Fecal Transmission of Spodoptera frugiperda Multiple Nucleopolyhedrovirus (SfMNPV; Baculoviridae)
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Colony and Virus
2.2. Production of Virus-Contaminated Feces
2.3. Presence of Virus in Fecal Samples: Insect Bioassay
2.4. Presence of Virus in Fecal Samples: Quantitative PCR
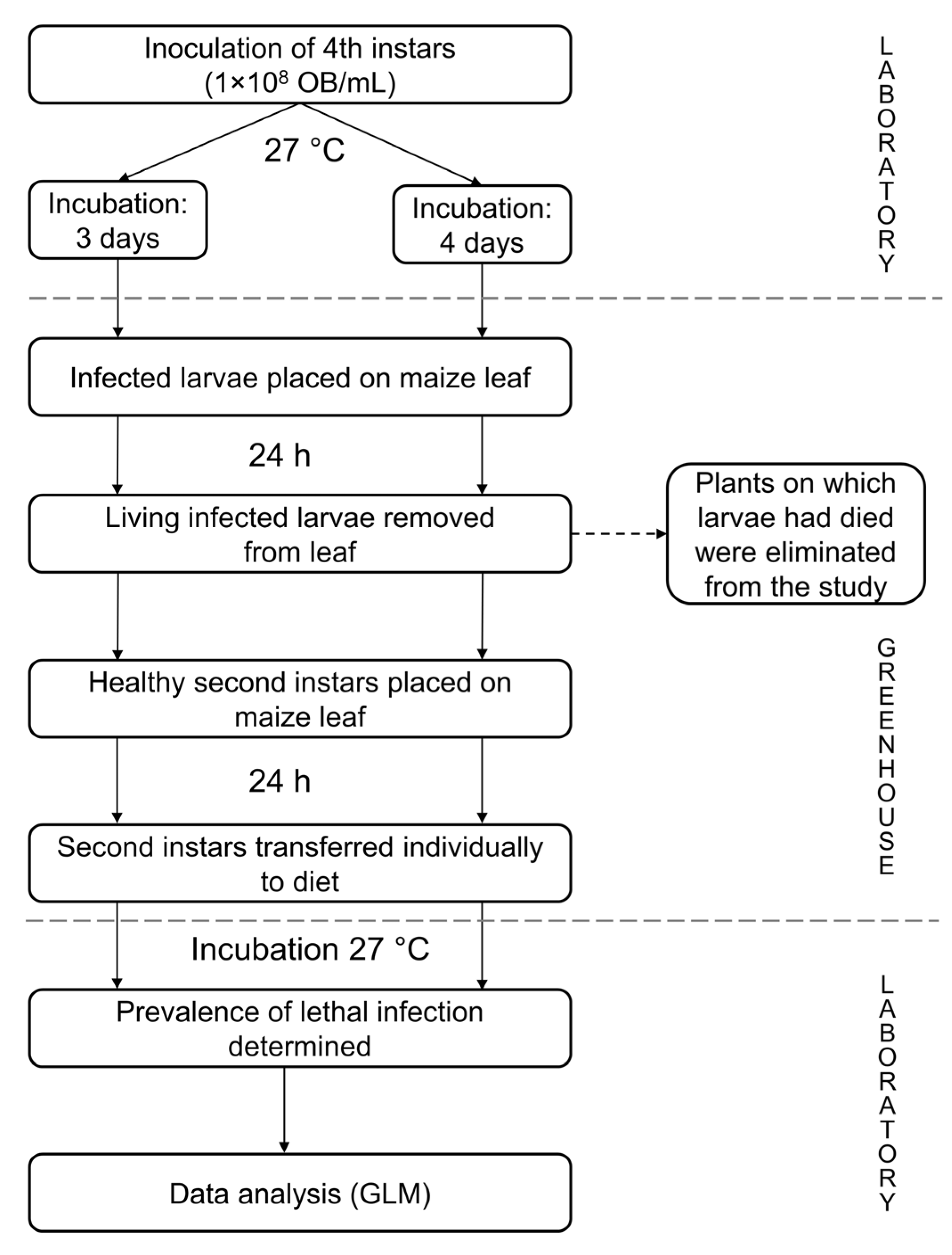
2.5. Transmission Under Greenhouse Conditions
2.6. Statistical Analyses
3. Results
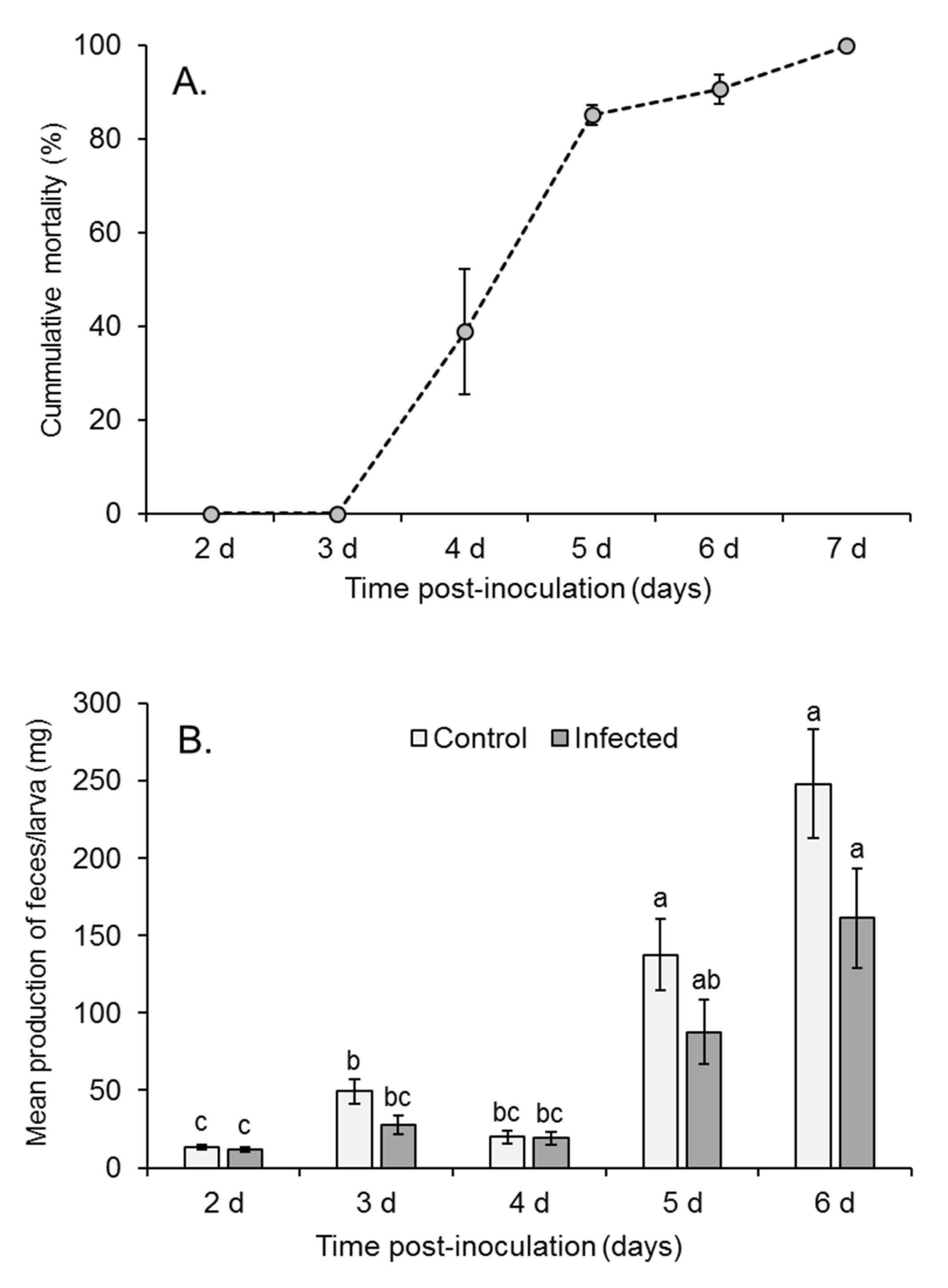
