Managing HIV-Associated Hodgkin Lymphoma During the COVID-19 Pandemic: Case Report and Literature Review
Abstract
:1. Introduction
2. Case Presentation
2.1. Demographic Data
2.2. Medical History
2.3. Clinical Presentation and Evaluation
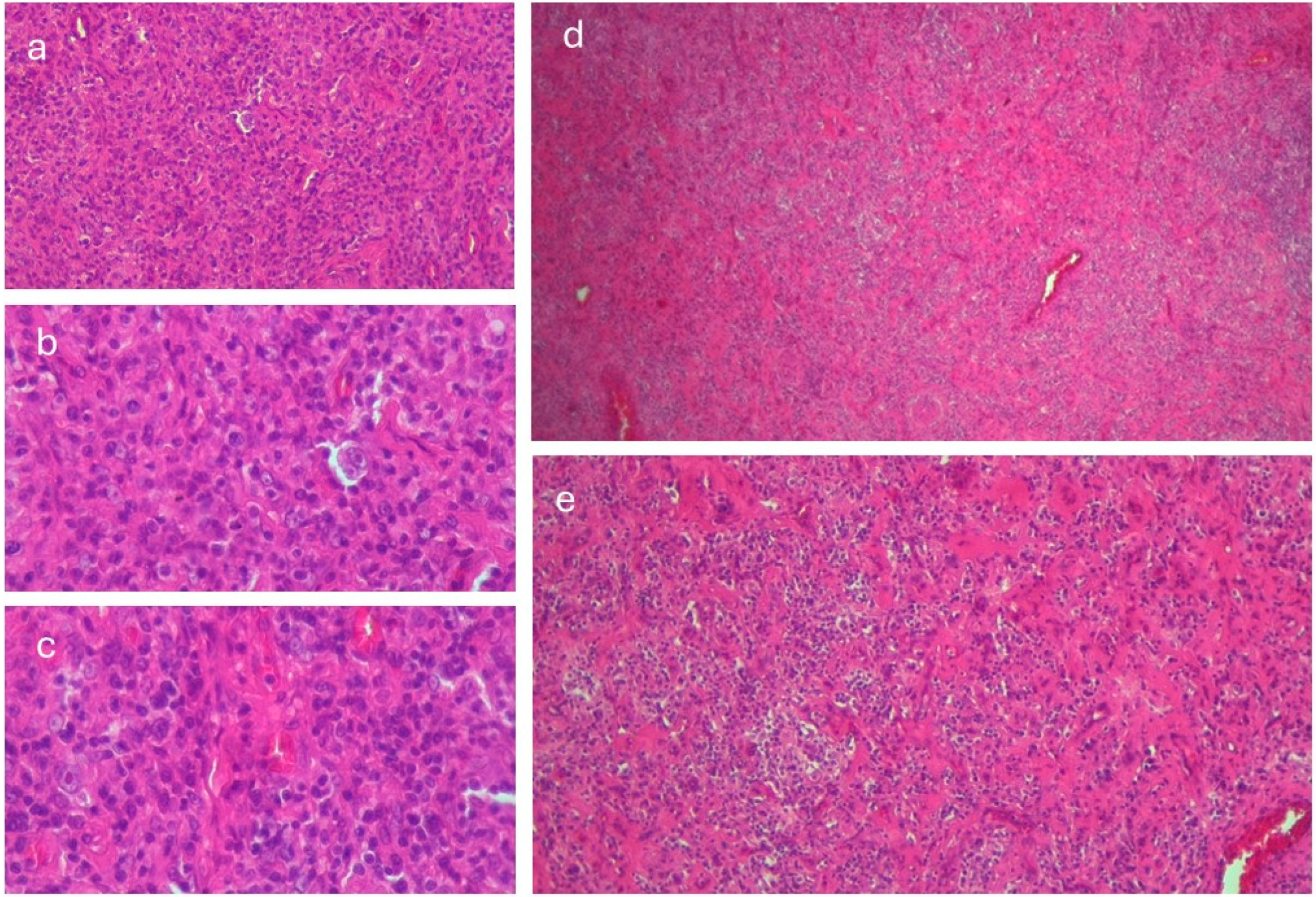
2.4. Diagnostic Investigations
2.5. Therapeutic Interventions and Outcome
3. Discussion
3.1. HIV and COVID-19
3.2. Hodgkin Lymphoma and COVID-19
3.3. HIV and Cancer
3.4. Immunophenotypic Profiling of Lymphoma in Patients with HIV
3.5. COVID-19 Vaccination in People with HIV or Lymphoma
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AIDS | Acquired immunodeficiency syndrome |
| ALT | Alanin aminotransferase |
| ARN-HIV | HIV viral load |
| AST | Aspartate aminotransferase |
| BP | Blood pressure |
| CBC | Complete blood count |
| COVID-19 | Coronavirus Disease 2019 |
| CT | Computed tomography |
| ECOG | Eastern Cooperative Oncology Group |
| ELISA | Enzyme-linked immunosorbent assay |
| GSC | Glasgow scale coma |
| HL | Hodgkin’s lymphoma |
| HIV | Human immunodeficiency virus |
| HBc Ab | Hepatitis B core antibody |
| HBs-Ag | Hepatitis B surface antigen |
| HCV-Ab | Hepatitis C virus antibody |
| HP | Histopathology |
| HR | Heart rate |
| IHC | Immunohistochemy |
| NET | Neutrophil extracellular trap |
| PLWH | People living with HIV |
| RT-PCR | Real-time polymerase chain reaction |
| SARS-CoV-2 | Severe Acute Respiratory Syndrome Coronavirus 2 |
| SO2 | Saturation of oxygen |
Appendix A
| Test Name | Week 0 | Week 2 | Week 3 | Week 4 | Week 6 | Week 8 | Week 9 |
|---|---|---|---|---|---|---|---|
| WBC ×109 (4–9) | 3.8 | 3.3 | 4.2 | 3.7 | 1.5 | 1.7 | 1.5 |
| N ×106 (2000–6300) | 2150 | 1370 | 2610 | 2990 | 1140 | 1300 | 1340 |
| CD4/mmc (430–1800) | 112 | 73 | 46 | 21 | |||
| Hb g/L (130–170) | 111 | 97 | 106 | 73 | 63 | 83 | 66 |
| PTL ×109 (150–450) | 117 | 139 | 155.6 | 111 | 47 | 21 | 4 |
| CRP mg/L (0–10) | 6.16 | 42.9 | 109.1 | 114.9 | 65.1 | 113.9 | 115.9 |
