Proinflammatory Biomarkers and Clinical Factors Associated with Long-Term Mortality in People with HIV
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Assessments
2.3. Long-Term Evaluation
2.4. Statistical Analysis
2.5. Ethical Approval
3. Results
3.1. Patients
3.2. Death
3.3. Factors Associated with the Risk of Death
3.4. Factors Associated with Mortality of PWID
3.5. Factors Associated with Cancer Incidence
3.6. Differences in Analyzed Parameters in PWH with HCV Coinfection
4. Discussion
4.1. Cause of Death Among PWH
4.2. Possible Mechanisms of Premature Death Among PWH
4.2.1. Proinflammatory Biomarkers
4.2.2. HCV Coinfection
4.2.3. CVD and Lipid Profile
4.2.4. Other Evaluated Risk Factors for Premature Death in PWH
4.3. Significance and Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PWH | People with HIV |
| ART | Antiretroviral therapy |
| HIV | Human immunodeficiency virus |
| AIDS | Acquired immunodeficiency syndrome |
| CVD | Cardiovascular disease |
| CRP | C-reactive protein |
| PCT | Procalcitonin |
| TNF-α | Tumor necrosis factor alpha |
| VCAM-1 | Vascular cell adhesion molecule 1 |
| ICAM-1 | Intercellular adhesion molecule 1 |
| HCV | Hepatitis C virus |
| HBV | Hepatitis B virus |
| PLT | Platelets |
| PWID | People who inject drugs |
| TGF-β | Transforming growth factor beta |
References
- Barre-Sinoussi, F.F.; Chermann, J.C.; Rey, F.; Nugeyre, M.T.; Chamaret, S.; Gruest, J.; Dauguet, C.; Axler-Blin, C.; Vezinet-Brun, F.; Rouzioux, C.; et al. Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS). Science 1983, 220, 868–871. [Google Scholar] [CrossRef] [PubMed]
- Gallo, R.C.; Salahuddin, S.Z.; Popovic, M.; Shearer, G.M.; Kaplan, M.; Haynes, B.F.; Palker, T.J.; Redfield, R.; Oleske, J.; Safai, B.; et al. Frequent detection and isolation of cytopathic retroviruses (HTLV-III) from patients with AIDS and at risk for AIDS. Science 1984, 224, 500–503. [Google Scholar] [CrossRef] [PubMed]
- Fact Sheet—Latest Statistics on the Status of the AIDS Epidemic. UNAIDS. 2021. Available online: https://www.unaids.org/en/resources/fact-sheet (accessed on 30 December 2024).
- Smith, C.; Sabin, C.A.; Lundgren, J.D.; Thiebaut, R.; Weber, R.; Law, M.; Monforte, A.; Kirk, O.; Friis-Moller, N.; Phillips, A.; et al. Factors associated with specific causes of death amongst HIV-positive individuals in the D:A:D study. AIDS 2010, 24, 1537–1548. [Google Scholar] [PubMed]
- Marcus, J.L.; Leyden, W.A.; Alexeeff, S.E.; Anderson, A.N.; Hechter, R.C.; Hu, H.; Lam, J.O.; Towner, W.J.; Yuan, Q.; Horberg, M.A.; et al. Comparison of Overall and Comorbidity-Free Life Expectancy Between Insured Adults With and Without HIV Infection, 2000–2016. JAMA Netw. Open 2020, 3, e207954. [Google Scholar] [CrossRef]
- Singh, S.; Giron, L.B.; Shaikh, M.W.; Shankaran, S.; Engen, P.A.; Bogin, Z.R.; Bambi, S.A.; Goldman, A.R.; Azevedo, J.L.L.C.; Orgaz, L.; et al. Distinct intestinal microbial signatures linked to accelerated systemic and intestinal biological aging. Microbiome 2024, 12, 31. [Google Scholar] [CrossRef]
- Hinton, A.O., Jr.; N’Jai, A.U.; Vue, Z.; Wanjalla, C. Connection between HIV and mitochondria in cardiovascular disease and implications for treatments. Circul. Res. 2024, 134, 1581–1606. [Google Scholar] [CrossRef]
- De Francesco, D.; Wit, F.W.; Burkle, A.; Oehlke, S.; Kootstra, N.A.; Winston, A.; Franceschi, C.; Garagnani, P.; Pirazzini, C.; Libert, C.; et al. Do people living with HIV experience greater age advancement than their HIV-negative counterparts? AIDS 2019, 33, 259–268. [Google Scholar] [CrossRef]
- Martínez-Ayala, P.; Alanis-Sánchez, G.A.; Álvarez-Zavala, M.; Sánchez-Reyes, K.; Ruiz-Herrera, V.V.; Cabrera-Silva, R.I.; González-Hernández, L.A.; Ramos-Becerra, C.; Cardona Muñoz, E.; Andrade-Villanueva, J.F. Effect of antiretroviral therapy on decreasing arterial stiffness, metabolic profile, vascular and systemic inflammatory cytokines in treatment-naïve HIV: A one-year prospective study. PLoS ONE 2023, 18, e0282728. [Google Scholar] [CrossRef]
- Borges, Á.H.; O’Connor, J.L.; Phillips, A.N.; Rönsholt, F.F.; Pett, S.; Vjecha, M.J.; French, M.A.; Lundgren, J.D.; INSIGHT SMART and ESPRIT Study Groups and the SILCAAT Scientific Committee. Factors Associated with plasma IL-6 levels during HIV Infection. J. Infect. Dis. 2015, 212, 585–595. [Google Scholar] [CrossRef]
- Guo, H.; Gao, J.; Taxman, D.J.; Ting, J.P.; Su, L. HIV-1 Infection induces interleukin-1β production via TLR8 protein-dependent and NLRP3 inflammasome mechanisms in human monocytes. J. Biol. Chem. 2014, 289, 21716–21726. [Google Scholar] [CrossRef]
- Collora, J.A.; Liu, R.; Pinto-Santini, D.; Ravindra, N.; Ganoza, C.; Lama, J.R.; Alfaro, R.; Chiarella, J.; Spudich, S.; Mounzer, K.; et al. Single-cell multiomics reveals persistence of HIV-1 in expanded cytotoxic T cell clones. Immunity 2022, 55, 1013–1031.e7. [Google Scholar] [CrossRef] [PubMed]
