Understanding HIV-Exposed Uninfected Children: A Narrative Review
Abstract
1. Introduction
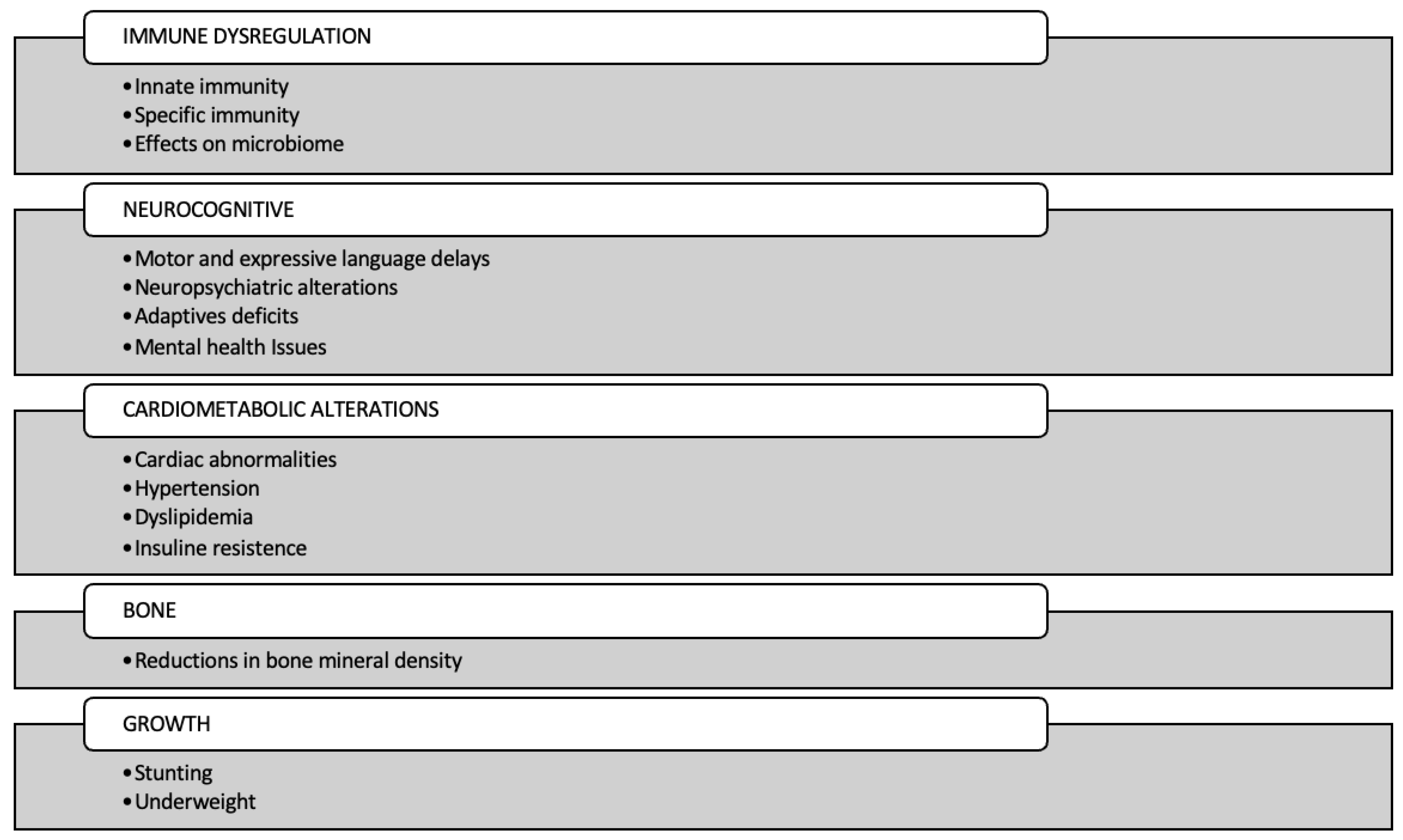
2. Immune Dysregulation
2.1. Innate Immunity
2.2. Adaptive Immunity
2.3. Effects on the Microbiome
2.4. Inflammation
3. Cardiometabolic Alterations
3.1. Cardiac Abnormalities
3.2. Hypertension
3.3. Dyslipidemia
3.4. Insulin Resistance
4. Neurocognitive
5. Bone
6. Growth
6.1. Linear and Ponderal Growth
6.2. Stunting and Underweight
7. Conclusions
8. Search Strategy, Selection Criteria
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Bulterys, M.A.; Njuguna, I.; Mahy, M.; Gulaid, L.A.; Powis, K.M.; Wedderburn, C.J.; John-Stewart, G. Neurodevelopment among Children Exposed to HIV and Uninfected in Sub-Saharan Africa. J. Int. AIDS Soc. 2023, 26 (Suppl. S4), e26159. [Google Scholar] [CrossRef] [PubMed]
- du Toit, L.D.V.; Prinsloo, A.; Steel, H.C.; Feucht, U.; Louw, R.; Rossouw, T.M. Immune and Metabolic Alterations in Children with Perinatal HIV Exposure. Viruses 2023, 15, 279. [Google Scholar] [CrossRef] [PubMed]
- Byrne, A.; Diener, C.; Brown, B.P.; Maust, B.S.; Feng, C.; Alinde, B.L.; Gibbons, S.M.; Koch, M.; Gray, C.M.; Jaspan, H.B.; et al. Neonates Exposed to HIV but Uninfected Exhibit an Altered Gut Microbiota and Inflammation Associated with Impaired Breast Milk Antibody Function. Microbiome 2024, 12, 261. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.S.; Hughes, M.D.; Riddler, S.A.; Haubrich, R.H.; Aids Clinical Trials Group A5142 Study Team. Bone Mineral Density Effects of Randomized Regimen and Nucleoside Reverse Transcriptase Inhibitor Selection from ACTG A5142. HIV Clin. Trials 2013, 14, 224–234. [Google Scholar] [CrossRef]
- Conesa-Buendía, F.M.; Llamas-Granda, P.; Larrañaga-Vera, A.; Wilder, T.; Largo, R.; Herrero-Beaumont, G.; Cronstein, B.; Mediero, A. Tenofovir Causes Bone Loss via Decreased Bone Formation and Increased Bone Resorption, Which Can Be Counteracted by Dipyridamole in Mice. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2019, 34, 923–938. [Google Scholar] [CrossRef]
- Mukherjee, S.B.; Devamare, S.; Seth, A.; Sapra, S. Development, Cognition, Adaptive Function and Maladaptive Behavior in HIV-Infected and HIV-Exposed Uninfected Children Aged 2-9 Years. Indian Pediatr. 2019, 56, 933–937. [Google Scholar] [CrossRef]
- Wedderburn, C.J.; Weldon, E.; Bertran-Cobo, C.; Rehman, A.M.; Stein, D.J.; Gibb, D.M.; Yeung, S.; Prendergast, A.J.; Donald, K.A. Early Neurodevelopment of HIV-Exposed Uninfected Children in the Era of Antiretroviral Therapy: A Systematic Review and Meta-Analysis. Lancet Child Adolesc. Health 2022, 6, 393–408. [Google Scholar] [CrossRef]
- Slogrove, A.L.; Powis, K.M.; Johnson, L.F.; Stover, J.; Mahy, M. Estimates of the Global Population of Children Who Are HIV-Exposed and Uninfected, 2000-18: A Modelling Study. Lancet Glob. Health 2020, 8, e67–e75. [Google Scholar] [CrossRef]
- Maloupazoa Siawaya, A.C.; Mveang-Nzoghe, A.; Mvoundza Ndjindji, O.; Mintsa Ndong, A.; Essone, P.N.; Djoba Siawaya, J.F. Cases of Impaired Oxidative Burst in HIV-Exposed Uninfected Infants’ Neutrophils-A Pilot Study. Front. Immunol. 2017, 8, 262. [Google Scholar] [CrossRef]
- Abu-Raya, B.; Kollmann, T.R.; Marchant, A.; MacGillivray, D.M. The Immune System of HIV-Exposed Uninfected Infants. Front. Immunol. 2016, 7, 383. [Google Scholar] [CrossRef]
- Dirajlal-Fargo, S.; Mussi-Pinhata, M.M.; Weinberg, A.; Yu, Q.; Cohen, R.; Harris, D.R.; Bowman, E.; Gabriel, J.; Kulkarni, M.; Funderburg, N.; et al. HIV-Exposed-Uninfected Infants Have Increased Inflammation and Monocyte Activation. AIDS Lond. Engl. 2019, 33, 845–853. [Google Scholar] [CrossRef] [PubMed]
