Prevalence of EBV, HHV6, HCMV, HAdV, SARS-CoV-2, and Autoantibodies to Type I Interferon in Sputum from Myalgic Encephalomyelitis/Chronic Fatigue Syndrome Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Ethical Permit
2.3. Sputum Collection
2.4. Analysis of EBV, HCMV, HHV6, HAdV, and SARS-CoV-2 in Sputum
2.5. ELISA Analysis of IgG autoAbs to Type-I IFN
2.6. Statistical Analyses
3. Results
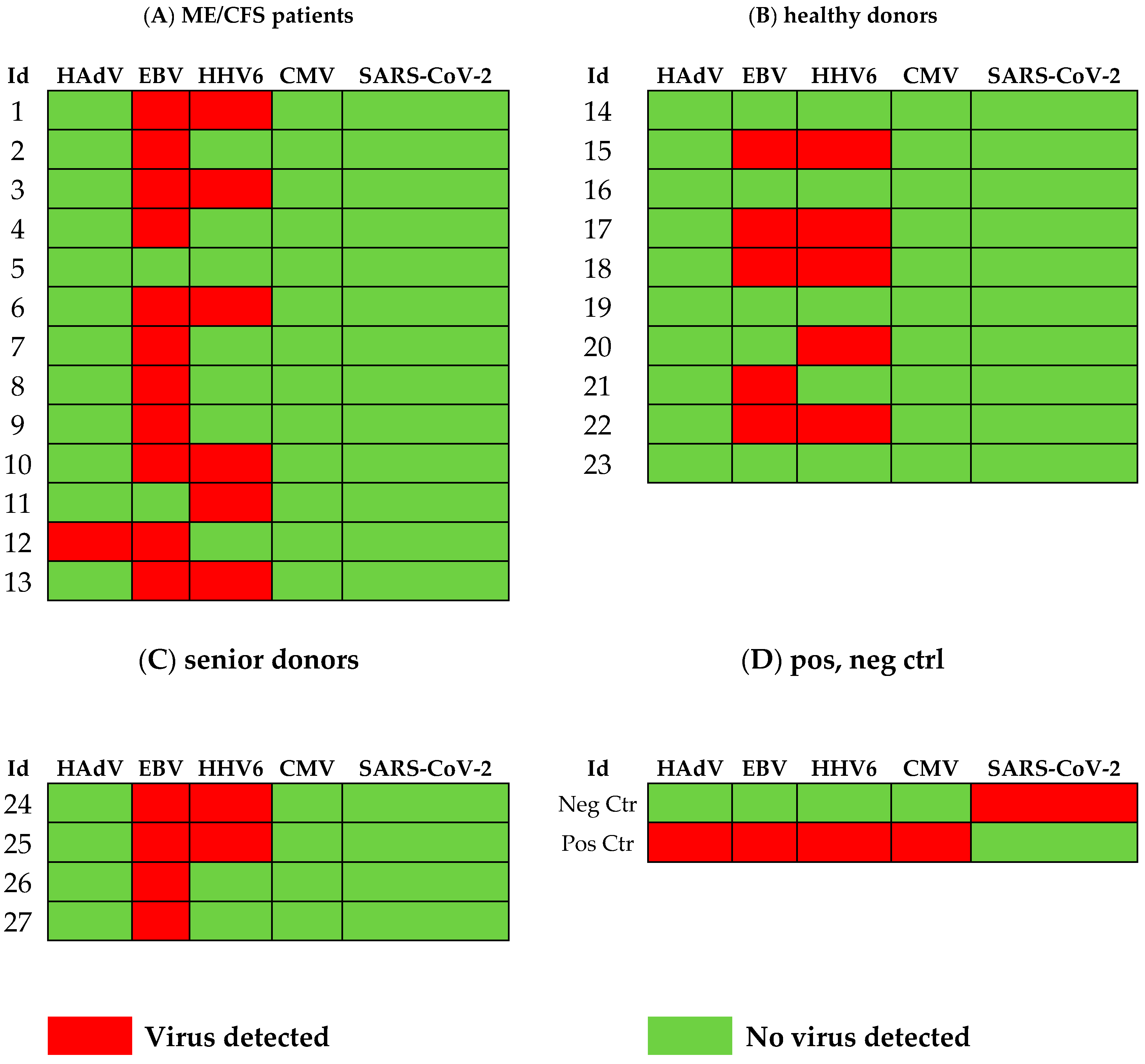
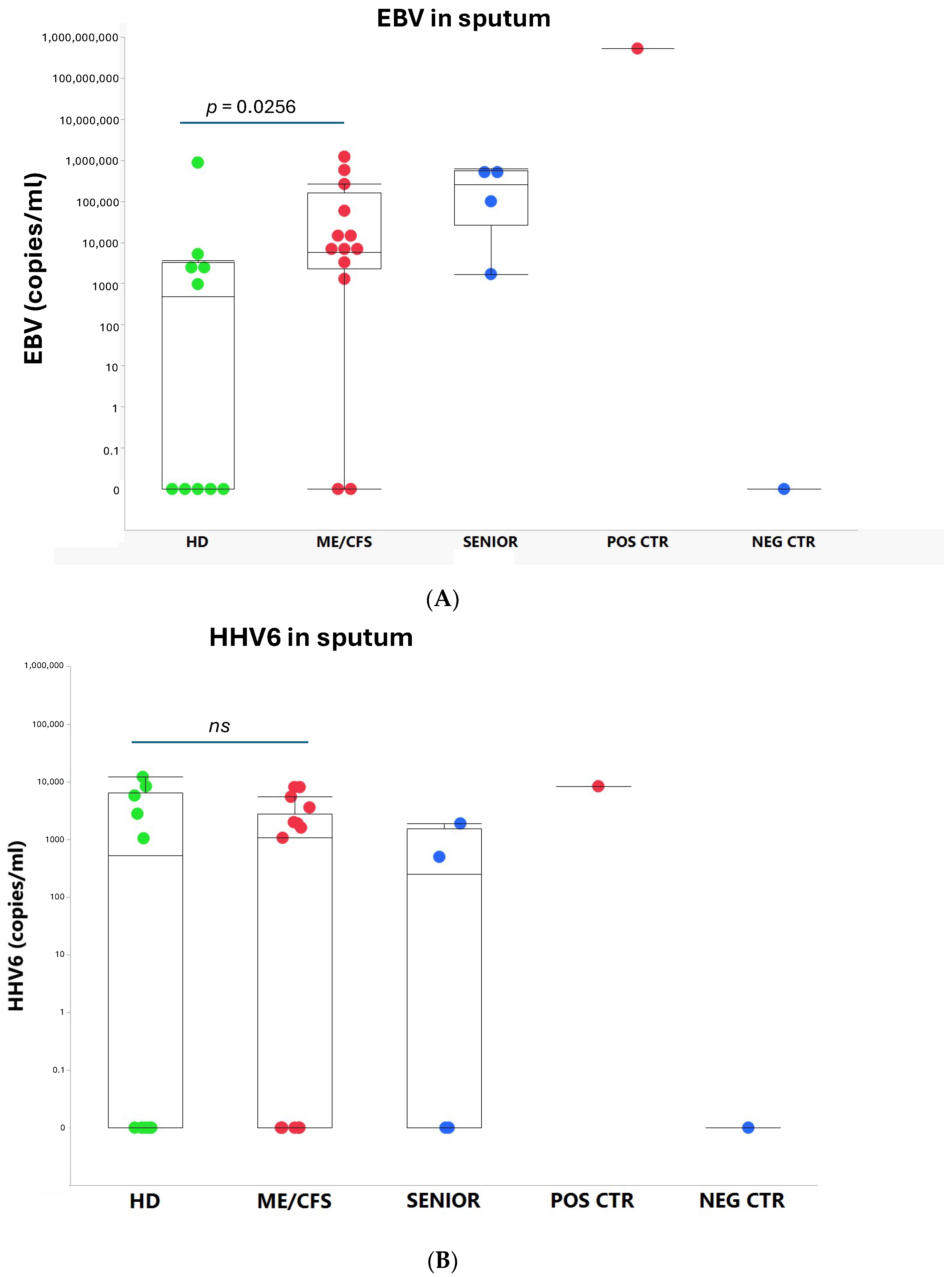
3.1. Viral Load of EBV, HCMV, HHV6, HAdV, and SARS-CoV-2 in Sputum
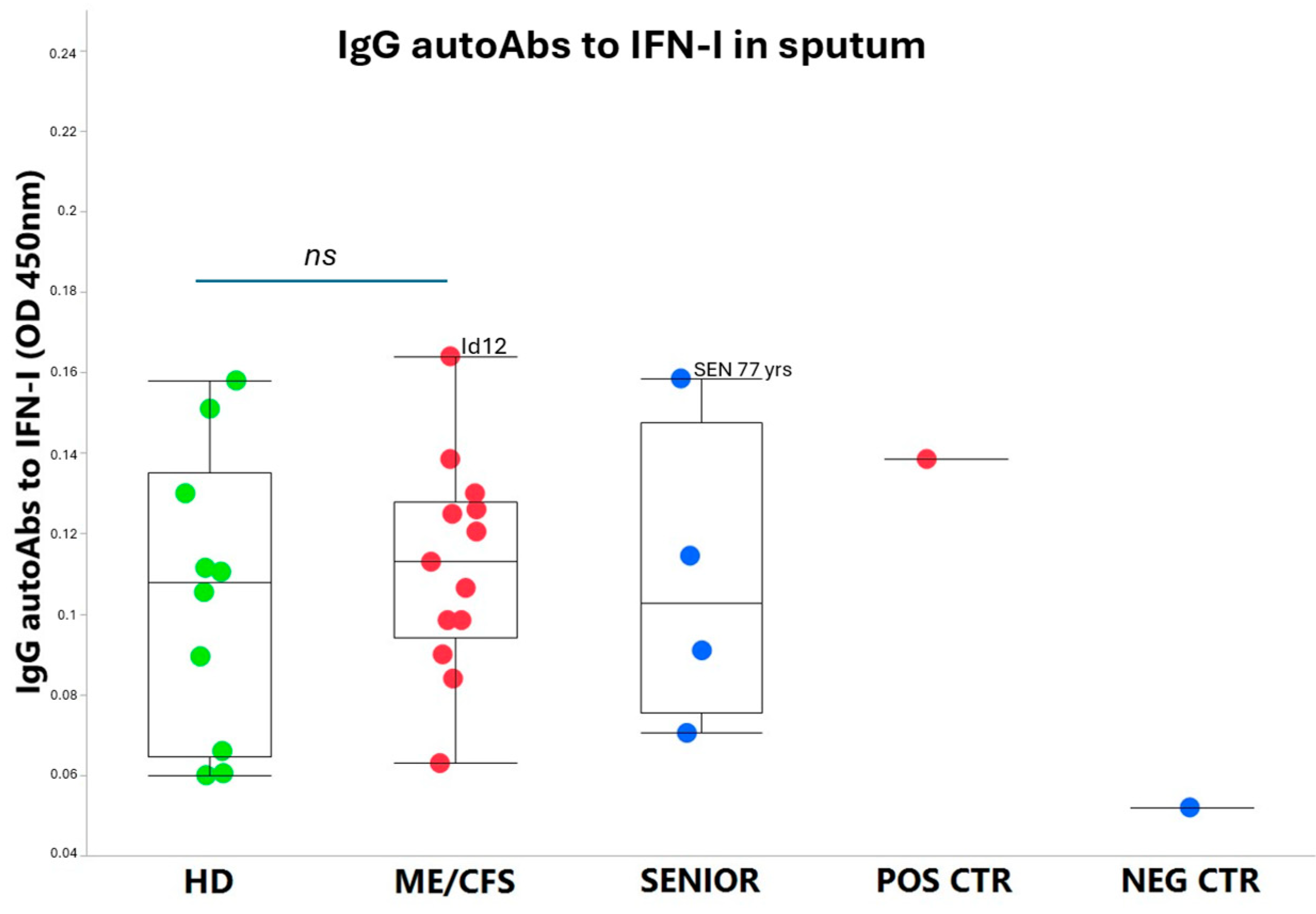
3.2. IgG autoAbs Against IFN-I in ME/CFS Patients
4. Discussion
4.1. ME/CFS Immune and Antiviral Dysregulation-Unknown Mechanisms
4.2. Overload of Epstein–Barr Virus in ME/CFS
4.3. Human Adenovirus and Herpesvirus Reactivation in Airways
4.4. Latent Virus–Host Immune Balance
4.5. Limitations of the Study
4.6. Future Perspectives and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vahratian, A.; Lin, J.S.; Bertolli, J.; Unger, E.R. Myalgic Encephalomyelitis/Chronic Fatigue Syndrome in Adults: United States, 2021–2022. NCHS Data Brief. 2023, 488, 1–8. [Google Scholar]
- Valdez, A.R.; Hancock, E.E.; Adebayo, S.; Kiernicki, D.J.; Proskauer, D.; Attewell, J.R.; Bateman, L.; DeMaria, A., Jr.; Lapp, C.W.; Rowe, P.C.; et al. Estimating Prevalence, Demographics, and Costs of ME/CFS Using Large Scale Medical Claims Data and Machine Learning. Front. Pediatr. 2018, 6, 412. [Google Scholar] [CrossRef]
- Bateman, L.; Bested, A.C.; Bonilla, H.F.; Chheda, B.V.; Chu, L.; Curtin, J.M.; Dempsey, T.T.; Dimmock, M.E.; Dowell, T.G.; Felsenstein, D.; et al. Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: Essentials of Diagnosis and Management. Mayo Clin. Proc. 2021, 96, 2861–2878. [Google Scholar] [CrossRef]
- Hanson, M.R. The viral origin of myalgic encephalomyelitis/chronic fatigue syndrome. PLoS Pathog. 2023, 19, e1011523. [Google Scholar] [CrossRef]
- Hickie, I.; Davenport, T.; Wakefield, D.; Vollmer-Conna, U.; Cameron, B.; Vernon, S.D.; Reeves, W.C.; Lloyd, A.; Dubbo Infection Outcomes Study Group. Post-infective and chronic fatigue syndromes precipitated by viral and non-viral pathogens: Prospective cohort study. BMJ 2006, 333, 575. [Google Scholar] [CrossRef]