| ERS mm/h (0–20) | 80 | 110 | 110 | 100 | 120 | 56 | 34 |
| Cr. mg/dL (0.7–1.3) | 0.76 | 0.68 | 1.36 | 0.71 | 0.76 | 0.53 | 0.52 |
| ALT U/L (0–40) | 84 | 32.4 | 185.7 | 58.3 | 32.5 | 113.2 | 135.4 |
| Bt mg/dL (0.3–1.2) | 0.91 | 1,24 | 8.2 | 12.4 | 21.5 | ||
| Albumin g/L (38–54) | 26.4 | 16.4 | 18.6 | 21.1 | 33.3 | ||
| D-Drs ug/L (˂500) | 4616 | 2445 | 1999.9 | 2075 | 940 | 1007 | 632 |
| LA mg/dL (5–20) | 18.28 | 24.13 | 23.9 | 31.7 | 18.7 | 38.2 | |
| SARS-CoV-2 | positive | positive | positive | positive | |||
| HCV-Ab | negative | ||||||
| HBsAg HBcAb | negative negative | ||||||
| Tumoral markers | Safety limits CA 19.9 = 21 U/mL (1.2–30.9), CEA = 0.5 ng/mL (0.5–5), PSA = −0.19 (0–4 ng/mL), AFP = 0.5 ng/mL (1.3–8.1) | ||||||
| Bone marrow aspiration | Erythrocyte series: normal percentage; erythroid precursors with moderate dysplastic appearance. Granulocyte series: increased percentage; granulocyte precursors with dysplastic appearance. Lymphoplasmocyte series: decrease percentage. Platelet series: normal; megakaryocytes with dysplastic appearance. | ||||||
References
- Alfano, V.; Ercolano, S. The Efficacy of Lockdown Against COVID-19: A Cross-Country Panel Analysis. Appl. Health Econ. Health Policy 2020, 18, 509–517. [Google Scholar] [CrossRef] [PubMed]
- De Vincentiis, L.; Carr, R.A.; Mariani, M.P.; Ferrara, G. Cancer diagnostic rates during the 2020 ‘lockdown’, due to COVID-19 pandemic, compared with the 2018-2019: An audit study from cellular pathology. J. Clin. Pathol. 2021, 74, 187–189. [Google Scholar] [CrossRef]
- Maringe, C.; Spicer, J.; Morris, M.; Purushotham, A.; Nolte, E.; Sullivan, R.; Rachet, B.; Aggarwal, A. The impact of the COVID-19 pandemic on cancer deaths due to delays in diagnosis in England, UK: A national, population-based, modelling study. Lancet Oncol. 2020, 21, 1023–1034. [Google Scholar] [CrossRef]
- Siegler, J.E.; Heslin, M.E.; Thau, L.; Smith, A.; Jovin, T.G. Falling stroke rates during COVID-19 pandemic at a comprehensive stroke center. J. Stroke Cerebrovasc. Dis. 2020, 29, 104953. [Google Scholar] [CrossRef]
