HIV-Helminth Co-Infections and Immune Checkpoints: Implications for Cancer Risk in South Africa
Abstract
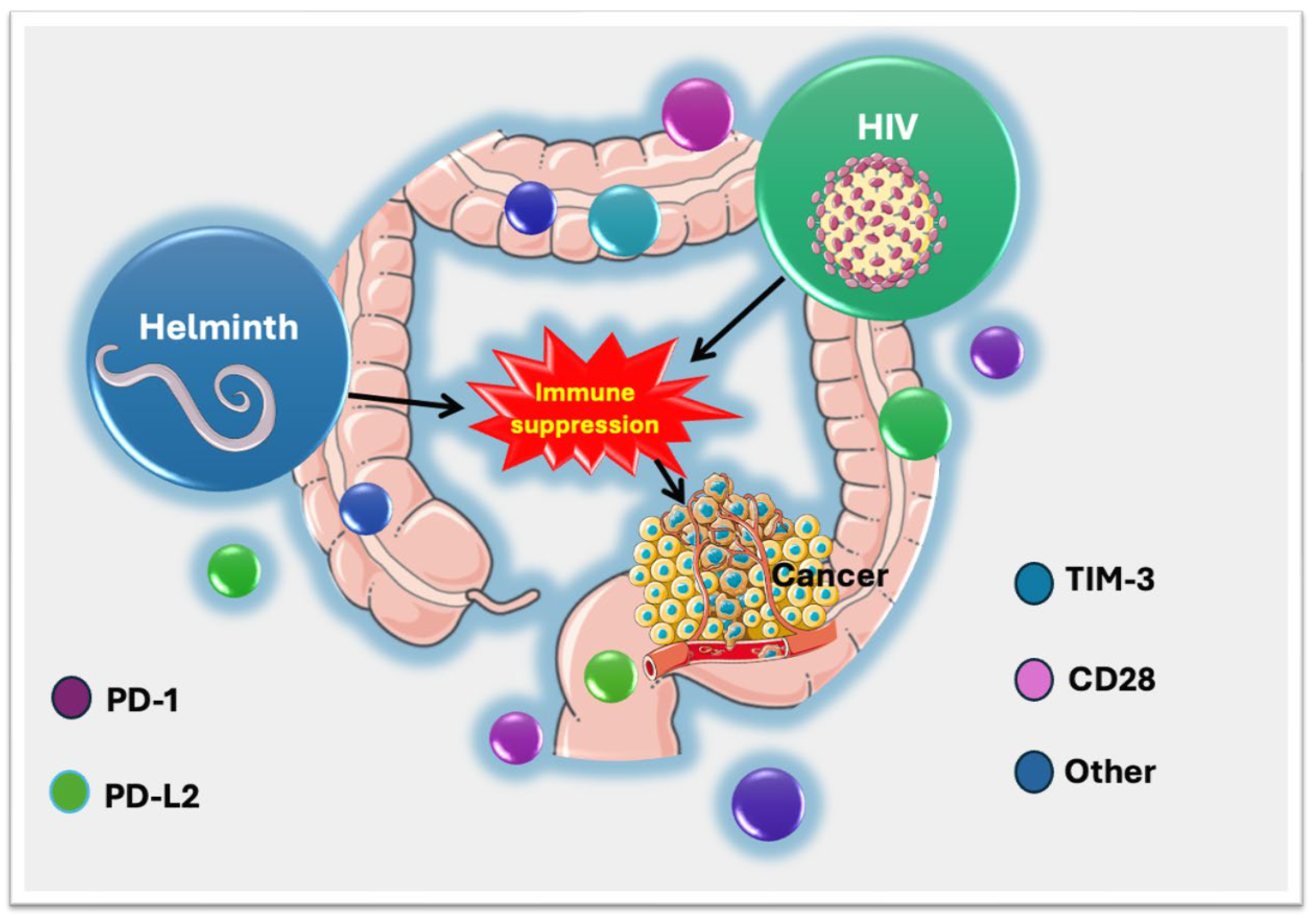
1. Introduction
| Mechanism | Helminths | Cancer | References |
|---|---|---|---|
| PD-1/PD-L1 Upregulation | Helminths upregulate PD-1 and PD-L1 on T cells and antigen-presenting cells, leading to immune suppression. | Tumors express PD-L1 to evade immune detection. | [24,27] |
| Treg Expansion and Th2 Skewing | Helminths induce Treg expansion and shift immune responses from Th1 to Th2, dampening pro-inflammatory responses. | Tumors recruit Tregs to suppress anti-tumor immunity. | [28,29] |
| MDSC and M2 Macrophage Induction | Helminths promote MDSC accumulation and M2 macrophage polarization, which suppress immune responses. | Tumors recruit MDSCs and polarize macrophages to M2 phenotype, aiding tumor progression. | [16,30] |
| Secretion of Immunomodulatory Molecules (IL-10, TGF-β) | Helminths secrete IL-10 and TGF-β, leading to immune suppression and tissue remodeling. | Tumors secrete IL-10 and TGF-β to create an immunosuppressive microenvironment. | [31,32] |
| CTLA-4 Expression | CTLA-4 expression in helminth-infected individuals, suggesting a role in immune regulation might be mainly from immunosuppressive cells such as Tregs. | Tumors exploit CTLA-4 checkpoints to suppress T-cell activation. | [27,32,33] |
2. Materials and Methods
2.1. Study Design
2.2. Study Population and Sampling
2.3. Parasite Detection
2.4. Full Blood Cell Count and Detection of HIV Status
2.5. Analysis of Immune Regulatory Molecules
2.6. Statistical Analysis
3. Results
3.1. Patient Demographics
3.2. The Prevalence of Parasites
3.3. The Profile of Immune Checkpoints/Co-Inhibitory Molecules, Immune Co-Stimulatory Molecules, and Immunosuppressive Enzymes
4. Discussion
4.1. CD28 Dysregulation and PD-1 Expression as Potential Pathways to Oncogenesis
4.2. PD-L2 Expression in a HIV-Helminth Co-Infected Group and Its Potential Role in Oncogenesis
4.3. TIM-3 Increase and Its Implications for Cancer Onset in HIV- and Helminth-Infected Groups
4.4. Implications for Colorectal Cancer (CRC) Risk
5. Challenges and Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Darvishian, M.; Butt, Z.A.; Wong, S.; Yoshida, E.M.; Khinda, J.; Otterstatter, M.; Yu, A.; Binka, M.; Rossi, C.; McKee, G.; et al. Elevated risk of colorectal, liver, and pancreatic cancers among HCV, HBV and/or HIV (co)infected individuals in a population based cohort in Canada. Ther. Adv. Med. Oncol. 2021, 13, 1758835921992987. [Google Scholar] [CrossRef] [PubMed]
- Walson, J.L.; Stewart, B.T.; Sangaré, L.; Mbogo, L.W.; Otieno, P.A.; Piper, B.K.S.; Richardson, B.A.; John-Stewart, G. Prevalence and correlates of helminth co-infection in Kenyan HIV-1 infected adults. PLoS Negl. Trop. Dis. 2010, 4, e644. [Google Scholar] [CrossRef]