3.1. Production of Virus-Contaminated Feces
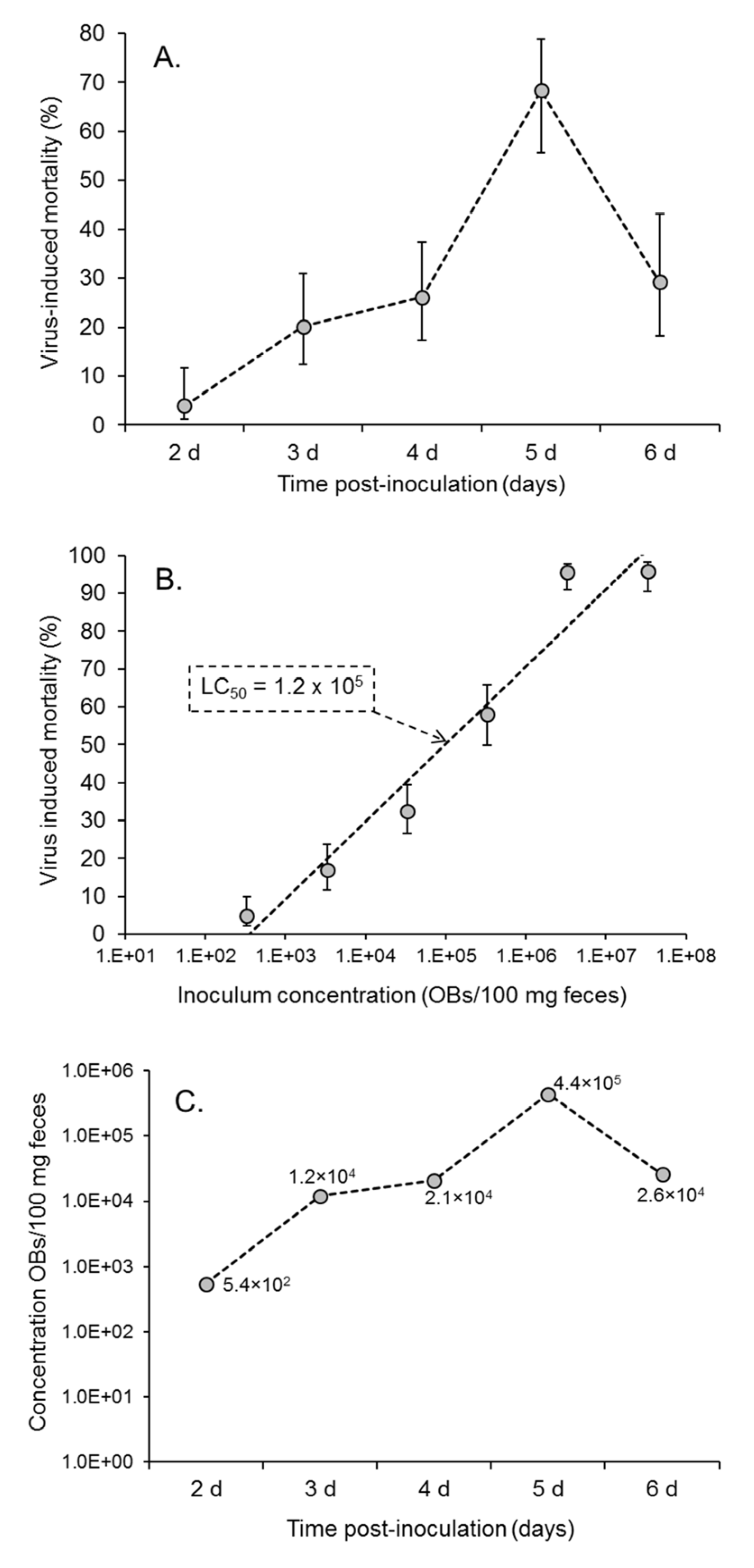
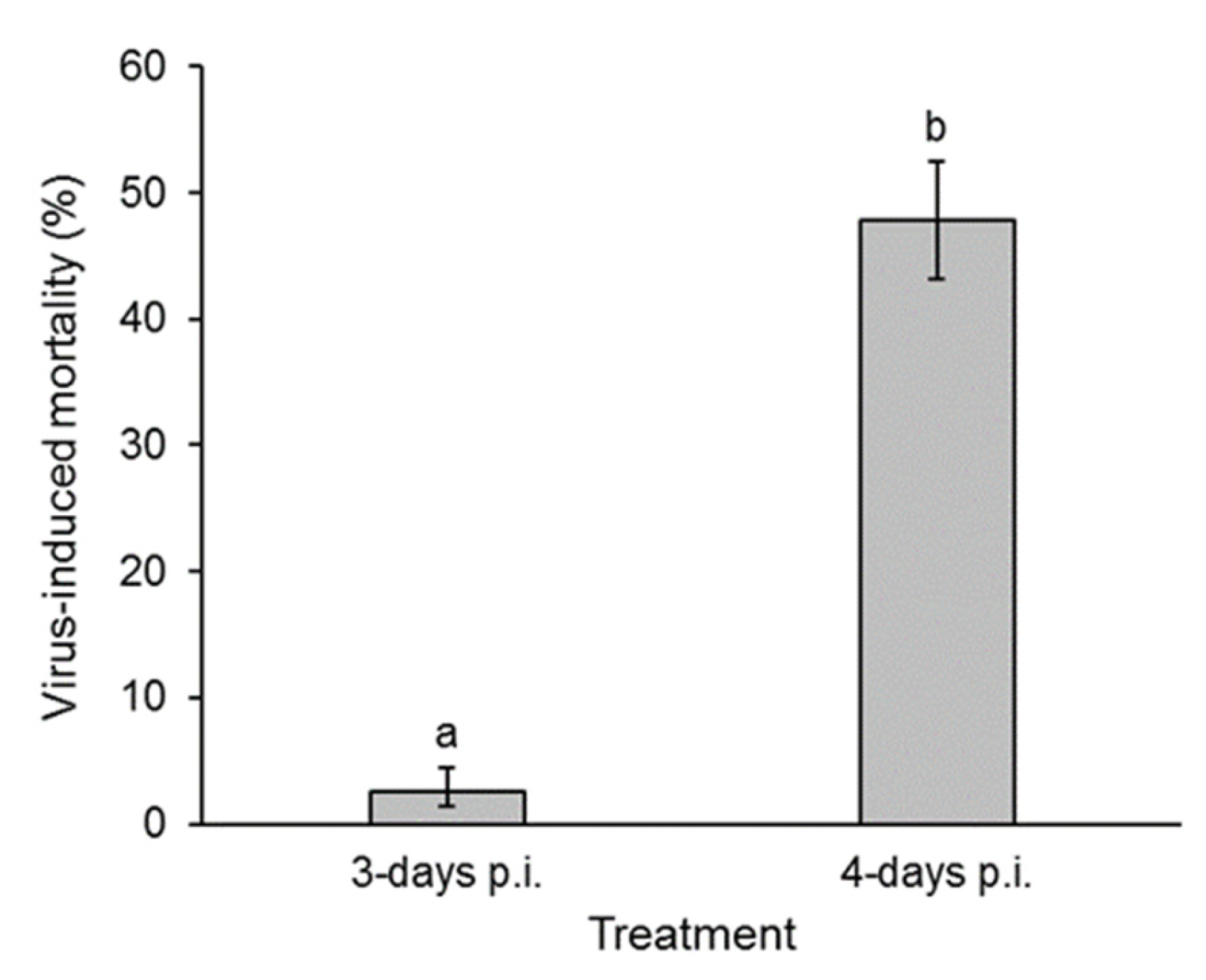
3.2. Presence of Virus in Fecal Samples: Insect Bioassay
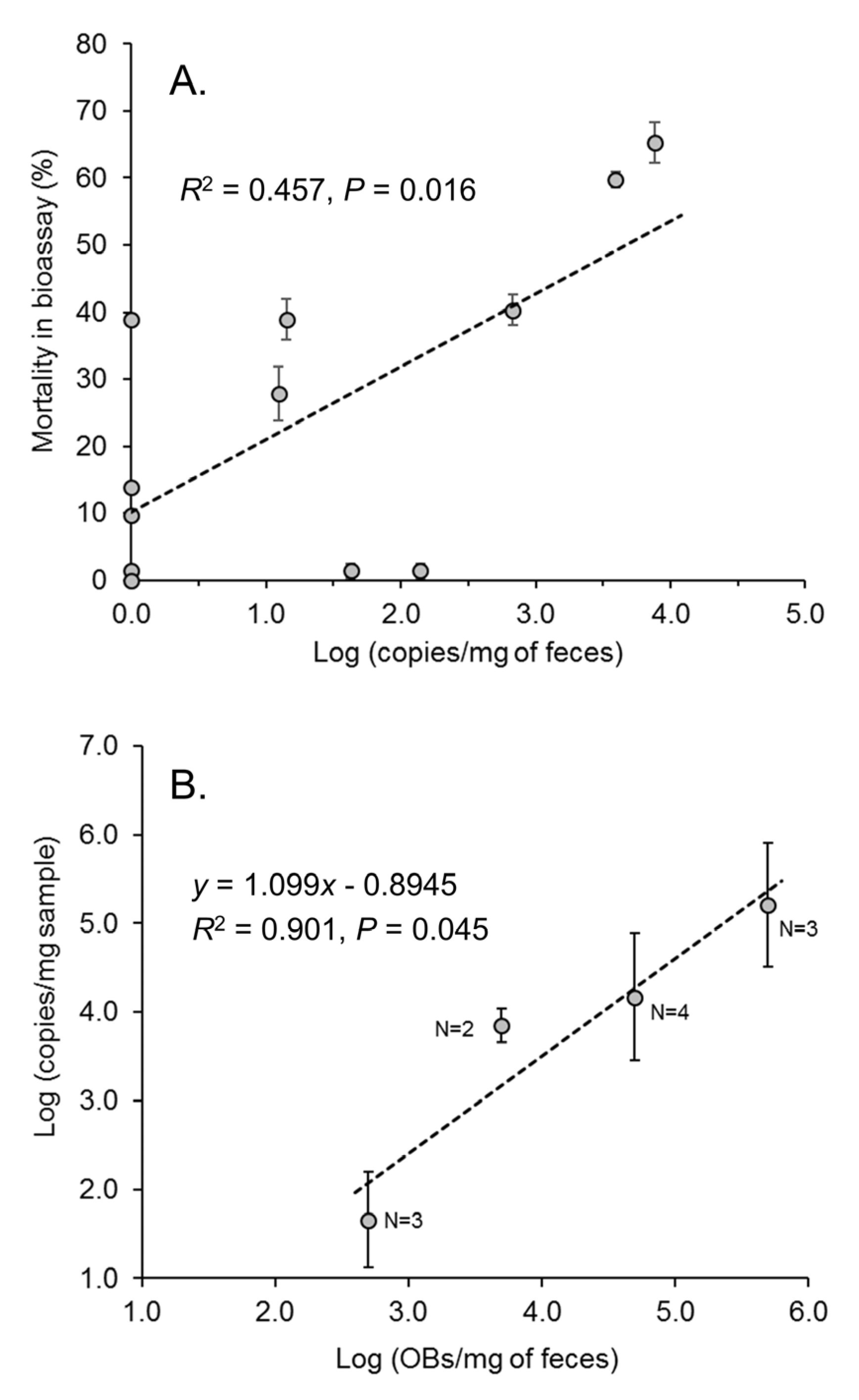
3.3. Presence of Virus in Fecal Samples: Quantitative PCR
3.4. Transmission Under Greenhouse Conditions
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Elderd, B.D. Developing models of disease transmission: Insights from ecological studies of insects and their baculoviruses. PLoS Path. 2013, 9, e1003372. [Google Scholar] [CrossRef] [PubMed]
- Cory, J.S. Insect virus transmission: Different routes to persistence. Curr. Opin. Insect Sci. 2015, 8, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Williams, T. Viruses. In Ecology of Invertebrate Diseases; Hajek, A.E., Shapiro-Ilan, D.I., Eds.; Wiley: Chichester, UK, 2018; pp. 215–285. [Google Scholar] [CrossRef]
- Erlandson, M.A.; Toprak, U.; Hegedus, D.D. Role of the peritrophic matrix in insect-pathogen interactions. J. Insect Physiol. 2019, 117, 103894. [Google Scholar] [CrossRef] [PubMed]
- Boogaard, B.; Van Oers, M.M.; Van Lent, J.W. An advanced view on baculovirus per os infectivity factors. Insects 2018, 9, 84. [Google Scholar] [CrossRef]
- Rohrmann, G.F. Baculovirus Molecular Biology, 4th ed.; National Center for Biotechnology Information: Bethesda, MD, USA, 2019. Available online: https://www.ncbi.nlm.nih.gov/books/NBK543458 (accessed on 23 September 2024).