- Evans, C.; Humphrey, J.H.; Ntozini, R.; Prendergast, A.J. HIV-Exposed Uninfected Infants in Zimbabwe: Insights into Health Outcomes in the Pre-Antiretroviral Therapy Era. Front. Immunol. 2016, 7, 190. [Google Scholar] [CrossRef] [PubMed]
- Slyker, J.A.; Lohman-Payne, B.; John-Stewart, G.C.; Dong, T.; Mbori-Ngacha, D.; Tapia, K.; Atzberger, A.; Taylor, S.; Rowland-Jones, S.L.; Blish, C.A. The Impact of HIV-1 Infection and Exposure on Natural Killer (NK) Cell Phenotype in Kenyan Infants during the First Year of Life. Front. Immunol. 2012, 3, 399. [Google Scholar] [CrossRef] [PubMed]
- Mataramvura, H.; Jӓger, J.; Jordan-Paiz, A.; Mazengera, L.R.; Gumbo, F.Z.; Bunders, M.J.; Duri, K. Phenotypic Characterization of NK Cells in 5-Year-Old Children Exposed to Maternal HIV and Antiretroviral Therapy in Early-Life. BMC Immunol. 2024, 25, 82. [Google Scholar] [CrossRef]
- Madzime, M.; Rossouw, T.M.; Theron, A.J.; Anderson, R.; Steel, H.C. Interactions of HIV and Antiretroviral Therapy With Neutrophils and Platelets. Front. Immunol. 2021, 12, 634386. [Google Scholar] [CrossRef]
- Yin, L.; Venturi, G.M.; Barfield, R.; Fischer, B.M.; Kim-Chang, J.J.; Chan, C.; De Paris, K.; Goodenow, M.M.; Sleasman, J.W. Maternal Immunity Shapes Biomarkers of Germinal Center Development in HIV-Exposed Uninfected Infants. Front. Immunol. 2024, 15, 1443886. [Google Scholar] [CrossRef]
- Selvam, A.; Buhimschi, I.A.; Makin, J.D.; Pattinson, R.C.; Anderson, R.; Forsyth, B.W. Hyperferritinemia and Markers of Inflammation and Oxidative Stress in the Cord Blood of HIV-Exposed, Uninfected (HEU) Infants. HIV Med. 2015, 16, 375–380. [Google Scholar] [CrossRef]
- Brito-Pérez, Y.; Camacho-Pacheco, R.T.; Plazola-Camacho, N.; Soriano-Becerril, D.; Coronado-Zarco, I.A.; Arreola-Ramírez, G.; González-Pérez, G.; Herrera-Salazar, A.; Flores-González, J.; Bermejo-Haro, M.Y.; et al. Impaired T Helper Cell Responses in Human Immunodeficiency Virus-Exposed Uninfected Newborns. Immun. Inflamm. Dis. 2021, 9, 1541–1553. [Google Scholar] [CrossRef]
- Miyamoto, M.; Pessoa, S.D.; Ono, E.; Machado, D.M.; Salomão, R.; Succi, R.C.d.M.; Pahwa, S.; de Moraes-Pinto, M.I. Low CD4+ T-Cell Levels and B-Cell Apoptosis in Vertically HIV-Exposed Noninfected Children and Adolescents. J. Trop. Pediatr. 2010, 56, 427–432. [Google Scholar] [CrossRef]
- European Collaborative Study. Levels and Patterns of Neutrophil Cell Counts over the First 8 Years of Life in Children of HIV-1-Infected Mothers. AIDS Lond. Engl. 2004, 18, 2009–2017. [Google Scholar] [CrossRef]
- Afran, L.; Jambo, K.C.; Nedi, W.; Miles, D.J.C.; Kiran, A.; Banda, D.H.; Kamg’ona, R.; Tembo, D.; Pachnio, A.; Nastouli, E.; et al. Defective Monocyte Enzymatic Function and an Inhibitory Immune Phenotype in Human Immunodeficiency Virus-Exposed Uninfected African Infants in the Era of Antiretroviral Therapy. J. Infect. Dis. 2022, 226, 1243–1255. [Google Scholar] [CrossRef] [PubMed]
- Machiavelli, A.; Duarte, R.T.D.; Pires, M.M.d.S.; Zárate-Bladés, C.R.; Pinto, A.R. The Impact of in Utero HIV Exposure on Gut Microbiota, Inflammation, and Microbial Translocation. Gut Microbes 2019, 10, 599–614. [Google Scholar] [CrossRef] [PubMed]
- Reikie, B.A.; Adams, R.C.M.; Leligdowicz, A.; Ho, K.; Naidoo, S.; Rusk, C.E.; de Beer, C.; Preiser, W.; Cotton, M.F.; Speert, D.P.; et al. Altered Innate Immune Development in HIV-Exposed Uninfected Infants. J. Acquir. Immune Defic. Syndr. 1999 2014, 66, 245–255. [Google Scholar] [CrossRef] [PubMed]
- White, M.; Feucht, U.D.; Duffley, E.; Molokoane, F.; Durandt, C.; Cassol, E.; Rossouw, T.; Connor, K.L. Does in Utero HIV Exposure and the Early Nutritional Environment Influence Infant Development and Immune Outcomes? Findings from a Pilot Study in Pretoria, South Africa. Pilot Feasibility Stud. 2020, 6, 192. [Google Scholar] [CrossRef]