- Mpaka-Mbatha, M.N.; Naidoo, P.; Islam, M.; Singh, R.; Mkhize-Kwitshana, Z.L. Demographic profile of HIV and helminth-coinfected adults in Kwazulu-natal, South Africa. S. Afr. J. Infect. Dis. 2023, 38, 466. [Google Scholar] [CrossRef]
- Adeleke, O.A.; Yogeswaran, P.; Wright, G. Intestinal helminth infections amongst HIV-infected adults in Mthatha General Hospital, South Africa. Afr. J. Prim. Health Care Fam. Med. 2015, 7, 910. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.; Mawa, P.A.; Kaleebu, P.; Elliott, A.M. Helminths and HIV infection: Epidemiological observations on immunological hypotheses. Parasite Immunol. 2006, 28, 613–623. [Google Scholar] [CrossRef]
- Mkhize-Kwitshana, Z.L.; Taylor, M.; Jooste, P.; Mabaso, M.L.; Walzl, G. The influence of different helminth infection phenotypes on immune responses against HIV in co-infected adults in South Africa. BMC Infect. Dis. 2011, 11, 273. [Google Scholar] [CrossRef]
- Mulu, A.; Anagaw, B.; Gelaw, A.; Ota, F.; Kassu, A.; Yifru, S. Effect of deworming on Th2 immune response during HIV-helminths co-infection. J. Transl. Med. 2015, 13, 236. [Google Scholar] [CrossRef]
- Esperante, D.; Gutiérrez, M.I.M.; Issa, M.E.; Schcolnik-Cabrera, A.; Mendlovic, F. Similarities and divergences in the metabolism of immune cells in cancer and helminthic infections. Front. Oncol. 2023, 13, 1251355. [Google Scholar] [CrossRef]
- Noyes, D.; Bag, A.; Oseni, S.; Semidey-Hurtado, J.; Cen, L.; Sarnaik, A.A.; Sondak, V.K.; Adeegbe, D. Tumor-associated Tregs obstruct antitumor immunity by promoting T cell dysfunction and restricting clonal diversity in tumor-infiltrating CD8+ T cells. J. Immunother. Cancer 2022, 10, e004605. [Google Scholar] [CrossRef]
- Alghanmi, M.; Minshawi, F.; Altorki, T.A.; Zawawi, A.; Alsaady, I.; Naser, A.Y.; Alwafi, H.; Alsulami, S.M.; Azhari, A.A.; Hashem, A.M.; et al. Helminth-derived proteins as immune system regulators: A systematic review of their promise in alleviating colitis. BMC Immunol. 2024, 25, 21. [Google Scholar] [CrossRef]
- Vacca, F.; Le Gros, G. Tissue-specific immunity in helminth infections. Mucosal Immunol. 2022, 15, 1212–1223. [Google Scholar] [CrossRef] [PubMed]
- Nutman, T.B. Looking beyond the induction of Th2 responses to explain immunomodulation by helminths. Parasite Immunol. 2015, 37, 304–313. [Google Scholar] [CrossRef] [PubMed]
- Maizels, R.M.; Pearce, E.J.; Artis, D.; Yazdanbakhsh, M.; Wynn, T.A. Regulation of pathogenesis and immunity in helminth infections. J. Exp. Med. 2009, 206, 2059–2066. [Google Scholar] [CrossRef] [PubMed]
- Zakeri, A.; Hansen, E.P.; Andersen, S.D.; Williams, A.R.; Nejsum, P. Immunomodulation by helminths: Intracellular pathways and extracellular vesicles. Front. Immunol. 2018, 9, 2349. [Google Scholar] [CrossRef]
- Sun, S.; Wang, X.; Wu, X.; Zhao, Y.; Wang, F.; Liu, X.; Song, Y.; Wu, Z.; Liu, M. Toll-like receptor activation by helminths or helminth products to alleviate inflammatory bowel disease. Parasites Vectors 2011, 4, 186. [Google Scholar] [CrossRef]
