A New Human SCARB2 Knock-In Mouse Model for Studying Coxsackievirus A16 and Its Neurotoxicity
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Cell and Virus
2.3. Virus Enrichment and Titration
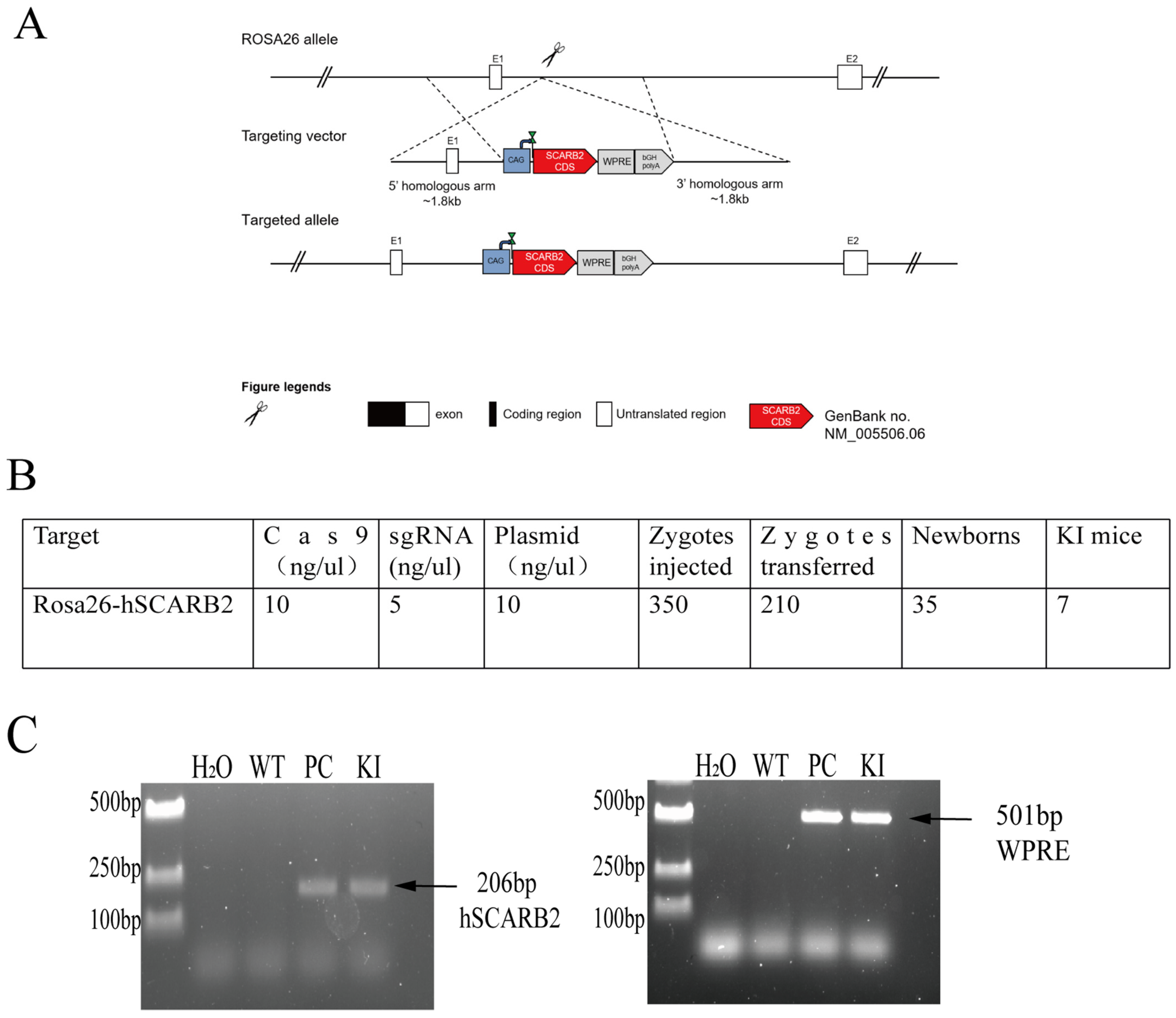
2.4. Generation of ROSA26-Targeted hSCARB2 KI F0 Mice via Cytoplasmic Microinjection
2.5. Genotyping
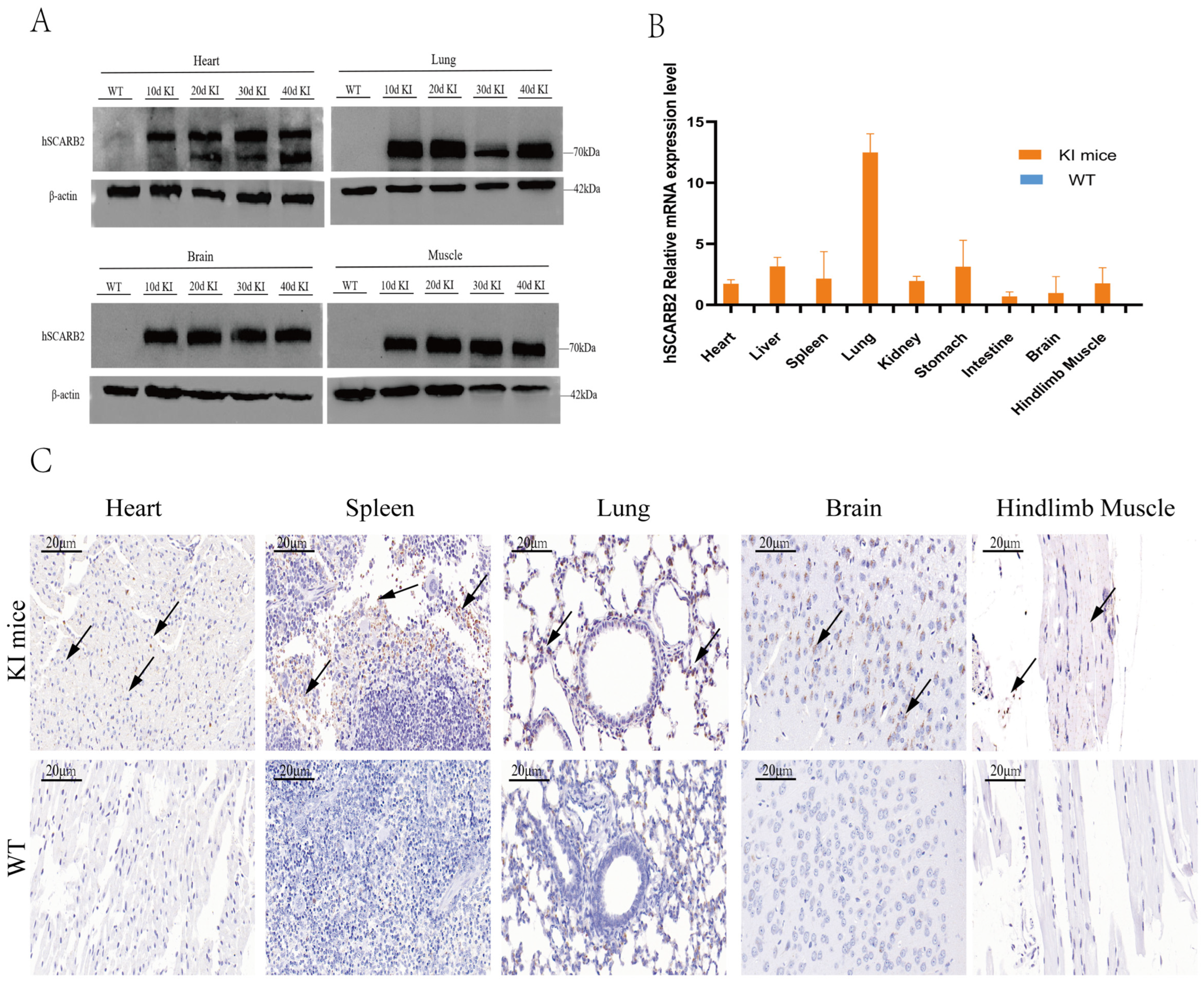
2.6. Western Blotting
2.7. CVA16 Infection
2.8. Real-Time PCR
2.9. Histopathological and Immunohistochemical Staining
2.10. Immunofluorescence Staining
2.11. Statistical Analysis
3. Results
3.1. Generation of ROSA26-hSCARB2 KI Mice
3.2. The Expression of hSCARB2 in KI Mice
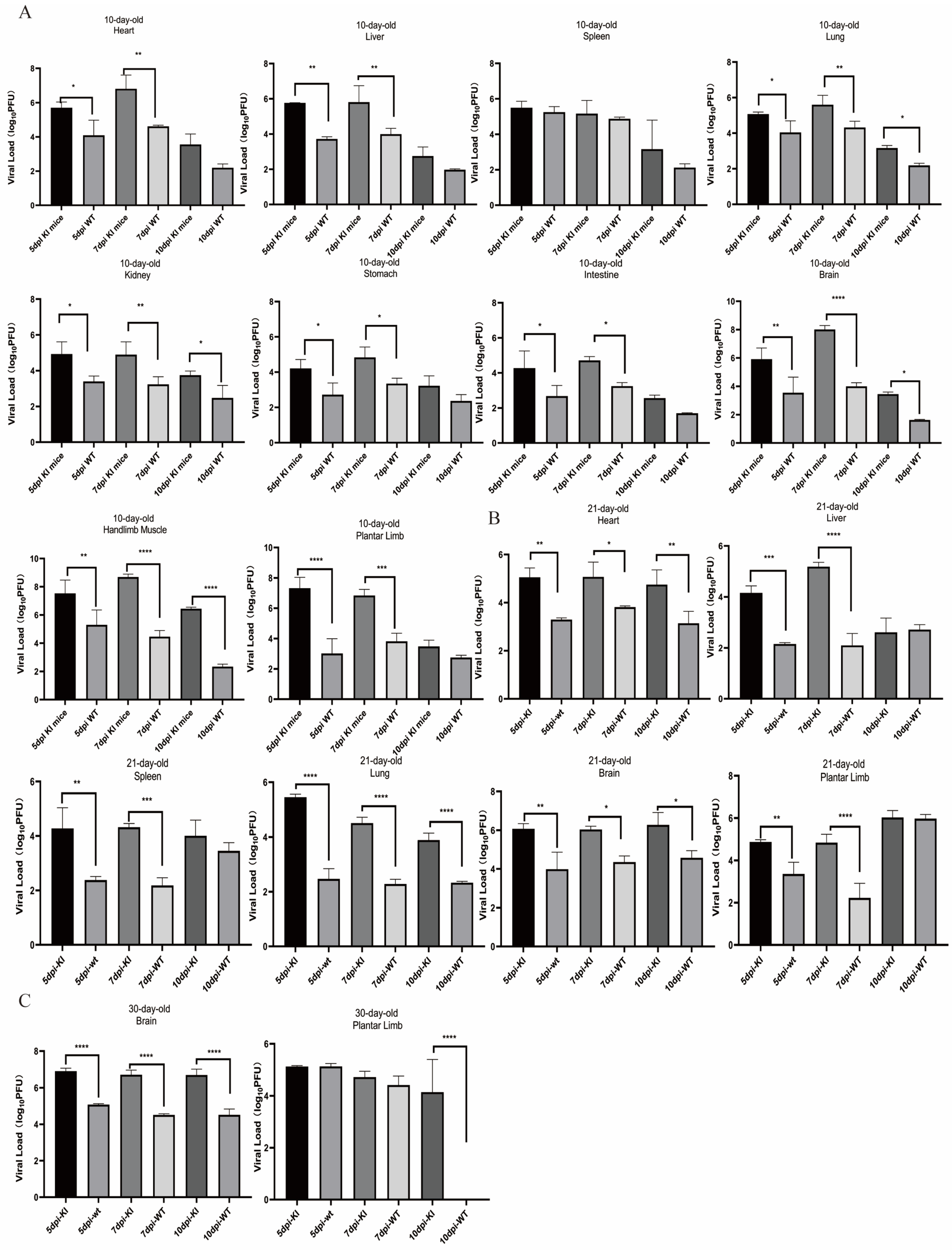
3.3. Susceptibility of hSCARB2 Mice to CVA16 Infection
3.4. Dynamic Changes in the CVA16 Viral Load and Detection of Viral Expression in Infected KI Mice
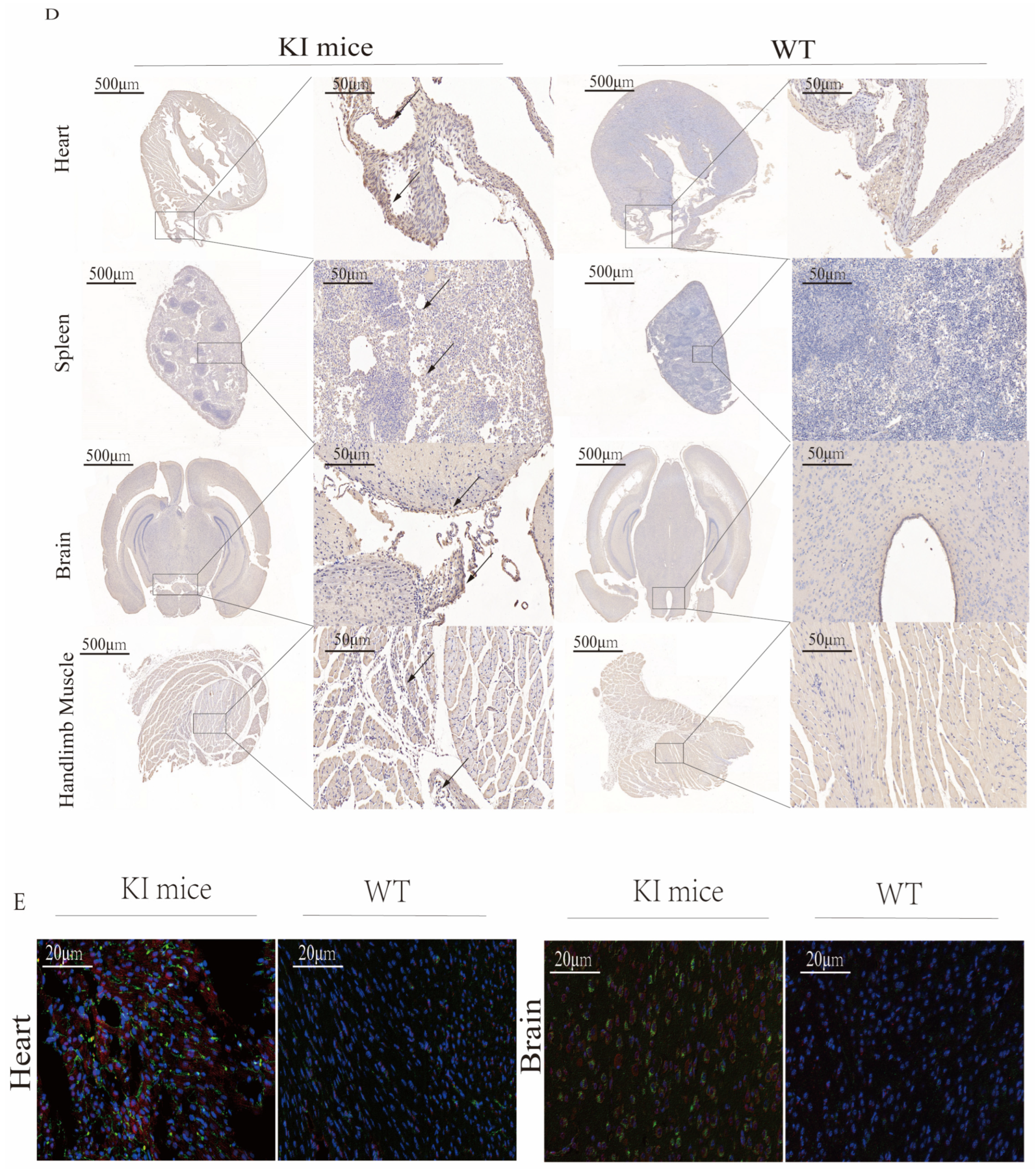
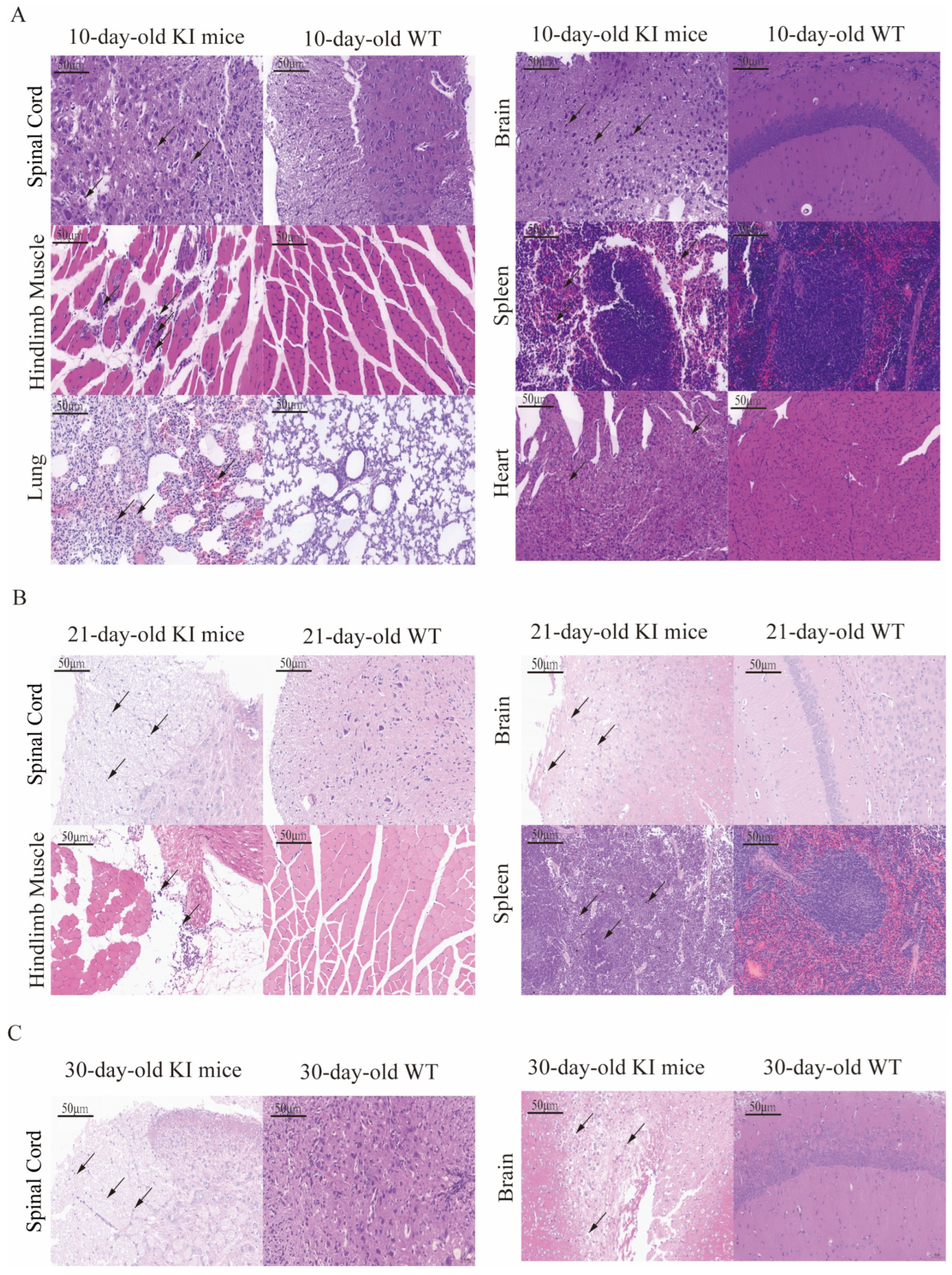
3.5. Histopathological Examination of CVA16-Infected Mice
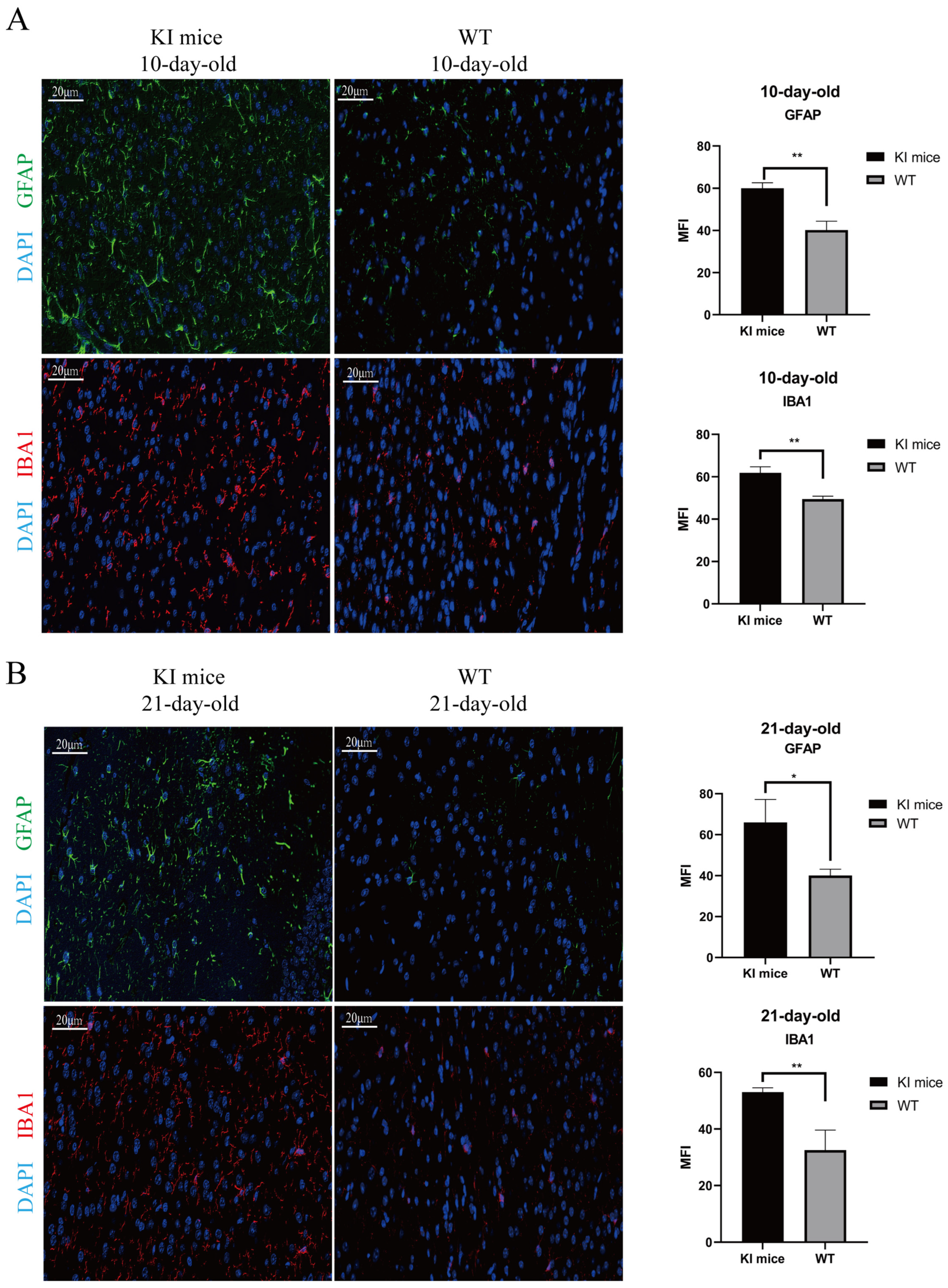
3.6. Activation of Glial Cells in CVA16-Infected Brains
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhu, P.; Ji, W.; Li, D.; Li, Z.; Chen, Y.; Dai, B.; Han, S.; Chen, S.; Jin, Y.; Duan, G. Current Status of Hand-Foot-and-Mouth Disease. J. Biomed. Sci. 2023, 30, 15. [Google Scholar] [CrossRef]
- Tikute, S.; Lavania, M. Hand, Foot, and Mouth Disease (HFMD) in India: A Review on Clinical Manifestations, Molecular Epidemiology, Pathogenesis, and Prevention. Indian Dermatol. Online J. 2023, 14, 475–481. [Google Scholar] [CrossRef] [PubMed]
