Histopathological Changes and Immune Response Profile in the Brains of Non-Human Primates Naturally Infected with Yellow Fever Virus
Abstract
1. Introduction
2. Materials and Methods
2.1. NHP Samples
2.2. Histopathology
2.3. Immunohistochemistry for YFV Antigen Detection
2.4. Immunohistochemistry for the Immune Response
2.5. Quantitative Analysis and Photodocumentation
2.6. Statistical Analysis
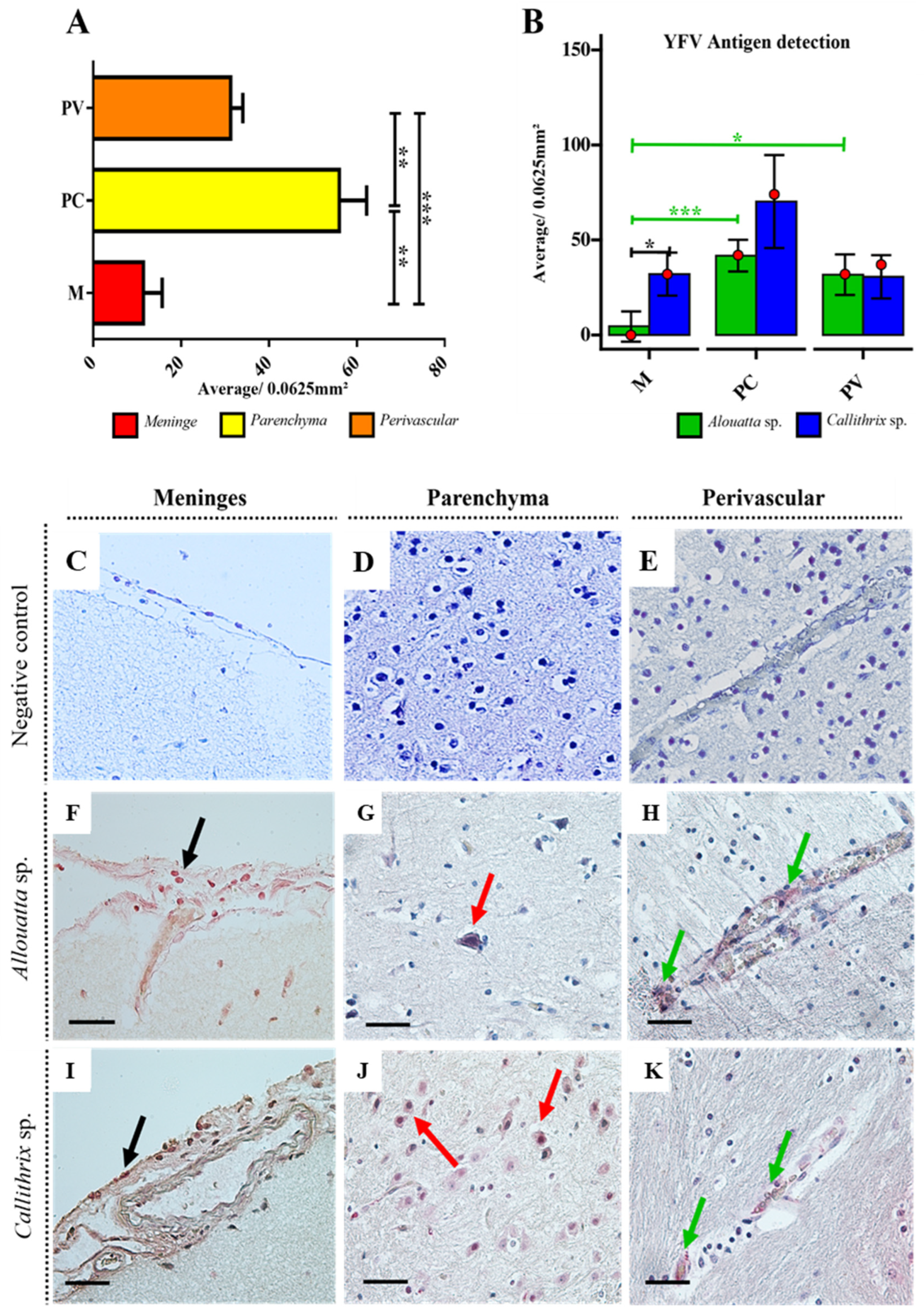
3. Results
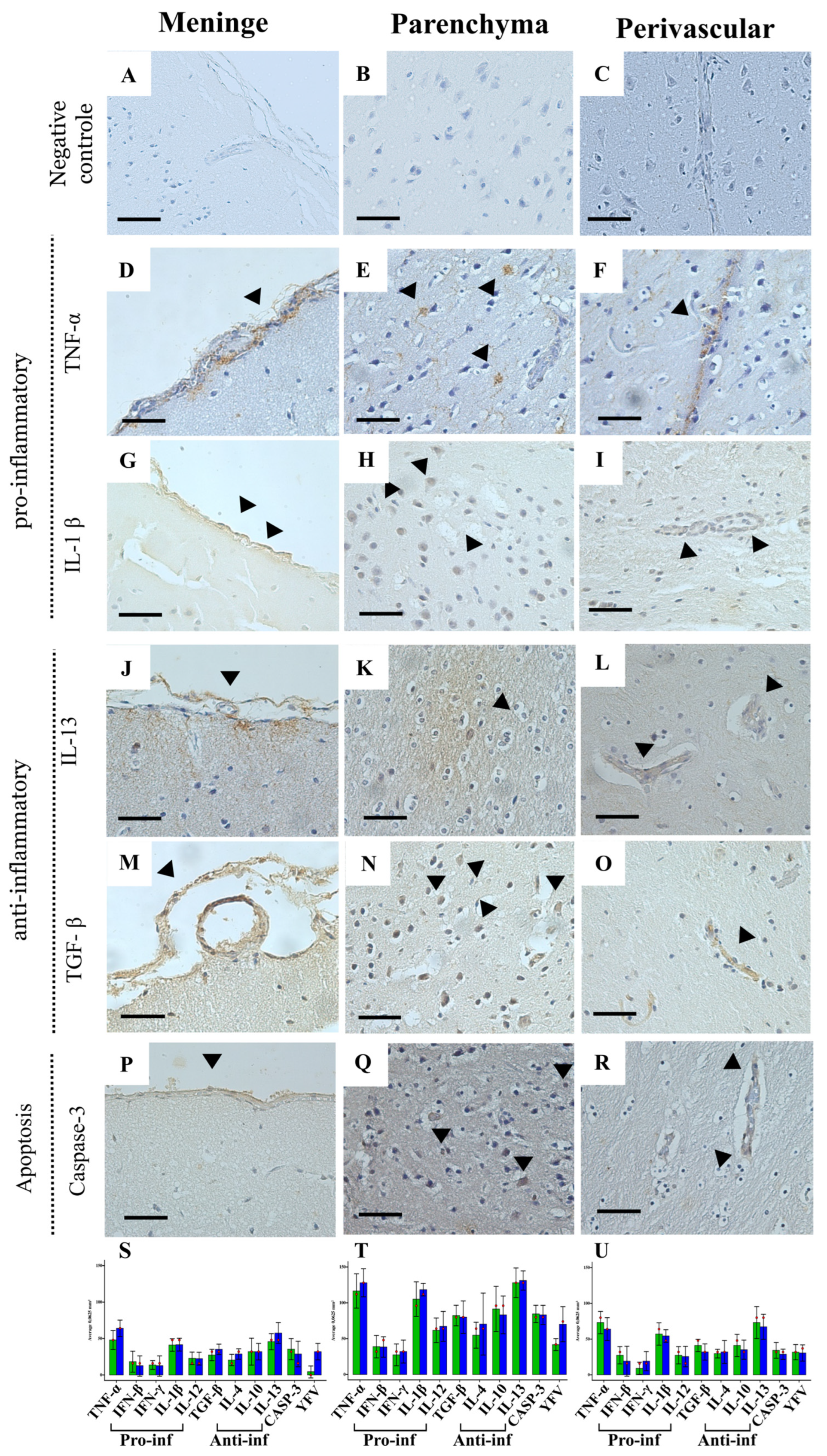
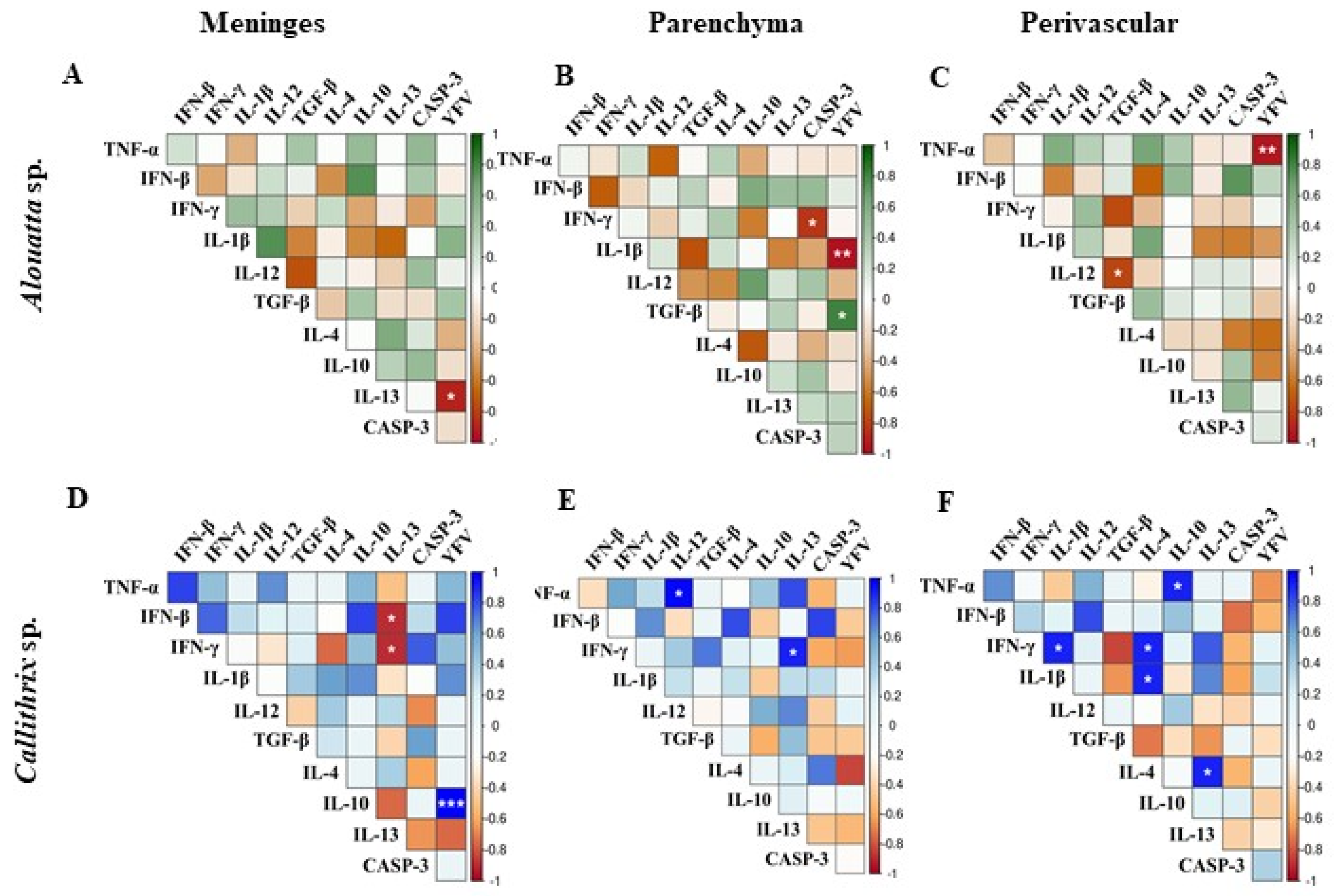
Profile of the Immune Response in the CNS
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kraemer, M.U.G.; Faria, N.R.; Reiner, R.C.; Golding, N.; Nikolay, B.; Stasse, S.; Johansson, M.A.; Salje, H.; Faye, O.; Wint, G.R.W.; et al. Spread of Yellow Fever Virus Outbreak in Angola and the Democratic Republic of the Congo 2015–16: A Modelling Study. Lancet Infect. Dis. 2017, 17, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Romano, A.P.M.; Costa, Z.G.A.; Ramos, D.G.; Andrade, M.A.; de Jayme, V.S.; de Almeida, M.A.B.; Vettorello, K.C.; Mascheretti, M.; Flannery, B. Yellow Fever Outbreaks in Unvaccinated Populations, Brazil, 2008-2009. PLoS Negl. Trop. Dis. 2014, 8, 18–21. [Google Scholar] [CrossRef]
- de Moraes, A.L.G. Participação de Primatas Não-Humanos Como Fonte de Infecção Da Febre Amarela (Ciclo Silvestre), (Trabalho de Conclusão de Curso, graduação em Medicina Veterinária); Faculdades Metropolitanas Unidas: São Paulo, Brasil, 2009. [Google Scholar]