- Stevenson, M.M.; Valanparambil, R.M.; Tam, M. Myeloid-derived suppressor cells: The expanding world of helminth modulation of the immune system. Front. Immunol. 2022, 13, 874308. [Google Scholar] [CrossRef]
- Kreider, T.; Anthony, R.M.; Urban, J.F., Jr.; Gause, W.C. Alternatively activated macrophages in helminth infections. Curr. Opin. Immunol. 2007, 19, 448–453. [Google Scholar] [CrossRef]
- Coakley, G.; Harris, N.L. Interactions between macrophages and helminths. Parasite Immunol. 2020, 42, e12717. [Google Scholar] [CrossRef]
- Christensen, J.E.; Christensen, J.P.; Kristensen, N.N.; Hansen, N.J.V.; Stryhn, A.; Thomsen, A.R. Role of CD28 co-stimulation in generation and maintenance of virus-specific T cells. Int. Immunol. 2002, 14, 701–711. [Google Scholar] [CrossRef]
- Linterman, M.A.; Denton, A.E.; Divekar, D.P.; Zvetkova, I.; Kane, L.; Ferreira, C.; Veldhoen, M.; Clare, S.; Dougan, G.; Espéli, M.; et al. CD28 expression is required after T cell priming for helper T cell responses and protective immunity to infection. eLife 2014, 3, e03180. [Google Scholar] [CrossRef]
- Borkow, G.; Bentwich, Z. Chronic immune activation associated with chronic helminthic and human immunodeficiency virus infections: Role of hyporesponsiveness and anergy. Clin. Microbiol. Rev. 2004, 17, 1012–1030. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.-J.; Zhao, J.-W.; Zhang, D.-H.; Zheng, A.-H.; Wu, G.-Q. Immunotherapy of cancer by targeting regulatory T cells. Int. Immunopharmacol. 2022, 104, 108469. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Wang, C.; Yang, L.; Wu, J.; Li, M.; Xiao, P.; Xu, Z.; Xu, Y.; Wang, K. Targeting immune checkpoints on tumor-associated macrophages in tumor immunotherapy. Front. Immunol. 2023, 14, 1199631. [Google Scholar] [CrossRef] [PubMed]
- Stempin, C.C.; Motrán, C.C.; Aoki, M.P.; Falcón, C.R.; Cerbán, F.M.; Cervi, L. PD-L2 negatively regulates Th1-mediated immunopathology during fasciola hepatica infection. Oncotarget 2016, 7, 77721–77731. [Google Scholar] [CrossRef]
- Yu, X.; Zheng, Y.; Mao, R.; Su, Z.; Zhang, J. BTLA/HVEM signaling: Milestones in research and role in chronic hepatitis b virus infection. Front. Immunol. 2019, 10, 617. [Google Scholar] [CrossRef]
- Breloer, M.; Hartmann, W.; Blankenhaus, B.; Eschbach, M.-L.; Pfeffer, K.; Jacobs, T. Cutting edge: The BTLA-HVEM regulatory pathway interferes with protective immunity to intestinal helminth infection. J. Immunol. 2015, 194, 1413–1416. [Google Scholar] [CrossRef]
- Rajamanickam, A.; Munisankar, S.; Dolla, C.; Nutman, T.B.; Babu, S. Cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4)- and programmed death 1 (PD-1)-mediated regulation of monofunctional and dual functional CD4+ and CD8+ T-cell responses in a chronic helminth infection. Infect. Immun. 2019, 87, e00469-19. [Google Scholar] [CrossRef]