- Naess, H.; Sundal, E.; Myhr, K.M.; Nyland, H.I. Postinfectious and chronic fatigue syndromes: Clinical experience from a tertiary-referral centre in Norway. Vivo 2010, 24, 185–188. [Google Scholar]
- Blomberg, J.; Gottfries, C.G.; Elfaitouri, A.; Rizwan, M.; Rosén, A. Infection Elicited Autoimmunity and Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: An Explanatory Model. Front. Immunol. 2018, 9, 229. [Google Scholar] [CrossRef] [PubMed]
- Komaroff, A.L.; Lipkin, W.I. Insights from myalgic encephalomyelitis/chronic fatigue syndrome may help unravel the pathogenesis of postacute COVID-19 syndrome. Trends Mol. Med. 2021, 27, 895–906. [Google Scholar] [CrossRef]
- Apostolou, E.; Rizwan, M.; Moustardas, P.; Sjögren, P.; Bertilson, B.C.; Bragée, B.; Polo, O.; Rosén, A. Saliva antibody-fingerprint of reactivated latent viruses after mild/asymptomatic COVID-19 is unique in patients with myalgic-encephalomyelitis/chronic fatigue syndrome. Front. Immunol. 2022, 13, 949787. [Google Scholar] [CrossRef]
- Hannestad, U.; Apostolou, E.; Sjögren, P.; Bragée, B.; Polo, O.; Bertilson, B.C.; Rosén, A. Post-COVID sequelae effect in chronic fatigue syndrome: SARS-CoV-2 triggers latent adenovirus in the oral mucosa. Front. Med. 2023, 10, 1208181. [Google Scholar] [CrossRef]
- Schindele, A.; Holm, A.; Kraft, S.; Nylander, K.; Allard, A.; Olofsson, K. Cross-evaluating Epstein-Barr virus, human papilloma virus, human cytomegalovirus and human adenovirus in nasal polyps and turbinate mucosa. Acta Otolaryngol. 2025, 145, 164–167. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.T.; Lidsky, P.V.; Xiao, Y.; Cheng, R.; Lee, I.T.; Nakayama, T.; Jiang, S.; He, W.; Demeter, J.; Knight, M.G.; et al. SARS-CoV-2 replication in airway epithelia requires motile cilia and microvillar reprogramming. Cell 2023, 186, 112–130.e20. [Google Scholar] [CrossRef] [PubMed]