- Vasconcelos, P.F.C. Yellow Fever. Rev. Soc. Bras. Med. Trop. 2003, 36, 275–293. [Google Scholar] [CrossRef]
- Ortiz-Martínez, Y.; Patiño-Barbosa, A.M.; Rodriguez-Morales, A.J. Yellow Fever in the Americas: The Growing Concern about New Epidemics. F1000Research 2017, 6, 398. [Google Scholar] [CrossRef]
- Figueiredo, P.d.O.; Stoffella-Dutra, A.G.; Costa, G.B.; de Oliveira, J.S.; Amaral, C.D.; Santos, J.D.; Rocha, K.L.S.; Júnior, J.P.A.; Nogueira, M.L.; Borges, M.A.Z.; et al. Re-Emergence of Yellow Fever in Brazil during 2016–2019: Challenges, Lessons Learned, and Perspectives. Viruses 2020, 12, 1233. [Google Scholar] [CrossRef] [PubMed]
- Callender, D.M. Management and Control of Yellow Fever Virus: Brazilian Outbreak January-April, 2018. Glob. Public Health 2019, 14, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Cunha, M.S.; da Costa, A.C.; de Azevedo Fernandes, N.C.C.; Guerra, J.M.; dos Santos, F.C.P.; Nogueira, J.S.; D’Agostino, L.G.; Komninakis, S.V.; Witkin, S.S.; Ressio, R.A.; et al. Epizootics Due to Yellow Fever Virus in São Paulo State, Brazil: Viral Dissemination to New Areas (2016–2017). Sci. Rep. 2019, 9, 5474. [Google Scholar] [CrossRef]
- Fernandes, N.C.C.d.A.; Cunha, M.S.; Guerra, J.M.; Réssio, R.A.; Cirqueira, C.d.S.; D’Andretta Iglezias, S.; de Carvalho, J.; Araujo, E.L.L.; Catão-Dias, J.L.; Díaz-Delgado, J. Outbreak of Yellow Fever among Nonhuman Primates, Espirito Santo, Brazil, 2017. Emerg. Infect. Dis. 2017, 23, 2038–2041. [Google Scholar] [CrossRef]