- Keddie, B.A.; Aponte, G.W.; Volkman, L.E. The pathway of infection of Autographa californica nuclear polyhedrosis virus in an insect host. Science 1989, 243, 1728–1730. [Google Scholar] [CrossRef]
- Washburn, J.O.; Lyons, E.H.; Haas-Stapleton, E.J.; Volkman, L.E. Multiple nucleocapsid packaging of Autographa californica nucleopolyhedrovirus accelerates the onset of systemic infection in Trichoplusia ni. J. Virol. 1999, 73, 411–416. [Google Scholar] [CrossRef]
- Simón, O.; Williams, T.; López-Ferber, M.; Caballero, P. Virus entry or the primary infection cycle are not the principal determinants of host specificity of Spodoptera spp. nucleopolyhedroviruses. J. Gen. Virol. 2004, 85, 2845–2855. [Google Scholar] [CrossRef]
- Jakubowska, A.K.; Lynn, D.E.; Herrero, S.; Vlak, J.M.; van Oers, M.M. Host-range expansion of Spodoptera exigua multiple nucleopolyhedrovirus to Agrotis segetum larvae when the midgut is bypassed. J. Gen. Virol. 2010, 91, 898–906. [Google Scholar] [CrossRef]
- Iwata, K.; Haas-Stapleton, E.; Kunimi, Y.; Inoue, M.N.; Nakai, M. Midgut-based resistance to oral infection by a nucleopolyhedrovirus in the laboratory-selected strain of the smaller tea tortrix, Adoxophyes honmai (Lepidoptera: Tortricidae). J. Gen. Virol. 2017, 98, 296–304. [Google Scholar] [CrossRef]
- Heimpel, A.M.; Adams, J.R. A new nuclear polyhedrosis of the cabbage looper, Trichoplusia ni. J. Invertebr. Pathol. 1966, 8, 340–346. [Google Scholar] [CrossRef]
- Shreesam, S.; Mathad, S.B.; Reddy, J.S. Histopathology of the armyworm, Mythimna (Pseudaletia) separata (Walker) (Lepidoptera: Noctuidae) infected with nuclear polyhedrosis virus. Entomon 1983, 8, 155–162. [Google Scholar]
- Matos, T.G.; Giugliano, L.G.; Ribeiro, B.M.; Báo, S.N. Structural and ultrastructural studies of Anticarsia gemmatalis midgut cells infected with the baculovirus A. gemmatalis nucleopolyhedrovirus. Int. J. Insect Morphol. Embryol. 1999, 28, 195–201. [Google Scholar] [CrossRef]
- Flipsen, J.T.; van Lent, J.W.; Goldbach, R.W.; Vlak, J.M. Expression of polyhedrin and p10 in the midgut of AcMNPV-infected Spodoptera exigua larvae: An immunoelectron microscopic investigation. J. Invertebr. Pathol. 1993, 61, 17–23. [Google Scholar] [CrossRef]
- Washburn, J.O.; Trudeau, D.; Wong, J.F.; Volkman, L.E. Early pathogenesis of Autographa californica multiple nucleopolyhedrovirus and Helicoverpa zea single nucleopolyhedrovirus in Heliothis virescens: A comparison of the ‘M’ and ‘S’ strategies for establishing fatal infection. J. Gen. Virol. 2003, 84, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Chikhalya, A.; Luu, D.D.; Carrera, M.; De La Cruz, A.; Torres, M.; Martinez, E.N.; Chen, T.; Stephens, K.D.; Haas-Stapleton, E.J. Pathogenesis of Autographa californica multiple nucleopolyhedrovirus in fifth-instar Anticarsia gemmatalis larvae. J. Gen. Virol. 2009, 90, 2023–2032. [Google Scholar] [CrossRef]
- Donly, B.C.; Kaplanoglu, E.; Theilmann, D.A.; Baldwin, D.; Sieminska, E.; Hegedus, D.D.; Erlandson, M.A. MacoNPV baculovirus midgut-specific gene expression during infection of the bertha armyworm, Mamestra configurata. Virology 2016, 499, 1–8. [Google Scholar] [CrossRef]
- Shrestha, A.; Bao, K.; Chen, Y.R.; Chen, W.; Wang, P.; Fei, Z.; Blissard, G.W. Global analysis of baculovirus Autographa californica multiple nucleopolyhedrovirus gene expression in the midgut of the lepidopteran host Trichoplusia ni. J. Virol. 2018, 92, e01277-18. [Google Scholar] [CrossRef]
- Ali, M.I.; Young, S.Y.; Yearian, W.C. Nuclear polyhedrosis virus transmission by infected Heliothis zea (Boddie)(Lepidoptera: Noctuidae) prior to death. J. Entomol. Sci. 1987, 22, 289–294. [Google Scholar] [CrossRef]
- Vasconcelos, S.D. Alternative routes for the horizontal transmission of a nucleopolyhedrovirus. J. Invertebr. Pathol. 1996, 68, 269–274. [Google Scholar] [CrossRef]
