Development and Application of a Multiplex PCR Assay for Simultaneous Detection of Tomato Yellow Leaf Curl Virus and Tomato Leaf Curl New Delhi Virus
Abstract
1. Introduction
2. Materials and Methods
2.1. Virus and Plant Material
2.1.1. Virus Resources
2.1.2. Field Plant Sampling Showing Viral Symptoms
2.2. DNA Isolation
2.3. Primer Used for Multiplex PCR Assay
2.4. Construction of Recombinant Plasmids Containing the Target DNA for Analysis of the Sensitivity of Multiplex PCR Assay
2.5. Optimization of Multiplex PCR Assay
2.6. Sensitivity and Specificity of Multiplex PCR Assay
2.7. Validation of Multiplex PCR Assay in Field Samples
3. Results
3.1. Development of Multiplex PCR Assay to Simultaneously Detect TYLCV and ToLCNDV
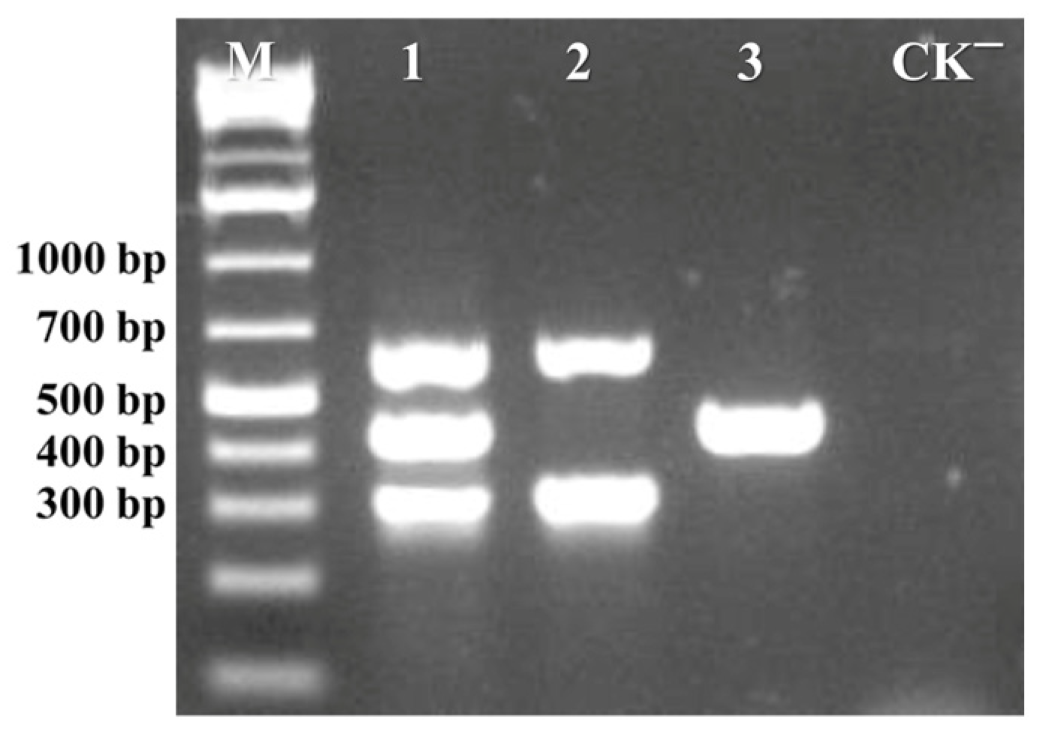
3.1.1. Development of Multiplex PCR Assay
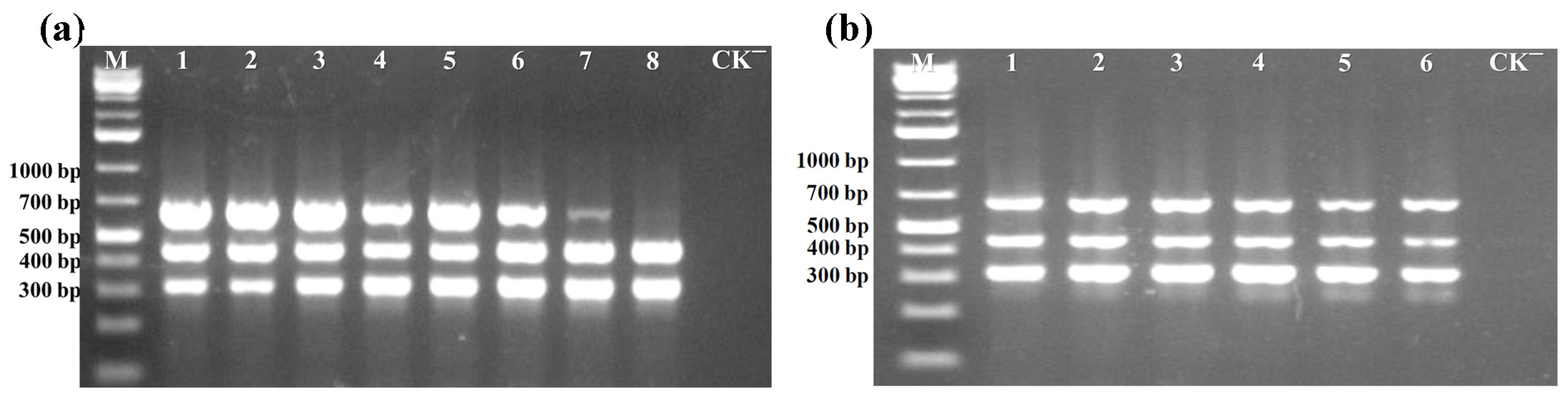
3.1.2. Primer Concentration Optimization of Multiplex PCR Assay
3.1.3. Annealing Temperature Optimization of Multiplex PCR Assay
3.2. Sensitivity and Specificity Detection of Multiplex PCR Assay
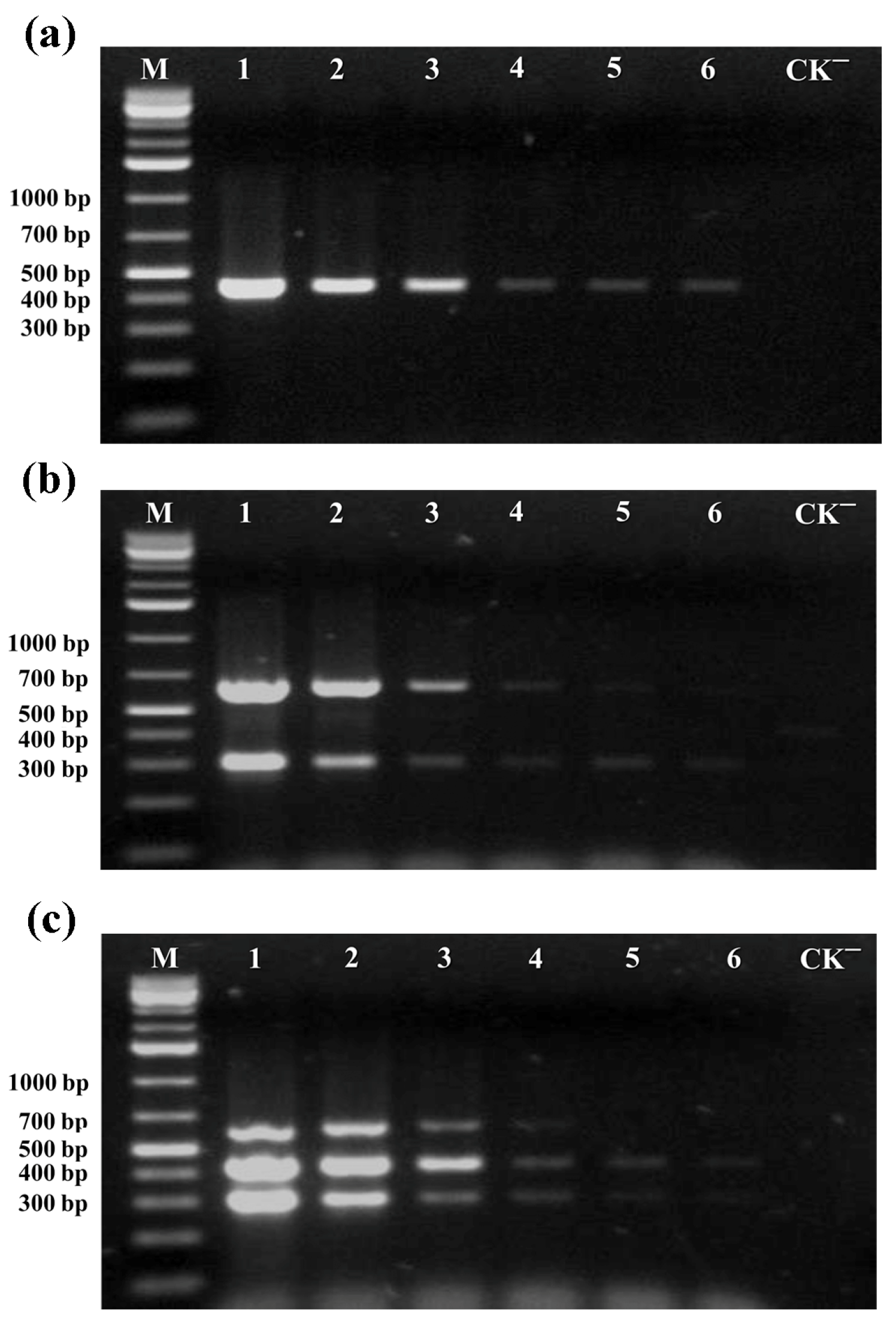
3.2.1. Sensitivity Detection
3.2.2. Specificity Detection
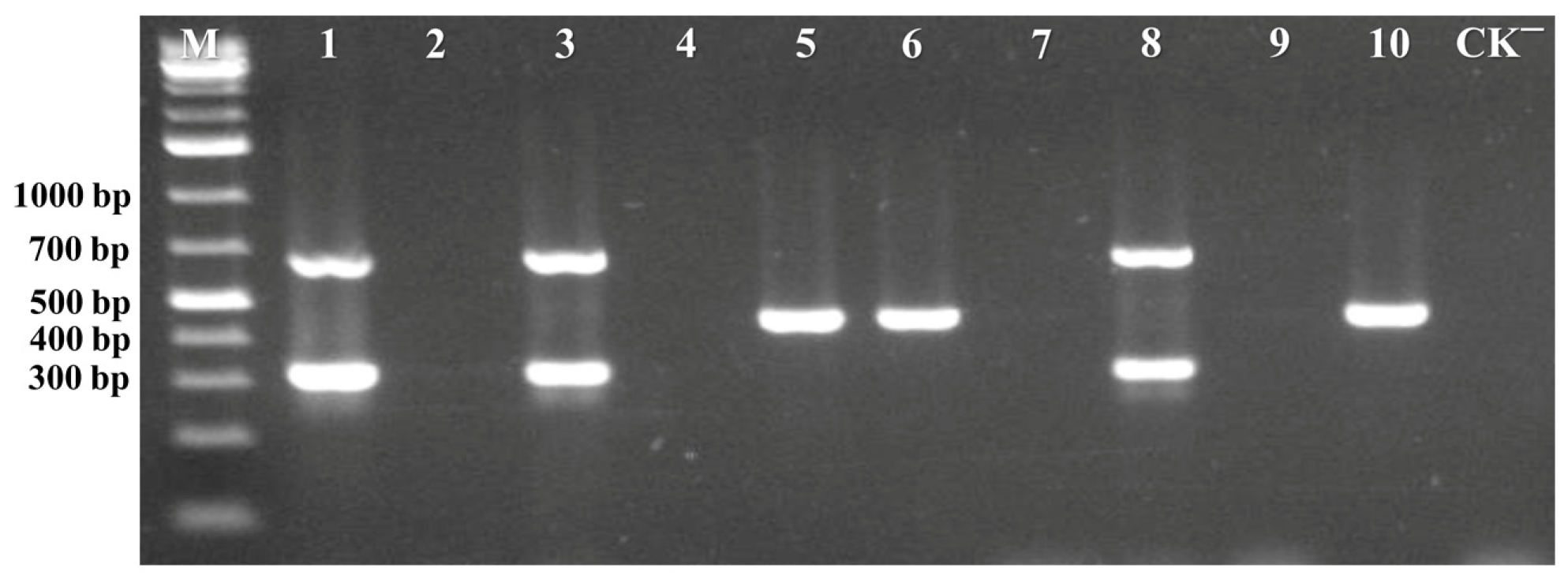
3.3. Validation of the Multiplex PCR Assay on Field Plant Samples
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Frascati, F.; Rotunno, S.; Accotto, G.P.; Noris, E.; Vaira, A.M.; Miozzi, L. Exogenous application of dsRNA for protection against tomato leaf curl New Delhi virus. Viruses 2024, 16, 436. [Google Scholar] [CrossRef]
- Prasad, A.; Prasad, M. Interaction of ToLCNDV TrAP with SlATG8f marks it susceptible to degradation by autophagy. Cell. Mol. Life Sci. 2022, 79, 241. [Google Scholar] [CrossRef] [PubMed]
- Padidam, M.; Beachy, R.N.; Fauquet, C.M. Tomato leaf curl geminivirus from India has a bipartite genome and coat protein is not essential for infectivity. J. Gen. Virol. 1995, 76 Pt 1, 25–35. [Google Scholar] [CrossRef]
