The Virus Entry Pathway Determines Sensitivity to the Antiviral Peptide TAT-I24
Abstract
1. Introduction
2. Materials and Methods
2.1. Peptides and Compounds
2.2. Viruses
2.2.1. SARS-CoV-2
2.2.2. Baculovirus
2.3. Cell Culture
2.4. Virus Neutralization Assay and Quantification of Virus RNA in the Supernatants
2.5. Detection of Viral RNA from Cell Lysates
2.6. Immunohistochemical Staining
2.7. Immunofluorescence
2.8. Luciferase Reporter Gene Assays
2.9. RNA Binding Assay
2.10. Fluorescence Microscopy of COS-7 and Vero E6 Cells Treated with Rho-TAT-I24
2.11. Plasmid Cloning and Transfection
2.12. Statistical Analysis
2.13. Image Analysis
3. Results and Discussion
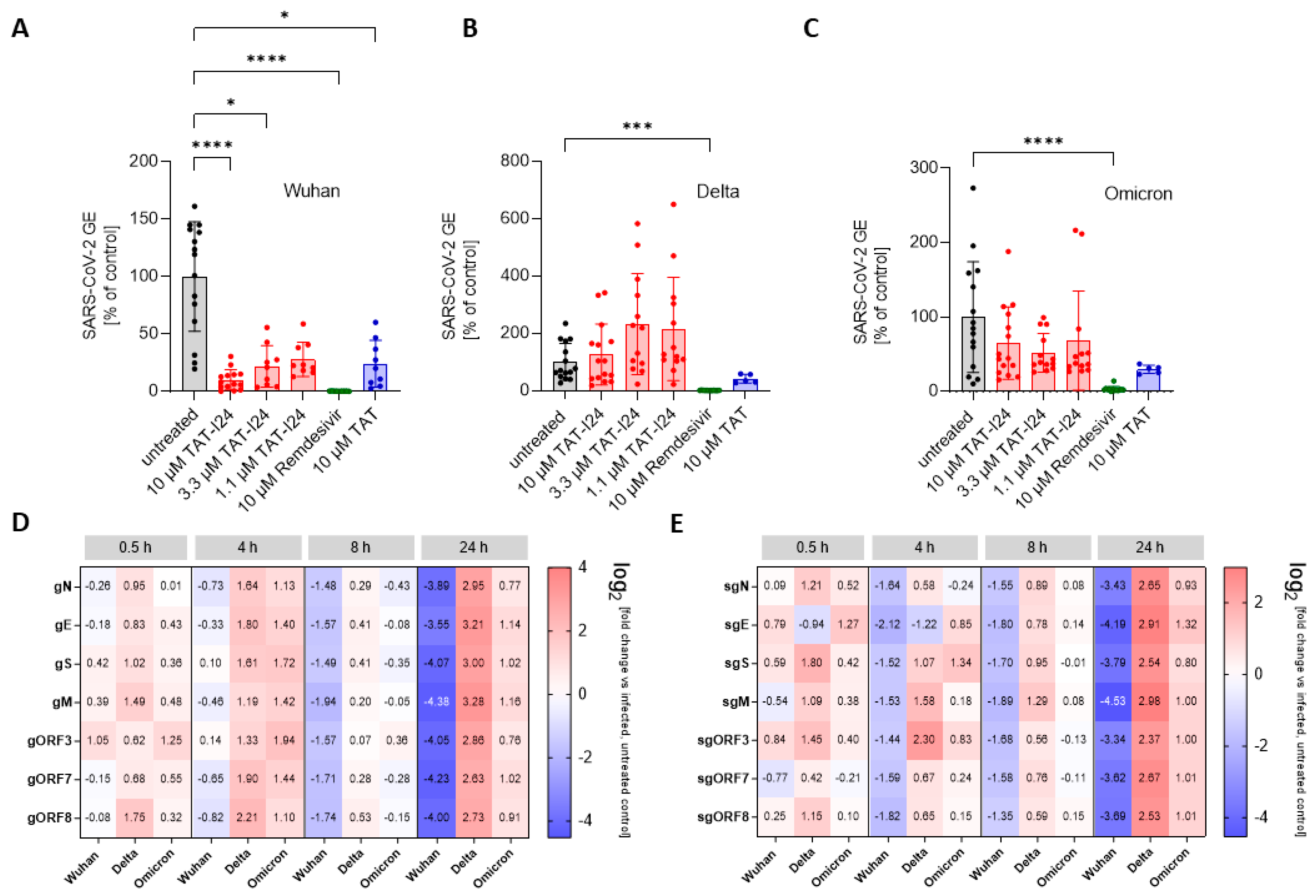
3.1. TAT-I24 Neutralizes the SARS-CoV-2 Wuhan Variant in Vero E6 Cells
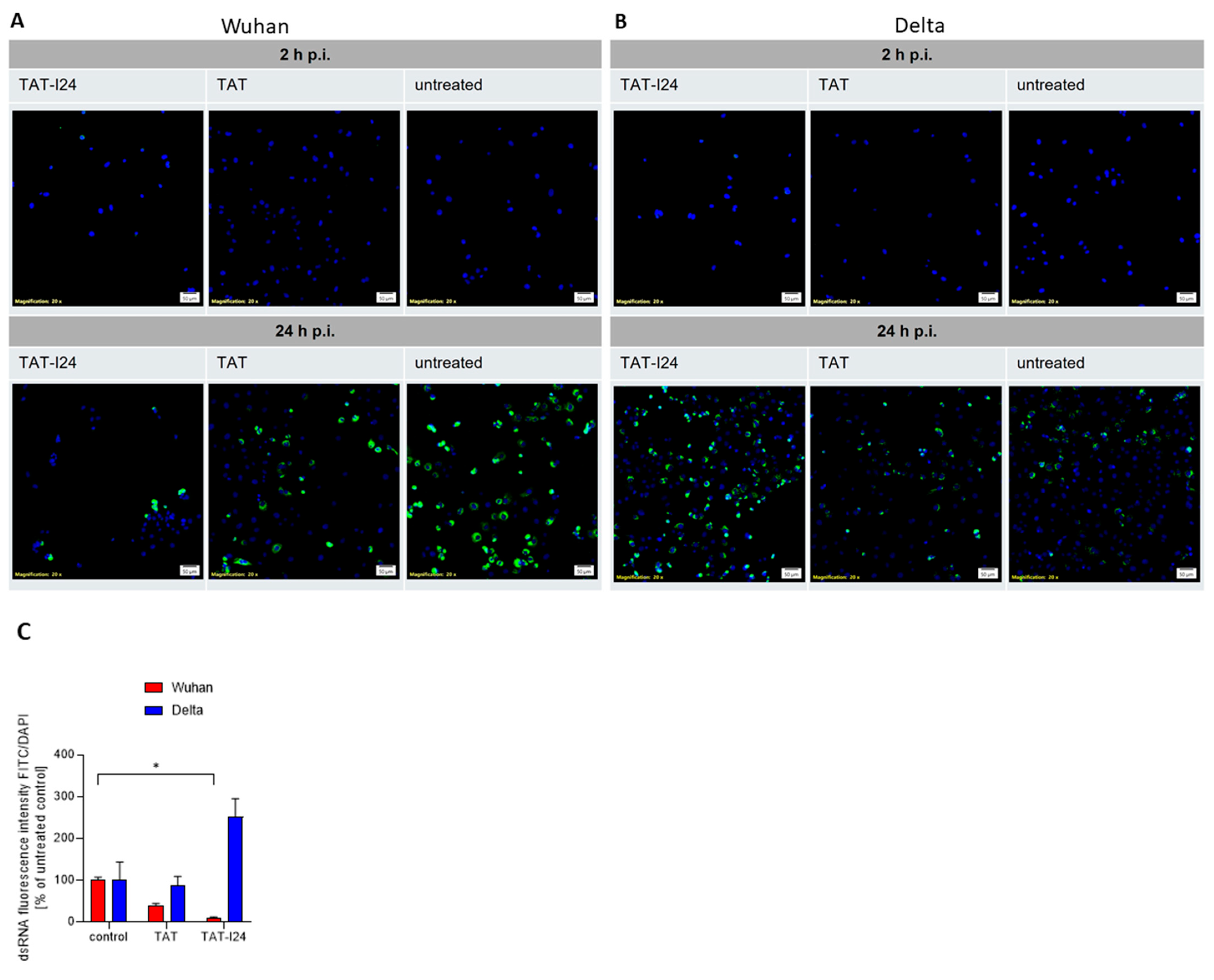
3.2. Sensitivity of SARS-CoV-2 to TAT-I24 Is Dependent on Virus Variant in Vero E6 Cells
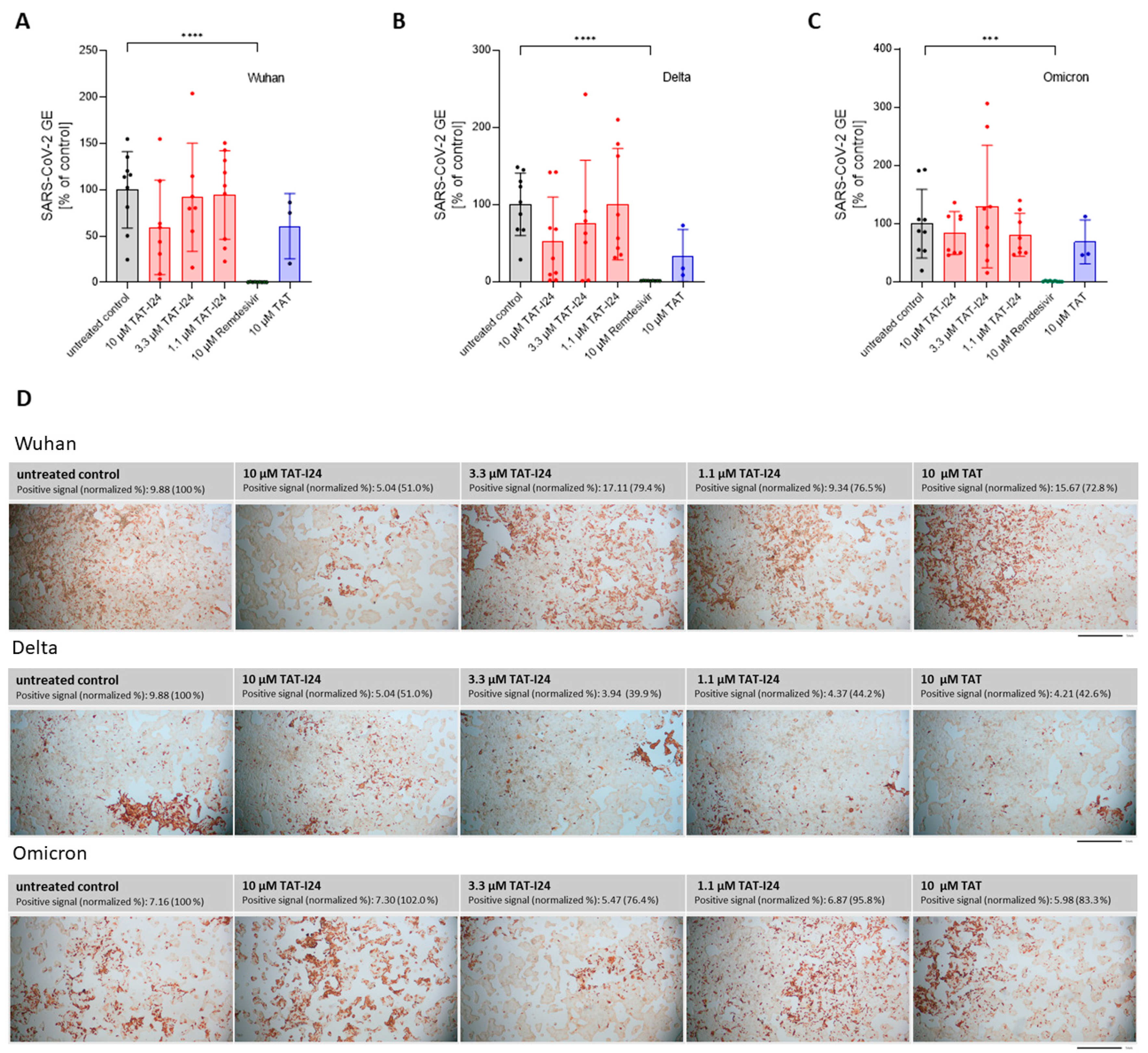
3.3. Lack of Sensitivity to TAT-I24 in SARS-CoV-2-Infected Calu-3 Cells
3.4. Differential Sensitivities of SARS-CoV-2 Wuhan and Delta Variants to CQ
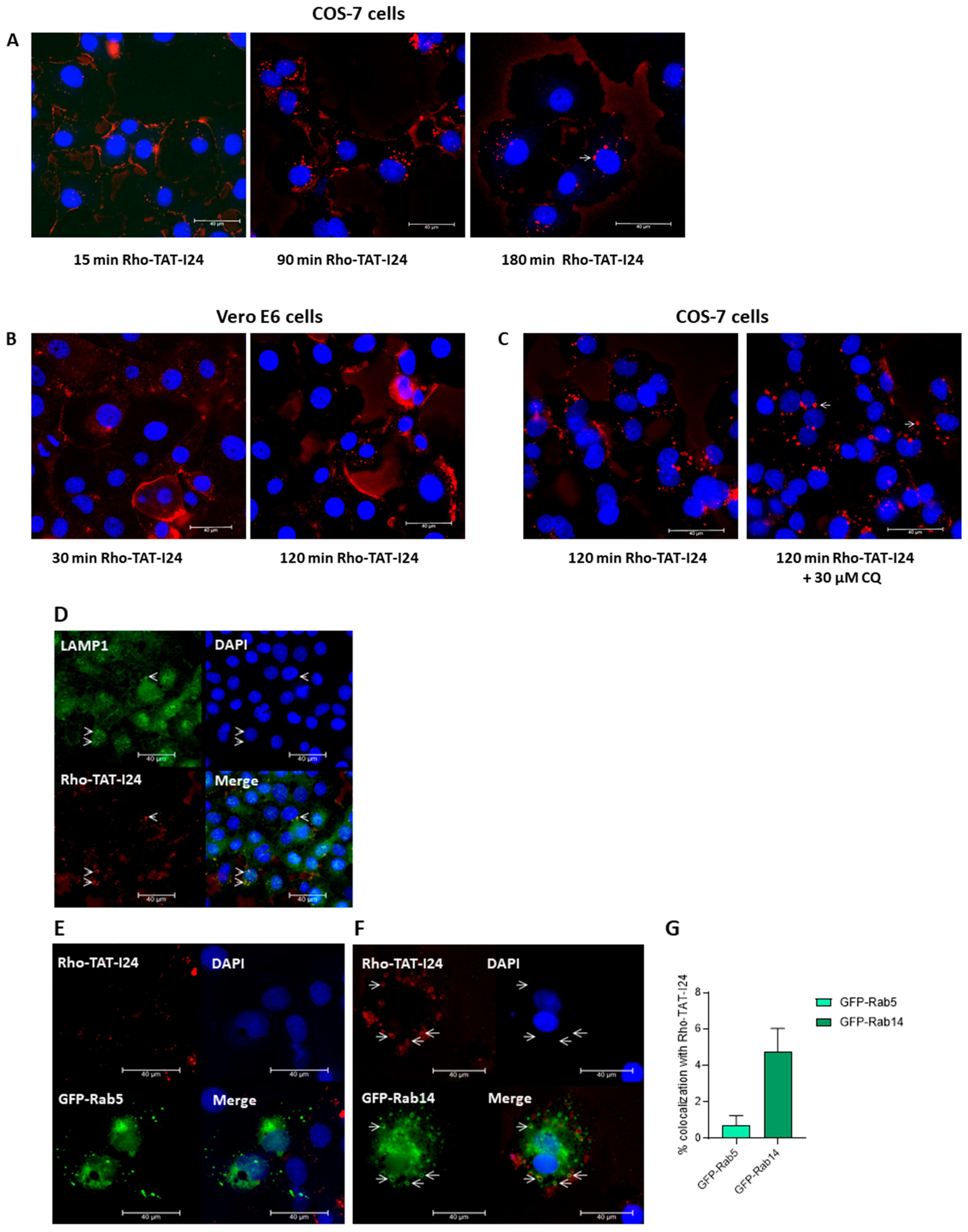
3.5. TAT-I24 Localizes to Endosomal Compartments
4. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lytras, S.; Xia, W.; Hughes, J.; Jiang, X.; Robertson, D.L. The Animal Origin of SARS-CoV-2. Science 2021, 373, 968–970. [Google Scholar] [PubMed]
- Hao, Y.J.; Wang, Y.L.; Wang, M.Y.; Zhou, L.; Shi, J.Y.; Cao, J.M.; Wang, D.P. The Origins of COVID-19 Pandemic: A Brief Overview. Transbound. Emerg. Dis. 2022, 69, 3181–3197. [Google Scholar] [CrossRef] [PubMed]
