Disparities in Influenza Control and Surveillance in Latin America and the Caribbean
Abstract
1. Introduction
2. Materials and Methods
2.1. Approach and Data Sources
2.2. Processing and Exclusion Criteria
3. Results
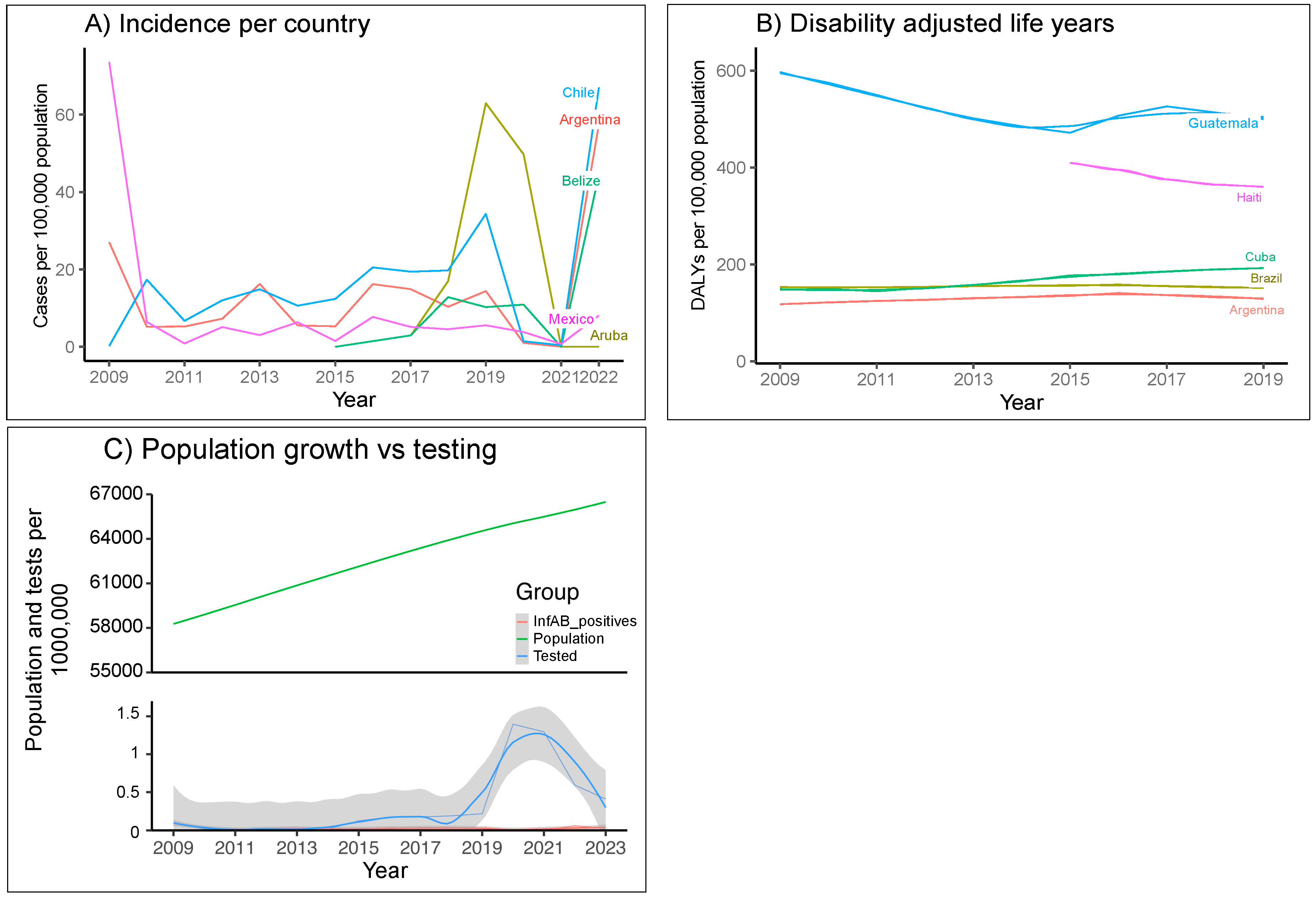
3.1. Burdens of Influenza
3.1.1. Burdens of Influenza in LAC Medical Systems
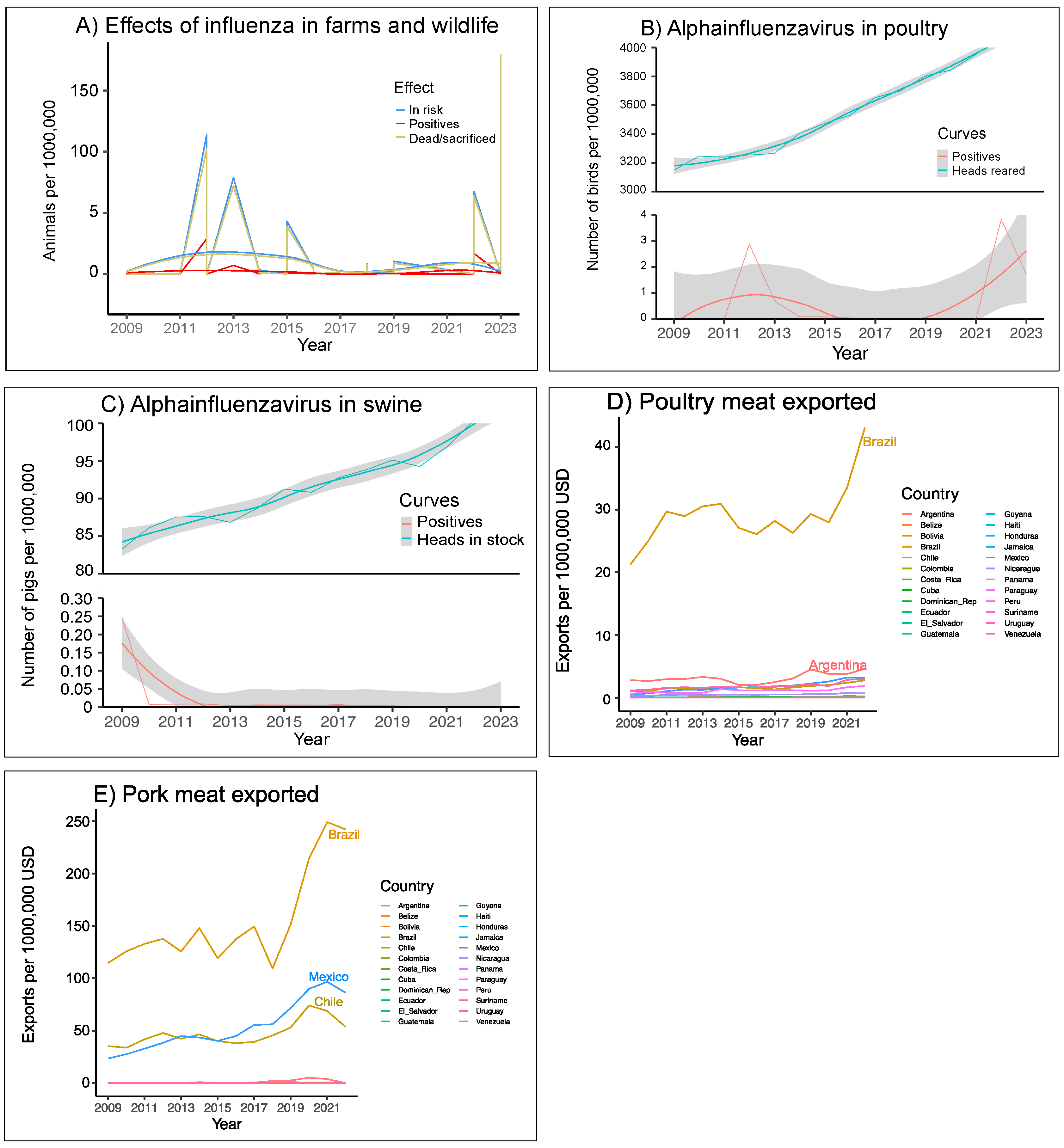
3.1.2. Burdens of Influenza in LAC Production Systems
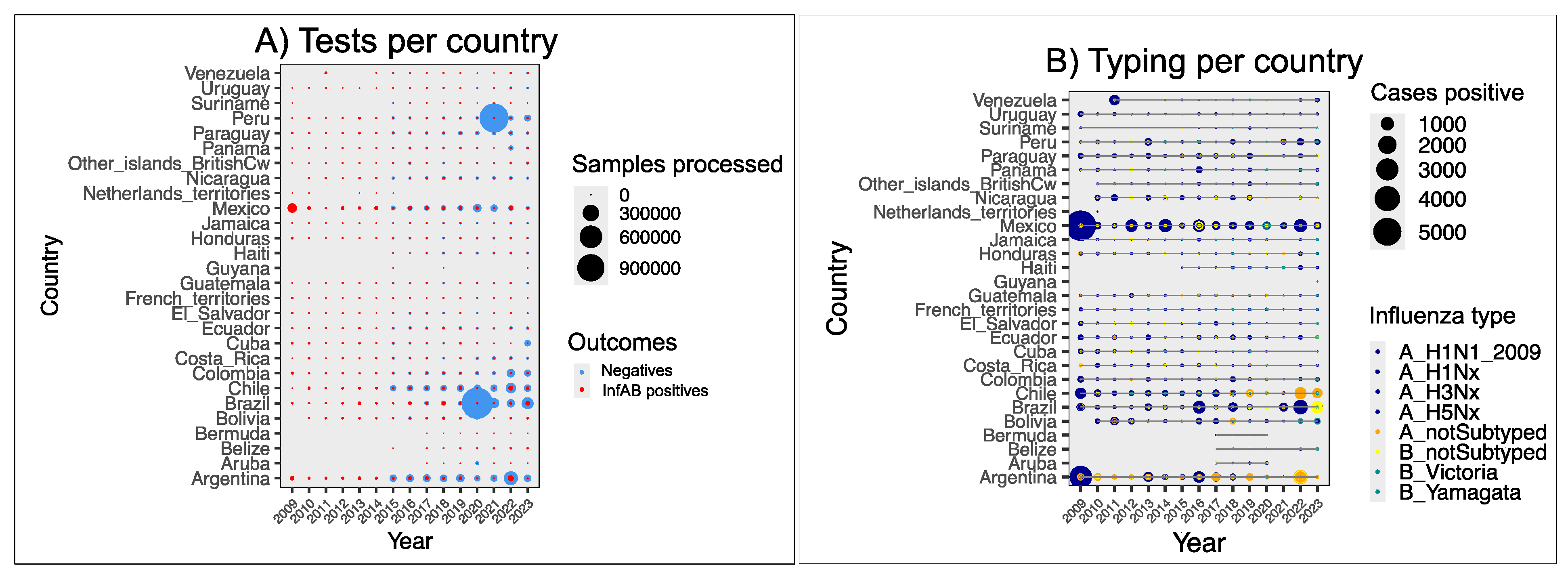
3.2. Screening and Typing of Influenzaviruses Within LAC
3.2.1. Screening and Typing Within Medical Networks
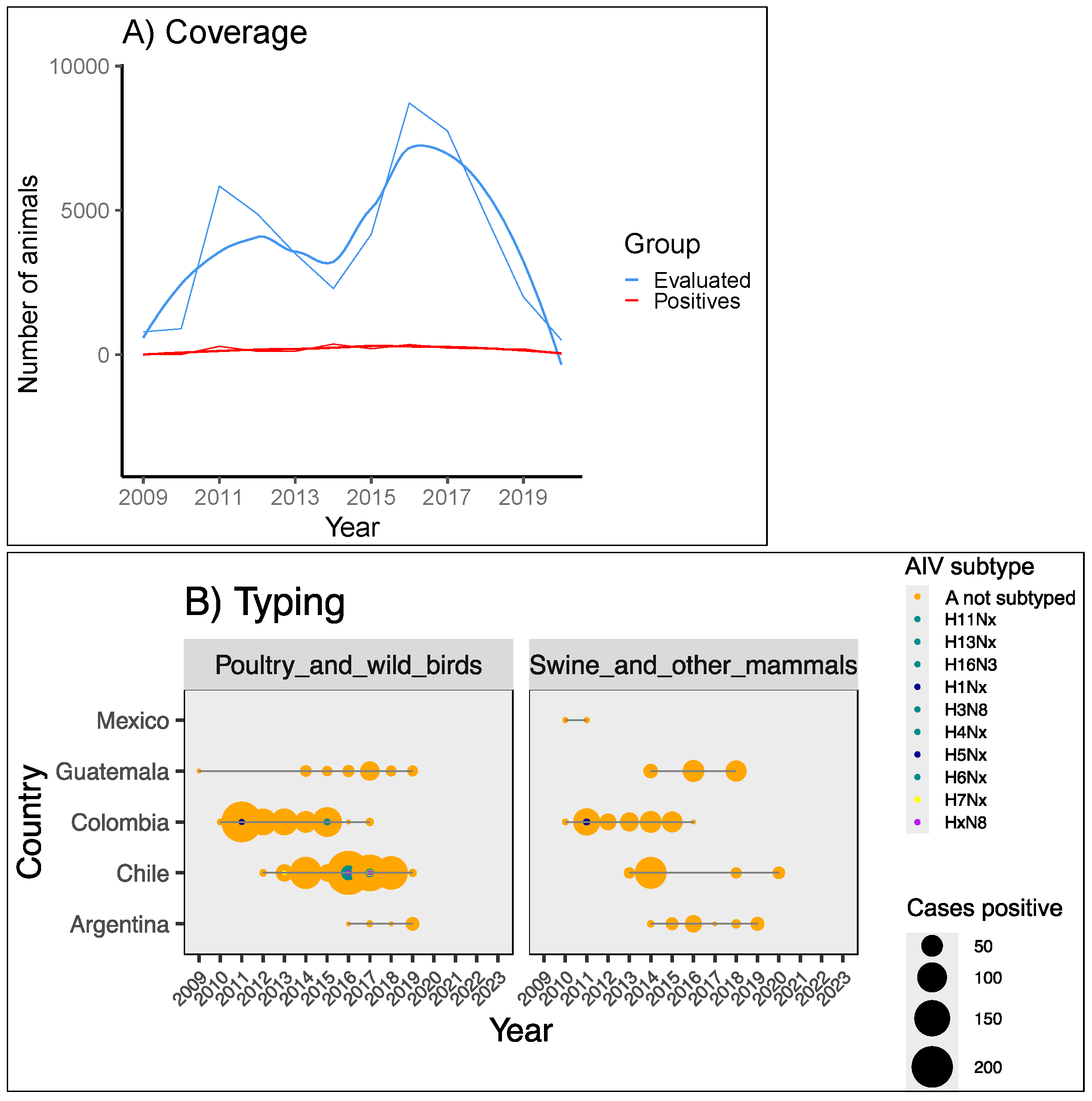
3.2.2. Screening and Typing in Farms and Wildlife
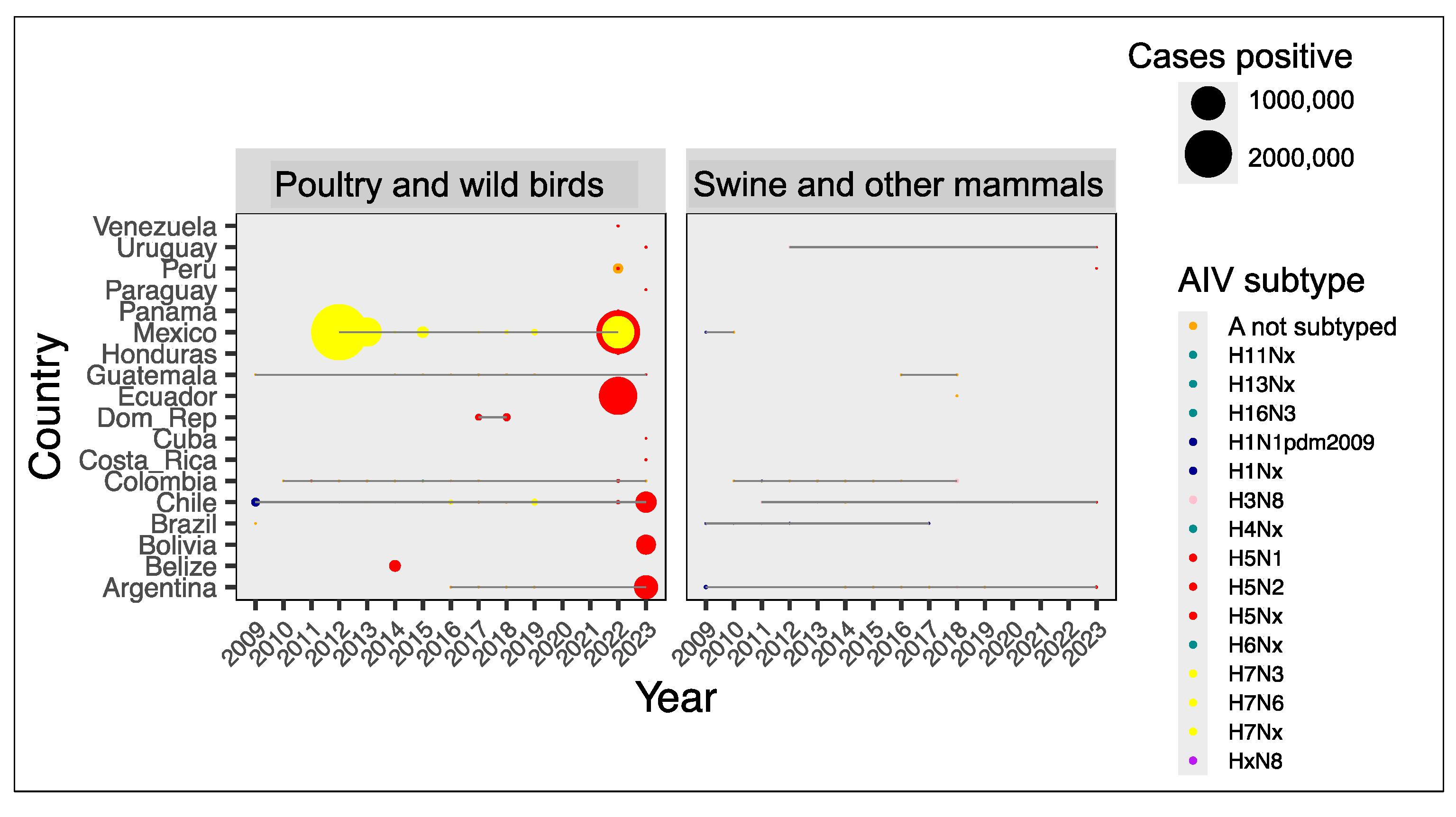
3.3. The Menace of HPAI Viruses
3.4. Strengths and Weaknesses in the LAC Influenza Control and Surveillance System for Animal Health
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Krammer, F.; Smith, G.J.D.; Fouchier, R.A.M.; Peiris, M.; Kedzierska, K.; Doherty, P.C.; Palese, P.; Shaw, M.L.; Treanor, J.; Webster, R.G.; et al. Influenza. Nat. Rev. Dis. Primers 2018, 4, 3. [Google Scholar] [CrossRef] [PubMed]