- Mei, Y.; Cai, L.; Wang, Y.; Li, F.; Yang, X.; Yang, J.; Zhou, X. Molecular characterization and pathogenicity of an infectious clone of tomato leaf curl New Delhi virus isolate infecting Cucumis melo. Stress Biol. 2023, 3, 51. [Google Scholar] [CrossRef]
- Panno, S.; Iacono, G.; Davino, M.; Marchione, S.; Zappardo, V.; Bella, P.; Tomassoli, L.; Accotto, G.P.; Davino, S. First report of tomato leaf curl New Delhi virus affecting zucchini squash in an important horticultural area of southern Italy. New Dis. Rep. 2016, 33, 6. [Google Scholar] [CrossRef]
- Donati, L.; Bertin, S.; Gentili, A.; Luigi, M.; Taglienti, A.; Manglli, A.; Tiberini, A.; Brasili, E.; Sciubba, F.; Pasqua, G.; et al. Effects of organic biostimulants added with zeolite on zucchini squash plants infected by tomato leaf curl New Delhi virus. Viruses 2022, 14, 607. [Google Scholar] [CrossRef] [PubMed]
- Vo, T.T.B.; Cho, W.K.; Jo, Y.; Lal, A.; Nattanong, B.; Qureshi, M.A.; Tabssum, M.; Troiano, E.; Parrella, G.; Kil, E.J.; et al. Transcriptional analysis of the differences between ToLCNDV-India and ToLCNDV-ES leading to contrary symptom development in cucumber. Int. J. Mol. Sci. 2023, 24, 2181. [Google Scholar] [CrossRef] [PubMed]
- Venkataravanappa, V.; Ashwathappa, K.V.; Reddy, C.N.; Shankarappa, K.S.; Reddy, M.K. Characterization of tomato leaf curl New Delhi virus associated with leaf curl and yellowing disease of watermelon and development of LAMP assay for its detection. 3 Biotech 2020, 10, 282. [Google Scholar] [CrossRef]
- Yamamoto, H.; Wakita, Y.; Kitaoka, T.; Fujishiro, K.; Kesumawati, E.; Koeda, S. Southeast Asian isolate of the tomato leaf curl New Delhi virus shows higher pathogenicity against tomato and cucurbit crops compared to that of the Mediterranean isolate. Horticult. J. 2021, 90, 314–325. [Google Scholar] [CrossRef]
- Bandaranayake, W.; Wickramarachchi, W.; Wickramasinghe, H.; Rajapakshe, R.; Dissanayake, D. Molecular detection and characterization of begomoviruses associated with cucurbitaceae vegetables in Sri Lanka. J. Natl. Sci. Found. Sri. 2014, 42, 239–245. [Google Scholar] [CrossRef]