- Leal, S.G.; Romano, A.P.M.; Monteiro, R.V.; de Melo, C.B.; Vasconcelos, P.F.d.C.; de Castro, M.B. Frequency of Histopathological Changes in Howler Monkeys (Alouatta Sp.) Naturally Infected with Yellow Fever Virus in Brazil. Rev. Soc. Bras. Med. Trop. 2016, 49, 29–33. [Google Scholar] [CrossRef]
- Vieira, W.T.; Gayotto, L.C.; Lima, C.P.; Brito, T. Histopathology of the Human Liver in Yellow Fever with Special Emphasis on the Diagnostic Role of the Councilman Body. Histopathology 1983, 7, 195–208. [Google Scholar] [CrossRef]
- Quaresma, J.A.S.; Barros, V.L.R.S.; Pagliari, C.; Fernandes, E.R.; Guedes, F.; Takakura, C.F.H.; Andrade, H.F.; Vasconcelos, P.F.C.; Duarte, M.I.S. Revisiting the Liver in Human Yellow Fever: Virus-Induced Apoptosis in Hepatocytes Associated with TGF-β, TNF-α and NK Cells Activity. Virology 2006, 345, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Almeida, M.A.B.; Santos, E.; Cruz Cardoso, J.; Fonseca, D.F.; Noll, C.A.; Silveira, V.R.; Maeda, A.Y.; Souza, R.P.; Kanamura, C.; Brasil, R.A. Yellow Fever Outbreak Affecting Alouatta Populations in Southern Brazil (Rio Grande Do Sul State), 2008–2009. Am. J. Primatol. 2012, 74, 68–76. [Google Scholar] [CrossRef]
- Kaufmann, S.H.; Hengartner, M.O. Programmed Cell Death: Alive and Well in the New Millennium. Trends Cell Biol. 2001, 11, 526–534. [Google Scholar] [CrossRef] [PubMed]