- Aswathyraj, S.; Arunkumar, G.; Alidjinou, E.K.; Hober, D. Hand, Foot and Mouth Disease (HFMD): Emerging Epidemiology and the Need for a Vaccine Strategy. Med. Microbiol. Immunol. 2016, 205, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.-Y.; Liu, C.-C. Recent Development of Enterovirus A Vaccine Candidates for the Prevention of Hand, Foot, and Mouth Disease. Expert Rev. Vaccines 2018, 17, 819–831. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Liu, F.; Chang, L.; Lai, S.; Teng, J.; Duan, J.; Jian, H.; Liu, T.; Che, G. Molecular Epidemiology and Clinical Characteristics of Enteroviruses Associated HFMD in Chengdu, China, 2013–2022. Virol. J. 2023, 20, 202. [Google Scholar] [CrossRef]
- Chiu, M.-L.; Luo, S.-T.; Chen, Y.-Y.; Chung, W.Y.; Duong, V.; Dussart, P.; Chan, Y.-F.; Perera, D.; Ooi, M.H.; Thao, N.T.T.; et al. Establishment of Asia-Pacific Network for Enterovirus Surveillance. Vaccine 2020, 38, 1–9. [Google Scholar] [CrossRef]
- Bello, A.M.; Roshorm, Y.M. Recent Progress and Advances towards Developing Enterovirus 71 Vaccines for Effective Protection against Human Hand, Foot and Mouth Disease (HFMD). Biologicals 2022, 79, 1–9. [Google Scholar] [CrossRef]
- Chakraborty, S.; Mohapatra, R.K.; Chandran, D.; Rana, R.; Nainu, F.; Chakraborty, C.; Chaicumpa, W.; Dhama, K. Hand-Foot-and-Mouth Disease (HFMD) in Children. Current Scenario, and Advancements in Developing Vaccines and Therapeutics: An Update–Correspondence. Int. J. Surg. 2022, 105, 106834. [Google Scholar] [CrossRef]