- White, M.P.J.; McManus, C.M.; Maizels, R.M. Regulatory T-cells in helminth infection: Induction, function and therapeutic potential. Immunology 2020, 160, 248–260. [Google Scholar] [CrossRef]
- Yap, G.S.; Gause, W.C. Helminth infections induce tissue tolerance mitigating immunopathology but enhancing microbial pathogen susceptibility. Front. Immunol. 2018, 9, 2135. [Google Scholar] [CrossRef]
- Van Ginderachter, J.A.; Beschin, A.; De Baetselier, P.; Raes, G. Myeloid-derived suppressor cells in parasitic infections. Eur. J. Immunol. 2010, 40, 2976–2985. [Google Scholar] [CrossRef]
- Labani-Motlagh, A.; Ashja-Mahdavi, M.; Loskog, A. The tumor microenvironment: A milieu hindering and obstructing antitumor immune responses. Front. Immunol. 2020, 11, 940. [Google Scholar] [CrossRef] [PubMed]
- Gazzinelli-Guimaraes, P.H.; Nutman, T.B. Helminth parasites and immune regulation. F1000Research 2018, 7, F1000 Faculty Rev-1685. [Google Scholar] [CrossRef] [PubMed]
- Buchbinder, E.I.; Desai, A. CTLA-4 and PD-1 pathways: Similarities, differences, and implications of their inhibition. Am. J. Clin. Oncol. 2016, 39, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.X.; Istl, A.C.; Quan, D.; Skaro, A.; Tang, E.; Zheng, X. PD-1 and PD-L1 inhibitors in cold colorectal cancer: Challenges and strategies. Cancer Immunol. Immunother. 2023, 72, 3875–3893. [Google Scholar] [CrossRef]
- Mpaka-Mbatha, M.N.; Naidoo, P.; Bhengu, K.N.; Islam, M.; Singh, R.; Nembe-Mafa, N.; Mkhize-Kwitshana, Z.L. Cytokine gene expression profiles during HIV and helminth coinfection in underprivileged peri-urban South African adults. Diagnostics 2023, 13, 2475. [Google Scholar] [CrossRef]
- Weisman, Z.; Kalinkovich, A.; Stein, M.; Greenberg, Z.; Borkow, G.; Adlerstein, D.; Mahdi, J.A.; Bentwich, Z. Effects of helminth eradication on the immune system. Pathog. Immun. 2017, 2, 293–307. [Google Scholar] [CrossRef]
- Ndlovu, H.H. Investigating the Role of CD28 Costimulation and IL-4/IL-13 Responsive Myeloid and Lymphoid Cells During Helminth Infections in Mice. Ph.D. Thesis, University of Cape Town, Cape Town, South Africa, 2013. [Google Scholar]
- Lai, Y.-P.; Kuo, L.-C.; Lin, B.-R.; Lin, H.-J.; Lin, C.-Y.; Chen, Y.-T.; Hsiao, P.-W.; Chang, H.-T.; Ko, P.C.-I.; Chen, H.-C.; et al. CD28 engagement inhibits CD73-mediated regulatory activity of CD8+ T cells. Commun. Biol. 2021, 4, 595. [Google Scholar] [CrossRef]
- Slaets, H.; Veeningen, N.; de Keizer, P.L.J.; Hellings, N.; Hendrix, S. Are immunosenescent T cells really senescent? Aging Cell 2024, 23, e14300. [Google Scholar] [CrossRef]
- Zhang, J.; He, T.; Xue, L.; Guo, H. Senescent T cells: A potential biomarker and target for cancer therapy. EBioMedicine 2021, 68, 103409. [Google Scholar] [CrossRef]
- Gardner, D.; Jeffery, L.E.; Sansom, D.M. Understanding the CD28/CTLA-4 (CD152) pathway and its implications for costimulatory blockade. Am. J. Transplant. 2014, 14, 1985–1991. [Google Scholar] [CrossRef]