- Hillary, V.E.; Ceasar, S.A. An Update on COVID-19: SARS-CoV-2 Variants, Antiviral Drugs, and Vaccines. Heliyon 2023, 9, e13952. [Google Scholar] [CrossRef] [PubMed]
- Markov, P.V.; Ghafari, M.; Beer, M.; Lythgoe, K.; Simmonds, P.; Stilianakis, N.I.; Katzourakis, A. The Evolution of SARS-CoV-2. Nat. Rev. Microbiol. 2023, 21, 361–379. [Google Scholar] [CrossRef]
- Carabelli, A.M.; Peacock, T.P.; Thorne, L.G.; Harvey, W.T.; Hughes, J.; de Silva, T.I.; Peacock, S.J.; Barclay, W.S.; de Silva, T.I.; Towers, G.J.; et al. SARS-CoV-2 Variant Biology: Immune Escape, Transmission and Fitness. Nat. Rev. Microbiol. 2023, 21, 162–177. [Google Scholar] [CrossRef]
- Pozzi, C.; Vanet, A.; Francesconi, V.; Tagliazucchi, L.; Tassone, G.; Venturelli, A.; Spyrakis, F.; Mazzorana, M.; Costi, M.P.; Tonelli, M. Antitarget, Anti-SARS-CoV-2 Leads, Drugs, and the Drug Discovery-Genetics Alliance Perspective. J. Med. Chem. 2023, 66, 3664–3702. [Google Scholar] [CrossRef]
- Karim, M.; Lo, C.W.; Einav, S. Preparing for the next Viral Threat with Broad-Spectrum Antivirals. J. Clin. Investig. 2023, 133, e170236. [Google Scholar] [CrossRef]
- Adamson, C.S.; Chibale, K.; Goss, R.J.M.; Jaspars, M.; Newman, D.J.; Dorrington, R.A. Antiviral Drug Discovery: Preparing for the next Pandemic. Chem. Soc. Rev. 2021, 50, 3647–3655. [Google Scholar] [CrossRef]
- Adalja, A.; Inglesby, T. Broad-Spectrum Antiviral Agents: A Crucial Pandemic Tool. Expert. Rev. Anti Infect. Ther. 2019, 17, 467–470. [Google Scholar] [CrossRef]