- Yi, E.-J.; Shin, Y.-J.; Kim, J.-H.; Kim, T.-G.; Chang, S.-Y. Enterovirus 71 Infection and Vaccines. Clin. Exp. Vaccine Res. 2017, 6, 4–14. [Google Scholar] [CrossRef]
- Phanthong, S.; Densumite, J.; Seesuay, W.; Thanongsaksrikul, J.; Teimoori, S.; Sookrung, N.; Poovorawan, Y.; Onvimala, N.; Guntapong, R.; Pattanapanyasat, K.; et al. Human Antibodies to VP4 Inhibit Replication of Enteroviruses Across Subgenotypes and Serotypes, and Enhance Host Innate Immunity. Front. Microbiol. 2020, 11, 562768. [Google Scholar] [CrossRef]
- Qiao, X.; Liu, X.; Wang, Y.; Li, Y.; Wang, L.; Yang, Q.; Wang, H.; Shen, H. Analysis of the Epidemiological Trends of Enterovirus A in Asia and Europe. J. Infect. Chemother. 2023, 29, 316–321. [Google Scholar] [CrossRef] [PubMed]
- Bian, L.; Gao, F.; Mao, Q.; Sun, S.; Wu, X.; Liu, S.; Yang, X.; Liang, Z. Hand, Foot, and Mouth Disease Associated with Coxsackievirus A10: More Serious than It Seems. Expert. Rev. Anti-Infect. Ther. 2019, 17, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, Y.; Li, H.; Liu, L. Hand-Foot-and-Mouth Disease-Associated Enterovirus and the Development of Multivalent HFMD Vaccines. Int. J. Mol. Sci. 2023, 24, 169. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-F.; Yu, C.-K. Animal Models of Enterovirus 71 Infection: Applications and Limitations. J. Biomed. Sci. 2014, 21, 31. [Google Scholar] [CrossRef]
- Yao, P.-P.; Miao, Z.-P.; Xu, F.; Lu, H.-J.; Sun, Y.-S.; Xia, Y.; Chen, C.; Yang, Z.-N.; Xia, S.-C.; Jiang, J.-M.; et al. An Adult Gerbil Model for Evaluating Potential Coxsackievirus A16 Vaccine Candidates. Vaccine 2019, 37, 5341–5349. [Google Scholar] [CrossRef]