- Ikumi, N.M.; Pillay, K.; Tilburgs, T.; Malaba, T.R.; Dzanibe, S.; Enninga, E.A.L.; Chakraborty, R.; Lamorde, M.; Myer, L.; Khoo, S.; et al. T-Cell Homeostatic Imbalance in Placentas From Women With Human Immunodeficiency Virus in the Absence of Vertical Transmission. J. Infect. Dis. 2021, 224 (Suppl. S2), S670–S682. [Google Scholar] [CrossRef]
- Kolte, L.; Rosenfeldt, V.; Vang, L.; Jeppesen, D.; Karlsson, I.; Ryder, L.P.; Skogstrand, K.; Dam Nielsen, S. Reduced Thymic Size but No Evidence of Impaired Thymic Function in Uninfected Children Born to Human Immunodeficiency Virus-Infected Mothers. Pediatr. Infect. Dis. J. 2011, 30, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Knight, M.A.; Nduati, E.; Hassan, A.S.; Gambo, F.; Odera, D.; Etyang, T.J.; Hajj, N.J.; Berkley, J.A.; Urban, B.C.; Rowland-Jones, S.L. Altered Memory T-Cell Responses to Bacillus Calmette-Guerin and Tetanus Toxoid Vaccination and Altered Cytokine Responses to Polyclonal Stimulation in HIV-Exposed Uninfected Kenyan Infants. PLoS ONE 2015, 10, e0143043. [Google Scholar] [CrossRef]
- Dauby, N.; Chamekh, M.; Melin, P.; Slogrove, A.L.; Goetghebuer, T. Increased Risk of Group B Streptococcus Invasive Infection in HIV-Exposed but Uninfected Infants: A Review of the Evidence and Possible Mechanisms. Front. Immunol. 2016, 7, 505. [Google Scholar] [CrossRef]
- Filteau, S.; Kasonka, L.; Wells, J.C.K.; Munthali, G.; Chisenga, M.; Rehman, A.M. Anthropometry, Body Composition, Early Growth and Chronic Disease Risk Factors among Zambian Adolescents Exposed or Not to Perinatal Maternal HIV. Br. J. Nutr. 2022, 129, 678–689. [Google Scholar] [CrossRef]
- Bengtson, A.M.; Pellowski, J.; McGarvey, S.; McGinty, R.; Botha, M.; Burd, T.; Burgner, D.; Mansell, T.; Zar, H.J. In-Utero HIV Exposure and Cardiometabolic Health among Children 5-8 Years: Findings from a Prospective Birth Cohort in South Africa. AIDS Lond. Engl. 2023, 37, 173–182. [Google Scholar] [CrossRef]
- García-Otero, L.; López, M.; Guitart-Mampel, M.; Morén, C.; Goncé, A.; Esteve, C.; Salazar, L.; Gómez, O.; Martínez, J.M.; Torres, B.; et al. Cardiac and Mitochondrial Function in HIV-Uninfected Fetuses Exposed to Antiretroviral Treatment. PLoS ONE 2019, 14, e0213279. [Google Scholar] [CrossRef]
- Ursache, A.; Tibeica, A.M.; Luca, A.; Onofriescu, M.; Matasariu, D.R.; Nemescu, D. Fetal Cardiac Evaluation in HIV-Positive Women under HAART Therapy in a Romanian Hospital. Exp. Ther. Med. 2021, 21, 606. [Google Scholar] [CrossRef] [PubMed]
- Lipshultz, S.E.; Sasaki, N.; Thompson, B.; Eidem, B.W.; Cheng, I.; Colan, S.D.; O’Brien, S.E.; Amdani, S.; Shearer, W.T.; Orav, E.J.; et al. Left Ventricular Diastolic Dysfunction in HIV-Uninfected Infants Exposed in Utero to Antiretroviral Therapy. AIDS Lond. Engl. 2020, 34, 529–537. [Google Scholar] [CrossRef] [PubMed]
- Cade, W.T.; Waggoner, A.D.; Hubert, S.; Krauss, M.J.; Singh, G.K.; Overton, E.T. Reduced Diastolic Function and Left Ventricular Mass in HIV-Negative Preadolescent Children Exposed to Antiretroviral Therapy in Utero. AIDS Lond. Engl. 2012, 26, 2053–2058. [Google Scholar] [CrossRef] [PubMed]
- Lipshultz, S.E.; Shearer, W.T.; Thompson, B.; Rich, K.C.; Cheng, I.; Orav, E.J.; Kumar, S.; Pignatelli, R.H.; Bezold, L.I.; LaRussa, P.; et al. Cardiac Effects of Antiretroviral Therapy in HIV-Negative Infants Born to HIV-Positive Mothers: NHLBI CHAART-1 (National Heart, Lung, and Blood Institute Cardiovascular Status of HAART Therapy in HIV-Exposed Infants and Children Cohort Study). J. Am. Coll. Cardiol. 2011, 57, 76–85. [Google Scholar] [CrossRef]