- Quaresma, J.A.S.; Barros, V.L.R.S.; Fernandes, E.R.; Pagliari, C.; Takakura, C.; Vasconcelos, P.F.d.C.; Andrade, H.F.; Duarte, M.I.S. Reconsideration of Histopathology and Ultrastructural Aspects of the Human Liver in Yellow Fever. Acta Trop. 2005, 94, 116–127. [Google Scholar] [CrossRef] [PubMed]
- Santos, D.O.; Oliveira, A.G.; de Lucena, F.P.; de Mattos, S.A.; de Carvalho, T.P.; Costa, F.B.; Moreira, L.G.A.; da Paixão, T.A.; Santos, R.L. Histopathological Patterns and Susceptibility of Neotropical Primates Naturally Infected with Yellow Fever Virus. Vet Pathol. 2020, 57, 681–686. [Google Scholar] [CrossRef]
- Laemmert, H.W., Jr.; Ferreira, L.C. The Isolation of Yellow Fever Virus from Wild-Caught Marmosets. Am. J. Trop. Med. 1945, 25, 231–232. [Google Scholar] [CrossRef]
- Bates, B.C. Territorial Behavior in Primates: A Review of Recent Field Studies. Primates 1970, 11, 271–284. [Google Scholar] [CrossRef]
- Moreno, E.S.; Spinola, R.; Tengan, C.H.; Brasil, R.A.; Siciliano, M.M. Yellow Fever Epizootics in Non-Human Primates, São Paulo State, Brazil, 2008–2009. Rev. Inst. Med. Trop. Sao Paulo 2013, 55, 45–50. [Google Scholar] [CrossRef]
- Davis, N.C. The Transmission of Yellow Fever: Experiments With the “Woolly Monkey” (Lagothrix Lago-Tricha Humboldt), the “Spider Monkey” (Ateleus Ater F. Cuvier), and the “Squirrel Monkey” (Saimiri Scireus Linnaeus). J. Exp. Med. 1930, 51, 703–720. [Google Scholar] [CrossRef]
- Davis, N.C.; Shannon, R.C. Studies on South American Yellow Fever: III. Transmission of the Virus to Brazilian Monkeys Preliminary Observations. J. Exp. Med. 1929, 50, 81–85. [Google Scholar] [CrossRef]