- Caine, E.A.; Fuchs, J.; Das, S.C.; Partidos, C.D.; Osorio, J.E. Efficacy of a Trivalent Hand, Foot, and Mouth Disease Vaccine against Enterovirus 71 and Coxsackieviruses A16 and A6 in Mice. Viruses 2015, 7, 5919–5932. [Google Scholar] [CrossRef]
- Liu, Q.; Shi, J.; Huang, X.; Liu, F.; Cai, Y.; Lan, K.; Huang, Z. A Murine Model of Coxsackievirus A16 Infection for Anti-Viral Evaluation. Antiviral Res. 2014, 105, 26–31. [Google Scholar] [CrossRef]
- Shih, C.; Liao, C.-C.; Chang, Y.-S.; Wu, S.-Y.; Chang, C.-S.; Liou, A.-T. Immunocompetent and Immunodeficient Mouse Models for Enterovirus 71 Pathogenesis and Therapy. Viruses 2018, 10, 674. [Google Scholar] [CrossRef]
- Fujii, K.; Nagata, N.; Sato, Y.; Ong, K.C.; Wong, K.T.; Yamayoshi, S.; Shimanuki, M.; Shitara, H.; Taya, C.; Koike, S. Transgenic Mouse Model for the Study of Enterovirus 71 Neuropathogenesis. Proc. Natl. Acad. Sci. USA 2013, 110, 14753–14758. [Google Scholar] [CrossRef]
- Song, J.M. Experimental Animal Models for Development of Human Enterovirus Vaccine. Clin. Exp. Vaccine Res. 2023, 12, 291–297. [Google Scholar] [CrossRef]
- Lin, J.-Y.; Shih, S.-R. Cell and Tissue Tropism of Enterovirus 71 and Other Enteroviruses Infections. J. Biomed. Sci. 2014, 21, 18. [Google Scholar] [CrossRef] [PubMed]
- Yamayoshi, S.; Iizuka, S.; Yamashita, T.; Minagawa, H.; Mizuta, K.; Okamoto, M.; Nishimura, H.; Sanjoh, K.; Katsushima, N.; Itagaki, T.; et al. Human SCARB2-Dependent Infection by Coxsackievirus A7, A14, and A16 and Enterovirus 71. J. Virol. 2012, 86, 5686–5696. [Google Scholar] [CrossRef]