- Petrova, V.; Russell, C. The evolution of seasonal influenza viruses. Nat. Rev. Microbiol. 2018, 16, 47–60. [Google Scholar] [CrossRef]
- Walker, P.J.; Siddell, S.G.; Lefkowitz, E.J.; Mushegian, A.R.; Adriaenssens, E.M.; Dempsey, D.M.; Dutilh, B.E.; Harrach, B.; Harrison, R.L.; Hendrickson, R.C.; et al. Changes to virus taxonomy and the Statutes ratified by the International Committee on Taxonomy of Viruses. Arch. Virol. 2020, 165, 2737–2748. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Influenza (Seasonal)—Fact Sheet. 2018. Available online: https://www.who.int/en/news-room/fact-sheets/detail/influenza-(seasonal) (accessed on 25 July 2023).
- Ghebrehewet, S.; MacPherson, P.; Ho, A. Influenza. BMJ (Clin. Res. Ed.) 2016, 355, i6258. [Google Scholar] [CrossRef] [PubMed]
- White, S.K.; Ma, W.; McDaniel, C.J.; Gray, G.C.; Lednicky, J.A. Serologic evidence of exposure to influenza D virus among persons with occupational contact with cattle. J. Clin. Virol. 2016, 81, 31–33. [Google Scholar] [CrossRef] [PubMed]