- Colivicchi, F.; Di Fusco, S.A.; Magnanti, M.; Cipriani, M.; Imperoli, G. The Impact of the Coronavirus Disease-2019 Pandemic and Italian Lockdown Measures on Clinical Presentation and Management of Acute Heart Failure. J. Card. Fail. 2020, 26, 464–465. [Google Scholar] [CrossRef]
- Nopp, S.; Janata-Schwatczek, K.; Prosch, H.; Shulym, I.; Königsbrügge, O.; Pabinger, I.; Ay, C. Pulmonary embolism during the COVID-19 pandemic: Decline in diagnostic procedures and incidence at a university hospital. Res. Pract. Thromb. Haemost. 2020, 4, 835–841. [Google Scholar] [CrossRef]
- Darcis, G.; Vaira, D.; Moutschen, M. Impact of coronavirus pandemic and containment measures on HIV diagnosis. Epidemiol. Infect. 2020, 148, e185. [Google Scholar] [CrossRef] [PubMed]
- Arbune, M.; Padurariu-Covit, M.D.; Tiutiuca, C.; Mihailov, R.; Niculet, E.; Arbune, A.A.; Tatu, A.L. Unusual Localization of AIDS-Related Kaposi’s Sarcoma in a Heterosexual Male during the COVID-19 Pandemic: A Case Report. Trop. Med. Infect. Dis. 2024, 9, 47. [Google Scholar] [CrossRef] [PubMed]
- Gaddey, H.L.; Riegel, A.M. Unexplained Lymphadenopathy: Evaluation and Differential Diagnosis. Am. Fam. Physician 2016, 94, 896–903. [Google Scholar]
- Oken, M.M.; Creech, R.H.; Tormey, D.C.; Horton, J.; Davis, T.E.; McFadden, E.T.; Carbone, P.P. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am. J. Clin. Oncol. 1982, 5, 649–655. [Google Scholar] [CrossRef]
- Xu, W.; Gu, B.; Lotter, W.E.; Kehl, K.L. Extraction and Imputation of Eastern Cooperative Oncology Group Performance Status From Unstructured Oncology Notes Using Language Models. JCO Clin. Cancer Inform. 2024, 8, e2300269. [Google Scholar] [CrossRef] [PubMed]
- CDC. 1994 Revised classification system for human immunodeficiency virus infection in children less than 13 years of age. Morb. Mortal. Wkly. Rep. 1994, 43, 1–10. Available online: https://www.cdc.gov/mmwr/pdf/rr/rr6303.pdf (accessed on 12 January 2024).