- McCoy, K.; Camberis, M.; Gros, G.L. Protective immunity to nematode infection is induced by CTLA-4 blockade. J. Exp. Med. 1997, 186, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, R.; Sugiura, D.; Shimizu, K.; Maruhashi, T.; Watada, M.; Okazaki, I.-M.; Okazaki, T. PD-1 primarily targets TCR signal in the inhibition of functional T cell activation. Front. Immunol. 2019, 10, 630. [Google Scholar] [CrossRef]
- Velu, V.; Shetty, R.D.; Larsson, M.; Shankar, E.M. Role of PD-1 co-inhibitory pathway in HIV infection and potential therapeutic options. Retrovirology 2015, 12, 14. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Kang, K.; Chen, P.; Zeng, Z.; Li, G.; Xiong, W.; Yi, M.; Xiang, B. Regulatory mechanisms of PD-1/PD-L1 in cancers. Mol. Cancer 2024, 23, 108. [Google Scholar] [CrossRef]
- Vivar, N.; Ruffin, N.; Sammicheli, S.; Hejdeman, B.; Rethi, B.; Chiodi, F. Survival and proliferation of CD28-T cells during HIV-1 infection relate to the amplitude of viral replication. J. Infect. Dis. 2011, 203, 1658–1667. [Google Scholar]
- Yonkers, N.L.; Rodriguez, B.; Post, A.B.; Asaad, R.; Jones, L.; Lederman, M.M.; Lehmann, P.V.; Anthony, D.D. HIV coinfection impairs CD28-mediated costimulation of hepatitis c virus-specific CD8 cells. J. Infect. Dis. 2006, 194, 391–400. [Google Scholar] [CrossRef]
- Wu, D.; Tang, R.; Qi, Q.; Zhou, X.; Zhou, H.; Mao, Y.; Li, R.; Liu, C.; Wang, W.; Hua, D.; et al. Five functional polymorphisms of B7/CD28 co-signaling molecules alter susceptibility to colorectal cancer. Cell. Immunol. 2015, 293, 41–48. [Google Scholar] [CrossRef]
- Imai, M.; Nakamura, Y.; Denda, T.; Komatsu, Y.; Yuki, S.; Nishina, T.; Hamamoto, Y.; Hara, H.; Esaki, T.; Kawakami, H.; et al. Association of PD-L1 and PD-L2 expression and tumor-infiltrating lymphocytes in BRAF v600e-mutated metastatic colorectal cancer: Gi-screen post-hoc analysis. ESMO Gastrointest. Oncol. 2023, 2, 100008. [Google Scholar] [CrossRef]
- Yuki, S.; Nakamura, Y.; Taniguchi, H.; Denda, T.; Nishina, T.; Hamamoto, Y.; Hara, H.; Esaki, T.; Kawakami, H.; Takashima, A.; et al. Expression of PD-L1 and PD-L2 in colorectal cancer (CRC): A post-hoc integrated analysis of scrum-japan gi-screen CRC. J. Clin. Oncol. 2021, 39, 120. [Google Scholar] [CrossRef]
- Huber, S.; Hoffmann, R.; Muskens, F.; Voehringer, D. Alternatively activated macrophages inhibit T-cell proliferation by STAT6-dependent expression of PD-L2. Blood 2010, 116, 3311–3320. [Google Scholar] [CrossRef]
- Prévost, J.; Edgar, C.R.; Richard, J.; Trothen, S.M.; Jacob, R.A.; Mumby, M.J.; Pickering, S.; Dubé, M.; Kaufmann, D.E.; Kirchhoff, F.; et al. HIV-1 vpu downregulates TIM-3 from the surface of infected CD4+ T cells. J. Virol. 2020, 94, e01999-19. [Google Scholar] [CrossRef] [PubMed]
- Hou, N.; Jiang, N.; Zou, Y.; Piao, X.; Liu, S.; Li, S.; Chen, Q. Down-regulation of TIM-3 in monocytes and macrophages in plasmodium infection and its association with parasite clearance. Front. Microbiol. 2017, 8, 1431. [Google Scholar] [CrossRef] [PubMed]