- Chitalia, V.C.; Munawar, A.H. A Painful Lesson from the COVID-19 Pandemic: The Need for Broad-Spectrum, Host-Directed Antivirals. J. Transl. Med. 2020, 18, 390. [Google Scholar] [CrossRef]
- Vilas Boas, L.C.P.; Campos, M.L.; Berlanda, R.L.A.; de Carvalho Neves, N.; Franco, O.L. Antiviral Peptides as Promising Therapeutic Drugs. Cell. Mol. Life Sci. 2019, 76, 3525–3542. [Google Scholar] [CrossRef] [PubMed]
- Skalickova, S.; Heger, Z.; Krejcova, L.; Pekarik, V.; Bastl, K.; Janda, J.; Kostolansky, F.; Vareckova, E.; Zitka, O.; Adam, V.; et al. Perspective of Use of Antiviral Peptides against Influenza Virus. Viruses 2015, 7, 5428–5442. [Google Scholar] [CrossRef] [PubMed]
- Matthews, T.; Salgo, M.; Greenberg, M.; Chung, J.; DeMasi, R.; Bolognesi, D. Enfuvirtide: The First Therapy to Inhibit the Entry of HIV-1 into Host CD4 Lymphocytes. Nat. Rev. Drug Discov. 2004, 3, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Blank, A.; Markert, C.; Hohmann, N.; Carls, A.; Mikus, G.; Lehr, T.; Alexandrov, A.; Haag, M.; Schwab, M.; Urban, S.; et al. First-in-Human Application of the Novel Hepatitis B and Hepatitis D Virus Entry Inhibitor Myrcludex B. J. Hepatol. 2016, 65, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Xia, S.; Lan, Q.; Pu, J.; Wang, C.; Liu, Z.; Xu, W.; Wang, Q.; Liu, H.; Jiang, S.; Lu, L. Potent MERS-CoV Fusion Inhibitory Peptides Identified from HR2 Domain in Spike Protein of Bat Coronavirus HKU4. Viruses 2019, 11, 56. [Google Scholar] [CrossRef]
- Xia, S.; Zhu, Y.; Liu, M.; Lan, Q.; Xu, W.; Wu, Y.; Ying, T.; Liu, S.; Shi, Z.; Jiang, S.; et al. Fusion Mechanism of 2019-NCoV and Fusion Inhibitors Targeting HR1 Domain in Spike Protein. Cell. Mol. Immunol. 2020, 17, 765–767. [Google Scholar] [CrossRef]