- Kuronita, T.; Eskelinen, E.-L.; Fujita, H.; Saftig, P.; Himeno, M.; Tanaka, Y. A Role for the Lysosomal Membrane Protein LGP85 in the Biogenesis and Maintenance of Endosomal and Lysosomal Morphology. J. Cell Sci. 2002, 115, 4117–4131. [Google Scholar] [CrossRef] [PubMed]
- Reczek, D.; Schwake, M.; Schröder, J.; Hughes, H.; Blanz, J.; Jin, X.; Brondyk, W.; Van Patten, S.; Edmunds, T.; Saftig, P. LIMP-2 Is a Receptor for Lysosomal Mannose-6-Phosphate-Independent Targeting of β-Glucocerebrosidase. Cell 2007, 131, 770–783. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Liu, Q.; Wu, X.; Chen, P.; Wu, X.; Guo, Y.; Liu, S.; Liang, Z.; Fan, C.; Wang, Y. A Safe and Sensitive Enterovirus A71 Infection Model Based on Human SCARB2 Knock-in Mice. Vaccine 2016, 34, 2729–2736. [Google Scholar] [CrossRef]
- Chen, Y.; Li, H.; Yang, J.; Zheng, H.; Guo, L.; Li, W.; Yang, Z.; Song, J.; Liu, L. A hSCARB2-Transgenic Mouse Model for Coxsackievirus A16 Pathogenesis. Virol. J. 2021, 18, 84. [Google Scholar] [CrossRef]
- Lanigan, T.M.; Kopera, H.C.; Saunders, T.L. Principles of Genetic Engineering. Genes 2020, 11, 291. [Google Scholar] [CrossRef]
- Kobayashi, K.; Koike, S. Cellular Receptors for Enterovirus A71. J. Biomed. Sci. 2020, 27, 23. [Google Scholar] [CrossRef]
- Miwatashi, W.; Ishida, M.; Takashino, A.; Kobayashi, K.; Yamaguchi, M.; Shitara, H.; Koike, S. Mouse Scarb2 Modulates EV-A71 Pathogenicity in Neonatal Mice. J. Virol. 2022, 96, e0056122. [Google Scholar] [CrossRef]