- Lister, T.A.; Crowther, D.; Sutcliffe, S.B.; Glatstein, E.; Canellos, G.P.; Young, R.C.; Rosenberg, S.A.; Coltman, C.A.; Tubiana, M. Report of a committee convened to discuss the evaluation and staging of patients with Hodgkin’s disease: Cotswolds meeting. J. Clin. Oncol. 1989, 7, 1630–1636. [Google Scholar] [CrossRef]
- Shi, Y.; Mi, L.; Lai, Y.; Zhao, M.; Jia, L.; Du, T.; Song, Y.; Li, X. PD-L1 immunohistochemistry assay optimization to provide more comprehensive pathological information in classic Hodgkin lymphoma. J. Hematop. 2023, 16, 7–16. [Google Scholar] [CrossRef]
- Li, X. Pitfalls in the pathological diagnosis of lymphoma. Chin. Clin. Oncol. 2015, 4, 3. [Google Scholar] [CrossRef]
- Hoppe, R.T.; Advani, R.H.; Ai, W.Z.; Ambinder, R.F.; Armand, P.; Bello, C.M.; Benitez, C.M.; Chen, W.; Dabaja, B.; Daly, M.E.; et al. NCCN Guidelines® Insights: Hodgkin Lymphoma, Version 2.2022. J. Natl. Compr. Cancer Netw. 2022, 20, 322–334. [Google Scholar] [CrossRef]
- Hasenclever, D.; Diehl, V. A prognostic score for advanced Hodgkin’s disease. International Prognostic Factors Project on Advanced Hodgkin’s Disease. N. Engl. J. Med. 1998, 339, 1506–1514. [Google Scholar] [CrossRef] [PubMed]
- Noy, A. Optimizing treatment of HIV-associated lymphoma. Blood 2019, 134, 1385–1394. [Google Scholar] [CrossRef]
- Mehta, P.; McAuley, D.F.; Brown, M.; Sanchez, E.; Tattersall, R.S.; Manson, J.J.; HLH Across Speciality Collaboration, U.K. COVID-19: Consider cytokine storm syndromes and immunosuppression. Lancet 2020, 395, 1033–1034. [Google Scholar] [CrossRef]
- Mozzini, C.; Girelli, D. The role of Neutrophil Extracellular Traps in COVID-19: Only an hypothesis or a potential new field of research? Thromb. Res. 2020, 191, 26–27. [Google Scholar] [CrossRef]
- Tesoriero, J.M.; Swain, C.E.; Pierce, J.L.; Zamboni, L.; Wu, M.; Holtgrave, D.R.; Gonzalez, C.J.; Udo, T.; Morne, J.E.; Hart-Malloy, R.; et al. COVID-19 Outcomes Among Persons Living With or Without Diagnosed HIV Infection in New York State. JAMA Netw. Open 2021, 4, e2037069. [Google Scholar] [CrossRef] [PubMed]
- Ssentongo, P.; Heilbrunn, E.S.; Ssentongo, A.E.; Advani, S.; Chinchilli, V.M.; Nunez, J.J.; Du, P. Epidemiology and outcomes of COVID-19 in HIV-infected individuals: A systematic review and meta-analysis. Sci. Rep. 2021, 11, 6283. [Google Scholar] [CrossRef] [PubMed]
- Viguerie, A.; Jacobson, E.U.; Hicks, K.A.; Bates, L.; Carrico, J.; Honeycutt, A.; Lyles, C.; Farnham, P.G. Assessing the Impact of COVID-19 on HIV Outcomes in the United States: A Modeling Study. Sex. Transm. Dis. 2024, 51, 299–304. [Google Scholar] [CrossRef]
- He, W.; Chen, L.; Yuan, G.; Fang, Y.; Chen, W.; Wu, D.; Liang, B.; Lu, X.; Ma, Y.; Li, L.; et al. COVID-19 in persons with haematological cancers. Leukemia 2020, 34, 1637–1645. [Google Scholar] [CrossRef]
- Regalado-Artamendi, I.; Jiménez-Ubieto, A.; Hernández-Rivas, J.; Navarro, B.; Núñez, L.; Alaez, C.; Córdoba, R.; Peñalver, F.J.; Cannata, J.; Estival, P.; et al. Risk Factors and Mortality of COVID-19 in Patients with Lymphoma: A Multicenter Study. Hemasphere 2021, 5, e538. [Google Scholar] [CrossRef] [PubMed]
- Pagano, L.; Salmanton-García, J.; Marchesi, F.; Busca, A.; Corradini, P.; Hoenigl, M.; Klimko, N.; Koehler, P.; Pagliuca, A.; Passamonti, F.; et al. COVID-19 infection in adult patients with hematological malignancies: A European Hematology Association Survey (EPICOVIDEHA). J. Hematol. Oncol. 2021, 14, 168. [Google Scholar] [CrossRef]