- Chinnapaiyan, S.; Dutta, R.K.; Nair, M.; Chand, H.S.; Rahman, I.; Unwalla, H.J. TGF-β1 increases viral burden and promotes HIV-1 latency in primary differentiated human bronchial epithelial cells. Sci. Rep. 2019, 9, 12552. [Google Scholar] [CrossRef] [PubMed]
- Pu, H.; Tian, J.; Flora, G.; Lee, Y.W.; Nath, A.; Hennig, B.; Toborek, M. HIV-1 Tat protein upregulates inflamma tory mediators and induces monocyte invasion into the brain. Mol. Cell. Neurosci. 2003, 24, 224–237. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Wang, J.; Yang, Q.; Xie, X.; Huang, Y. The role of CD38 in HIV infection. AIDS Res. Ther. 2021, 18, 11. [Google Scholar] [CrossRef]
- Horsburgh, B.A.; Lee, E.; Hiener, B.; Eden, J.S.; Schlub, T.E.; von Stockenstrom, S.; Odevall, L.; Milush, J.M.; Liegler, T.; Sinclair, E.; et al. High levels of genetically intact HIV in HLA-DR+ memory T cells indicates their value for reservoir studies. AIDS 2020, 4, 659–668. [Google Scholar] [CrossRef]
- Achhra, A.C.; Lyass, A.; Borowsky, L.; Bogorodskaya, M.; Plutzky, J.; Massaro, J.M.; D’Agostino, R.B., Sr.; Triant, V.A. Assessing Cardiovascular Risk in People Living with HIV: Current Tools and Limitations. Curr. HIV/AIDS Rep. 2021, 18, 271–279. [Google Scholar] [CrossRef]
- Larney, S.; Peacock, A.; Leung, J.; Colledge, S.; Hickman, M.; Vickerman, P.; Grebely, J.; Dumchev, K.V.; Griffiths, P.; Hines, L.; et al. Global, regional, and country-level coverage of interventions to prevent and manage HIV and hepatitis C among people who inject drugs: A systematic review. Lancet Glob Heal. 2017, 5, e1208–e1220. [Google Scholar] [CrossRef]
- Bangsberg, D.R.; Hecht, F.M.; Clague, H.; Charlebois, E.D.; Ciccarone, D.; Chesney, M.; Moss, A. Provider assessment of adherence to HIV antiretroviral therapy. J. Acquir. Immune Defic. Syndr. 2001, 26, 435–442. [Google Scholar] [CrossRef]
- Freiberg, M.S.; Chang, C.H.; Skanderson, M.; Patterson, O.V.; DuVall, S.L.; Brandt, C.A.; So-Armah, K.A.; Vasan, R.S.; Oursler, K.A.; Gottdiener, J.; et al. Association Between HIV Infection and the Risk of Heart Failure With Reduced Ejection Fraction and Preserved Ejection Fraction in the Antiretroviral Therapy Era: Results From the Veterans Aging Cohort Study. JAMA Cardiol. 2017, 2, 536–546. [Google Scholar] [CrossRef]
- Perkins, M.V.; Joseph, S.B.; Dittmer, D.P.; Mackman, N. Cardiovascular Disease and Thrombosis in HIV Infection. Arterioscler. Thromb. Vasc. Biol. 2023, 43, 175–191. [Google Scholar] [CrossRef]
- Feinstein, M.J.; Bahiru, E.; Achenbach, C.; Longenecker, C.T.; Hsue, P.; So-Armah, K.; Freiberg, M.S.; Lloyd-Jones, D. Patterns of Cardiovascular Mortality for HIV-Infected Adults in the United States: 1999 to 2013. Am. J. Cardiol. 2016, 117, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Alonso, A.; Barnes, A.E.; Guest, J.L.; Shah, A.; Shao, I.Y.; Marconi, V. HIV Infection and Incidence of Cardiovascular Diseases: An Analysis of a Large Healthcare Database. J. Am. Hear. Assoc. 2019, 8, e012241. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Westra, J.R.; Giordano, T.P.; Berenson, A.B.; Baillargeon, J.G.; Kuo, Y.F. Assessing comorbidities and survival in HIV-infected and uninfected matched Medicare enrollees. AIDS 2021, 35, 1667–1675. [Google Scholar] [CrossRef]
- Trickey, A.; McGinnis, K.; Gill, M.J.; Abgrall, S.; Berenguer, J.; Wyen, C.; Hessamfar, M.; Reiss, P.; Kusejko, K.; Silverberg, M.J.; et al. Longitudinal trends in causes of death among adults with HIV on antiretroviral therapy in Europe and North America from 1996 to 2020: A collaboration of cohort studies. Lancet HIV 2024, 11, e176–e185. [Google Scholar] [CrossRef]
- Zhou, Q.; Lu, X.; Qian, L.; Yu, C.; Xie, J.; Kong, D. Procalcitonin, C-reactive protein, and white blood cell count levels in end-stage cancer patients: A retrospective study on inflammatory markers and their prognostic value. Medicine 2024, 103, e40792. [Google Scholar] [CrossRef] [PubMed]
- Soeroso, N.N.; Tanjung, M.F.; Afiani, D.; Pradana, A.; Tarigan, S.P.; Wahyuni, A.S. Procalcitonin Level in Non-Small Cell Lung Cancer Patients among Indonesian Population. Open Access Maced. J. Med. Sci. 2018, 6, 2123–2127. [Google Scholar] [CrossRef]
- Censi, S.; Manso, J.; Benvenuti, T.; Piva, I.; Iacobone, M.; Mondin, A.; Torresan, F.; Basso, D.; Crivellari, G.; Zovato, S.; et al. The role of procalcitonin in the follow-up of medullary thyroid cancer. Eur. Thyroid J. 2023, 12, e220161. [Google Scholar] [CrossRef]
- Kong, D.H.; Kim, Y.K.; Kim, M.R.; Jang, J.H.; Lee, S. Emerging Roles of Vascular Cell Adhesion Molecule-1 (VCAM-1) in Immunological Disorders and Cancer. Int. J. Mol. Sci. 2018, 19, 1057. [Google Scholar] [CrossRef]
- VanHeyst, K.A.; Choi, S.H.; Kingsley, D.T.; Huang, A.Y. Ectopic Tumor VCAM-1 Expression in Cancer Metastasis and Therapy Resistance. Cells 2022, 11, 3922. [Google Scholar] [CrossRef]
- Metcalfe, R.; Fraser, R.; Trayner, K.M.A.; Glancy, M.; Yeung, A.; Sills, L.; Ritchie, T.; Priyadarshi, S.; Peters, S.E.; McAuley, A.; et al. Rising mortality among people who inject drugs living with HIV in Scotland, UK: A 20-year retrospective cohort study. HIV Med. 2024, 18, 265–274. [Google Scholar] [CrossRef]