- Marinho, P.E.S.; Alvarenga, P.P.M.; Crispim, A.P.C.; Candiani, T.M.S.; Alvarenga, A.M.; Bechler, I.M.; Alves, P.A.; Dornas, F.P.; De Oliveira, D.B.; Bentes, A.A.; et al. Wild-Type Yellow Fever Virus Rna in Cerebrospinal Fluid of Child. Emerg. Infect. Dis. 2019, 25, 1567–1570. [Google Scholar] [CrossRef]
- Jennings, A.D.; Gibson, C.A.; Miller, B.R.; Mathews, J.H.; Mitchell, C.J.; Roehrig, J.T.; Wood, D.J.; Taffs, F.; Sil, B.K.; Whitby, S.N.; et al. Analysis of a Yellow Fever Virus Isolated from a Fatal Case of Vaccine-Associated Human Encephalitis. J. Infect. Dis. 1994, 169, 512–518. [Google Scholar] [CrossRef]
- Monath, T.P.; Barrett, A.D.T. Pathogenesis and Pathophysiology of Yellow Fever. Adv. Virus Res. 2003, 60, 343–395. [Google Scholar] [CrossRef] [PubMed]
- Traiber, C.; Coelho Amaral, P.; Ritter, V.R.F.; Winge, A. Infant meningoencephalitis caused by yellow fever vaccine virus transmitted via breastmilk. J. Pediatr. 2011, 87, 269–272. [Google Scholar] [CrossRef] [PubMed]
- Alcantara, B.N.; Imbeloni, A.A.; Durans, D.B.S.; Araújo, M.T.F.; Cruz, E.R.M.; Carvalho, C.A.M.; Mendonça, M.H.R.; Sousa, J.R.; Moraes, A.F.; Martins Filho, A.J.; et al. Histopathological Lesions of Congenital Zika Syndrome in Newborn Squirrel Monkeys. Sci. Rep. 2021, 11, 6099. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing (version 4.4.3); R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 10 October 2024).