- Vojdani, A.; Vojdani, E.; Saidara, E.; Maes, M. Persistent SARS-CoV-2 Infection, EBV, HHV-6 and Other Factors May Contribute to Inflammation and Autoimmunity in Long COVID. Viruses 2023, 15, 400. [Google Scholar] [CrossRef]
- Albanese, M.; Tagawa, T.; Hammerschmidt, W. Strategies of Epstein-Barr virus to evade innate antiviral immunity of its human host. Front. Microbiol. 2022, 13, 955603. [Google Scholar] [CrossRef]
- Hale, B.G. Autoantibodies targeting type I interferons: Prevalence, mechanisms of induction, and association with viral disease susceptibility. Eur. J. Immunol. 2023, 53, e2250164. [Google Scholar] [CrossRef]
- Carruthers, B.M. Definitions and aetiology of myalgic encephalomyelitis: How the Canadian consensus clinical definition of myalgic encephalomyelitis works. J. Clin. Pathol. 2007, 60, 117–119. [Google Scholar] [CrossRef] [PubMed]
- Carruthers, B.M.; van de Sande, M.I.; De Meirleir, K.L.; Klimas, N.G.; Broderick, G.; Mitchell, T.; Staines, D.; Powles, A.C.; Speight, N.; Vallings, R.; et al. Myalgic encephalomyelitis: International Consensus Criteria. J. Intern. Med. 2011, 270, 327–338. [Google Scholar] [CrossRef]
- Hernroth, B.E.; Conden-Hansson, A.C.; Rehnstam-Holm, A.S.; Girones, R.; Allard, A.K. Environmental factors influencing human viral pathogens and their potential indicator organisms in the blue mussel, Mytilus edulis: The first Scandinavian report. Appl. Environ. Microbiol. 2002, 68, 4523–4533. [Google Scholar] [CrossRef]
- Schindele, A.; Holm, A.; Nylander, K.; Allard, A.; Olofsson, K. Mapping human papillomavirus, Epstein-Barr virus, cytomegalovirus, adenovirus, and p16 in laryngeal cancer. Discov. Oncol. 2022, 13, 18. [Google Scholar] [CrossRef]
- Corman, V.M.; Landt, O.; Kaiser, M.; Molenkamp, R.; Meijer, A.; Chu, D.K.; Bleicker, T.; Brunink, S.; Schneider, J.; Schmidt, M.L.; et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Eurosurveillance 2020, 25, 2000045. [Google Scholar] [CrossRef]
- Gervais, A.; Rovida, F.; Avanzini, M.A.; Croce, S.; Marchal, A.; Lin, S.C.; Ferrari, A.; Thorball, C.W.; Constant, O.; Le Voyer, T.; et al. Autoantibodies neutralizing type I IFNs underlie West Nile virus encephalitis in approximately 40% of patients. J. Exp. Med. 2023, 220, e20230661. [Google Scholar] [CrossRef] [PubMed]