- Degenhardt, L.; Peacock, A.; College, S.; Leung, J.; Grebely, J.; Vickerman, P.; Stone, J.; Cunningham, E.B.; Trickey, A.; Dumchev, K.; et al. Global prevalence of injecting drug use and sociodemographic characteristics and prevalence of HIV, HBV, and HCV in people who inject drugs: A multistage systematic review. Lancet Glob. Health 2017, 5, e1192–e1207. [Google Scholar] [CrossRef]
- Chalouni, M.; Pol, S.; Sogni, P.; Fontaine, H.; Lacombe, K.; Marc-Lacombe, J.; Esterle, L.; Dorival, C.; Bourlière, M.; Bani-Sadr, F.; et al. ANRS CO13 HEPAVIH and ANRS CO22 HEPATHER cohort study groups. Increased mortality in HIV/HCV-coinfected compared to HCV-monoinfected patients in the DAA era due to non-liver-related death. J. Hepatol. 2021, 74, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Sazonova, Y.; Kulchynska, R.; Azarskova, M.; Liulchuk, M.; Salyuk, T.; Doan, I.; Barzilay, E. Population-level prevalence of detectable HIV viremia in people who inject drugs (PWID) in Ukraine: Implications for HIV treatment and case finding interventions. PLoS ONE 2023, 18, e0290661. [Google Scholar] [CrossRef] [PubMed]
- Affi, R.; Gabillard, D.; Kouame, G.M.; Ntakpe, J.B.; Moh, R.; Badje, A.; Danel, C.; Inwoley, A.; Eholié, S.P.; Anglaret, X.; et al. Plasma sVCAM-1, antiretroviral therapy and mortality in HIV-1-infected west African adults. HIV Med. 2022, 23, 717–726. [Google Scholar] [CrossRef] [PubMed]
- Okay, G.; Koc, M.M.; Guler, E.M.; Yabaci, A.; Kocyigit, A.; Akkoyunlu, Y. The effect of antiretroviral therapy on IL-6, IL-1β, TNF-α, IFN-γ levels and their relationship with HIV-RNA and CD4 + T cells in HIV patients. Curr. HIV Res. 2020, 18, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Teasdale, C.A.; Hernandez, C.; Zerbe, A.; Chege, D.; Hawken, M.; El-Sadr, W.M. Changes in D-dimer after initiation of antiretroviral therapy in adults living with HIV in Kenya. BMC Inf. Dis. 2020, 20, 508. [Google Scholar] [CrossRef]
- Sharif, S.; Van der Graaf, Y.; Cramer, M.J.; Kapelle, L.J.; de Borst, G.J.; Visseren, F.L.J.; Westerink, J.; SMART study group. Low-grade inflammation as a risk factor for cardiovascular events and all-cause mortality in patients with type 2 Diabetes. Cardiovasc. Diabetol. 2021, 20, 220. [Google Scholar] [CrossRef]
- Reddy, K.S.S.; Varadaraj, P.; Nallusamy, G.; SenthilNathan, S. Correlation Between Hemoglobin A1c (HbA1c) and High-Sensitivity C-Reactive Protein (hs-CRP) in Myocardial Infarction Patients and Their Six-Month Mortality Follow-Up. Cureus 2024, 16, e67070. [Google Scholar] [CrossRef]
- Li, Y.; Zhong, X.; Cheng, G.; Zhao, C.; Zhang, L.; Hong, Y.; Wan, Q.; He, R.; Wang, Z. Hs-CRP and all-cause, cardiovascular, and cancer mortality risk: A meta-analysis. Atherosclerosis 2017, 259, 75–82. [Google Scholar] [CrossRef]
- Riitho, V.; Connon, R.; Gwela, A.; Namusanje, J.; Nhema, R.; Siika, A.; Bwakura-Dangarembizi, M.; Musiime, V.; Berkley, J.A.; Szubert, A.J.; et al. Biomarkers of mortality in adults and adolescents with advanced HIV in sub-Saharan Africa. Nat. Commun. 2024, 15, 5492. [Google Scholar] [CrossRef]
- Tien, P.C.; Choi, A.I.; Zolopa, A.R.; Benson, C.; Tracy, R.; Scherzer, R.; Bacchetti, P.; Shlipak, M.; Grunfeld, C. Inflammation and mortality in HIV-infected adults: Analysis of the FRAM study cohort. J. Acquir. Immune Defic. Syndr. 2010, 55, 316–322. [Google Scholar] [CrossRef]
- Salter, M.L.; Lau, B.; Mehta, S.H.; Go, V.F.; Leng, S.; Kirk, G.D. Correlates of elevated interleukin-6 and C-reactive protein in persons with or at high risk for HCV and HIV infections. J. Acquir. Immune Defic. Syndr. 2013, 64, 488–495. [Google Scholar] [CrossRef] [PubMed]
- Parrey, A.H.; Koka, M.; Kasana, B.; Ismail, M. Procalcitonin and qSOFA as a marker of mortality in sepsis. Rev. Recent Clin. Trials 2024, 19, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Sato, S.; Sato, S.; Tsuzura, H.; Ikeda, Y.; Hayashida, S.; Takahashi, S.; Amano, N.; Murata, A.; Shimada, Y.; Iijima, K.; et al. Elevated serum procalcitonin levels and their association with the prognosis of patients with liver cirrhosis. Eur. J. Gastroenterol. Hepatol. 2020, 32, 1222–1228. [Google Scholar] [CrossRef] [PubMed]
- Özel, A.; Yuce, S.; Ilbegi, E.N. Advancing Prognostic Prediction in Pediatric Trauma: The Role of Inflammatory Markers. Klin. Padiatr. 2024. [Google Scholar] [CrossRef]
- Osawa, T.; Watanabe, M.; Morimoto, K.; Okumura, M.; Yoshiyama, T.; Ogata, H.; Goto, H.; Kudoh, S.; Ohta, K.; Sasaki, Y. Serum Procalcitonin Levels Predict Mortality Risk in Patients With Pulmonary Tuberculosis: A Single-Center Prospective Observational Study. J. Infect. Dis. 2020, 222, 1651–1654. [Google Scholar] [CrossRef]
- Zhang, Y.; Gu, K.; Du, W.; Xu, A. Risk factors and prediction model for mortality in HIV/Talaromyces marneffei co-infection: A retrospective cohort study. Heliyon 2024, 10, e32560. [Google Scholar] [CrossRef]
- VCAM1 Vascular Cell Adhesion Molecule 1. Available online: https://www.ncbi.nlm.nih.gov/gene/7412 (accessed on 20 December 2024).