- Renukadevi, P.; Devi, R.G.; Jothika, C.; Karthikeyan, G.; Malathi, V.G.; Balakrishnan, N.; Rajagopal, B.; Nakkeeran, S.; Abd-Allah, E.F. Genomic distinctiveness and recombination in tomato leaf curl New Delhi virus (ToLCNDV-BG) isolates infecting bitter gourd. 3 Biotech 2024, 14, 184. [Google Scholar] [CrossRef] [PubMed]
- Sáez, C.; Flores-León, A.; Montero-Pau, J.; Sifres, A.; Dhillon, N.P.S.; López, C.; Picó, B. RNA-Seq transcriptome analysis provides candidate genes for resistance to tomato leaf curl New Delhi virus in melon. Front. Plant Sci. 2022, 12, 798858. [Google Scholar] [CrossRef]
- Wang, S.; Guo, H.; Ge, F.; Sun, Y. Apoptotic neurodegeneration in whitefly promotes the spread of TYLCV. Elife 2020, 9, e56168. [Google Scholar] [CrossRef]
- Shteinberg, M.; Mishra, R.; Anfoka, G.; Altaleb, M.; Brotman, Y.; Moshelion, M.; Gorovits, R.; Czosnek, H. Tomato yellow leaf curl virus (TYLCV) promotes plant tolerance to drought. Cells 2021, 10, 2875. [Google Scholar] [CrossRef]
- Cohen, S.; Harpaz, I. Periodic, rather than continual acquisition of a new tomato virus by its vector, the tobacco whitefly (Bemisia tabaci gennadius). Entomol. Exp. Appl. 1964, 7, 155–166. [Google Scholar] [CrossRef]
- Li, F.; Qiao, R.; Yang, X.; Gong, P.; Zhou, X. Occurrence, distribution, and management of tomato yellow leaf curl virus in China. Phytopathol. Res. 2022, 4, 28. [Google Scholar] [CrossRef]
- Kil, E.J.; Kim, S.; Lee, Y.J.; Byun, H.S.; Park, J.; Seo, H.; Kim, C.S.; Shim, J.K.; Lee, J.H.; Kim, J.K.; et al. Tomato yellow leaf curl virus (TYLCV-IL): A seed-transmissible geminivirus in tomatoes. Sci. Rep. 2016, 6, 19013. [Google Scholar] [CrossRef]
- Moriones, E.; Navas-Castillo, J. Tomato yellow leaf curl virus, an emerging virus complex causing epidemics worldwide. Virus Res. 2000, 71, 123–134. [Google Scholar] [CrossRef]
- Cohen, J.; Gera, A. Lisianthus leaf curl-a new disease of lisianthus caused by tomato yellow leaf curl virus. Plant Dis. 1995, 4, 416–420. [Google Scholar] [CrossRef]