- Eaton-Fitch, N.; Rudd, P.; Er, T.; Hool, L.; Herrero, L.; Marshall-Gradisnik, S. Immune exhaustion in ME/CFS and long COVID. JCI Insight 2024, 9, e183810. [Google Scholar] [CrossRef]
- Blomberg, J.; Rizwan, M.; Bohlin-Wiener, A.; Elfaitouri, A.; Julin, P.; Zachrisson, O.; Rosén, A.; Gottfries, C.G. Antibodies to Human Herpesviruses in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome Patients. Front. Immunol. 2019, 10, 1946. [Google Scholar] [CrossRef] [PubMed]
- Apostolou, E.; Rosén, A. Epigenetic reprograming in myalgic encephalomyelitis/chronic fatigue syndrome: A narrative of latent viruses. J. Intern. Med. 2024, 296, 93–115. [Google Scholar] [CrossRef] [PubMed]
- Choutka, J.; Jansari, V.; Hornig, M.; Iwasaki, A. Unexplained post-acute infection syndromes. Nat. Med. 2022, 28, 911–923. [Google Scholar] [CrossRef]
- Su, Y.; Yuan, D.; Chen, D.G.; Ng, R.H.; Wang, K.; Choi, J.; Li, S.; Hong, S.; Zhang, R.; Xie, J.; et al. Multiple early factors anticipate post-acute COVID-19 sequelae. Cell 2022, 185, 881–895.e20. [Google Scholar] [CrossRef]
- Le Voyer, T.; Parent, A.V.; Liu, X.; Cederholm, A.; Gervais, A.; Rosain, J.; Nguyen, T.; Perez Lorenzo, M.; Rackaityte, E.; Rinchai, D.; et al. Autoantibodies against type I IFNs in humans with alternative NF-kappaB pathway deficiency. Nature 2023, 623, 803–813. [Google Scholar] [CrossRef]
- Bastard, P.; Vazquez, S.E.; Liu, J.; Laurie, M.T.; Wang, C.Y.; Gervais, A.; Le Voyer, T.; Bizien, L.; Zamecnik, C.; Philippot, Q.; et al. Vaccine breakthrough hypoxemic COVID-19 pneumonia in patients with auto-Abs neutralizing type I IFNs. Sci. Immunol. 2023, 8, eabp8966. [Google Scholar] [CrossRef]
- Philippot, Q.; Fekkar, A.; Gervais, A.; Le Voyer, T.; Boers, L.S.; Conil, C.; Bizien, L.; de Brabander, J.; Duitman, J.W.; Romano, A.; et al. Autoantibodies Neutralizing Type I IFNs in the Bronchoalveolar Lavage of at Least 10% of Patients During Life-Threatening COVID-19 Pneumonia. J. Clin. Immunol. 2023, 43, 1093–1103. [Google Scholar] [CrossRef]