- Bindu, T.N.; Balakrishnan, P.; Sajeev, T.V.; Sudheendrakumar, V.V. Role of soil and larval excreta in the horizontal transmission of the baculovirus HpNPV and its implications in the management of teak defoliator Hyblaea puera. Curr. Sci. 2022, 122, 1321–1326. [Google Scholar] [CrossRef]
- Hewson, I.; Brown, J.M.; Gitlin, S.A.; Doud, D.F. Nucleopolyhedrovirus detection and distribution in terrestrial, freshwater, and marine habitats of Appledore Island, Gulf of Maine. Microb. Ecol. 2011, 62, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Del-Angel, C.; Lasa, R.; Mercado, G.; Rodríguez-del-Bosque, L.A.; Caballero, P.; Williams, T. Acquisition of lethal infection, hypermobility and modified climbing behavior in nucleopolyhedrovirus infected larvae of Anticarsia gemmatalis. Biol. Contr. 2018, 125, 90–97. [Google Scholar] [CrossRef]
- Tay, W.T.; Rane, R.V.; Padovan, A.; Walsh, T.K.; Elfekih, S.; Downes, S.; Nam, K.; d’Alençon, E.; Zhang, J.; Wu, Y.; et al. Global population genomic signature of Spodoptera frugiperda (fall armyworm) supports complex introduction events across the Old World. Comm. Biol. 2022, 5, 297. [Google Scholar] [CrossRef] [PubMed]
- Kenis, M.; Benelli, G.; Biondi, A.; Calatayud, P.A.; Day, R.; Desneux, N.; Harrison, R.D.; Kriticos, D.; Rwomushana, I.; Van den Berg, J.; et al. Invasiveness, biology, ecology, and management of the fall armyworm, Spodoptera frugiperda. Entomol. Gen. 2023, 43, 187–241. [Google Scholar] [CrossRef]
- Barrera, G.; Simón, O.; Villamizar, L.; Williams, T.; Caballero, P. Spodoptera frugiperda multiple nucleopolyhedrovirus as a potential biological insecticide: Genetic and phenotypic comparison of field isolates from Colombia. Biol. Contr. 2011, 58, 113–120. [Google Scholar] [CrossRef]
- Bentivenha, J.P.; Rodrigues, J.G.; Lima, M.F.; Marçon, P.; Popham, H.J.; Omoto, C. Baseline susceptibility of Spodoptera frugiperda (Lepidoptera: Noctuidae) to SfMNPV and evaluation of cross-resistance to major insecticides and Bt proteins. J. Econ. Entomol. 2019, 112, 91–98. [Google Scholar] [CrossRef]
- Lei, C.; Yang, J.; Wang, J.; Hu, J.; Sun, X. Molecular and biological characterization of Spodoptera frugiperda multiple nucleopolyhedrovirus field isolate and genotypes from China. Insects 2020, 11, 777. [Google Scholar] [CrossRef]
- Tepa-Yotto, G.T.; Douro-Kpindou, O.K.; Koussihouédé, P.S.B.; Adjaoké, A.M.; Winsou, J.K.; Tognigban, G.; Tamò, M. Control potential of multiple nucleopolyhedrovirus (SfMNPV) isolated from fall armyworm in Nigeria (West Africa). Insects 2024, 15, 225. [Google Scholar] [CrossRef]
- Simón, O.; Williams, T.; López-Ferber, M.; Caballero, P. Functional importance of deletion mutant genotypes in an insect nucleopolyhedrovirus population. Appl. Env. Microbiol. 2005, 71, 4254–4262. [Google Scholar] [CrossRef]
- Simón, O.; Palma, L.; Beperet, I.; Muñoz, D.; López-Ferber, M.; Caballero, P.; Williams, T. Sequence comparison between three geographically distinct Spodoptera frugiperda multiple nucleopolyhedrovirus isolates: Detecting positively selected genes. J. Invertebr. Pathol. 2011, 107, 33–42. [Google Scholar] [CrossRef]
- Masson, T.; Fabre, M.L.; Pidre, M.L.; Niz, J.M.; Berretta, M.F.; Romanowski, V.; Ferrelli, M.L. Genomic diversity in a population of Spodoptera frugiperda nucleopolyhedrovirus. Infect. Genet. Evol. 2021, 90, 104749. [Google Scholar] [CrossRef] [PubMed]
- Molina-Ruiz, C.S.; Zamora-Briseño, J.A.; Simón, O.; Lasa, R.; Williams, T. A qPCR assay for the quantification of selected genotypic variants of Spodoptera frugiperda multiple nucleopolyhedrovirus (Baculoviridae). Viruses 2024, 16, 881. [Google Scholar] [CrossRef]
- Williams, T.; Melo-Molina, G.d.C.; Jiménez-Fernández, J.A.; Weissenberger, H.; Gómez-Díaz, J.S.; Navarro-de-la-Fuente, L.; Richards, A.R. Presence of Spodoptera frugiperda multiple nucleopolyhedrovirus (SfMNPV) occlusion bodies in maize field soils of Mesoamerica. Insects 2023, 14, 80. [Google Scholar] [CrossRef] [PubMed]
- Jamovi. The Jamovi Project 2024. Statistical Software v.2.5. Available online: https://www.jamovi.org (accessed on 12 November 2024).