- Lipshultz, S.E.; Williams, P.L.; Zeldow, B.; Wilkinson, J.D.; Rich, K.C.; van Dyke, R.B.; Seage, G.R.; Dooley, L.B.; Kaltman, J.R.; Siberry, G.K.; et al. Cardiac Effects of In-Utero Exposure to Antiretroviral Therapy in HIV-Uninfected Children Born to HIV-Infected Mothers. AIDS Lond. Engl. 2015, 29, 91–100. [Google Scholar] [CrossRef]
- Sibiude, J.; Le Chenadec, J.; Bonnet, D.; Tubiana, R.; Faye, A.; Dollfus, C.; Mandelbrot, L.; Delmas, S.; Lelong, N.; Khoshnood, B.; et al. In Utero Exposure to Zidovudine and Heart Anomalies in the ANRS French Perinatal Cohort and the Nested PRIMEVA Randomized Trial. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2015, 61, 270–280. [Google Scholar] [CrossRef]
- Lipshultz, S.E.; Miller, T.L.; Wilkinson, J.D.; Scott, G.B.; Somarriba, G.; Cochran, T.R.; Fisher, S.D. Cardiac Effects in Perinatally HIV-Infected and HIV-Exposed but Uninfected Children and Adolescents: A View from the United States of America. J. Int. AIDS Soc. 2013, 16, 18597. [Google Scholar] [CrossRef]
- Wilkinson, J.D.; Williams, P.L.; Leister, E.; Zeldow, B.; Shearer, W.T.; Colan, S.D.; Siberry, G.K.; Dooley, L.B.; Scott, G.B.; Rich, K.C.; et al. Cardiac Biomarkers in HIV-Exposed Uninfected Children. AIDS Lond. Engl. 2013, 27, 1099–1108. [Google Scholar] [CrossRef]
- García-Otero, L.; López, M.; Goncé, A.; Fortuny, C.; Salazar, L.; Valenzuela-Alcaraz, B.; Guirado, L.; César, S.; Gratacós, E.; Crispi, F. Cardiac Remodeling and Hypertension in HIV-Uninfected Infants Exposed in Utero to Antiretroviral Therapy. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2021, 73, 586–593. [Google Scholar] [CrossRef]
- Jao, J.; Jacobson, D.L.; Yu, W.; Borkowsky, W.; Geffner, M.E.; McFarland, E.J.; Patel, K.; Williams, P.L.; Miller, T.; Pediatric HIV/AIDS Cohort Study. A Comparison of Metabolic Outcomes Between Obese HIV-Exposed Uninfected Youth From the PHACS SMARTT Study and HIV-Unexposed Youth from the NHANES Study in the United States. J. Acquir. Immune Defic. Syndr. 1999 2019, 81, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Claudio, C.C.; Patin, R.V.; Palchetti, C.Z.; Machado, D.M.; Succi, R.C.d.M.; Oliveira, F.L.C. Nutritional Status and Metabolic Disorders in HIV-Exposed Uninfected Prepubertal Children. Nutr. Burbank Los Angel. Cty. Calif 2013, 29, 1020–1023. [Google Scholar] [CrossRef] [PubMed]
- Jao, J.; Kirmse, B.; Yu, C.; Qiu, Y.; Powis, K.; Nshom, E.; Epie, F.; Tih, P.M.; Sperling, R.S.; Abrams, E.J.; et al. Lower Preprandial Insulin and Altered Fuel Use in HIV/Antiretroviral-Exposed Infants in Cameroon. J. Clin. Endocrinol. Metab. 2015, 100, 3260–3269. [Google Scholar] [CrossRef]
- Ibrahim, A.; Warton, F.L.; Fry, S.; Cotton, M.F.; Jacobson, S.W.; Jacobson, J.L.; Molteno, C.D.; Little, F.; van der Kouwe, A.J.W.; Laughton, B.; et al. Maternal ART throughout Gestation Prevents Caudate Volume Reductions in Neonates Who Are HIV Exposed but Uninfected. Front. Neurosci. 2023, 17, 1085589. [Google Scholar] [CrossRef] [PubMed]
- Green, F.; du Plooy, C.; Rehman, A.M.; Nhapi, R.T.; Lake, M.T.; Barnett, W.; Hoffman, N.; Zar, H.J.; Donald, K.A.; Stein, D.J.; et al. Language Outcomes of Preschool Children Who Are HIV-Exposed Uninfected: An Analysis of a South African Cohort. PLoS ONE 2024, 19, e0297471. [Google Scholar] [CrossRef]
- Toledo, G.; Côté, H.C.F.; Adler, C.; Thorne, C.; Goetghebuer, T. Neurological Development of Children Who Are HIV-Exposed and Uninfected. Dev. Med. Child Neurol. 2021, 63, 1161–1170. [Google Scholar] [CrossRef]
- Ezeamama, A.E.; Zalwango, S.K.; Sikorskii, A.; Tuke, R.; Musoke, P.M.; Giordani, B.; Boivin, M.J. In Utero and Peripartum Antiretroviral Exposure as Predictor of Cognition in 6- to 10-Year-Old HIV-Exposed Ugandan Children—A Prospective Cohort Study. HIV Med. 2021, 22, 592–604. [Google Scholar] [CrossRef]