- Hou, N.; Piao, X.; Liu, S.; Wu, C.; Chen, Q. TIM-3 induces Th2-biased immunity and alternative macrophage activation during Schistosoma japonicum infection. Infect. Immun. 2015, 83, 3074–3082. [Google Scholar] [CrossRef] [PubMed]
- Mokhtari, Z.; Rezaei, M.; Sanei, M.H.; Dehghanian, A.; Faghih, Z.; Heidari, Z.; Tavana, S. TIM3 and PD-1 as a therapeutic and prognostic targets in colorectal cancer: Relationship with sidedness, clinicopathological parameters, and survival. Front. Oncol. 2023, 13, 1069696. [Google Scholar] [CrossRef]
- Mak, G.; Zaunders, J.J.; Bailey, M.; Seddiki, N.; Rogers, G.; Leong, L.; Phan, T.G.; Kelleher, A.D.; Koelsch, K.K.; Boyd, M.A.; et al. Preservation of gastrointestinal mucosal barrier function and microbiome in patients with controlled HIV infection. Front. Immunol. 2021, 12, 688886. [Google Scholar] [CrossRef]
- McSorley, H.J.; Maizels, R.M. Helminth infections and host immune regulation. Clin. Microbiol. Rev. 2012, 25, 585–608. [Google Scholar] [CrossRef]
- Castelli, V.; Lombardi, A.; Palomba, E.; Bozzi, G.; Ungaro, R.; Alagna, L.; Mangioni, D.; Muscatello, A.; Bandera, A.; Gori, A. Immune checkpoint inhibitors in people living with HIV/AIDS: Facts and controversies. Cells 2021, 10, 2227. [Google Scholar] [CrossRef]
- Pastille, E.; Frede, A.; McSorley, H.J.; Gräb, J.; Adamczyk, A.; Kollenda, S.; Hansen, W.; Epple, M.; Buer, J.; Maizels, R.M.; et al. Intestinal helminth infection drives carcinogenesis in colitis-associated colon cancer. PLoS Pathog. 2017, 13, e1006649. [Google Scholar] [CrossRef]
- Kupritz, J.; Angelova, A.; Nutman, T.B.; Gazzinelli-Guimaraes, P.H. Helminth-induced human gastrointestinal dysbiosis: A systematic review and meta-analysis reveals insights into altered taxon diversity and microbial gradient collapse. mBio 2021, 12, e0289021. [Google Scholar] [CrossRef]
- Hou, X.; Zheng, Z.; Wei, J.; Zhao, L. Effects of gut microbiota on immune responses and immunotherapy in colorectal cancer. Front. Immunol. 2022, 13, 1030745. [Google Scholar] [CrossRef]
- Gamberg, J.; Pardoe, I.; Bowmer, M.I.; Howley, C.; Grant, M. Lack of CD28 expression on HIV-specific cytotoxic T lymphocytes is associated with disease progression. Immunol. Cell Biol. 2004, 82, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yao, H.; Li, C.; Liang, L.; Zhang, Y.; Shi, H.; Zhou, C.; Chen, Y.; Fang, J.-Y.; Xu, J. PD-L2 expression in colorectal cancer: Independent prognostic effect and targetability by deglycosylation. Oncoimmunology 2017, 6, e1327494. [Google Scholar] [CrossRef] [PubMed]
- South African National Department of Health (NDoH). The South African National Welcome Back Campaign Strategy; National Department of Health: Pretoria, South Africa, 2021. Available online: https://knowledgehub.health.gov.za/system/files/elibdownloads/2023-04/A5_Welcome%2520Back%2520Campaign_070222_2-%2520FINAL%2520%25281%2529.pdf (accessed on 6 March 2025).