- Andalibi, M.S.; Fields, J.A.; Iudicello, J.E.; Diaz, M.M.; Tang, B.; Letendre, S.L.; Ellis, R.J. Elevated Biomarkers of Inflammation and Vascular Dysfunction Are Associated with Distal Sensory Polyneuropathy in People with HIV. Int. J. Mol. Sci. 2024, 25, 4245. [Google Scholar] [CrossRef]
- Graham, S.M.; Rajwans, N.; Jaoko, W.; Estambale, B.B.; McClelland, R.S.; Overbaugh, J.; Liles, W.C. Endothelial activation biomarkers increase after HIV-1 acquisition: Plasma vascular cell adhesion molecule-1 predicts disease progression. AIDS 2013, 27, 1803–1813. [Google Scholar] [CrossRef]
- Affi, R.; Gabillard, D.; Dunyach-Remy, C.; Ntakpe, J.B.; Moh, R.; Badje, A.; Kouame, G.M.; Karcher, S.; Le Carrou, J.; Danel, C.; et al. Association of Plasma Soluble Vascular Cell Adhesion Molecule-1 and sCD14 With Mortality in HIV-1-Infected West African Adults With High CD4 Counts. J. Acquir. Immune Defic. Syndr. 2021, 86, 138–145. [Google Scholar] [CrossRef]
- He, J.; Duan, M.; Zhuang, H. ICAM1 and VCAM1 are associated with outcome in patients with sepsis: A systematic review and meta-analysis. Heliyon 2024, 10, e40003. [Google Scholar] [CrossRef]
- Juno, J.A.; Phetsouphanh, C.; Klenerman, P.; Kent, S.J. Perturbation of mucosal-associated invariant T cells and iNKT cells in HIV infection. Curr. Opin. HIV AIDS 2019, 14, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Kronfli, N.; Bhatnagar, S.R.; Hull, M.W.; Moodie, E.E.M.; Cox, J.; Walmsley, S.; Gill, J.; Cooper, C.; Martel-Laferrière, V.; Pick, N.; et al. Canadian Co-Infection Cohort Investigators. Trends in cause-specific mortality in HIV-hepatitis C coinfection following hepatitis C treatment scale-up. AIDS 2019, 33, 1013–1022. [Google Scholar] [CrossRef]
- Rosenthal, E.; Roussillon, C.; Salmon-Céron, D.; Georget, A.; Hénard, S.; Huleux, T.; Gueit, I.; Mortier, E.; Costagliola, D.; Morlat, P.; et al. Liver-related deaths in HIV-infected patients between 1995 and 2010 in France: The Mortavic 2010 study in collaboration with the Agence Nationale de Recherche sur le SIDA (ANRS) EN 20 Mortalité 2010 survey. HIV Med. 2015, 16, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Platt, L.; Easterbrook, P.; Gower, E.; McDonald, B.; Sabin, K.; McGowan, C.; Yanny, I.; Razavi, H.; Vickerman, P. Prevalence and burden of HCV co-infection in people living with HIV: A global systematic review and meta-analysis. Lancet Infect. Dis. 2016, 16, 797–808. [Google Scholar] [CrossRef] [PubMed]
- Mikuła, T.; Suchacz, M.; Sapuła, M.; Wiercińska-Drapało, A. Significance of Vascular Cell Adhesion Molecule-1 and Tumor Necrosis Factor-Alpha in HIV-Infected Patients. J. Clin. Med. 2022, 11, 514. [Google Scholar] [CrossRef] [PubMed]
- Aldámiz-Echevarría, T.; Berenguer, J.; Miralles, P.; Jiménez-Sousa, M.A.; Carrero, A.; Pineda-Tenor, D.; Díez, C.; Tejerina, F.; Pérez-Latorre, L.; Bellón, J.M.; et al. Soluble Adhesion Molecules in Patients Coinfected with HIV and HCV: A Predictor of Outcome. PLoS ONE 2016, 11, e0148537. [Google Scholar] [CrossRef][Green Version]
- Schulte-Hermann, K.; Schalk, H.; Haider, B.; Hutterer, J.; Gmeinhart, B.; Pichler, K.; Brath, H.; Dorner, T.E. Impaired lipid profile and insulin resistance in a cohort of Austrian HIV patients. J. Infect. Chemother. 2016, 22, 248–253. [Google Scholar] [CrossRef]
- Huang, H.; Kang, R.; Zhao, Z. Is hepatitis C associated with atherosclerotic burden? A systematic review and meta-analysis. PLoS ONE 2014, 9, e106376. [Google Scholar] [CrossRef]