- Kadam, R.U.; Juraszek, J.; Brandenburg, B.; Buyck, C.; Schepens, W.B.G.G.; Kesteleyn, B.; Stoops, B.; Vreeken, R.J.; Vermond, J.; Goutier, W.; et al. Potent Peptidic Fusion Inhibitors of Influenza Virus. Science 2017, 358, 496–502. [Google Scholar] [CrossRef]
- Hoffmann, A.R.; Guha, S.; Wu, E.; Ghimire, J.; Wang, Y.; He, J.; Garry, R.F.; Wimley, W.C. Broad-Spectrum Antiviral Entry Inhibition by Interfacially Active Peptides. J. Virol. 2020, 94, 1–20. [Google Scholar] [CrossRef]
- Ahmed, A.; Siman-Tov, G.; Keck, F.; Kortchak, S.; Bakovic, A.; Risner, K.; Lu, T.K.; Bhalla, N.; de la Fuente-Nunez, C.; Narayanan, A. Human Cathelicidin Peptide LL-37 as a Therapeutic Antiviral Targeting Venezuelan Equine Encephalitis Virus Infections. Antivir. Res. 2019, 164, 61–69. [Google Scholar] [CrossRef]
- Ridyard, K.E.; Overhage, J. The Potential of Human Peptide Ll-37 as an Antimicrobial and Anti-Biofilm Agent. Antibiotics 2021, 10, 650. [Google Scholar] [CrossRef]
- Zhao, H.; To, K.K.W.; Sze, K.-H.; Yung, T.T.-M.; Bian, M.; Lam, H.; Yeung, M.L.; Li, C.; Chu, H.; Yuen, K.-Y. A Broad-Spectrum Virus- and Host-Targeting Peptide against Respiratory Viruses Including Influenza Virus and SARS-CoV-2. Nat. Commun. 2020, 11, 4252. [Google Scholar] [CrossRef] [PubMed]
- Vivès, E.; Brodin, P.; Lebleu, B. A Truncated HIV-1 Tat Protein Basic Domain Rapidly Translocates through the Plasma Membrane and Accumulates in the Cell Nucleus. J. Biol. Chem. 1997, 272, 16010–16017. [Google Scholar] [CrossRef] [PubMed]