- Maluleka, L.M.; Hlongwane, N.; Mokgatle, M.M. Knowledge and Perceptions of Healthcare Workers about the Implementation of the Universal Test and Treat Guideline in Under-Resourced, High-HIV Prevalence Rural Settings. Healthcare 2023, 11, 968. [Google Scholar] [CrossRef] [PubMed]
- Waldman, A.D.; Fritz, J.M.; Lenardo, M.J. A guide to cancer immunotherapy: From T cell basic science to clinical practice. Nat. Rev. Immunol. 2020, 20, 651–668. [Google Scholar] [CrossRef]
- Dyck, L.; Mills, K.H.G. Immune checkpoints and their inhibition in cancer and infectious diseases. Eur. J. Immunol. 2017, 47, 765–779. [Google Scholar] [CrossRef]
- Bailly, C. Contribution of the TIM-3/GAL-9 immune checkpoint to tropical parasitic diseases. Acta Trop. 2023, 238, 106792. [Google Scholar] [CrossRef]
- Arora, N.; Kaur, R.; Anjum, F.; Tripathi, S.; Mishra, A.; Kumar, R.; Prasad, A. Neglected agent eminent disease: Linking human helminthic infection, inflammation, and malignancy. Front. Cell. Infect. Microbiol. 2019, 9, 402. [Google Scholar] [CrossRef]
- Scholte, L.L.S.; Pascoal-Xavier, M.A.; Nahum, L.A. Helminths and cancers from the evolutionary perspective. Front. Med. 2018, 5, 90. [Google Scholar] [CrossRef]
- Siegel, M.O.; Simon, G.L. Is human immunodeficiency virus infection a risk factor for Strongyloides stercoralis hyperinfection and dissemination. PLoS Negl. Trop. Dis. 2012, 6, e1581. [Google Scholar] [CrossRef]
- Grossi, P.A.; Lombardi, D.; Petrolo, A.; Rovelli, C.; Di Rosa, Z.; Perriccioli, G.; Rossi, A.; Minoja, G.; Scaglione, F.; Dalla Gasperina, D. Strongyloides stercoralis Hyperinfection in an HIV-Infected Patient Successfully Treated with Subcutaneous Ivermectin. Trop. Med. Infect. Dis. 2018, 3, 46. [Google Scholar] [CrossRef]

| Parameters | Uninfected Controls (n = 20) | HIV-Infected Only (n = 20) | Helminth-Infected Only (n = 20) | HIV + Helminth Co-Infection (n = 18) | p-Value |
|---|---|---|---|---|---|
| Age (years) | 46.4 ± 17.3 | 42.9 ± 12.3 | 37.1 ± 16.4 | 39.4 ± 10.0 | 0.200 |
| Gender, n (%) | |||||
| Males | 6 (30) | 9 (45) | 7 (35) | 6 (30) | 0.725 |
| Females | 14 (70) | 11 (55) | 13 (65) | 14 (70) | |
| BMI (kg/m2) | 28.0 ± 6.9 | 26.0 ± 7.0 | 29.6 ± 8.3 | 27.0 ± 5.4 | 0.418 |
| CD4 count (u/L) | 996 ± 375 * | 629 ± 448 # | 892 ± 197 * | 579 ± 370 # | 0.001 |
| CD8 (u/L) | 781 ± 341 | 910 ± 455 | 724 ± 299 | 742 ± 354 | 0.3801 |
| CD4/CD8 ratio | 1.57 ± 0.92 * | 0.75 ± 0.44 $ | 1.43 ± 0.60 # | 0.90 ± 0.62 #,$ | 0.0004 |
| Viral load (copies/mL) | |||||
| n (%) | |||||
| <20 | 13 (65) | 11 (61) | 1.000 | ||
| >20 | 7 (35) | 7 (39) | |||
| White cell count (×109/L) | 6.52 ± 1.96 | 5.65 ± 2.06 | 6.07 ± 1.83 | 5.16 ± 1.81 | 0.163 |
| Neutrophils (×109/L) | 3.61 ± 1.50 | 3.05 ± 1.41 | 3.19 ± 1.34 | 2.65 ± 1.04 | 0.180 |
| Lymphocytes (×109/L) | 2.27 ± 0.61 | 2.02 ± 1.00 | 2.21 ± 0.50 | 1.79 ± 0.66 | 0.164 |
| Monocytes (×109/L) | 0.42 ± 0.12 | 0.40 ± 0.12 | 0.47 ± 0.15 | 0.39 ± 0.11 | 0.206 |
| Unstandardized β–Coefficient Values (Reference Group: Uninfected Controls) | |||||||
|---|---|---|---|---|---|---|---|
| Parameters | HIV Infected | Helminth Infected | HIV and Helminth Co-Infected | ||||
| β (95% CI) | p | β (95% CI) | p | β (95% CI) | p | ||
| Immune Checkpoints/Co-Inhibitory Molecules | |||||||
| Programmed cell death (PD)-1 | A | 0.05 (−0.04–0.14) | 0.251 | 0.13 (−0.11–0.37) | 0.266 | 0.18 (0.00–0.35) | 0.046 |