- Yonal-Hindilerden, I.; Hindilerden, F.; Mastanzade, M.; Tiryaki, T.O.; Tasan-Yenigun, S.; Bilen, Y.; Aksoz, S.; Cagatay, A.A.; Nalcaci, M. Case Report: Severe COVID-19 Pneumonia in a Patient With Relapsed/Refractory Hodgkin’s Lymphoma. Front. Oncol. 2021, 11, 601709. [Google Scholar] [CrossRef]
- Fakharian, A.; Ebrahimibagha, H.; Mirenayat, M.S.; Farahmandi, F. COVID-19 Reinfection in a Patient with Hodgkin Lymphoma: A Case Report. Tanaffos 2021, 20, 71–74. [Google Scholar]
- Hamed, M.; Alamoudi, D. Recurrent COVID-19 Infection in a Refractory/Classical Hodgkin’s Lymphoma Patient Undergoing Autologous Stem Cell Transplantation: A Case Report. Cureus 2023, 15, e46950. [Google Scholar] [CrossRef]
- Cartas, U.S.; González, J.L.V.; Hernandez, W.; Ríos, C.A.G. Lymphoma as a Complication of Recurrent COVID-19 Infection in Patients with Rheumatic Disease. Ann. Case Rep. 2022, 7, 1042. [Google Scholar] [CrossRef]
- Veyri, M.; Lavolé, A.; Choquet, S.; Costagliola, D.; Solas, C.; Katlama, C.; Poizot-Martin, I.; Spano, J.P. Do people living with HIV face more secondary cancers than general population: From the French CANCERVIH network. Bull. Cancer 2021, 108, 908–914. [Google Scholar] [CrossRef] [PubMed]
- Berhan, A.; Bayleyegn, B.; Getaneh, Z. HIV/AIDS Associated Lymphoma: Review. Blood Lymphat. Cancer 2022, 12, 31–45. [Google Scholar] [CrossRef]
- Dolcetti, R.; Gloghini, A.; Caruso, A.; Carbone, A. A lymphomagenic role for HIV beyond immune suppression? Blood 2016, 127, 1403–1409. [Google Scholar] [CrossRef] [PubMed]
- Navarro, J.T.; Moltó, J.; Tapia, G.; Ribera, J.M. Hodgkin Lymphoma in People Living with HIV. Cancers 2021, 13, 4366. [Google Scholar] [CrossRef]
- European AIDS Clinical Society (EACS). Guideline Version 12.0 October 2023. Available online: https://www.eacsociety.org/media/guidelines-12.0.pdf (accessed on 10 December 2024).
- Branch, C.; Parson-Martinez, J.; Cory, T.J. Drug-drug interactions in HIV-infected patients receiving chemotherapy. Expert. Opin. Drug Metab. Toxicol. 2025, 21, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Zelenetz, A.D.; Gordon, L.I.; Chang, J.E.; Christian, B.; Abramson, J.S.; Advani, R.H.; Bartlett, N.L.; Budde, L.E.; Caimi, P.F.; De Vos, S.; et al. NCCN Guidelines® Insights: B-Cell Lymphomas, Version 5.2021. J. Natl. Compr. Cancer Netw. 2021, 19, 1218–1230. [Google Scholar] [CrossRef]
- Konkay, K.; Paul, T.R.; Uppin, S.G.; Rao, D.R. Hodgkin lymphoma: A clinicopathological and immunophenotypic study. Indian J. Med. Paediatr. Oncol. 2016, 37, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xie, X.; Li, J.; Xiao, Q.; He, S.; Fu, H.; Zhang, X. Immune Characteristics and Immunotherapy of HIV-Associated Lymphoma. Curr. Issues Mol. Biol. 2024, 46, 9984–9997. [Google Scholar] [CrossRef]
- Mao, X.; Li, Y.; Liu, S.; He, C.; Yi, S.; Kuang, D.; Xiao, M.; Zhu, L.; Wang, C. Multicolor flow cytometric assessment of Ki67 expression and its diagnostic value in mature B-cell neoplasms. Front. Oncol. 2023, 13, 1108837. [Google Scholar] [CrossRef]
- Verdu-Bou, M.; Tapia, G.; Hernandez-Rodriguez, A.; Navarro, J.T. Clinical and Therapeutic Implications of Epstein-Barr Virus in HIV-Related Lymphomas. Cancers 2021, 13, 5534. [Google Scholar] [CrossRef]
- Muncunill, J.; Baptista, M.J.; Hernandez-Rodríguez, Á.; Dalmau, J.; Garcia, O.; Tapia, G.; Moreno, M.; Sancho, J.M.; Martínez-Picado, J.; Feliu, E.; et al. Plasma Epstein-Barr Virus Load as an Early Biomarker and Prognostic Factor of Human Immunodeficiency Virus-related Lymphomas. Clin. Infect. Dis. 2019, 68, 834–843. [Google Scholar] [CrossRef] [PubMed]