- Smith, M.L.; Puka, K.; Sehra, R.; Read, S.E.; Bitnun, A. Longitudinal Development of Cognitive, Visuomotor and Adaptive Behavior Skills in HIV Uninfected Children, Aged 3-5 Years of Age, Exposed Pre- and Perinatally to Anti-Retroviral Medications. AIDS Care 2017, 29, 1302–1308. [Google Scholar] [CrossRef]
- Young, J.M.; Bitnun, A.; Read, S.E.; Smith, M.L. Early Academic Achievement of HIV-Exposed Uninfected Children Compared to HIV-Unexposed Uninfected Children at 5 Years of Age. Child Neuropsychol. J. Norm. Abnorm. Dev. Child. Adolesc. 2021, 27, 532–547. [Google Scholar] [CrossRef]
- Steventon Roberts, K.J.; Sherr, L.; Haag, K.; Smith, C.; Jochim, J.; Toska, E.; Marlow, M.; Cluver, L. Adolescent Parenthood and HIV-Infection in South Africa-Associations with Child Cognitive Development. PLOS Glob. Public Health 2022, 2, e0000238. [Google Scholar] [CrossRef]
- Kerr, S.J.; Puthanakit, T.; Malee, K.M.; Thongpibul, K.; Ly, P.S.; Sophonphan, J.; Suwanlerk, T.; Kosalaraksa, P.; Ounchanum, P.; Aurpibul, L.; et al. Increased Risk of Executive Function and Emotional Behavioral Problems Among Virologically Well-Controlled Perinatally HIV-Infected Adolescents in Thailand and Cambodia. J. Acquir. Immune Defic. Syndr. 1999 2019, 82, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.K.; Gupta, R.K.; Hashem, S.; Nisar, S.; Azeem, T.; Bhat, A.A.; Syed, N.; Garg, R.K.; Venkatesh, V.; Kamal, M.; et al. Brain Microstructural Changes Support Cognitive Deficits in HIV Uninfected Children Born to HIV Infected Mothers. Brain Behav. Immun.—Health 2020, 2, 100039. [Google Scholar] [CrossRef] [PubMed]
- Young, J.M.; Chen, V.; Bitnun, A.; Read, S.E.; Smith, M.L. Attention and Neurodevelopment in Young Children Who Are HIV-Exposed Uninfected. AIDS Care 2024, 36, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Rotheram-Borus, M.J.; Christodoulou, J.; Hayati Rezvan, P.; Comulada, W.S.; Gordon, S.; Skeen, S.; Stewart, J.; Almirol, E.; Tomlinson, M. Maternal HIV Does Not Affect Resiliency among Uninfected/HIV Exposed South African Children from Birth to 5 Years of Age. AIDS Lond. Engl. 2019, 33 (Suppl. S1), S5–S16. [Google Scholar] [CrossRef]
- Crowell, C.S.; Williams, P.L.; Yildirim, C.; Van Dyke, R.B.; Smith, R.; Chadwick, E.G.; Seage, G.R.; Diperna, A.; Hazra, R.; Pediatric HIV/AIDS Cohort Study. Safety of In-Utero Antiretroviral Exposure: Neurologic Outcomes in Children Who Are HIV-Exposed but Uninfected. AIDS Lond. Engl. 2020, 34, 1377–1387. [Google Scholar] [CrossRef]
- Graham, A.S.; Holmes, M.J.; Little, F.; Dobbels, E.; Cotton, M.F.; Laughton, B.; van der Kouwe, A.; Meintjes, E.M.; Robertson, F.C. MRS Suggests Multi-Regional Inflammation and White Matter Axonal Damage at 11 Years Following Perinatal HIV Infection. NeuroImage Clin. 2020, 28, 102505. [Google Scholar] [CrossRef]
- Wedderburn, C.J.; Yeung, S.; Subramoney, S.; Fouche, J.-P.; Joshi, S.H.; Narr, K.L.; Rehman, A.M.; Roos, A.; Gibb, D.M.; Zar, H.J.; et al. Association of in Utero HIV Exposure with Child Brain Structure and Language Development: A South African Birth Cohort Study. BMC Med. 2024, 22, 129. [Google Scholar] [CrossRef]
- van Wyhe, K.S.; Laughton, B.; Cotton, M.F.; Meintjes, E.M.; van der Kouwe, A.J.; Boivin, M.J.; Kidd, M.; Thomas, K.G. Cognitive Outcomes at Ages Seven and Nine Years in South African Children from the Children with HIV Early Antiretroviral (CHER) Trial: A Longitudinal Investigation. J. Int. AIDS Soc. 2021, 24, e25734. [Google Scholar] [CrossRef]