- Nikolopoulos, G.K.; Paraskevis, D.; Psichogiou, M.; Hatzakis, A. HBV-DNA levels predict overall mortality in HIV/HBV coinfected individuals. J. Med. Virol. 2016, 88, 466–473. [Google Scholar] [CrossRef]
- Castilho, J.L.; Turner, M.; Shepherd, B.E.; Koethe, J.R.; Furukawa, S.S.; Bofill, C.E.; Raffanti, S.; Sterling, T.R. CD4/CD8 Ratio and CD4 Nadir Predict Mortality Following Noncommunicable Disease Diagnosis in Adults Living with HIV. AIDS Res. Hum. Retroviruses 2019, 35, 960–967. [Google Scholar] [CrossRef] [PubMed]
- Scherlinger, M.; Richez, C.; Tsokos, G.C.; Boilard, E.; Blanco, P. The role of platelets in immune-mediated inflammatory diseases. Nat. Rev. Immunol. 2023, 23, 495–510. [Google Scholar] [CrossRef] [PubMed]
- Zhu, A.; Real, F.; Zhu, J.; Greffe, S.; de Truchis, P.; Rouveix, E.; Bomsel, M.; Capron, C. HIV-Sheltering Platelets From Immunological Non-Responders Induce a Dysfunctional Glycolytic CD4+ T-Cell Profile. Front. Immunol. 2022, 12, 781923. [Google Scholar] [CrossRef] [PubMed]
- Madzime, M.; Rossouw, T.M.; Theron, A.J.; Anderson, R.; Steel, H.C. Interactions of HIV and Antiretroviral Therapy With Neutrophils and Platelets. Front. Immunol. 2021, 12, 634386. [Google Scholar] [CrossRef]
- De Pablo-Bernal, R.S.; Ruiz-Mateos, E.; Rosado, I.; Dominguez-Molina, B.; Alvarez-Ríos, A.I.; Carrillo-Vico, A.; De La Rosa, R.; Delgado, J.; Muñoz-Fernández, M.A.; Leal, M.; et al. TNF-α levels in HIV-infected patients after long-term suppressive cART persist as high as in elderly, HIV-uninfected subjects. J. Antimicrob. Chemother. 2014, 69, 3041–3046. [Google Scholar] [CrossRef]
- Aikpitanyi-Iduitua, G.A.; Ibeh, I.N.; Idemudia, N.L.; Aikpitanyi-Iduitua, R.O.; Omoregie, R. Interferon gamma, interleukin 6 and tissue necrosis factor alpha levels among asymptomatic HIV patients in Benin City, Nigeria. Hum. Antibodies 2022, 30, 177–182. [Google Scholar] [CrossRef]
- Kuller, L.H.; Tracy, R.; Belloso, W.; De Wit, S.; Drummond, F.; Lane, H.C.; Ledergerber, B.; Lundgren, J.; Neuhaus, J.; Nixon, D.; et al. INSIGHT SMART Study Group. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med. 2008, 5, e203. [Google Scholar] [CrossRef]
- Andrade, B.B.; Hullsiek, K.H.; Boulware, D.R.; Rupert, A.; French, M.A.; Ruxrungtham, K.; Montes, M.L.; Price, H.; Barreiro, P.; Audsley, J.; et al. Biomarkers of inflammation and coagulation are associated with mortality and hepatitis flares in persons coinfected with HIV and hepatitis viruses. J. Infect. Dis. 2013, 207, 1379–1388. [Google Scholar] [CrossRef]
- Wada, N.I.; Bream, J.H.; Martínez-Maza, O.; Macatangay, B.; Galvin, S.R.; Margolick, J.B.; Jacobson, L.P. Inflammatory Biomarkers and Mortality Risk Among HIV-Suppressed Men: A Multisite Prospective Cohort Study. Clin. Infect. Dis. 2016, 63, 984–990. [Google Scholar] [CrossRef]
- Li, J.; Shen, C.; Wang, X.; Lai, Y.; Zhou, K.; Li, P.; Liu, L.; Che, G. Prognostic value of TGF-β in lung cancer: Systematic review and meta-analysis. BMC Cancer 2019, 19, 691. [Google Scholar] [CrossRef]
- Poggi, A.; Zocchi, M.R. HIV-1 Tat triggers TGF-beta production and NK cell apoptosis that is prevented by pertussis toxin B. Clin. Dev. Immunol. 2006, 13, 369–372. [Google Scholar]
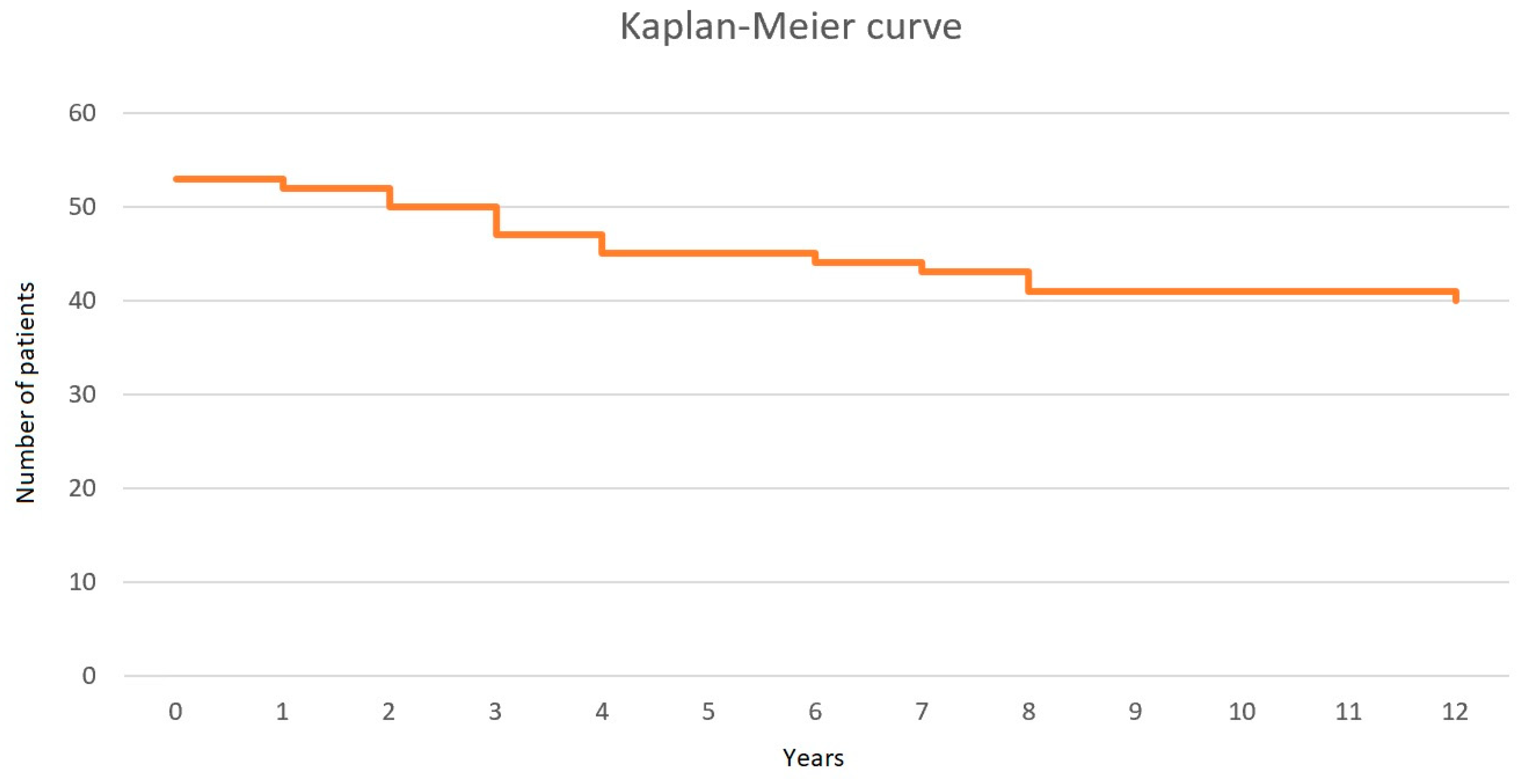
| Variable | All Patients (n = 72) | Patients Who Lived (n = 40) | Patients Who Died (n = 13) | p |
|---|---|---|---|---|