- Wickham, H. Tidyverse: Easily Install and Load Tidyverse Packages. R Package Version 1.3.0. 2017. Available online: https://CRAN.R-project.org/package=tidyverse (accessed on 10 October 2024).
- Kassambara, A. Rstatix: Pipe-Friendly Framework for Basic Statistical Tests. R Package Version 0.7.0. Available online: https://cran.r-project.org/web/packages/rstatix/index.html (accessed on 20 October 2024).
- Harrell, F.E. Hmisc: Harrell Miscellaneous. R Package Version 4.5-0. 2024. Available online: https://CRAN.R-project.org/package=Hmisc (accessed on 10 October 2024).
- Wei, T.; Simko, W. Package ‘corrplot’. Statistician 2017, 56, 316–324. [Google Scholar]
- Mitelman, A.K.; Buccheri, V.; Pracchia, L.F.; Rubens, C.V.; Poppe, S.; Cavalcante, A.M.M.P.; Monteiro, S.M. Quantificação das citocinas séricas Th1/Th2 por citometria de fluxo no linfoma de Hodgkin clássico (Measurement of Th1/Th2 serum cytokines by flow cytometry in classical Hodgkin lymphoma). Rev. Bras. Hematol. Hemoter. 2009, 31, 260–266. [Google Scholar] [CrossRef]
- Brasil. Ministério da Saúde. Secretaria de Vigilância em Saúde. Situação Epidemiológica Da Febre Amarela No Monitoramento 2019/2020. Bol. Epidemiológico 2020, 51, 1–19. Available online: https://www.gov.br/saude/pt-br/centrais-de-conteudo/publicacoes/boletins/epidemiologicos/edicoes/2020/boletim-epidemiologico-vol-51-no-01/view (accessed on 13 November 2023).
- Gómez, M.M.; de Abreu, F.V.S.; Dos Santos, A.A.C.; de Mello, I.S.; Santos, M.P.; Ribeiro, I.P.; Ferreira-de-Brito, A.; de Miranda, R.M.; de Castro, M.G.; Ribeiro, M.S.; et al. Genomic and Structural Features of the Yellow Fever Virus from the 2016-2017 Brazilian Outbreak. J. Gen. Virol. 2018, 99, 536–548. [Google Scholar] [CrossRef]
- Rezende, I.M.; Sacchetto, L.; Munhoz, E.; Alves, P.A.; Iani, F.C.d.M.; Adelino, M.R.; Duarte, M.M.; Cury, A.L.F.; Bernardes, A.F.L.; Santos, T.A.; et al. Persistence of Yellow Fever Virus Outside the Amazon Basin, Causing Epidemics in Southeast Brazil, from 2016 to 2018. PLoS Negl. Trop. Dis. 2018, 12, e0006538. [Google Scholar] [CrossRef]
- Delatorre, E.; Santos De Abreu, F.V.; Ribeiro, I.P.; Gómez, M.M.; Cunha Dos Santos, A.A.; Ferreira-De-Brito, A.; Alberto Santos Neves, M.S.; Bonelly, I.; De Miranda, R.M.; Furtado, N.D.; et al. Distinct YFV Lineages Co-Circulated in the Central-Western and Southeastern Brazilian Regions from 2015 to 2018. Front. Microbiol. 2019, 10, 1079. [Google Scholar] [CrossRef] [PubMed]
- Abreu, F.V.S.; dos Santos, E.; Gomes, M.Q.; Vargas, W.P.; de Oliveira Passos, P.H.; Nunes e Silva, C.; Araújo, P.C.; Pires, J.R.; Romano, A.P.M.; Teixeira, D.S.; et al. Capture of Alouatta Guariba Clamitans for the Surveillance of Sylvatic Yellow Fever and Zoonotic Malaria: Which Is the Best Strategy in the Tropical Atlantic Forest? Am. J. Primatol. 2019, 81, e23000. [Google Scholar] [CrossRef]
- Figueiredo, P.O.; Silva, A.T.S.; Oliveira, J.S.; Marinho, P.E.; Rocha, F.T.; Domingos, G.P.; Poblete, P.C.P.; Oliveira, L.B.S.; Duarte, D.C.; Bonjardim, C.A.; et al. Detection and Molecular Characterization of Yellow Fever Virus, 2017, Brazil. Ecohealth 2018, 15, 864–870. [Google Scholar] [CrossRef]