- Yao, T.-J.; Malee, K.; Zhang, J.; Smith, R.; Redmond, S.; Rice, M.L.; Frederick, T.; Torre, P.; Mellins, C.A.; Hoffman, H.J.; et al. In Utero Antiretroviral Exposure and Risk of Neurodevelopmental Problems in HIV-Exposed Uninfected 5-Year-Old Children. AIDS Patient Care STDs 2023, 37, 119–130. [Google Scholar] [CrossRef]
- Bulterys, M.A.; Njuguna, I.; King’e, M.; Chebet, D.; Moraa, H.; Gomez, L.; Onyango, A.; Malavi, K.; Nzia, G.; Chege, M.; et al. Neurodevelopment of Children Who Are HIV-Exposed and Uninfected in Kenya. J. Int. AIDS Soc. 2023, 26 (Suppl. S4), e26149. [Google Scholar] [CrossRef]
- Njuguna, I.N.; King’e, M.; Moraa, H.; Kumar, M.; Benki-Nugent, S.; Wagner, A.D.; McGrath, C.J.; Dorsey, S.; Ndegwa, S.; Onyango, A.; et al. Cohort Profile: Longitudinal and Population Comparison of Children Who Are HIV-Exposed Uninfected and Children Who Are HIV Unexposed in Kenya (HOPE Study). BMJ Open 2024, 14, e081975. [Google Scholar] [CrossRef] [PubMed]
- Dhume, S.H.; Balogun, K.; Sarkar, A.; Acosta, S.; Mount, H.T.J.; Cahill, L.S.; Sled, J.G.; Serghides, L. Perinatal Exposure to Atazanavir-Based Antiretroviral Regimens in a Mouse Model Leads to Differential Long-Term Motor and Cognitive Deficits Dependent on the NRTI Backbone. Front. Mol. Neurosci. 2024, 17, 1376681. [Google Scholar] [CrossRef] [PubMed]
- Familiar, I.; Majumder, A.; Sikorskii, A.; Boivin, M.; Nakasujja, N.; Bass, J. Longitudinal Dyadic Interdependence in Depression Symptoms of Caregivers Living with HIV in Uganda and Their Dependent Children’s Neurodevelopment and Executive Behavior Outcomes. AIDS Behav. 2021, 25, 3828–3835. [Google Scholar] [CrossRef] [PubMed]
- Ntozini, R.; Chandna, J.; Evans, C.; Chasekwa, B.; Majo, F.D.; Kandawasvika, G.; Tavengwa, N.V.; Mutasa, B.; Mutasa, K.; Moulton, L.H.; et al. Early Child Development in Children Who Are HIV-Exposed Uninfected Compared to Children Who Are HIV-Unexposed: Observational Sub-Study of a Cluster-Randomized Trial in Rural Zimbabwe. J. Int. AIDS Soc. 2020, 23, e25456. [Google Scholar] [CrossRef]
- Purswani, M.U.; Russell, J.S.; Dietrich, M.; Malee, K.; Spector, S.A.; Williams, P.L.; Frederick, T.; Burchett, S.; Redmond, S.; Hoffman, H.J.; et al. Birth Prevalence of Congenital Cytomegalovirus Infection in HIV-Exposed Uninfected Children in the Era of Combination Antiretroviral Therapy. J. Pediatr. 2020, 216, 82–87.e2. [Google Scholar] [CrossRef]
- Nachega, J.B.; Uthman, O.A.; Mofenson, L.M.; Anderson, J.R.; Kanters, S.; Renaud, F.; Ford, N.; Essajee, S.; Doherty, M.C.; Mills, E.J. Safety of Tenofovir Disoproxil Fumarate-Based Antiretroviral Therapy Regimens in Pregnancy for HIV-Infected Women and Their Infants: A Systematic Review and Meta-Analysis. J. Acquir. Immune Defic. Syndr. 1999 2017, 76, 1–12. [Google Scholar] [CrossRef]
- Salvadori, N.; Fan, B.; Teeyasoontranon, W.; Ngo-Giang-Huong, N.; Phanomcheong, S.; Luvira, A.; Puangsombat, A.; Suwannarat, A.; Srirompotong, U.; Putiyanun, C.; et al. Maternal and Infant Bone Mineral Density 1 Year After Delivery in a Randomized, Controlled Trial of Maternal Tenofovir Disoproxil Fumarate to Prevent Mother-to-Child Transmission of Hepatitis B Virus. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2019, 69, 144–146. [Google Scholar] [CrossRef]
- Floridia, M.; Liotta, G.; Andreotti, M.; Galluzzo, C.M.; Amici, R.; Jere, H.; Sagno, J.-B.; Marazzi, M.C.; Buonomo, E.; Scarcella, P.; et al. Levels of Bone Markers in a Population of Infants Exposed in Utero and during Breastfeeding to Tenofovir within an Option B+ Programme in Malawi. J. Antimicrob. Chemother. 2016, 71, 3206–3211. [Google Scholar] [CrossRef]
- Osorio, L.E.; Boechat, M.I.; Mirochnick, M.; Kumwenda, N.; Kreitchmann, R.; Emel, L.; Pinto, J.; Joao, E.; Santos, B.; Swenson, M.; et al. Bone Age and Mineral Density Assessments Using Plain Roentgenograms in Tenofovir-Exposed Infants in Malawi and Brazil Enrolled in HIV Prevention Trials Network 057. Pediatr. Infect. Dis. J. 2017, 36, 184–188. [Google Scholar] [CrossRef]