| Age (years) (mean (SD)) | 38.68 (11.04) | 37.73 (11.42) | 40.85 (11.52) | 0.153 |
| Male gender (n (%)) | 56 (77.78) | 31 (77.50) | 11 (84.62) | 0.711 |
| Female gender (n (%)) | 16 (22.22) | 9 (22.50) | 2 (15.38) | 0.711 |
| Intravenous drug use (n (%)) | 39 (54.17) | 18 (45.00) | 11 (84.62) | 0.013 |
| HCV coinfection (n (%)) | 26 (36.11) | 10 (25.00) | 8 (61.54) | 0.022 |
| HBV coinfection (n (%)) | 13 (18.06) | 6 (15.00) | 4 (30.77) | 0.237 |
| Duration of HIV infection (years) (mean (SD)) | 4.48 (5.21) | 2.80 (3.11) | 7.54 (6.80) | 0.001 |
| Duration of HIV treatment (weeks) (mean (SD)) | 93.50 (142.28) | 76.28 (126.44) | 116.61 (181.23) | 0.355 |
| PLT (G/L) (mean (SD)) | 217.82 (99.59) | 225.88 (111.34) | 182.46 (90.98) | 0.209 |
| CRP (mg/L) (mean (SD)) | 22.10 (44.61) | 10.75 (15.15) | 39.17 (50.42) | 0.003 |
| PCT (ng/mL) (mean (SD)) | 0.72 (3.98) | 0.05 (0.01) | 0.36 (0.60) | 0.002 |
| VCAM-1 (ng/mL) (mean (SD)) | 912.00 (68.52) | 729.10 (556.28) | 1574.54 (770.91) | <0.001 |
| TNF-α (pg/mL) (mean (SD)) | 64.55 (64.99) | 67.96 (79.82) | 48.71 (24.96) | 0.113 |
| CD4+ cell count (cells/μL) (mean (SD)) | 294.47 (210.60) | 280.95 (204.23) | 191.31 (111.84) | 0.085 |
| Total cholesterol (mmol/L) (mean (SD)) | 4.18 (1.20) | 4.27 (0.95) | 3.41 (1.28) | 0.011 |
| LDL cholesterol (mmol/L) (mean (SD)) | 2.43 (1.00) | 1.58 (0.83) | 1.69 (0.76) | 0.316 |
| HDL cholesterol (mmol/L) (mean (SD)) | 1.15 (0.41) | 2.30 (0.82) | 1.95 (0.93) | 0.120 |
| Triglycerides (mmol/L) (mean (SD)) | 1.70 (0.86) | 1.18 (0.35) | 0.82 (0.28) | 0.002 |
| Parameter | OR (95% CI) | p | aOR * (95% CI) | p |
|---|---|---|---|---|
| Age | ||||
| <35 years (n = 23) | Ref. | |||
| ≥35 years (n = 30) | 6.08 (1.19–31.01) | 0.030 | 4.01 (0.75–21.49) | 0.104 |
| Gender | ||||
| Male (n = 42) | Ref. | |||
| Female (n = 11) | 2.86 (0.32–25.80) | 0.350 | 2.84 (0.31–26.76) | 0.362 |
| Intravenous drug use | ||||
| No (n = 24) | Ref. | |||
| Yes (n = 29) | 6.72 (1.32–34.32) | 0.009 | 6.59 (1.22–35.66) | 0.029 |
| HCV infection | ||||
| No (n = 35) | Ref. | |||
| Yes (n = 18) | 4.80 (1.27–18.09) | 0.021 | 5.64 (1.17–27.22) | 0.031 |
| HBV infection | ||||
| No (n = 43) | Ref. | |||
| Yes (n = 10) | 2.52 (0.58–10.88) | 0.216 | 2.46 (0.51–11.85) | 0.262 |
| CD4+ cell count | ||||
| ≥200 cells/μL (n = 35) | Ref. | |||
| <200 cells/μL (n = 17) | 1.46 (0.40–5.38) | 0.571 | 1.70 (0.37–7.91) | 0.498 |
| PLT | ||||
| ≥140 G/L (n = 40) | Ref. | |||
| <140 G/L (n = 13) | 1.53 (0.38–6.16) | 0.549 | 2.63 (0.52–13.29) | 0.242 |
| CRP | ||||
| <5 mg/L (n = 28) | Ref. | |||
| ≥5 mg/L (n = 25) | 5.56 (1.32–23.46) | 0.020 | 20.65 (1.88–227.24) | 0.013 |
| PCT | ||||
| <0.05 ng/mL (n = 44) | Ref. | |||
| ≥0.05 ng/mL (n = 9) | 22.17 (3.69–133.02) | <0.001 | 16.60 (2.43–113.20) | 0.004 |
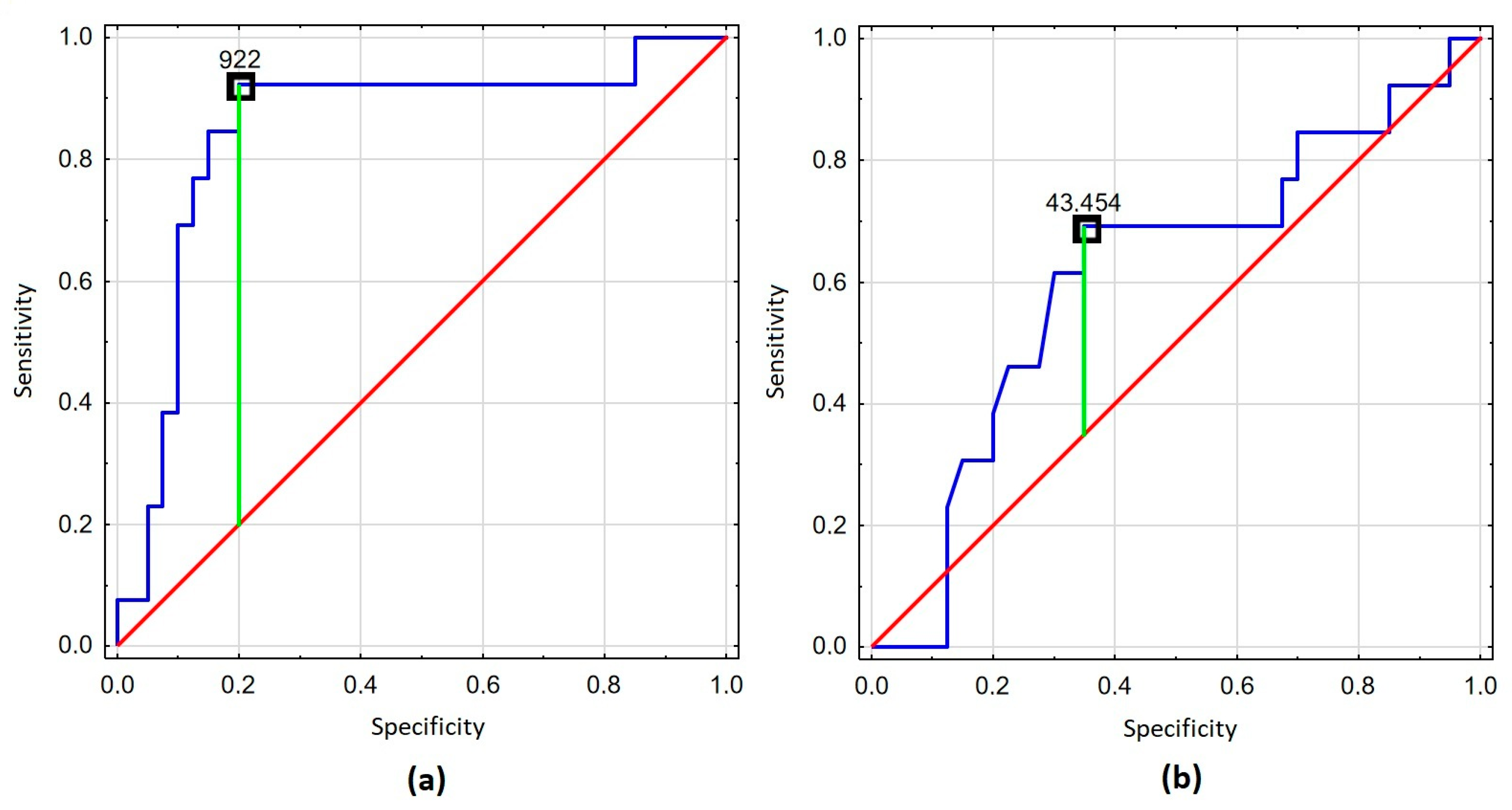
| VCAM-1 | ||||
| <922 ng/mL (n = 33) | Ref. | |||
| ≥922 ng/mL (n = 20) | 48.00 (5.41–425.56) | <0.001 | 33.49 (3.54–316.98) | 0.002 |
| TNF-α | ||||
| <43.45 pg/mL (n = 22) | Ref. | |||
| ≥43.45 pg/mL (n = 31) | 0.34 (0.09–1.23) | 0.099 | 0.30 (0.07–1.35) | 0.116 |
| Total cholesterol | ||||
| <4.91 mmol/L (n = 44) | Ref. | |||
| ≥4.91 mmol/L (n = 9) | 0.33 (0.04–2.96) | 0.324 | 0.24 (0.00–5.64) | 0.997 |
| HDL cholesterol | ||||
| >1 mmol/L (n = 34) | Ref. | |||
| ≤1 mmol/L (n = 19) | 4.22 (1.13–15.72) | 0.032 | 2.67 (0.62–11.56) | 0.188 |
| LDL cholesterol | ||||
| <2.6 mmol/L (n = 40) | Ref. | |||