- Gaudino, M.; Moreno, A.; Snoeck, C.J.; Zohari, S.; Saegerman, C.; O’donovan, T.; Ryan, E.; Zanni, I.; Foni, E.; Sausy, A.; et al. Emerging Influenza D virus infection in European livestock as determined in serology studies: Are we underestimating its spread over the continent? Transbound Emerg. Dis. 2021, 68, 1125–1135. [Google Scholar] [CrossRef] [PubMed]
- Sanogo, I.N.; Kouakou, C.; Batawui, K.; Djegui, F.; Byarugaba, D.K.; Adjin, R.; Adjabli, K.; Wabwire-Mangen, F.; Erima, B.; Atim, G.; et al. Serological Surveillance of Influenza D Virus in Ruminants and Swine in West and East Africa, 2017–2020. Viruses 2021, 13, 1749. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gaymard, A.; Le Briand, N.; Frobert, E.; Lina, B.; Escuret, V. Functional balance between neuraminidase and haemagglutinin in influenza viruses. Clin. Microbiol. Infect. 2016, 22, 975–983. [Google Scholar] [CrossRef]
- Nobusawa, E.; Sato, K. Comparison of the mutation rates of human influenza A and B viruses. J. Virol. 2006, 80, 3675–3678. [Google Scholar] [CrossRef]