- Kourtis, A.P.; Wiener, J.; Wang, L.; Fan, B.; Shepherd, J.A.; Chen, L.; Liu, W.; Shepard, C.; Wang, L.; Wang, A.; et al. Tenofovir Disoproxil Fumarate Use during Pregnancy and Infant Bone Health: The Tenofovir in Pregnancy Pilot Study. Pediatr. Infect. Dis. J. 2018, 37, e264–e268. [Google Scholar] [CrossRef]
- Siberry, G.K.; Jacobson, D.L.; Kalkwarf, H.J.; Wu, J.W.; DiMeglio, L.A.; Yogev, R.; Knapp, K.M.; Wheeler, J.J.; Butler, L.; Hazra, R.; et al. Lower Newborn Bone Mineral Content Associated With Maternal Use of Tenofovir Disoproxil Fumarate During Pregnancy. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2015, 61, 996–1003. [Google Scholar] [CrossRef] [PubMed]
- Ellis, R.P.; Evans, C.; Wedderburn, C.J.; Prendergast, A.J. Children Who Are HIV Exposed-Uninfected: Does Maternal ART Regimen Matter? Curr. Opin. HIV AIDS 2024, 19, 316–322. [Google Scholar] [CrossRef] [PubMed]
- Purswani, M.U.; Jacobson, D.L.; DiMeglio, L.A.; Yao, T.-J.; Kopp, J.B.; Van Dyke, R.B.; Yu, W.; Siberry, G.K.; Pediatric HIV/AIDS Cohort Study (PHACS). Phosphaturia in HIV-Exposed Uninfected Neonates Associated with Maternal Use of Tenofovir Disoproxil Fumarate in Late Pregnancy. J. Pediatr. Infect. Dis. Soc. 2024, 13, 396–405. [Google Scholar] [CrossRef] [PubMed]
- Kapito-Tembo, A.P.; Bauleni, A.; Wesevich, A.; Ongubo, D.; Hosseinipour, M.C.; Dube, Q.; Mwale, P.; Corbett, A.; Mwapasa, V.; Phiri, S. Growth and Neurodevelopment Outcomes in HIV-, Tenofovir-, and Efavirenz-Exposed Breastfed Infants in the PMTCT Option B+ Program in Malawi. J. Acquir. Immune Defic. Syndr. 1999 2021, 86, 81–90. [Google Scholar] [CrossRef]
- Eckard, A.R.; Mora, S. Bone Health in HIV-Infected Children and Adolescents. Curr. Opin. HIV AIDS 2016, 11, 294–300. [Google Scholar] [CrossRef]
- Jumare, J.; Datong, P.; Osawe, S.; Okolo, F.; Mohammed, S.; Inyang, B.; Abimiku, A.; INFANT Study Team. Compromised Growth Among HIV-Exposed Uninfected Compared With Unexposed Children in Nigeria. Pediatr. Infect. Dis. J. 2019, 38, 280–286. [Google Scholar] [CrossRef]
- Aizire, J.; Sikorskii, A.; Ogwang, L.W.; Kawalazira, R.; Mutebe, A.; Familiar-Lopez, I.; Mallewa, M.; Taha, T.; Boivin, M.J.; Fowler, M.G.; et al. Decreased Growth among Antiretroviral Drug and HIV-Exposed Uninfected versus Unexposed Children in Malawi and Uganda. AIDS Lond. Engl. 2020, 34, 215–225. [Google Scholar] [CrossRef]
- Evans, C.; Chasekwa, B.; Ntozini, R.; Majo, F.D.; Mutasa, K.; Tavengwa, N.; Mutasa, B.; Mbuya, M.N.N.; Smith, L.E.; Stoltzfus, R.J.; et al. Mortality, Human Immunodeficiency Virus (HIV) Transmission, and Growth in Children Exposed to HIV in Rural Zimbabwe. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2021, 72, 586–594. [Google Scholar] [CrossRef]
- Mabaya, L.; Matarira, H.T.; Tanyanyiwa, D.M.; Musarurwa, C.; Mukwembi, J. Growth Trajectories of HIV Exposed and HIV Unexposed Infants. A Prospective Study in Gweru, Zimbabwe. Glob. Pediatr. Health 2021, 8, 2333794X21990338. [Google Scholar] [CrossRef]
- Rosala-Hallas, A.; Bartlett, J.W.; Filteau, S. Growth of HIV-Exposed Uninfected, Compared with HIV-Unexposed, Zambian Children: A Longitudinal Analysis from Infancy to School Age. BMC Pediatr. 2017, 17, 80. [Google Scholar] [CrossRef]
- Lane, C.E.; Widen, E.M.; Collins, S.M.; Young, S.L. HIV-Exposed, Uninfected Infants in Uganda Experience Poorer Growth and Body Composition Trajectories than HIV-Unexposed Infants. J. Acquir. Immune Defic. Syndr. 1999 2020, 85, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Neary, J.; Langat, A.; Singa, B.; Kinuthia, J.; Itindi, J.; Nyaboe, E.; Ng’anga’, L.W.; Katana, A.; John-Stewart, G.C.; McGrath, C.J. Higher Prevalence of Stunting and Poor Growth Outcomes in HIV-Exposed Uninfected than HIV-Unexposed Infants in Kenya. AIDS Lond. Engl. 2022, 36, 605–610. [Google Scholar] [CrossRef] [PubMed]