| ≥2.6 mmol/L (n = 13) | 0.19 (0.02–1.67) | 0.135 | 0.17 (0.00–3.78) | 0.998 |
| Triglyceride | ||||
| <1.7 mmol/L (n = 38) | Ref. | |||
| ≥1.7 mmol/L (n = 15) | 1.88 (0.50–7.07) | 0.353 | 1.60 (0.35–7.28) | 0.543 |
| Parameter | OR (95% CI) | p | aOR * (95% CI) | p |
|---|---|---|---|---|
| Age | ||||
| <35 years (n = 11) | Ref. | |||
| ≥35 years (n = 18) | 4.50 (0.75–26.93) | 0.100 | 4.93 (0.73–33.21) | 0.764 |
| Gender | ||||
| Male (n = 23) | Ref. | |||
| Female (n = 7) | 1.50 (0.43–5.21) | 0.523 | 1.72 (0.08–6.18) | 0.362 |
| HCV infection | ||||
| No (n = 15) | Ref. | |||
| Yes (n = 14) | 5.33 (1.03–27.76) | 0.047 | 6.18 (1.03–36.89) | 0.046 |
| HBV infection | ||||
| No (n = 22) | Ref. | |||
| Yes (n = 7) | 1.31 (0.23–7.41) | 0.758 | 1.01 (0.14–7.46) | 0.996 |
| CD4+ cell count | ||||
| ≥200 cells/μL (n = 22) | Ref. | |||
| <200 cells/μL (n = 7) | 2.86 (0.50–16.36) | 0.238 | 2.84 (0.37–21.57) | 0.314 |
| PLT | ||||
| ≥140 G/L (n = 24) | Ref. | |||
| <140 G/L (n = 5) | 3.00 (0.41–21.74) | 0.277 | 2.44 (0.27–21.97) | 0.426 |
| CRP | ||||
| <5 mg/L (n = 13) | Ref. | |||
| ≥5 mg/L (n = 16) | 7.07 (1.17–42.85) | 0.033 | 9.42 (1.30–68.01) | 0.026 |
| PCT | ||||
| <0.05 ng/mL (n = 6) | Ref. | |||
| ≥0.05 ng/mL (n = 23) | 7.06 (0.00–250.09) | 0.997 | 25.09 (0.00–286.89) | 0.997 |
| VCAM-1 | ||||
| <922 ng/mL (n = 14) | Ref. | |||
| ≥922 ng/mL (n = 15) | 4.50 (0.75–26.93) | 0.099 | 3.28 (0.36–35.78) | 0.994 |
| TNF-α | ||||
| <43.45 pg/mL (n = 19) | Ref. | |||
| ≥43.45 pg/mL (n = 10) | 0.47 (0.14–1.56) | 0.218 | 0.29 (0.05–1.74) | 0.117 |
| Total cholesterol | ||||
| <4.91 mmol/L (n = 25) | Ref. | |||
| ≥4.91 mmol/L (n = 4) | 0.50 (0.05–5.51) | 0.571 | 0.26 (0.02–3.17) | 0.288 |
| HDL cholesterol | ||||
| >1 mmol/L (n = 17) | Ref. | |||
| ≤1 mmol/L (n = 12) | 4.55 (0.92–22.63) | 0.064 | 3.28 (0.60–17.98) | 0.172 |
| LDL cholesterol | ||||
| <2.6 mmol/L (n = 21) | Ref. | |||
| ≥2.6 mmol/L (n = 8) | 0.16 (0.02–1.51) | 0.109 | 0.06 (0.01–0.69) | 0.052 |
| Triglyceride | ||||
| <1.7 mmol/L (n = 21) | Ref. | |||
| ≥1.7 mmol/L (n = 8) | 2.00 (0.38–10.48) | 0.412 | 1.89 (0.30–11.94) | 0.497 |
| Parameter | OR (95% CI) | p | aOR * (95% CI) | p |
|---|---|---|---|---|
| Age | ||||
| <35 years (n = 22) | Ref. | |||
| ≥35 years (n = 25) | 6.63 (0.73–60.22) | 0.093 | 7.86 (0.80–77.16) | 0.077 |
| Gender | ||||
| Male (n = 36) | Ref. | |||
| Female (n = 11) | 0.73 (0.12–4.39) | 0.726 | 0.45 (0.06–3.29) | 0.434 |
| Intravenous drug use | ||||
| No (n = 24) | Ref. | |||
| Yes (n = 23) | 3.06 (0.53–17.66) | 0.212 | 3.00 (0.48–18.73) | 0.239 |
| HCV infection | ||||
| No (n = 33) | Ref. | |||
| Yes (n = 14) | 4.00 (0.76–21.02) | 0.102 | 6.89 (1.00–46.69) | 0.051 |
| HBV infection | ||||
| No (n = 39) | Ref. | |||
| Yes (n = 8) | 2.27 (0.35–14.49) | 0.387 | 1.37 (0.18–10.27) | 0.759 |
| CD4+ cell count | ||||
| ≥200 cells/μL (n = 31) | Ref. | |||
| <200 cells/μL (n = 16) | 4.67 (0.75–29.01) | 0.098 | 4.39 (0.65–29.53) | 0.128 |
| PLT | ||||
| ≥140 G/L (n = 34) | Ref. | |||
| <140 G/L (n = 13) | 4.59 (0.86–24.42) | 0.074 | 5.15 (0.76–34.84) | 0.093 |
| CRP | ||||
| <5 mg/L (n = 27) | Ref. | |||
| ≥5 mg/L (n = 20) | 4.17 (0.72–24.23) | 0.112 | 4.41 (0.70–27.84) | 0.115 |
| PCT | ||||
| <0.05 ng/mL (n = 41) | Ref. | |||
| ≥0.05 ng/mL (n = 6) | 25.33 (3.21–199.69) | 0.002 | 18.41 (1.84–183.98) | 0.013 |
| VCAM-1 | ||||
| <922 ng/mL (n = 33) | Ref. | |||