- Silva, N.I.O.; Sacchetto, L.; De Rezende, I.M.; Trindade, G.D.S.; Labeaud, A.D.; De Thoisy, B.; Drumond, B.P. Recent Sylvatic Yellow Fever Virus Transmission in Brazil: The News from an Old Disease. Virol. J. 2020, 17, 9. [Google Scholar] [CrossRef]
- Passos, P.O.; Ramos, D.G.R.; Romano, A.P.; Cavalcante, K.R.L.J.; Miranda, L.H.M.; Coelho, J.M.C.O.; Barros, R.C.; Filho, A.J.M.; Quaresma, J.A.S.; Macêdo, I.L.; et al. Hepato-pathological hallmarks for the surveillance of Yellow Fever in South American non-human primates. Acta Trop. 2022, 231, 106468. [Google Scholar] [CrossRef] [PubMed]
- Laemmert, H.J.; Kumm, H. The Susceptibility of Howler Monkeys to Yellow Fever Virus. Am. J. Trop. Med. Hyg. 1950, 30, 723–731. [Google Scholar] [CrossRef]
- Pinheiro, F.P.; Travassos da Rosa, A.P.A.; Moraes, M.A.P. An Epidemic of Yellow Fever in Central Brazil, 1972-1973. II. Ecological Studies. Am. J. Trop. Med. Hyg. 1981, 30, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Thoisy, B.; Vogel, I.; Reynes, J.M.; Pouliquen, J.F.; Carme, B.; Kazanji, M.; Vié, J.C. Health Evaluation of Translocated Free-Ranging Primates in French Guiana. Am. J. Primatol. 2001, 54, 1–16. [Google Scholar] [CrossRef]
- Escosteguy, C.C.; Pereira, A.G.L.; Marques, M.R.V.E.; Lima, T.R.d.A.; Galliez, R.M.; Medronho, R. de A. Febre Amarela: Perfil Dos Casos e Fatores Associados Ao Óbito Em Hospital Referência No Estado Do Rio de Janeiro, 2017–2018. Rev. Saude Publica 2019, 53, 89. [Google Scholar] [CrossRef]
- Stokes, A.; Hudson, P. Transmission of Yellow Fever to Macacus Rhesus. JAMA 1928, 90, 253–254. [Google Scholar] [CrossRef]
- Engelmann, F.; Josset, L.; Girke, T.; Park, B.; Barron, A.; Dewane, J.; Hammarlund, E.; Lewis, A.; Axthelm, M.K.; Slifka, M.K.; et al. Pathophysiologic and Transcriptomic Analyses of Viscerotropic Yellow Fever in a Rhesus Macaque Model. PLoS Negl. Trop. Dis. 2014, 8, e3295. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.Y.; Zhang, H.; Guzman, H.; Tesh, R.B. Experimental yellow fever vírus infection in the Golden hamster (Mesocricetus auratus). II. Pathology. J. Infect. Dis. 2001, 183, 1437–1444. [Google Scholar] [CrossRef]
- Silva, G.M.M.; Veríssimo, K.C.S.; Oliveira, M.A.B. Orçamento das atividades diárias de dois grupos de Callithrix jacchus em área urbana. Rev. Etol. 2011, 10, 57–63. [Google Scholar]
- Flores, E.F.; Weiblen, R. O Vírus do Nilo Ocidental. Ciência Rural 2008, 39, 604–612. [Google Scholar] [CrossRef]
- Raika, M.; Lobo, G.; Da, S.; Furtado, C.; Ribas, J.; Júnior, G.; De Paula, L.; Fernando, J.; Barcellos, M. Citocinas Na Dengue: Inovações Do Sistema Imune 1. Sci. Amaz. 2014, 1, 25–40. [Google Scholar]
- Noronha, T.G. Duração Da Imunidade Pós-Vacinação Contra Febre Amarela Em Crianças: Estudo Complementar Sobre Imunidade Humoral. Ph.D. Thesis, Escola Nacional de Saúde Pública Sergio Arouca, Rio de Janeiro, Brazil, 2016. [Google Scholar]
- Galea, I.; Bechmann, I.; Perry, V.H. What Is Immune Privilege (Not)? Trends Immunol. 2007, 28, 12–18. [Google Scholar] [CrossRef]