- Biere, B.; Bauer, B.; Schweiger, B. Differentiation of influenza B virus lineages Yamagata and Victoria by real-time PCR. J. Clin. Microbiol. 2010, 48, 1425–1427. [Google Scholar] [CrossRef] [PubMed]
- Blagodatski, A.; Trutneva, K.; Glazova, O.; Mityaeva, O.; Shevkova, L.; Kegeles, E.; Onyanov, N.; Fede, K.; Maznina, A.; Khavina, E.; et al. Avian Influenza in Wild Birds and Poultry: Dissemination Pathways, Monitoring Methods, and Virus Ecology. Pathogens 2021, 10, 630. [Google Scholar] [CrossRef]
- Kumlin, U.; Olofsson, S.; Dimock, K.; Arnberg, N. Sialic acid tissue distribution and influenza virus tropism. Influenza Other Respir Viruses 2008, 2, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Horimoto, T.; Kawaoka, Y. Influenza: Lessons from past pandemics, warnings from current incidents. Nat. Rev. Microbiol. 2005, 3, 591–600. [Google Scholar] [CrossRef]
- Quantius, J.; Schmoldt, C.; Vazquez-Armendariz, A.I.; Becker, C.; El Agha, E.; Wilhelm, J.; Morty, R.E.; Vadász, I.; Mayer, K.; Gattenloehner, S.; et al. Influenza Virus Infects Epithelial Stem/Progenitor Cells of the Distal Lung: Impact on Fgfr2b-Driven Epithelial Repair. PLoS Pathog. 2016, 12, e1005544. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.C.; Wilson, I.A. Influenza Hemagglutinin Structures and Antibody Recognition. Cold Spring Harb. Perspect. Med. 2020, 10, a038778. [Google Scholar] [CrossRef] [PubMed]
- Poulson, R.L.; Tompkins, S.M.; Berghaus, R.D.; Brown, J.D.; Stallknecht, D.E. Environmental Stability of Swine and Human Pandemic Influenza Viruses in Water under Variable Conditions of Temperature, Salinity, and pH. Appl. Environ. Microbiol. 2016, 82, 3721–3726. [Google Scholar] [CrossRef]
- Labadie, T.; Batéjat, C.; Manuguerra, J.-C.; Leclercq, I. Influenza Virus Segment Composition Influences Viral Stability in the Environment. Front. Microbiol. 2018, 9, 1496. [Google Scholar] [CrossRef] [PubMed]