| ≥922 ng/mL (n = 14) | 24.00 (2.52–228.70) | 0.006 | 19.09 (1.92–189.82) | 0.012 |
| TNF-α | ||||
| <43.45 pg/mL (n = 20) | Ref. | |||
| ≥43.45 pg/mL (n = 27) | 0.09 (0.01–0.82) | 0.033 | 0.11 (0.01–1.08) | 0.058 |
| Total cholesterol | ||||
| <4.91 mmol/L (n = 38) | Ref. | |||
| ≥4.91 mmol/L (n = 9) | 0.67 (0.07–6.35) | 0.724 | 9.18 (0.90–93.99) | 0.465 |
| HDL cholesterol | ||||
| >1 mmol/L (n = 32) | Ref. | |||
| ≤1 mmol/L (n = 15) | 3.52 (0.68–18.30) | 0.135 | 2.97 (0.51–17.17) | 0.225 |
| LDL cholesterol | ||||
| <2.6 mmol/L (n = 34) | Ref. | |||
| ≥2.6 mmol/L (n = 13) | 0.39 (0.04–3.59) | 0.405 | 0.22 (0.02–2.26) | 0.205 |
| Triglyceride | ||||
| <1.7 mmol/L (n = 34) | Ref. | |||
| ≥1.7 mmol/L (n = 13) | 2.25 (0.43–11.83) | 0.338 | 2.32 (0.35–15.30) | 0.380 |
| Variable | All HIV/HCV-Coinfected Patients (n = 18) | Patients Who Lived (n = 10) | Patients Who Died (n = 8) | p |
|---|---|---|---|---|
| Age (years) (mean (SD)) | 35.94 (6.66) | 33.80 (6.81) | 38.63 (5.78) | 0.130 |
| Male gender (n (%)) | 14 (77.78) | 8 (80.00) | 6 (75.00) | 0.800 |
| Female gender (n (%)) | 4 (22.22) | 2 (20.00) | 2 (25.00) | 0.800 |
| Intravenous drug use (n (%)) | 14 (77.78) | 6 (60.00) | 8 (100.00) | 0.196 |
| HBV coinfection (n (%)) | 3 (16.67) | 1 (10.00) | 2 (25.00) | 0.466 |
| Duration of HIV infection (years) (mean (SD)) | 5.42 (6.53) | 2.65 (3.73) | 8.88 (7.81) | 0.322 |
| Duration of HIV treatment (weeks) (mean (SD)) | 86.39 (168.59) | 82.50 (176.06) | 91.25 (170.67) | 0.427 |
| PLT (G/L) (mean (SD)) | 215.17 (128.37) | 236.20 (156.64) | 188.88 (83.96) | 0.454 |
| CRP (mg/L) (mean (SD)) | 18.83 (23.32) | 14.30 (23.36) | 24.50 (24.66) | 0.252 |
| PCT (ng/mL) (mean (SD)) | 0.11 (0.20) | 0.05 (0.00) | 0.17 (0.30) | 0.056 |
| VCAM-1 (ng/mL) (mean (SD)) | 1234.67 (925.33) | 797.60 (786.00) | 1781.00 (819.19) | 0.002 |
| TNF-α (pg/mL) (mean (SD)) | 54.87 (23.46) | 56.49 (21.11) | 52.84 (27.49) | 0.374 |
| CD4+ cell count (cells/μL) (mean (SD)) | 253.89 (203.67) | 313.00 (258.00) | 180.00 (62.66) | 0.504 |
| Total cholesterol (mmol/L) (mean (SD)) | 3.88 (1.02) | 4.07 (0.82) | 3.65 (1.24) | 0.395 |
| LDL cholesterol (mmol/L) (mean (SD)) | 2.14 (0.74) | 2.06 (0.59) | 2.23 (0.93) | 0.924 |
| HDL cholesterol (mmol/L) (mean (SD)) | 1.04 (0.36) | 1.25 (0.28) | 0.78 (0.27) | 0.002 |
| Triglycerides (mmol/L) (mean (SD)) | 1.72 (0.89) | 1.65 (0.91) | 1.80 (0.91) | 0.964 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lembas, A.; Załęski, A.; Mikuła, T.; Kozłowska, J.; Wiercińska-Drapało, A. Proinflammatory Biomarkers and Clinical Factors Associated with Long-Term Mortality in People with HIV. Viruses 2025, 17, 243. https://doi.org/10.3390/v17020243
Lembas A, Załęski A, Mikuła T, Kozłowska J, Wiercińska-Drapało A. Proinflammatory Biomarkers and Clinical Factors Associated with Long-Term Mortality in People with HIV. Viruses. 2025; 17(2):243. https://doi.org/10.3390/v17020243
Chicago/Turabian StyleLembas, Agnieszka, Andrzej Załęski, Tomasz Mikuła, Joanna Kozłowska, and Alicja Wiercińska-Drapało. 2025. "Proinflammatory Biomarkers and Clinical Factors Associated with Long-Term Mortality in People with HIV" Viruses 17, no. 2: 243. https://doi.org/10.3390/v17020243
APA StyleLembas, A., Załęski, A., Mikuła, T., Kozłowska, J., & Wiercińska-Drapało, A. (2025). Proinflammatory Biomarkers and Clinical Factors Associated with Long-Term Mortality in People with HIV. Viruses, 17(2), 243. https://doi.org/10.3390/v17020243