- Navas-Castillo, J.; Sánchez-Campos, S.; Díaz, J.A.; Sáez-Alonso, E.; Moriones, E. Tomato yellow leaf curl virus-Is causes a novel disease of common bean and severe epidemics in tomato in Spain. Plant Dis. 1999, 1, 29–32. [Google Scholar] [CrossRef] [PubMed]
- Reina, J.; Morilla, G.; Bejarano, E.R.; Rodríguez, M.D.; Janssen, D. First report of Capsicum annuum plants infected by tomato yellow leaf curl virus. Plant Dis. 1999, 12, 1176. [Google Scholar] [CrossRef]
- Anfoka, G.; Haj Ahmad, F.; Abhary, M.; Hussein, A. Detection and molecular characterization of viruses associated with tomato yellow leaf curl disease in cucurbit crops in Jordan. Plant Pathol. 2009, 4, 754–762. [Google Scholar] [CrossRef]
- Zhou, Y.; Zheng, H.; Jiang, D.; Liu, M.; Zhang, W.; Yan, J. A rapid detection of tomato yellow leaf curl virus using recombinase polymerase amplification-lateral flow dipstick assay. Lett. Appl. Microbiol. 2022, 74, 640–646. [Google Scholar] [CrossRef] [PubMed]
- Kil, E.J.; Park, J.; Choi, E.Y.; Byun, H.; Lee, K.; An, C.G.; Lee, J.; Lee, G.; Choi, H.S.; Kim, C.S.; et al. Seed transmission of tomato yellow leaf curl virus in sweet pepper (Capsicum annuum). Eur. J. Plant Pathol. 2018, 150, 759–764. [Google Scholar] [CrossRef]
- Akbar, A.; Al Hashash, H.; Al-Ali, E. Tomato yellow leaf curl virus (TYLCV) in Kuwait and global analysis of the population structure and evolutionary pattern of TYLCV. Virol. J. 2024, 21, 308. [Google Scholar] [CrossRef] [PubMed]
- Prasad, A.; Sharma, N.; Hari-Gowthem, G.; Muthamilarasan, M.; Prasad, M. Tomato yellow leaf curl virus: Impact, challenges, and management. Trends Plant Sci. 2020, 25, 897–911. [Google Scholar] [CrossRef]
- Martinelli, F.; Scalenghe, R.; Davino, S.; Panno, S.; Scuderi, G.; Ruisi, P.; Villa, P.; Stroppiana, D.; Boschetti, M.; Goulart, L.R.; et al. Advanced methods of plant disease detection. A review. Agron. Sustain. Dev. 2015, 35, 1–25. [Google Scholar] [CrossRef]