- Granados, R.R.; Williams, K.A. In vivo replication of baculoviruses. In The Biology of Baculoviruses; Granados, R.R., Federici, B.A., Eds.; CRC Press: Boca Raton, FL, USA, 1986; Volume I, pp. 89–108. [Google Scholar]
- Engelhard, E.K.; Kam-Morgan, L.N.; Washburn, J.O.; Volkman, L.E. The insect tracheal system: A conduit for the systemic spread of Autographa californica M nuclear polyhedrosis virus. Proc. Natl. Acad. Sci. USA 1994, 91, 3224–3227. [Google Scholar] [CrossRef] [PubMed]
- Barrett, J.W.; Brownwright, A.J.; Primavera, M.J.; Retnakaran, A.; Palli, S.R. Concomitant primary infection of the midgut epithelial cells and the hemocytes of Trichoplusia ni by Autographa californica nucleopolyhedrovirus. Tissue Cell 1998, 30, 602–616. [Google Scholar] [CrossRef]
- Alam, M.Z.; Yearian, W.C.; Young, S.Y.; Mueller, A.J. Soybean foliage consumption by Pseudoplusia includens (Walker) (Lepidoptera: Noctuidae) larvae infected with nuclear polyhedrosis virus. J. Entomol. Sci. 1987, 22, 212–223. [Google Scholar] [CrossRef]
- Harper, J.D. Food consumption by cabbage loopers infected with nuclear polyhedrosis virus. J. Invertebr. Pathol. 1973, 21, 191–197. [Google Scholar] [CrossRef]
- Vasconcelos, S.D.; Hails, R.S.; Speight, M.R.; Cory, J.S. Differential crop damage by healthy and nucleopolyhedrovirus-infected Mamestra brassicae L. (Lepidoptera: Noctuidae) larvae: A field examination. J. Invertebr. Pathol. 2005, 88, 177–179. [Google Scholar] [CrossRef]
- Zhao, X.; Geng, Y.; Qiao, H.; Liu, Y.; Hu, T.; Xu, W.; Hao, D. Nutrition of host plants influence the infectivity of nucleopolyhedrovirus to polyphagous caterpillar, Hyphantria cunea. Chem. Biol. Technol. Agric. 2024, 11, 17. [Google Scholar] [CrossRef]
- Ramírez-Arias, F.G.; Lasa, R.; Murillo, R.; Navarro-de-la-Fuente, L.; Mercado, G.; Williams, T. Post-mortem incubation influences occlusion body production in nucleopolyhedrovirus-infected larvae of Spodoptera frugiperda. Biol. Contr. 2019, 135, 33–40. [Google Scholar] [CrossRef]
- Mathad, S.B.; Splittstoesser, C.M.; McEwen, F.L. Histopathology of the cabbage looper, Trichoplusia ni, infected with a nuclear polyhedrosis. J. Invertebr. Pathol. 1968, 11, 456–464. [Google Scholar] [CrossRef]
- Tinsley, T.W.; Harrap, K.A. Viruses of Invertebrates. In Newly Characterized Protist and Invertebrate Viruses. Comprehensive Virology; Fraenkel-Conrat, H., Wagner, R.R., Eds.; Plenum Press: New York, NY, USA, 1978; Volume 12, pp. 1–102. [Google Scholar]
- Teakle, R.E. A nuclear-polyhedrosis virus from Heliothis punctigera Wallengren (Lepidoptera: Noctuidae). Queensland J. Agric. Anim. Sci. 1973, 30, 161–177. [Google Scholar]
- Xia, J.; Fei, S.; Huang, Y.; Lai, W.; Yu, Y.; Liang, L.; Wu, H.; Swevers, L.; Sun, J.; Feng, M. Single-nucleus sequencing of silkworm larval midgut reveals the immune escape strategy of BmNPV in the midgut during the late stage of infection. Insect Biochem. Mol. Biol. 2024, 164, 104043. [Google Scholar] [CrossRef] [PubMed]
- Awais, M.M.; Fei, S.; Xia, J.; Feng, M.; Sun, J. Insights into midgut cell types and their crucial role in antiviral immunity in the lepidopteran model Bombyx mori. Front. Immunol. 2024, 15, 1349428. [Google Scholar] [CrossRef]
- Gomes, F.M.; Carvalho, D.B.; Machado, E.A.; Miranda, K. Ultrastructural and functional analysis of secretory goblet cells in the midgut of the lepidopteran Anticarsia gemmatalis. Cell Tissue Res. 2013, 352, 313–326. [Google Scholar] [CrossRef]
- Caccia, S.; Casartelli, M.; Tettamanti, G. The amazing complexity of insect midgut cells: Types, peculiarities, and functions. Cell Tissue Res. 2019, 377, 505–525. [Google Scholar] [CrossRef]
