecca Yee. 2025. "Laboratory Characterization of Co-Infections in Individuals Infected with HHV-8" Viruses 17, no. 4: 460. https://doi.org/10.3390/v17040460
APA StyleJett, A., Tariq, Z., & Yee, R. (2025). Laboratory Characterization of Co-Infections in Individuals Infected with HHV-8. Viruses, 17(4), 460. https://doi.org/10.3390/v17040460