- Dee, S.A.; Bauermann, F.V.; Niederwerder, M.C.; Singrey, A.; Clement, T.; de Lima, M.; Long, C.; Patterson, G.; Sheahan, M.A.; Stoian, A.M.M.; et al. Survival of viral pathogens in animal feed ingredients under transboundary shipping models. PLoS ONE 2018, 13, e0194509. [Google Scholar] [CrossRef]
- Desrosiers, R. Survival and transmission of swine influenza A virus within and between farms. J. Swine Health Prod. 2021, 29, 133–138. [Google Scholar] [CrossRef]
- Weinstein, R.A.; Bridges, C.B.; Kuehnert, M.J.; Hall, C.B. Transmission of Influenza: Implications for Control in Health Care. Clin. Infect. Dis. 2013, 37, 1094–1101. [Google Scholar] [CrossRef]
- Moghadami, M. A Narrative Review of Influenza: A Seasonal and Pandemic Disease. Iranian J. Med. Sci. 2017, 42, 2–13. [Google Scholar]
- Yang, G.; Zhou, K.; Yi, Y.; Qiu, C. Epidemiology. In Avian Influenza in Human; Qiu, C., Shi, Y., Lu, P., Eds.; Springer: Singapore, 2021. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Update: Human Infection with Highly Pathogenic Avian Influenza A(H5N1) Virus in Texas. Available online: https://www.cdc.gov/ncird/whats-new/human-infection-H5N1-bird-flu.html#print (accessed on 8 April 2024).