- Jaybhaye, S.G.; Chavhan, R.L.; Hinge, V.R.; Deshmukh, A.S.; Kadam, U.S. CRISPR-Cas assisted diagnostics of plant viruses and challenges. Virology 2024, 597, 110160. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Jiao, X.; Zhou, X.; Liu, H.; Ni, Y.; Wu, J. Highly sensitive serological methods for detecting tomato yellow leaf curl virus in tomato plants and whiteflies. Virol. J. 2013, 10, 142. [Google Scholar] [CrossRef] [PubMed]
- Londoño, M.A.; Harmon, C.L.; Polston, J.E. Evaluation of recombinase polymerase amplification for detection of begomoviruses by plant diagnostic clinics. Virol. J. 2016, 13, 48. [Google Scholar] [CrossRef]
- Caruso, A.G.; Ragona, A.; Bertacca, S.; Montoya, M.A.M.; Panno, S.; Davino, S. Development of an in-field real-time LAMP assay for rapid detection of tomato leaf curl New Delhi virus. Plants 2023, 12, 1487. [Google Scholar] [CrossRef]
- Bang, B.; Lee, J.; Kim, S.; Park, J.; Nguyen, T.T.; Seo, Y.S. A Rapid and Efficient Method for Construction of an Infectious Clone of Tomato yellow leaf curl virus. Plant Pathol. J. 2014, 30, 310–315. [Google Scholar] [CrossRef]
- Sun, A.; Wang, L.; Zhang, Y.; Yang, X.; Su, Y.; Wu, X. Development and application of a duplex RT-RPA assay for the simultaneous detection of cymbidium mosaic virus and odontoglossum ringspot virus. Viruses 2024, 16, 543. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Yang, J. Visual DNA diagnosis of tomato yellow leaf curl virus with integrated recombinase polymerase amplification and a gold-nanoparticle probe. Sci. Rep. 2019, 9, 15146. [Google Scholar] [CrossRef]
- Bakheit, M.A.; Torra, D.; Palomino, L.A.; Thekisoe, O.M.; Mbati, P.A.; Ongerth, J.; Karanis, P. Sensitive and specific detection of Cryptosporidium species in PCR-negative samples by loop-mediated isothermal DNA amplification and confirmation of generated LAMP products by sequencing. Vet. Parasitol. 2008, 158, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Venbrux, M.; Crauwels, S.; Rediers, H. Current and emerging trends in techniques for plant pathogen detection. Front. Plant Sci. 2023, 14, 1120968. [Google Scholar] [CrossRef]