- Wu, Y.; Qu, Z.; Xiong, R.; Yang, Y.; Liu, S.; Nie, J.; Liang, C.; Huang, W.; Wang, Y.; Fan, C. A Practical Method for Evaluating the in Vivo Efficacy of EVA-71 Vaccine Using a hSCARB2 Knock-in Mouse Model. Emerg. Microbes Infect. 2021, 10, 1180–1190. [Google Scholar] [CrossRef]
- Imura, A.; Sudaka, Y.; Takashino, A.; Tamura, K.; Kobayashi, K.; Nagata, N.; Nishimura, H.; Mizuta, K.; Koike, S. Development of an Enterovirus 71 Vaccine Efficacy Test Using Human Scavenger Receptor B2 Transgenic Mice. J. Virol. 2020, 94, e01921-19. [Google Scholar] [CrossRef] [PubMed]
- Ozawa, M.; Emori, C.; Ikawa, M. CRISPR/Cas9-Mediated Highly Efficient Gene Targeting in Embryonic Stem Cells for Developing Gene-Manipulated Mouse Models. J. Vis. Exp. 2022, 186. [Google Scholar] [CrossRef]
- Major, L.; McClements, M.E.; MacLaren, R.E. A Review of CRISPR Tools for Treating Usher Syndrome: Applicability, Safety, Efficiency, and In Vivo Delivery. Int. J. Mol. Sci. 2023, 24, 7603. [Google Scholar] [CrossRef]
- Janik, E.; Niemcewicz, M.; Ceremuga, M.; Krzowski, L.; Saluk-Bijak, J.; Bijak, M. Various Aspects of a Gene Editing System-CRISPR-Cas9. Int. J. Mol. Sci. 2020, 21, 9604. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, Y. Recent Progress on Functional Genomics Research of Enterovirus 71. Virol. Sin. 2019, 34, 9–21. [Google Scholar] [CrossRef]
- Hooi, Y.T.; Ong, K.C.; Tan, S.H.; Perera, D.; Wong, K.T. Coxsackievirus A16 in a 1-Day-Old Mouse Model of Central Nervous System Infection Shows Lower Neurovirulence than Enterovirus A71. J. Comp. Pathol. 2020, 176, 19–32. [Google Scholar] [CrossRef]