- Quaresma, J.A.S.; Vasconcelos, P.F.C.; da Costa Vasconcelos, P.F.; de Sousa, J.R.; da Silva, A.N.L.; da Silva, A.N.L.; de Almeida, G.M.F.; Pagliari, C.; Andrade, H.F.; Duarte, M.I.S. New Insights into the Mechanism of Immune-Mediated Tissue Injury in Yellow Fever. Viruses 2022, 14, 2379. [Google Scholar] [CrossRef]
- Carvalho, M.L.G.; Falcão, L.F.M.; Lopes, J.d.C.; Mendes, C.C.H.; Olímpio, F.A.; Miranda, V.d.S.C.; Santos, L.C.d.; de Moraes, D.D.P.; Bertonsin Filho, M.V.; da Costa, L.D.; et al. Role of Th17 Cytokines in the Liver’s Immune Response during Fatal Yellow Fever: Triggering Cell Damage Mechanisms. Cells 2022, 11, 2053. [Google Scholar] [CrossRef] [PubMed]
- de Sousa, J.R.; da Silva, A.N.L.; de Almeida, G.M.F.; da Silva, A.N.L.; de Almeida, G.M.F.; Pagliari, C.; Andrade, H.F.; Duarte, M.I.S.; Quaresma, J.A.S. Activation of the Cytokine Network and Unfavorable Outcome in Human Yellow Fever Cases. J. Infect. Dis. 2004, 190, 1821–1827. [Google Scholar] [CrossRef]
- da Costa Lopes, J.; Quaresma, J.A.S.; Falcão, L.F.M.; Martins Filho, A.J.; de Sousa, J.R.; Vasconcelos, P.F.C. Factors Involved in the Apoptotic Cell Death Mechanism in Yellow Fever Hepatitis. Viruses 2022, 14, 1204. [Google Scholar] [CrossRef]
- Holanda, G.M.; Casseb, S.M.; Quaresma, J.A.S.; Vasconcelos, P.F.C.; Cruz, A.C.R. Yellow Fever Virus Modulates Cytokine mRNA Expression and Induces Activation of Caspase 3/7 in the Human Hepatocarcinoma Cell Line HepG2. Arch. Virol. 2019, 164, 1187–1192. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliveira, S.R.d.M.; Cruz, E.d.R.M.d.; Prestes, N.G.d.O.; da Silva, F.S.; de Araújo, M.T.F.; Amador Neto, O.P.; Lima, M.d.L.G.; de Alcântara, B.N.; Dias, D.D.; Sousa, J.R.d.; et al. Histopathological Changes and Immune Response Profile in the Brains of Non-Human Primates Naturally Infected with Yellow Fever Virus. Viruses 2025, 17, 386. https://doi.org/10.3390/v17030386
Oliveira SRdM, Cruz EdRMd, Prestes NGdO, da Silva FS, de Araújo MTF, Amador Neto OP, Lima MdLG, de Alcântara BN, Dias DD, Sousa JRd, et al. Histopathological Changes and Immune Response Profile in the Brains of Non-Human Primates Naturally Infected with Yellow Fever Virus. Viruses. 2025; 17(3):386. https://doi.org/10.3390/v17030386
Chicago/Turabian StyleOliveira, Suzana Ribeiro de Melo, Ermelinda do Rosário Moutinho da Cruz, Nelielma Garcia de Oliveira Prestes, Fábio Silva da Silva, Marialva Tereza Ferreira de Araújo, Orlando Pereira Amador Neto, Maria de Lourdes Gomes Lima, Bianca Nascimento de Alcântara, Daniel Damous Dias, Jorge Rodrigues de Sousa, and et al. 2025. "Histopathological Changes and Immune Response Profile in the Brains of Non-Human Primates Naturally Infected with Yellow Fever Virus" Viruses 17, no. 3: 386. https://doi.org/10.3390/v17030386
APA StyleOliveira, S. R. d. M., Cruz, E. d. R. M. d., Prestes, N. G. d. O., da Silva, F. S., de Araújo, M. T. F., Amador Neto, O. P., Lima, M. d. L. G., de Alcântara, B. N., Dias, D. D., Sousa, J. R. d., Filho, A. J. M., Casseb, L. M. N., & Medeiros, D. B. d. A. (2025). Histopathological Changes and Immune Response Profile in the Brains of Non-Human Primates Naturally Infected with Yellow Fever Virus. Viruses, 17(3), 386. https://doi.org/10.3390/v17030386