- Gervais, A.; Marchal, A.; Fortova, A.; Berankova, M.; Krbkova, L.; Pychova, M.; Salat, J.; Zhao, S.; Kerrouche, N.; Le Voyer, T.; et al. Autoantibodies neutralizing type I IFNs underlie severe tick-borne encephalitis in approximately 10% of patients. J. Exp. Med. 2024, 221, e20240637. [Google Scholar] [CrossRef]
- Feng, A.; Yang, E.Y.; Moore, A.R.; Dhingra, S.; Chang, S.E.; Yin, X.; Pi, R.; Mack, E.K.; Volkel, S.; Gessner, R.; et al. Autoantibodies are highly prevalent in non-SARS-CoV-2 respiratory infections and critical illness. JCI Insight 2023, 8, e163150. [Google Scholar] [CrossRef] [PubMed]
- Walter, J.E.; Rosen, L.B.; Csomos, K.; Rosenberg, J.M.; Mathew, D.; Keszei, M.; Ujhazi, B.; Chen, K.; Lee, Y.N.; Tirosh, I.; et al. Broad-spectrum antibodies against self-antigens and cytokines in RAG deficiency. J. Clin. Investig. 2015, 125, 4135–4148. [Google Scholar] [CrossRef] [PubMed]
- Ariza, M.E. Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: The Human Herpesviruses Are Back! Biomolecules 2021, 11, 185. [Google Scholar] [CrossRef]
- Shikova, E.; Reshkova, V.; Kumanova, A.; Raleva, S.; Alexandrova, D.; Capo, N.; Murovska, M.; On Behalf of the European Network on ME/CFS. Cytomegalovirus, Epstein-Barr virus, and human herpesvirus-6 infections in patients with myalgic small ie, Cyrillicncephalomyelitis/chronic fatigue syndrome. J. Med. Virol. 2020, 92, 3682–3688. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.H.; Kim, K.H.; Jeong, S.H.; Park, J.W.; Lee, S.M.; Seo, Y.H. Comparison of sputum and nasopharyngeal swabs for detection of respiratory viruses. J. Med. Virol. 2014, 86, 2122–2127. [Google Scholar] [CrossRef]
- Lion, T. Adenovirus persistence, reactivation, and clinical management. FEBS Lett. 2019, 593, 3571–3582. [Google Scholar] [CrossRef]
- Garnett, C.T.; Erdman, D.; Xu, W.; Gooding, L.R. Prevalence and quantitation of species C adenovirus DNA in human mucosal lymphocytes. J. Virol. 2002, 76, 10608–10616. [Google Scholar] [CrossRef]
- Lion, T. Adenovirus infections in immunocompetent and immunocompromised patients. Clin. Microbiol. Rev. 2014, 27, 441–462. [Google Scholar] [CrossRef]