- Green, M.; Loewenstein, P.M. Autonomous Functional Domains of Chemically Synthesized Human Immunodeficiency Virus Tat Trans-Activator Protein. Cell 1988, 55, 1179–1188. [Google Scholar] [CrossRef] [PubMed]
- Frankel, A.D.; Pabo, C.O. Cellular Uptake of the Tat Protein from Human Immunodeficiency Virus. Cell 1988, 55, 1189–1193. [Google Scholar] [CrossRef] [PubMed]
- Ruzsics, Z.; Hoffmann, K.; Riedl, A.; Krawczyk, A.; Widera, M.; Sertznig, H.; Schipper, L.; Kapper-Falcone, V.; Debreczeny, M.; Ernst, W.; et al. A Novel, Broad-Acting Peptide Inhibitor of Double-Stranded DNA Virus Gene Expression and Replication. Front. Microbiol. 2020, 11, 2934. [Google Scholar] [CrossRef]
- Harant, H.; Höfinger, S.; Kricek, F.; Ruf, C.; Ruzsics, Z.; Hengel, H.; Lindley, I.J.D. The Peptide TAT-I24 with Antiviral Activity against DNA Viruses Binds Double-Stranded DNA with High Affinity. Biologics 2021, 1, 41–60. [Google Scholar] [CrossRef]
- Ziu, T.; Sambur, E.; Ruzsics, Z.; Hengel, H.; Grabherr, R.; Höfinger, S.; Harant, H. In Vitro Profiling of the Antiviral Peptide TAT-I24. Int. J. Mol. Sci. 2024, 25, 10463. [Google Scholar] [CrossRef]
- Hardt, M.; Föderl-Höbenreich, E.; Freydl, S.; Kouros, A.; Loibner, M.; Zatloukal, K. Pre-Analytical Sample Stabilization by Different Sampling Devices for PCR-Based COVID-19 Diagnostics. N. Biotechnol. 2022, 70, 19–27. [Google Scholar] [CrossRef]
- Gisaid. Available online: https://gisaid.org/ (accessed on 6 October 2024).
- Covid-19. Available online: https://www.cdc.gov/covid/?CDC_AAref_Val=https://www.cdc.gov/coronavirus/2019-ncov/lab/rt-pcr-panel-primer-probes.html (accessed on 6 October 2024).