- Gentile, A.; Paget, J.; Bellei, N.; Torres, J.P.; Vazquez, C.; Laguna-Torres, V.A.; Plotkin, S. Influenza in Latin America: A report from the Global Influenza Initiative (GII). Vaccine 2019, 37, 2670–2678. [Google Scholar] [CrossRef]
- Savy, V.; Ciapponi, A.; Bardach, A.; Glujovsky, D.; Aruj, P.; Mazzoni, A.; Gibbons, L.; Ortega-Barría, E.; Colindres, R.E. Burden of influenza in Latin America and the Caribbean: A systematic review and meta-analysis. Influenza Other Respir Viruses 2013, 7, 1017–1032. [Google Scholar] [CrossRef]
- Vicari, A.S.; Olson, D.; Vilajeliu, A.; Andrus, J.K.; Ropero, A.M.; Morens, D.M.; Santos, I.J.; Azziz-Baumgartner, E.; Berman, S. Seasonal Influenza Prevention and Control Progress in Latin America and the Caribbean in the Context of the Global Influenza Strategy and the COVID-19 Pandemic. Am. J. Trop. Med. Hyg. 2021, 105, 93–101. [Google Scholar] [CrossRef]
- Gonzalez-Mosegui, G.B.; Antoñanzas-Villar, F.; De Mello-Vianna, C.M. Burden of disease attributed to acute respiratory infections in South America. J. Infect. Dev. Ctries 2022, 16, 1614–1622. [Google Scholar] [CrossRef] [PubMed]
- Afanador-Villamizar, A.; Gomez-Romero, C.; Diaz, A.; Ruiz-Saenz, J. Avian influenza in Latin America: A systematic review of serological and molecular studies from 2000–2015. PLoS ONE 2017, 12, e0179573. [Google Scholar] [CrossRef]
- Alvis-Guzmán, N.; Marín-Correa, C.; Castañeda-Orjuela, C.A.; Sánchez-Ruiz, C.; Carrasquilla-Sotomayor, M.; Sanchez-Largaespada Mena, R.; Mejía, H. Hospitalization costs due to severe acute respiratory infection (SARI) in three Central American countries. Infectio 2018, 22, 159–166. [Google Scholar] [CrossRef]
- Zhang, Y.; Aevermann, B.D.; Anderson, T.K.; Burke, D.F.; Dauphin, G.; Gu, Z.; He, S.; Kumar, S.; Larsen, C.N.; Lee, A.J.; et al. Influenza Research Database: An integrated bioinformatics resource for influenza virus research. Nucleic Acids Res. 2017, 45, D466–D474. [Google Scholar] [CrossRef]
- GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar] [CrossRef]
- Stevens, G.A.; Alkema, L.; E Black, R.; Boerma, J.T.; Collins, G.S.; Ezzati, M.; Grove, J.T.; Hogan, D.R.; Hogan, M.C.; Horton, R.; et al. Guidelines for accurate and transparent health estimates reporting: The GATHER statement. Lancet 2016, 388, e19–e23. [Google Scholar] [CrossRef]
- Murad, M.H.; Wang, Z. Guidelines for reporting meta-epidemiological methodology research. Evid. Based Med. 2017, 22, 139–142. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.F.; Rankin, S.C.; Schurer, J.M.; Cole, S.; Conti, L.; Rabinowitz, P.; Gray, G.; Kahn, L.; Machalaba, C.; Mazet, J.; et al. Checklist for One Health Epidemiological Reporting of Evidence (COHERE). One Health 2017, 4, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Hammond, A.; Kim, J.J.; Sadler, H.; Vandemaele, K. Influenza surveillance systems using traditional and alternative sources of data: A scoping review. Influenza Other Respir Viruses 2022, 16, 965–974. [Google Scholar] [CrossRef]
- Chauhan, R.P.; Gordon, M.L. A Systematic Review Analyzing the Prevalence and Circulation of Influenza Viruses in Swine Population Worldwide. Pathogens 2020, 9, 355. [Google Scholar] [CrossRef]
- Schaefer, R.; Rech, R.R.; Gava, D.; Cantão, M.E.; Da Silva, M.C.; Silveira, S.; Zanella, J.R.C. A human-like H1N2 influenza virus detected during an outbreak of acute respiratory disease in swine in Brazil. Arch. Virol. 2015, 160, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Ciacci-Zanella, J.R.; Schaefer, R.; Gava, D.; Haach, V.; Cantão, M.E.; Coldebella, A. Influenza A virus infection in Brazilian swine herds following the introduction of pandemic 2009 H1N1. Vet. Microbiol. 2015, 180, 118–122. [Google Scholar] [CrossRef]