- Pallás, V.; Sánchez-Navarro, J.A.; James, D. Recent advances on the multiplex molecular detection of plant viruses and viroids. Front. Microbiol. 2018, 9, 2087. [Google Scholar] [CrossRef]
- Devi, O.P.; Sharma, S.K.; Sanatombi, K.; Devi, K.S.; Pathaw, N.; Roy, S.S.; Chanu, N.T.; Sanabam, R.; Devi, H.C.; Singh, A.R.; et al. A simplified multiplex PCR assay for simultaneous detection of six viruses infecting diverse Chilli species in India and its application in field diagnosis. Pathogens 2022, 12, 6. [Google Scholar] [CrossRef]
- Lafrance, R.; Valdez-Torres, J.B.; Villicaña, C.; García-Estrada, R.S.; Esparza-Araiza, M.J.; León-Félix, J. Response surface methodology for optimization of multiplex-PCR protocols for detection of TYLCV, TSWV and Fol molecular markers: Analytical performance evaluation. Genes 2023, 14, 337. [Google Scholar] [CrossRef] [PubMed]
- Lefeuvre, P.; Hoareau, M.; Delatte, H.; Reynaud, B.; Lett, J.M. A multiplex PCR method discriminating between the TYLCV and TYLCV-Mld clades of tomato yellow leaf curl virus. J. Virol. Methods 2007, 144, 165–168. [Google Scholar] [CrossRef]
- Aloyce, R.C.; Tairo, F.; Sseruwagi, P.; Rey, M.E.; Ndunguru, J. A single-tube duplex and multiplex PCR for simultaneous detection of four cassava mosaic begomovirus species in cassava plants. J. Virol. Methods 2013, 189, 148–156. [Google Scholar] [CrossRef]
- Xue, B.; Shang, J.; Yang, J.; Zhang, L.; Du, J.; Yu, L.; Yang, W.; Naeem, M. Development of a multiplex RT-PCR assay for the detection of soybean mosaic virus, bean common mosaic virus and cucumber mosaic virus in field samples of soybean. J. Virol. Methods 2021, 298, 114278. [Google Scholar] [CrossRef]
- Fortes, I.M.; Sánchez-Campos, S.; Fiallo-Olivé, E.; Díaz-Pendón, J.A.; Navas-Castillo, J.; Moriones, E. A novel strain of tomato leaf curl New Delhi virus has spread to the mediterranean basin. Viruses 2016, 8, 307. [Google Scholar] [CrossRef]