- Telwatte, S.; Martin, H.A.; Marczak, R.; Fozouni, P.; Vallejo-Gracia, A.; Kumar, G.R.; Murray, V.; Lee, S.; Ott, M.; Wong, J.K.; et al. Novel RT-DdPCR Assays for Measuring the Levels of Subgenomic and Genomic SARS-CoV-2 Transcripts. Methods 2022, 201, 15–25. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Kicker, E.; Tittel, G.; Schaller, T.; Pferschy-Wenzig, E.M.; Zatloukal, K.; Bauer, R. SARS-CoV-2 Neutralizing Activity of Polyphenols in a Special Green Tea Extract Preparation. Phytomedicine 2022, 98, 153970. [Google Scholar] [CrossRef] [PubMed]
- Ignatovich, I.A.; Dizhe, E.B.; Pavlotskaya, A.V.; Akifiev, B.N.; Burov, S.V.; Orlov, S.V.; Perevozchikov, A.P. Complexes of Plasmid DNA with Basic Domain 47-57 of the HIV-1 Tat Protein Are Transferred to Mammalian Cells by Endocytosis-Mediated Pathways. J. Biol. Chem. 2003, 278, 42625–42636. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, A.; Seelig, J. High Affinity of the Cell-Penetrating Peptide HIV-1 Tat-PTD for DNA. Biochemistry 2007, 46, 8138–8145. [Google Scholar] [CrossRef] [PubMed]
- Pham, V.V.; Salguero, C.; Khan, S.N.; Meagher, J.L.; Brown, W.C.; Humbert, N.; de Rocquigny, H.; Smith, J.L.; D’Souza, V.M. HIV-1 Tat Interactions with Cellular 7SK and Viral TAR RNAs Identifies Dual Structural Mimicry. Nat. Commun. 2018, 9, 4266. [Google Scholar] [CrossRef] [PubMed]
- Rajpal, V.R.; Sharma, S.; Sehgal, D.; Singh, A.; Kumar, A.; Vaishnavi, S.; Tiwari, M.; Bhalla, H.; Goel, S.; Raina, S.N. A Comprehensive Account of SARS-CoV-2 Genome Structure, Incurred Mutations, Lineages and COVID-19 Vaccination Program. Future Virol. 2022, 17, 687–706. [Google Scholar] [CrossRef]
- Wang, M.; Cao, R.; Zhang, L.; Yang, X.; Liu, J.; Xu, M.; Shi, Z.; Hu, Z.; Zhong, W.; Xiao, G. Remdesivir and Chloroquine Effectively Inhibit the Recently Emerged Novel Coronavirus (2019-NCoV) in Vitro. Cell Res. 2020, 30, 269–271. [Google Scholar] [CrossRef]
- Li, K.; Melnychuk, S.; Sandstrom, P.; Ji, H. Tracking the Evolution of the SARS-CoV-2 Delta Variant of Concern: Analysis of Genetic Diversity and Selection across the Whole Viral Genome. Front. Microbiol. 2023, 14, 1222301. [Google Scholar] [CrossRef]
- Chakraborty, C.; Bhattacharya, M.; Sharma, A.R.; Mallik, B. Omicron (B.1.1.529)—A New Heavily Mutated Variant: Mapped Location and Probable Properties of Its Mutations with an Emphasis on S-Glycoprotein. Int. J. Biol. Macromol. 2022, 219, 980–997. [Google Scholar] [CrossRef]
- Chatterjee, S.; Bhattacharya, M.; Nag, S.; Dhama, K.; Chakraborty, C. A Detailed Overview of SARS-CoV-2 Omicron: Its Sub-Variants, Mutations and Pathophysiology, Clinical Characteristics, Immunological Landscape, Immune Escape, and Therapies. Viruses 2023, 15, 167. [Google Scholar] [CrossRef]
- Bultmann, H.; Teuton, J.; Brandt, C.R. Addition of a C-Terminal Cysteine Improves the Anti-Herpes Simplex Virus Activity of a Peptide Containing the Human Immunodeficiency Virus Type 1 TAT Protein Transduction Domain. Antimicrob. Agents Chemother. 2007, 51, 1596–1607. [Google Scholar] [CrossRef]
- Bultmann, H.; Brandt, C.R. Peptides Containing Membrane-Transiting Motifs Inhibit Virus Entry. J. Biol. Chem. 2002, 277, 36018–36023. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Tsutsumi, H.; Furuta, T.; Sakurai, M.; Mihara, H. Interaction of Amphiphilic α-Helical Cell-Penetrating Peptides with Heparan Sulfate. Org. Biomol. Chem. 2014, 12, 4673–4681. [Google Scholar] [CrossRef] [PubMed]
- Richard, J.P.; Melikov, K.; Brooks, H.; Prevot, P.; Lebleu, B.; Chernomordik, L.V. Cellular Uptake of Unconjugated TAT Peptide Involves Clathrin-Dependent Endocytosis and Heparan Sulfate Receptors. J. Biol. Chem. 2005, 280, 15300–15306. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, A.; Seelig, J. Interaction of the Protein Transduction Domain of HIV-1 TAT with Heparan Sulfate: Binding Mechanism and Thermodynamic Parameters. Biophys. J. 2004, 86, 254–263. [Google Scholar] [CrossRef]
- Christianson, H.C.; Belting, M. Heparan Sulfate Proteoglycan as a Cell-Surface Endocytosis Receptor. Matrix Biol. 2014, 35, 51–55. [Google Scholar] [CrossRef]
- Liu, L.; Chopra, P.; Li, X.; Bouwman, K.M.; Tompkins, S.M.; Wolfert, M.A.; De Vries, R.P.; Boons, G.J. Heparan Sulfate Proteoglycans as Attachment Factor for SARS-CoV-2. ACS Cent. Sci. 2021, 7, 1009–1018. [Google Scholar] [CrossRef]