- Kosulin, K.; Haberler, C.; Hainfellner, J.A.; Amann, G.; Lang, S.; Lion, T. Investigation of adenovirus occurrence in pediatric tumor entities. J. Virol. 2007, 81, 7629–7635. [Google Scholar] [CrossRef]
- Virgin, H.W.; Wherry, E.J.; Ahmed, R. Redefining chronic viral infection. Cell 2009, 138, 30–50. [Google Scholar] [CrossRef]
- Levin, D.; Schneider, W.M.; Hoffmann, H.H.; Yarden, G.; Busetto, A.G.; Manor, O.; Sharma, N.; Rice, C.M.; Schreiber, G. Multifaceted activities of type I interferon are revealed by a receptor antagonist. Sci. Signal 2014, 7, ra50. [Google Scholar] [CrossRef] [PubMed]
- Crow, Y.J.; Stetson, D.B. The type I interferonopathies: 10 years on. Nat. Rev. Immunol. 2022, 22, 471–483. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Q.; Zheng, C.; Wang, L. MAVS: The next STING in cancers and other diseases. Crit. Rev. Oncol. Hematol. 2024, 207, 104610. [Google Scholar] [CrossRef] [PubMed]
- Miyagi, S.; Watanabe, T.; Hara, Y.; Arata, M.; Uddin, M.K.; Mantoku, K.; Sago, K.; Yanagi, Y.; Suzuki, T.; Masud, H.; et al. A STING inhibitor suppresses EBV-induced B cell transformation and lymphomagenesis. Cancer Sci. 2021, 112, 5088–5099. [Google Scholar] [CrossRef]
- Pasquesi, G.I.M.; Allen, H.; Ivancevic, A.; Barbachano-Guerrero, A.; Joyner, O.; Guo, K.; Simpson, D.M.; Gapin, K.; Horton, I.; Nguyen, L.L.; et al. Regulation of human interferon signaling by transposon exonization. Cell 2024, 187, 7621–7636.e7619. [Google Scholar] [CrossRef] [PubMed]
- Peluso, M.J.; Deeks, S.G. Mechanisms of long COVID and the path toward therapeutics. Cell 2024, 187, 5500–5529. [Google Scholar] [CrossRef]
- Kosulin, K.; Geiger, E.; Vecsei, A.; Huber, W.D.; Rauch, M.; Brenner, E.; Wrba, F.; Hammer, K.; Innerhofer, A.; Potschger, U.; et al. Persistence and reactivation of human adenoviruses in the gastrointestinal tract. Clin. Microbiol. Infect. 2016, 22, 381.e1–381.e8. [Google Scholar] [CrossRef]
- Alvarez-Cardona, J.J.; Whited, L.K.; Chemaly, R.F. Brincidofovir: Understanding its unique profile and potential role against adenovirus and other viral infections. Future Microbiol. 2020, 15, 389–400. [Google Scholar] [CrossRef]