- le Roux, S.M.; Abrams, E.J.; Donald, K.A.; Brittain, K.; Phillips, T.K.; Nguyen, K.K.; Zerbe, A.; Kroon, M.; Myer, L. Growth Trajectories of Breastfed HIV-Exposed Uninfected and HIV-Unexposed Children under Conditions of Universal Maternal Antiretroviral Therapy: A Prospective Study. Lancet Child Adolesc. Health 2019, 3, 234–244. [Google Scholar] [CrossRef]
- Dewey, K.G.; Mayers, D.R. Early Child Growth: How Do Nutrition and Infection Interact? Matern. Child. Nutr. 2011, 7 (Suppl. S3), 129–142. [Google Scholar] [CrossRef] [PubMed]
- Toledo, G.; Landes, M.; van Lettow, M.; Tippett Barr, B.A.; Bailey, H.; Crichton, S.; Msungama, W.; Thorne, C. Risk Factors for Stunting in Children Who Are HIV-Exposed and Uninfected after Option B+ Implementation in Malawi. Matern. Child Nutr. 2023, 19, e13451. [Google Scholar] [CrossRef]
- Ejigu, Y.; Magnus, J.H.; Sundby, J.; Magnus, M.C. Differences in Growth of HIV-Exposed Uninfected Infants in Ethiopia According to Timing of In-Utero Antiretroviral Therapy Exposure. Pediatr. Infect. Dis. J. 2020, 39, 730–736. [Google Scholar] [CrossRef]
- Lwanga, C.; Aber, P.; Tickell, K.D.; Ngari, M.M.; Mukisa, J.; Atuhairwe, M.; Brown, L.; Mupere, E.; Potani, I.; Shahrin, L.; et al. Impact of HIV Exposure without Infection on Hospital Course and Mortality among Young Children in Sub-Saharan Africa: A Multi-Site Cohort Study. BMC Med. 2024, 22, 573. [Google Scholar] [CrossRef]
- Malawi Demographic and Health Survey 2015–16. Available online: https://dhsprogram.com/pubs/pdf/FR319/FR319.pdf (accessed on 15 January 2025).
- Powis, K.M.; Smeaton, L.; Hughes, M.D.; Tumbare, E.A.; Souda, S.; Jao, J.; Wirth, K.E.; Makhema, J.; Lockman, S.; Fawzi, W.; et al. In-Utero Triple Antiretroviral Exposure Associated with Decreased Growth among HIV-Exposed Uninfected Infants in Botswana. AIDS Lond. Engl. 2016, 30, 211–220. [Google Scholar] [CrossRef]
- Ruff, A.; Dlamini, X.; Nonyane, B.A.; Simmons, N.; Kochelani, D.; Burtt, F.; Mlotshwa, F.; Gama, N.; Scheepers, E.; Schmitz, K.; et al. A Trial of Nurturing Care among Children Who Are HIV-Exposed and Uninfected in eSwatini. J. Int. AIDS Soc. 2023, 26 (Suppl. S4), e26158. [Google Scholar] [CrossRef]
- Baethge, C.; Goldbeck-Wood, S.; Mertens, S. SANRA-a Scale for the Quality Assessment of Narrative Review Articles. Res. Integr. Peer Rev. 2019, 4, 5. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salvi, M.; Fioretti, B.; Alberti, M.; Scarvaglieri, I.; Arsuffi, S.; Tiecco, G.; Castelli, F.; Quiros-Roldan, E. Understanding HIV-Exposed Uninfected Children: A Narrative Review. Viruses 2025, 17, 442. https://doi.org/10.3390/v17030442
Salvi M, Fioretti B, Alberti M, Scarvaglieri I, Arsuffi S, Tiecco G, Castelli F, Quiros-Roldan E. Understanding HIV-Exposed Uninfected Children: A Narrative Review. Viruses. 2025; 17(3):442. https://doi.org/10.3390/v17030442
Chicago/Turabian StyleSalvi, Martina, Benedetta Fioretti, Maria Alberti, Irene Scarvaglieri, Stefania Arsuffi, Giorgio Tiecco, Francesco Castelli, and Eugenia Quiros-Roldan. 2025. "Understanding HIV-Exposed Uninfected Children: A Narrative Review" Viruses 17, no. 3: 442. https://doi.org/10.3390/v17030442
APA StyleSalvi, M., Fioretti, B., Alberti, M., Scarvaglieri, I., Arsuffi, S., Tiecco, G., Castelli, F., & Quiros-Roldan, E. (2025). Understanding HIV-Exposed Uninfected Children: A Narrative Review. Viruses, 17(3), 442. https://doi.org/10.3390/v17030442