- Kearns, F.L.; Sandoval, D.R.; Casalino, L.; Clausen, T.M.; Rosenfeld, M.A.; Spliid, C.B.; Amaro, R.E.; Esko, J.D. Spike-Heparan Sulfate Interactions in SARS-CoV-2 Infection. Curr. Opin. Struct. Biol. 2022, 76, 102439. [Google Scholar]
- Clausen, T.M.; Sandoval, D.R.; Spliid, C.B.; Pihl, J.; Perrett, H.R.; Painter, C.D.; Narayanan, A.; Majowicz, S.A.; Kwong, E.M.; McVicar, R.N.; et al. SARS-CoV-2 Infection Depends on Cellular Heparan Sulfate and ACE2. Cell 2020, 183, 1043–1057.e15. [Google Scholar] [CrossRef]
- Harant, H. Selective Inhibition of Murine Cytomegalovirus Viral Gene Expression by the Antiviral Peptide TAT-I24. Int. J. Mol. Sci. 2022, 23, 7246. [Google Scholar] [CrossRef]
- Li, Y.; Renner, D.M.; Comar, C.E.; Whelan, J.N.; Reyes, H.M.; Leonardo Cardenas-Diaz, F.; Truitt, R.; Hui Tan, L.; Dong, B.; Dionysios Alysandratos, K.; et al. SARS-CoV-2 Induces Double-Stranded RNA-Mediated Innate Immune Responses in Respiratory Epithelial-Derived Cells and Cardiomyocytes. Proc. Natl. Acad. Sci. USA 2021, 118, e2022643118. [Google Scholar] [CrossRef]
- Ogando, N.S.; Dalebout, T.J.; Zevenhoven-Dobbe, J.C.; Limpens, R.W.A.L.; van der Meer, Y.; Caly, L.; Druce, J.; de Vries, J.J.C.; Kikkert, M.; Barcena, M.; et al. SARS-Coronavirus-2 Replication in Vero E6 Cells: Replication Kinetics, Rapid Adaptation and Cytopathology. J. Gen. Virol. 2020, 101, 925–940. [Google Scholar] [CrossRef] [PubMed]
- Koch, J.; Uckeley, Z.M.; Doldan, P.; Stanifer, M.; Boulant, S.; Lozach, P. TMPRSS2 Expression Dictates the Entry Route Used by SARS-CoV-2 to Infect Host Cells. EMBO J. 2021, 40, e107821. [Google Scholar] [CrossRef] [PubMed]
- Jackson, C.B.; Farzan, M.; Chen, B.; Choe, H. Mechanisms of SARS-CoV-2 Entry into Cells. Nat. Rev. Mol. Cell Biol. 2022, 23, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Pizzato, M.; Baraldi, C.; Boscato Sopetto, G.; Finozzi, D.; Gentile, C.; Gentile, M.D.; Marconi, R.; Paladino, D.; Raoss, A.; Riedmiller, I.; et al. SARS-CoV-2 and the Host Cell: A Tale of Interactions. Front. Virol. 2021, 1, 815388. [Google Scholar] [CrossRef]
- Zhang, H.; Rostami, M.R.; Leopold, P.L.; Mezey, J.G.; O’Beirne, S.L.; Strulovici-Barel, Y.; Crystal, R.G. Expression of the SARS-CoV-2 ACE2 Receptor in the Human Airway Epithelium. Am. J. Respir. Crit. Care Med. 2020, 202, 219–229. [Google Scholar] [CrossRef]
- Jia, H.P.; Look, D.C.; Shi, L.; Hickey, M.; Pewe, L.; Netland, J.; Farzan, M.; Wohlford-Lenane, C.; Perlman, S.; McCray, P.B. ACE2 Receptor Expression and Severe Acute Respiratory Syndrome Coronavirus Infection Depend on Differentiation of Human Airway Epithelia. J. Virol. 2005, 79, 14614–14621. [Google Scholar] [CrossRef]
- Vankadari, N.; Ghosal, D.; Ketavarapu, V.; Mitnala, S.; Vishnubotla, R.; Reddy, D.N. Structure of Human TMPRSS2 in Complex with SARS-CoV-2 Spike Glycoprotein and Implications for Potential Therapeutics. J. Phys. Chem. Lett. 2022, 13, 5324–5333. [Google Scholar] [CrossRef]
- Hoffmann, M.; Mösbauer, K.; Hofmann-Winkler, H.; Kaul, A.; Kleine-Weber, H.; Krüger, N.; Gassen, N.C.; Müller, M.A.; Drosten, C.; Pöhlmann, S. Chloroquine Does Not Inhibit Infection of Human Lung Cells with SARS-CoV-2. Nature 2020, 585, 588–590. [Google Scholar] [CrossRef]
- Bhattacharya, M.; Chatterjee, S.; Sharma, A.R.; Lee, S.S.; Chakraborty, C. Delta Variant (B.1.617.2) of SARS-CoV-2: Current Understanding of Infection, Transmission, Immune Escape, and Mutational Landscape. Folia Microbiol. 2023, 68, 17–28. [Google Scholar] [CrossRef]
- Ozono, S.; Zhang, Y.; Ode, H.; Sano, K.; Tan, T.S.; Imai, K.; Miyoshi, K.; Kishigami, S.; Ueno, T.; Iwatani, Y.; et al. SARS-CoV-2 D614G Spike Mutation Increases Entry Efficiency with Enhanced ACE2-Binding Affinity. Nat. Commun. 2021, 12, 848. [Google Scholar] [CrossRef]
- Johnson, B.A.; Xie, X.; Bailey, A.L.; Kalveram, B.; Lokugamage, K.G.; Muruato, A.; Zou, J.; Zhang, X.; Juelich, T.; Smith, J.K.; et al. Loss of Furin Cleavage Site Attenuates SARS-CoV-2 Pathogenesis. Nature 2021, 591, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Peacock, T.P.; Goldhill, D.H.; Zhou, J.; Baillon, L.; Frise, R.; Swann, O.C.; Kugathasan, R.; Penn, R.; Brown, J.C.; Sanchez-David, R.Y.; et al. The Furin Cleavage Site in the SARS-CoV-2 Spike Protein Is Required for Transmission in Ferrets. Nat. Microbiol. 2021, 6, 899–909. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, J.; Johnson, B.A.; Xia, H.; Ku, Z.; Schindewolf, C.; Widen, S.G.; An, Z.; Weaver, S.C.; Menachery, V.D.; et al. Delta Spike P681R Mutation Enhances SARS-CoV-2 Fitness over Alpha Variant. Cell Rep. 2022, 39, 110829. [Google Scholar] [CrossRef] [PubMed]