- El-Haddad, D.; El Chaer, F.; Vanichanan, J.; Shah, D.P.; Ariza-Heredia, E.J.; Mulanovich, V.E.; Gulbis, A.M.; Shpall, E.J.; Chemaly, R.F. Brincidofovir (CMX-001) for refractory and resistant CMV and HSV infections in immunocompromised cancer patients: A single-center experience. Antiviral Res. 2016, 134, 58–62. [Google Scholar] [CrossRef]
- Camargo, J.F.; Morris, M.I.; Abbo, L.M.; Simkins, J.; Saneeymehri, S.; Alencar, M.C.; Lekakis, L.J.; Komanduri, K.V. The use of brincidofovir for the treatment of mixed dsDNA viral infection. J. Clin. Virol. 2016, 83, 1–4. [Google Scholar] [CrossRef]
- Hill, J.A.; Nichols, W.G.; Marty, F.M.; Papanicolaou, G.A.; Brundage, T.M.; Lanier, R.; Zerr, D.M.; Boeckh, M.J. Oral brincidofovir decreases the incidence of HHV-6B viremia after allogeneic HCT. Blood. 2020, 135, 1447–1451. [Google Scholar] [CrossRef] [PubMed]
- Grimley, M.S.; Maron, G. Preliminary Results of a Phase 2a Clinical Trial to Evaluate Safety, Tolerability and Antiviral Activity of Intravenous Brincidofovir (BCV IV) in Immunocompromised Patients with Adenovirus Infection. In Proceedings of the 65th ASH Annual Meeting, San Diego, CA, USA, 9–12 December 2023. [Google Scholar]
- Kosulin, K. Intestinal HAdV Infection: Tissue Specificity, Persistence, and Implications for Antiviral Therapy. Viruses 2019, 11, 804. [Google Scholar] [CrossRef] [PubMed]
- Radke, J.R.; Cook, J.L. Human adenovirus infections: Update and consideration of mechanisms of viral persistence. Curr. Opin. Infect. Dis. 2018, 31, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Calcedo, R.; Medina-Jaszek, A.; Keough, M.; Peng, H.; Wilson, J.M. Adenoviruses in lymphocytes of the human gastro-intestinal tract. PLoS ONE 2011, 6, e24859. [Google Scholar] [CrossRef]
| ME/CFS Patients | Healthy Controls | Senior Controls | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample | Duration of | Disease | Sex | Age | Sample | Duration of | Sex | Age | Sample | Duration of | Sex | Age |
| Id | ME/CFS (yrs) | Severity | yrs | Id | ME/CFS (yrs) | yrs | Id | ME/CFS (yrs) | yrs | |||