- Tikute, S.S.; Wangikar, P.B.; Varanasi, G. Pathological and Molecular Studies on Coxsackie Virus A-16 Isolated from Hand, Foot, and Mouth Disease Cases in India: Approach Using Neonatal Mouse Model. J. Med. Virol. 2019, 91, 1765–1775. [Google Scholar] [CrossRef]
- Hooi, Y.T.; Ong, K.C.; Tan, S.H.; Perera, D.; Wong, K.T. A Novel Orally Infected Hamster Model for Coxsackievirus A16 Hand-Foot-and-Mouth Disease and Encephalomyelitis. Lab. Investig. 2020, 100, 1262–1275. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, X.; Zhang, L.; Sun, Y.; Liang, Y.; Li, H.; Zhang, Y. Role of Trigger Receptor 2 Expressed on Myeloid Cells in Neuroinflammation-neglected Multidimensional Regulation of Microglia. Neurochem. Int. 2023, 171, 105639. [Google Scholar] [CrossRef]
- Patani, R.; Hardingham, G.E.; Liddelow, S.A. Functional Roles of Reactive Astrocytes in Neuroinflammation and Neurodegeneration. Nat. Rev. Neurol. 2023, 19, 395–409. [Google Scholar] [CrossRef]
- Kwon, H.S.; Koh, S.-H. Neuroinflammation in Neurodegenerative Disorders: The Roles of Microglia and Astrocytes. Transl. Neurodegener. 2020, 9, 42. [Google Scholar] [CrossRef] [PubMed]
- Bernier, L.-P.; York, E.M.; MacVicar, B.A. Immunometabolism in the Brain: How Metabolism Shapes Microglial Function. Trends Neurosci. 2020, 43, 854–869. [Google Scholar] [CrossRef] [PubMed]