- Volkman, L.E.; Summers, M.D. Autographa californica nuclear polyhedrosis virus: Comparative infectivity of the occluded, alkali-liberated, and nonoccluded forms. J. Invertebr. Pathol. 1977, 30, 102–103. [Google Scholar] [CrossRef]
- Blissard, G.W.; Theilmann, D.A. Baculovirus entry and egress from insect cells. Annu. Rev. Virol. 2018, 5, 113–139. [Google Scholar] [CrossRef]
- Granados, R.R.; Lawler, K.A. In vivo pathway of Autographa californica baculovirus invasion and infection. Virology 1981, 108, 297–308. [Google Scholar] [CrossRef]
- Rosinski, M.; Reid, S.; Nielsen, L.K. Kinetics of baculovirus replication and release using real-time quantitative polymerase chain reaction. Biotechnol. Bioeng. 2002, 77, 476–480. [Google Scholar] [CrossRef]
- Shrestha, A.; Bao, K.; Chen, W.; Wang, P.; Fei, Z.; Blissard, G.W. Transcriptional responses of the Trichoplusia ni midgut to oral infection by the baculovirus Autographa californica multiple nucleopolyhedrovirus. J. Virol. 2019, 93, e00353-19. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.W.; Yan, Y.L.; Hou, C.P.; Hong, T.; Wang, Y.S.; Xu, X.; Liu, S.H.; Xu, J.P. A novel digestive protease chymotrypsin-like serine contributes to anti-BmNPV activity in silkworm (Bombyx mori). Dev. Comp. Immunol. 2025, 162, 105301. [Google Scholar] [CrossRef]
- Selot, R.; Kumar, V.; Shukla, S.; Chandrakuntal, K.; Brahmaraju, M.; Dandin, S.B.; Laloraya, M.; Kumar, P.G. Identification of a soluble NADPH oxidoreductase (BmNOX) with antiviral activities in the gut juice of Bombyx mori. Biosci. Biotechnol. Biochem. 2007, 71, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Zhu, F.; Chen, K. The mechanisms of silkworm resistance to the baculovirus and antiviral breeding. Annu. Rev. Entomol. 2023, 68, 381–399. [Google Scholar] [CrossRef] [PubMed]
- Dow, J.A. pH gradients in lepidopteran midgut. J. Exp. Biol. 1992, 172, 355–375. [Google Scholar] [CrossRef]
- Klein, U.; Koch, A.; Moffett, D.F. Ion transport in Lepidoptera. In Biology of the Insect Midgut; Lehane, M.J., Billingsley, P.F., Eds.; Springer: Dordrecht, The Netherlands, 1996; pp. 236–264. [Google Scholar]
- Gringorten, J.L.; Crawford, D.N.; Harvey, W.R. High pH in the ectoperitrophic space of the larval lepidopteran midgut. J. Exp. Biol. 1993, 183, 353–359. [Google Scholar] [CrossRef]
- Mori, H.; Metcalf, P. Cypoviruses. In Insect Virology; Asgari, S., Johnson, K.N., Eds.; Caister Academic Press: Poole, UK, 2010; pp. 307–323. [Google Scholar]
- Sivaprasad, V.; Rahul, K.; Makwana, P. Immunodiagnosis of silkworm diseases. Meth. Microbiol. 2021, 49, 27–46. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ávila-Hernández, E.; Molina-Ruiz, C.S.; Gómez-Díaz, J.S.; Williams, T. Fecal Transmission of Spodoptera frugiperda Multiple Nucleopolyhedrovirus (SfMNPV; Baculoviridae). Viruses 2025, 17, 298. https://doi.org/10.3390/v17030298
Ávila-Hernández E, Molina-Ruiz CS, Gómez-Díaz JS, Williams T. Fecal Transmission of Spodoptera frugiperda Multiple Nucleopolyhedrovirus (SfMNPV; Baculoviridae). Viruses. 2025; 17(3):298. https://doi.org/10.3390/v17030298
Chicago/Turabian StyleÁvila-Hernández, Eduardo, Cindy S. Molina-Ruiz, Juan S. Gómez-Díaz, and Trevor Williams. 2025. "Fecal Transmission of Spodoptera frugiperda Multiple Nucleopolyhedrovirus (SfMNPV; Baculoviridae)" Viruses 17, no. 3: 298. https://doi.org/10.3390/v17030298
APA StyleÁvila-Hernández, E., Molina-Ruiz, C. S., Gómez-Díaz, J. S., & Williams, T. (2025). Fecal Transmission of Spodoptera frugiperda Multiple Nucleopolyhedrovirus (SfMNPV; Baculoviridae). Viruses, 17(3), 298. https://doi.org/10.3390/v17030298