- Grebenciucova, E.; VanHaerents, S. Interleukin 6: At the interface of human health and disease. Front. Immunol. 2023, 14, 1255533. [Google Scholar] [CrossRef] [PubMed]
- Ganapathi, K.A.; Nicolae, A.; Egan, C.; Geng, H.; Xi, L.; Pack, S.D.; McFadden, J.R.; Raffeld, M.; Jaffe, E.S.; Pittaluga, S. Peripheral T-cell lymphomas expressing CD30 and CD15 expand the spectrum of anaplastic large cell lymphoma, ALK-negative. Br. J. Haematol. 2024, 204, 1862–1871. [Google Scholar] [CrossRef]
- Zhao, H.; Cai, S.; Xiao, Y.; Xia, M.; Chen, H.; Xie, Z.; Tang, X.; He, H.; Peng, J.; Chen, J. Expression and prognostic significance of the PD-1/PD-L1 pathway in AIDS-related non-Hodgkin lymphoma. Cancer Med. 2024, 13, e7195. [Google Scholar] [CrossRef]
- Hübel, K.; Bower, M.; Aurer, I.; Bastos-Oreiro, M.; Besson, C.; Brunnberg, U.; Cattaneo, C.; Collins, S.; Cwynarski, K.; Pria, A.D.; et al. Human immunodeficiency virus-associated Lymphomas: EHA-ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Hemasphere 2024, 8, e150. [Google Scholar] [CrossRef] [PubMed]
- Mebratie, D.Y.; Dagnaw, G.G. Review of immunohistochemistry techniques: Applications, current status, and future perspectives. Semin. Diagn. Pathol. 2024, 41, 154–160. [Google Scholar] [CrossRef]
- Magaki, S.; Hojat, S.A.; Wei, B.; So, A.; Yong, W.H. An Introduction to the Performance of Immunohistochemistry. Methods Mol. Biol. 2019, 1897, 289–298. [Google Scholar] [CrossRef]
- Yimpak, P.; Bumroongkit, K.; Tantiworawit, A.; Rattanathammethee, T.; Aungsuchawan, S.; Daroontum, T. Immunohistochemistry-based investigation of MYC, BCL2, and Ki-67 protein expression and their clinical impact in diffuse large B-cell lymphoma in upper Northern Thailand. PLoS ONE 2024, 19, e0307253. [Google Scholar] [CrossRef]
- Höft, M.A.; Burgers, W.A.; Riou, C. The immune response to SARS-CoV-2 in people with HIV. Cell Mol. Immunol. 2024, 21, 184–196. [Google Scholar] [CrossRef]
- Narita, K.; Nakaji, S.; Tabata, R.; Terao, T.; Kuzume, A.; Tsushima, T.; Ikeda, D.; Fukumoto, A.; Miura, D.; Takeuchi, M.; et al. Antibody response to COVID-19 vaccination in patients with lymphoma. Int. J. Hematol. 2022, 115, 728–736. [Google Scholar] [CrossRef]
- Hall, V.G.; Teh, B.W. COVID-19 Vaccination in Patients with Cancer and Patients Receiving HSCT or CAR-T Therapy: Immune Response, Real-World Effectiveness, and Implications for the Future. J. Infect. Dis. 2023, 228 (Suppl. S1), S55–S69. [Google Scholar] [CrossRef] [PubMed]

|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Padurariu-Covit, M.-D.; Andreescu, M.; Niculet, E.; Plesea-Condratovici, A.; Arbune, M. Managing HIV-Associated Hodgkin Lymphoma During the COVID-19 Pandemic: Case Report and Literature Review. Viruses 2025, 17, 404. https://doi.org/10.3390/v17030404
Padurariu-Covit M-D, Andreescu M, Niculet E, Plesea-Condratovici A, Arbune M. Managing HIV-Associated Hodgkin Lymphoma During the COVID-19 Pandemic: Case Report and Literature Review. Viruses. 2025; 17(3):404. https://doi.org/10.3390/v17030404
Chicago/Turabian StylePadurariu-Covit, Monica-Daniela, Mihaela Andreescu, Elena Niculet, Alina Plesea-Condratovici, and Manuela Arbune. 2025. "Managing HIV-Associated Hodgkin Lymphoma During the COVID-19 Pandemic: Case Report and Literature Review" Viruses 17, no. 3: 404. https://doi.org/10.3390/v17030404
APA StylePadurariu-Covit, M.-D., Andreescu, M., Niculet, E., Plesea-Condratovici, A., & Arbune, M. (2025). Managing HIV-Associated Hodgkin Lymphoma During the COVID-19 Pandemic: Case Report and Literature Review. Viruses, 17(3), 404. https://doi.org/10.3390/v17030404