- Benichou Haziot, C.; Birak, K.S. Therapeutic Potential of Microbiota Modulation in Alzheimer’s Disease: A Review of Preclinical Studies. J. Alzheimers Dis. Rep. 2023, 7, 415–431. [Google Scholar] [CrossRef] [PubMed]
- Linnerbauer, M.; Wheeler, M.A.; Quintana, F.J. Astrocyte Crosstalk in CNS Inflammation. Neuron 2020, 108, 608–622. [Google Scholar] [CrossRef]
- Lee, H.-G.; Lee, J.-H.; Flausino, L.E.; Quintana, F.J. Neuroinflammation: An Astrocyte Perspective. Sci. Transl. Med. 2023, 15, eadi7828. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, H.; Wang, Z.; Zhang, Y.; Hu, L.; Yang, J.; Zhang, C.; Lou, M.; Pi, N.; Wang, Q.; Fan, S.; et al. A New Human SCARB2 Knock-In Mouse Model for Studying Coxsackievirus A16 and Its Neurotoxicity. Viruses 2025, 17, 423. https://doi.org/10.3390/v17030423
Wu H, Wang Z, Zhang Y, Hu L, Yang J, Zhang C, Lou M, Pi N, Wang Q, Fan S, et al. A New Human SCARB2 Knock-In Mouse Model for Studying Coxsackievirus A16 and Its Neurotoxicity. Viruses. 2025; 17(3):423. https://doi.org/10.3390/v17030423
Chicago/Turabian StyleWu, Haiting, Ziou Wang, Yiwei Zhang, Lingfeng Hu, Jinling Yang, Caixing Zhang, Mumeng Lou, Na Pi, Qiyan Wang, Shengtao Fan, and et al. 2025. "A New Human SCARB2 Knock-In Mouse Model for Studying Coxsackievirus A16 and Its Neurotoxicity" Viruses 17, no. 3: 423. https://doi.org/10.3390/v17030423
APA StyleWu, H., Wang, Z., Zhang, Y., Hu, L., Yang, J., Zhang, C., Lou, M., Pi, N., Wang, Q., Fan, S., & Huang, Z. (2025). A New Human SCARB2 Knock-In Mouse Model for Studying Coxsackievirus A16 and Its Neurotoxicity. Viruses, 17(3), 423. https://doi.org/10.3390/v17030423