| 1 | 8 | 2 | F | 61 | 14 | NA | F | 56 | 24 | NA | F | 65 |
| 2 | 13 | 2 | F | 60 | 15 | NA | F | 33 | 25 | NA | M | 77 |
| 3 | 10 | 2 | F | 58 | 16 | NA | F | 61 | 26 | NA | F | 72 |
| 4 | 12 | 1 | F | 56 | 17 | NA | F | 61 | 27 | NA | M | 75 |
| 5 | 28 | 1 | F | 54 | 18 | NA | F | 37 | Median | 73.5 | ||
| 6 | 12 | 1 | F | 54 | 19 | NA | F | 49 | Range | 65–77 | ||
| 7 | 10 | 1 | F | 53 | 20 | NA | F | 66 | ||||
| 8 | 14 | 3 | F | 49 | 21 | NA | M | 48 | ||||
| 9 | 14 | 3 | F | 48 | 22 | NA | F | 33 | Positive, negative control | |||
| 10 | 8 | 3 | F | 37 | 23 | NA | F | 66 | Sample | Duration of | Sex | Age |
| 11 | 17 | 2 | F | 37 | Median | 54 | Id | ME/CFS (yrs) | yrs | |||
| 12 | 12 | 3 | F | 22 | Range | 33–66 | NEG CTR | NA | F | 46 | ||
| 13 | 27 | 2 | F | 59 | POS CTR | NA | F | 54 | ||||
| Median | 12 | 52.5 | ||||||||||
| Range | 8- 28 | 22–61 | ||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hannestad, U.; Allard, A.; Nilsson, K.; Rosén, A. Prevalence of EBV, HHV6, HCMV, HAdV, SARS-CoV-2, and Autoantibodies to Type I Interferon in Sputum from Myalgic Encephalomyelitis/Chronic Fatigue Syndrome Patients. Viruses 2025, 17, 422. https://doi.org/10.3390/v17030422
Hannestad U, Allard A, Nilsson K, Rosén A. Prevalence of EBV, HHV6, HCMV, HAdV, SARS-CoV-2, and Autoantibodies to Type I Interferon in Sputum from Myalgic Encephalomyelitis/Chronic Fatigue Syndrome Patients. Viruses. 2025; 17(3):422. https://doi.org/10.3390/v17030422
Chicago/Turabian StyleHannestad, Ulf, Annika Allard, Kent Nilsson, and Anders Rosén. 2025. "Prevalence of EBV, HHV6, HCMV, HAdV, SARS-CoV-2, and Autoantibodies to Type I Interferon in Sputum from Myalgic Encephalomyelitis/Chronic Fatigue Syndrome Patients" Viruses 17, no. 3: 422. https://doi.org/10.3390/v17030422
APA StyleHannestad, U., Allard, A., Nilsson, K., & Rosén, A. (2025). Prevalence of EBV, HHV6, HCMV, HAdV, SARS-CoV-2, and Autoantibodies to Type I Interferon in Sputum from Myalgic Encephalomyelitis/Chronic Fatigue Syndrome Patients. Viruses, 17(3), 422. https://doi.org/10.3390/v17030422