| B | 0.12 (0.01–0.23) | 0.034 | 0.15 (−0.16–0.45) | 0.335 | 0.19 (−0.13–0.51) | 0.235 | |
| PD-ligand (L)2 | A | 0.57 (−12.41–13.54) | 0.929 | 6.29 (−9.95–22.52) | 0.436 | 7.95 (0.67–15.23) | 0.033 |
| B | 7.68 (−9.11–24.480 | 0.351 | 12.53 (−8.38–33.45) | 0.228 | 6.96 (−3.73–17.66) | 0.193 | |
| B and T lymphocyte attenuator (BTLA) | A | 0.79 (−1.24–2.83) | 0.431 | 2.26 (−1.70–6.22) | 0.253 | 15.15 (−17.03–47.33) | 0.342 |
| B | 1.96 (−0.51–4.42) | 0.114 | 3.66 (−1.07–8.39) | 0.124 | 44.05 (−10.47–98.57) | 0.107 | |
| Cytotoxic T-lymphocyte Antigen-4 (CTLA-4) | A | 4,03 (−20.65–28.71) | 0.743 | 4.59 (−16.74–25.93) | 0.665 | 12.03 (−33.70–57.77) | 0.597 |
| B | 3.99 (−31.11–39.09) | 0.813 | 5.05 (−15.93–26.04) | 0.626 | 33.97 (−37.38–105.33) | 0.339 | |
| T cell immunoglobulin and mucin domain containing molecule 3 (TIM-3) | A | 13.58 (−5.73–32.90) | 0.161 | 6.02 (−10.38–22.43) | 0.459 | 12.58 (−8.68–33.84) | 0.235 |
| B | 23.15 (−0.20–46.50) | 0.052 | 20.98 (3.52–38.45) | 0.020 | 18.11 (−13.02–49.25) | 0.238 | |
| Immunosuppressive Enzymes | |||||||
| Indoleamine 2,3-dioxygenase (IDO) | A | −9.47 (−31.92–12.98) | 0.394 | −5.84 (−27.65–15.97) | 0.589 | −10.39 (−32.94–12.16) | 0.352 |
| B | −9.44 (−39.65–20.77) | 0.522 | −2.40 (−26.65–21.84) | 0.840 | −6.64 (−47.18–33.89) | 0.735 | |
| Immune Co-Stimulatory Molecules | |||||||
| CD27 | A | 803.69 (−310.51–1917.89) | 0.150 | −23.60 (−544.54–497.34) | 0.927 | 213.92 (−403.73–831.57) | 0.483 |
| B | 911.70 (−535.26–2358.66) | 0.204 | 427.14 (−156.24–1010.53) | 0.144 | 511.32 (−349.16–1371.80) | 0.229 | |
| CD28 | A | −594.33 (−946.57–−242.10) | 0.002 | −590.42 (−902.25–−278.59) | 0.001 | −594.01 (−958.93–−229.08) | 0.003 |
| B | −651.95 (−1126.74–−177.15) | 0.010 | −674.32 (−1054.48–−294.16) | 0.001 | −671.55 (−1322.53–−20.58) | 0.044 | |
| CD80 | A | −0.93 (−2.44–0.59) | 0.220 | 0.81 (−2.58–4.21) | 0.629 | 2.32 (−2.84–7.48) | 0.363 |
| B | −0.86 (−2.94–1.22) | 0.398 | 1.06 (−3.15–5.28) | 0.608 | 5.92 (−3.42–15.26) | 0.200 | |
| CD137 | A | 0.27 (−0.14–0.689) | 0.190 | 1.40 (−4.63–3.26) | 0.136 | 1.18 (−0.14–2.50) | 0.077 |
| B | 0.51 (−0.43–1.06) | 0.069 | 1.48 (−0.94–3.90) | 0.219 | 2.31 (−0.129–4.74) | 0.062 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Damane, B.P.; Mulaudzi, T.V.; Kader, S.S.; Naidoo, P.; Dlamini, Z.; Mkhize-Kwitshana, Z.L. HIV-Helminth Co-Infections and Immune Checkpoints: Implications for Cancer Risk in South Africa. Viruses 2025, 17, 451. https://doi.org/10.3390/v17030451
Damane BP, Mulaudzi TV, Kader SS, Naidoo P, Dlamini Z, Mkhize-Kwitshana ZL. HIV-Helminth Co-Infections and Immune Checkpoints: Implications for Cancer Risk in South Africa. Viruses. 2025; 17(3):451. https://doi.org/10.3390/v17030451
Chicago/Turabian StyleDamane, Botle Precious, Thanyani Victor Mulaudzi, Sayed Shakeel Kader, Pragalathan Naidoo, Zodwa Dlamini, and Zilungile Lynette Mkhize-Kwitshana. 2025. "HIV-Helminth Co-Infections and Immune Checkpoints: Implications for Cancer Risk in South Africa" Viruses 17, no. 3: 451. https://doi.org/10.3390/v17030451
APA StyleDamane, B. P., Mulaudzi, T. V., Kader, S. S., Naidoo, P., Dlamini, Z., & Mkhize-Kwitshana, Z. L. (2025). HIV-Helminth Co-Infections and Immune Checkpoints: Implications for Cancer Risk in South Africa. Viruses, 17(3), 451. https://doi.org/10.3390/v17030451