- Trofimenko, E.; Homma, Y.; Fukuda, M.; Widmann, C. The Endocytic Pathway Taken by Cationic Substances Requires Rab14 but Not Rab5 and Rab7. Cell Rep. 2021, 37, 109945. [Google Scholar] [CrossRef]
- Liu, J.; Cao, R.; Xu, M.; Wang, X.; Zhang, H.; Hu, H.; Li, Y.; Hu, Z.; Zhong, W.; Wang, M. Hydroxychloroquine, a Less Toxic Derivative of Chloroquine, Is Effective in Inhibiting SARS-CoV-2 Infection in Vitro. Cell Discov. 2020, 6, 16. [Google Scholar]
- Carlsson, S.R.; Roth, J.; Piller, F.; Fukuda, M. Isolation and Characterization of Human Lysosomal Membrane Glycoproteins, h-Lamp-1 and h-Lamp-2. Major Sialoglycoproteins Carrying Polylactosaminoglycan. J. Biol. Chem. 1988, 263, 18911–18919. [Google Scholar] [CrossRef]
- Junutula, J.R.; De Maziére, A.M.; Peden, A.A.; Ervin, K.E.; Advani, R.J.; Van Dijk, S.M.; Klumperman, J.; Scheller, R.H. Rab14 Is Involved in Membrane Trafficking between the Golgi Complex and Endosomes. Mol. Biol. Cell 2004, 15, 2218–2229. [Google Scholar] [CrossRef]
- Reed, S.E.; Hodgson, L.R.; Song, S.; May, M.T.; Kelly, E.E.; McCaffrey, M.W.; Mastick, C.C.; Verkade, P.; Tavare, J.M. A Role for Rab14 in the Endocytic Trafficking of GLUT4 in 3T3-L1 Adipocytes. J. Cell Sci. 2013, 126, 1931–1941. [Google Scholar] [CrossRef]
- Lu, R.; Johnson, D.L.; Stewart, L.; Waite, K.; Elliott, D.; Wilson, J.M. Rab14 Regulation of Claudin-2 Trafficking Modulates Epithelial Permeability and Lumen Morphogenesis. Mol. Biol. Cell 2014, 25, 1744–1754. [Google Scholar] [CrossRef]
- Staring, J.; Raaben, M.; Brummelkamp, T.R. Viral Escape from Endosomes and Host Detection at a Glance. J. Cell Sci. 2018, 131, jcs216259. [Google Scholar] [CrossRef]
- Fan, J.; Liu, X.; Mao, F.; Yue, X.; Lee, I.; Xu, Y. Proximity Proteomics Identifies Novel Function of Rab14 in Trafficking of Ebola Virus Matrix Protein VP40. Biochem. Biophys. Res. Commun. 2020, 527, 387–392. [Google Scholar] [CrossRef] [PubMed]
- Qi, M.; Williams, J.A.; Chu, H.; Chen, X.; Wang, J.J.; Ding, L.; Akhirome, E.; Wen, X.; Lapierre, L.A.; Goldenring, J.R.; et al. Rab11-FIP1C and Rab14 Direct Plasma Membrane Sorting and Particle Incorporation of the HIV-1 Envelope Glycoprotein Complex. PLoS Pathog. 2013, 9, e1003278. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-Y.; Bai, J.S.; Liu, C.-C.; Zhou, J.-F.; Chen, J.; Cheng, Y.; Zhou, B. The Small GTPase Rab14 Regulates the Trafficking of Ceramide from Endoplasmic Reticulum to Golgi Apparatus and Facilitates Classical Swine Fever Virus Assembly. J. Virol. 2023, 97, e0036423. [Google Scholar] [CrossRef] [PubMed]
- Tebaldi, G.; Pritchard, S.M.; Nicola, A. V Herpes Simplex Virus Entry by a Nonconventional Endocytic Pathway. J. Virol. 2020, 94, 10.1128. [Google Scholar] [CrossRef]
- Kunz, S. Receptor Binding and Cell Entry of Old World Arenaviruses Reveal Novel Aspects of Virus-Host Interaction. Virology 2009, 387, 245–249. [Google Scholar] [CrossRef]
- Quirin, K.; Eschli, B.; Scheu, I.; Poort, L.; Kartenbeck, J.; Helenius, A. Lymphocytic Choriomeningitis Virus Uses a Novel Endocytic Pathway for Infectious Entry via Late Endosomes. Virology 2008, 378, 21–33. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kicker, E.; Kouros, A.; Zatloukal, K.; Harant, H. The Virus Entry Pathway Determines Sensitivity to the Antiviral Peptide TAT-I24. Viruses 2025, 17, 458. https://doi.org/10.3390/v17040458
Kicker E, Kouros A, Zatloukal K, Harant H. The Virus Entry Pathway Determines Sensitivity to the Antiviral Peptide TAT-I24. Viruses. 2025; 17(4):458. https://doi.org/10.3390/v17040458
Chicago/Turabian StyleKicker, Eva, Antonio Kouros, Kurt Zatloukal, and Hanna Harant. 2025. "The Virus Entry Pathway Determines Sensitivity to the Antiviral Peptide TAT-I24" Viruses 17, no. 4: 458. https://doi.org/10.3390/v17040458
APA StyleKicker, E., Kouros, A., Zatloukal, K., & Harant, H. (2025). The Virus Entry Pathway Determines Sensitivity to the Antiviral Peptide TAT-I24. Viruses, 17(4), 458. https://doi.org/10.3390/v17040458