- Marchant, W.G.; Brown, J.K.; Gautam, S.; Ghosh, S.; Simmons, A.M.; Srinivasan, R. Non-feeding transmission modes of the tomato yellow leaf curl virus by the whitefly Bemisia tabaci do not contribute to reoccurring leaf curl outbreaks in tomato. Insects 2024, 15, 760. [Google Scholar] [CrossRef]
- Kim, I.R.; Lim, H.S.; Choi, W.; Kang, D.I.; Lee, S.Y.; Lee, J.R. Monitoring living modified canola using an efficient multiplex PCR assay in natural environments in south Korea. Appl. Sci. 2020, 10, 7721. [Google Scholar] [CrossRef]

| Viruses | Primer Name | Sequences (5′–3′) | Size (bp) |
|---|---|---|---|
| ToLCNDV-DNA-A | ToLCNDV-DNA-A-F | CTATGGAGCTCGTGCAGTTGTC | 651 bp |
| ToLCNDV-DNA-A-R | GTAGCATATACAGGATTTGATGCGTGAG | ||
| TYLCV | TYLCV-F | AATCATTTCCACGCCCGTCTC | 442 bp |
| TYLCV-R | TCCAAAATCCATTGGGCTGTTTCC | ||
| ToLCNDV-DNA-B | ToLCNDV-DNA-B-F | CGTTCGGACCTATAGCCAGATAGG | 305 bp |
| ToLCNDV-DNA-B-R | CTTCTCTCTCAAGGATAAGAATCCTTGG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, H.; Zhang, J.; Wu, X.; Li, L.; Qian, Y. Development and Application of a Multiplex PCR Assay for Simultaneous Detection of Tomato Yellow Leaf Curl Virus and Tomato Leaf Curl New Delhi Virus. Viruses 2025, 17, 322. https://doi.org/10.3390/v17030322
Hu H, Zhang J, Wu X, Li L, Qian Y. Development and Application of a Multiplex PCR Assay for Simultaneous Detection of Tomato Yellow Leaf Curl Virus and Tomato Leaf Curl New Delhi Virus. Viruses. 2025; 17(3):322. https://doi.org/10.3390/v17030322
Chicago/Turabian StyleHu, Hongxia, Jie Zhang, Xiaoyin Wu, Li Li, and Yajuan Qian. 2025. "Development and Application of a Multiplex PCR Assay for Simultaneous Detection of Tomato Yellow Leaf Curl Virus and Tomato Leaf Curl New Delhi Virus" Viruses 17, no. 3: 322. https://doi.org/10.3390/v17030322
APA StyleHu, H., Zhang, J., Wu, X., Li, L., & Qian, Y. (2025). Development and Application of a Multiplex PCR Assay for Simultaneous Detection of Tomato Yellow Leaf Curl Virus and Tomato Leaf Curl New Delhi Virus. Viruses, 17(3), 322. https://doi.org/10.3390/v17030322