- Hurtado, R.; de Azevedo-Júnior, S.M.; Vanstreels, R.E.T.; Fabrizio, T.; Walker, D.; Rodrigues, R.C.; Seixas, M.M.M.; de Araújo, J.; Thomazelli, L.M.; Ometto, T.L.; et al. Surveillance of Avian Influenza Virus in Aquatic Birds on the Brazilian Amazon Coast. Ecohealth 2016, 13, 813–818. [Google Scholar] [CrossRef]
- Baudon, E.; Peyre, M.; Peiris, M.; Cowling, B.J. Epidemiological features of influenza circulation in swine populations: A systematic review and meta-analysis. PLoS ONE 2017, 12, e0179044. [Google Scholar] [CrossRef]
- Corrales, J.P.; Pineda-Ortiz, M.P.; Ortiz-Castro, L.F.; Moreno, D.C.Z. Association between seropositivity to the swine influenza virus (VIP) and the characteristics of technical pig farms in Colombia. CES Med. Veter Zootec. 2016, 11, 10–22. (In Spanish) [Google Scholar] [CrossRef]
- World Health Organization. Human Infection Caused by Avian Influenza A(H5)—Ecuador. 2023. Available online: https://www.who.int/emergencies/disease-outbreak-news/item/2023-DON434 (accessed on 8 March 2023).
- European Food Safety Authority; European Centre for Disease Prevention and Control; European Union Reference Laboratory for Avian Influenza; Adlhoch, C.; Fusaro, A.; Gonzales, J.L.; Kuiken, T.; Marangon, S.; Mirinaviciute, G.; Niqueux, E.; et al. Avian influenza overview December 2022–March 2023. EFSA J. 2023, 21, 7917. [Google Scholar] [CrossRef]
- Pan American Health Organization/World Health Organization. Epidemiological Update: Outbreaks of Avian Influenza Caused by Influenza A(H5N1) in the Region of the Americas. 17 May 2023; OPS/OMS: Washington, DC, USA, 2023. [Google Scholar]
- Castillo, A.; Fasce, R.; Parra, B.; Andrade, W.; Covarrubias, P.; Hueche, A.; Campano, C.; Tambley, C.; Rojas, M.; Araya, M.T.M.; et al. The first case of human infection with H5N1 avian Influenza A virus in Chile. J. Trav. Med. 2023, 30, taad083. [Google Scholar] [CrossRef]
- Jimenez-Bluhm, P.; Bravo-Vasquez, N.; Torchetti, M.K.; Killian, M.L.; Livingston, B.; Herrera, J.; Fuentes, M.; Schultz-Cherry, S.; Hamilton-West, C. Low pathogenic avian influenza (H7N6) virus causing an outbreak in commercial Turkey farms in Chile. Emerg. Microbes Infect. 2019, 8, 479–485. [Google Scholar] [CrossRef]
- Flórez, R.J.; Vera, A.V.; Lora, M.A.; Ramírez-Nieto, G. Molecular evaluation of the presence of influenza A virus in pigs in processing plants in Colombia. Rev. MVZ Córdoba 2018, 23, 2018. [Google Scholar] [CrossRef]
- Ayala, E.; Chapa, J. Impacto de la Influenza AH1N1 en el Mercado Mexicano de Carne de Cerdo; Estudios Economicos: Mexico City, Mexico, 2017; pp. 3–25. [Google Scholar] [CrossRef]
- Klein, H.; Vidal, F. La emergencia de Brasil como principal exportador mundial de carne de pollo. Historia Agraria de América Latina 2022, 3, 75–99. [Google Scholar] [CrossRef]
- Fraiha, A.L.S.; Matos, A.C.D.; Cunha, J.L.R.; Santos, B.S.Á.d.S.; Peixoto, M.V.C.; Oliveira, A.G.G.; Lobato, Z.I.P. Swine influenza A virus subtypes circulating in Brazilian commercial pig herds from 2012 to 2019. Braz. J. Microbiol. 2021, 52, 2421–2430. [Google Scholar] [CrossRef]
- Patiño-Quiroz, B.E.; Baldrich-Romero, N.E.; Duque-Patiño, S. Analysis of the regulatory model for health prevention of avian influenza in Colombia. Rev. Fac. Cien. Agrop. 2017, 9, 23–29. [Google Scholar]
- Pan American Health Organization/World Health Organization. Epidemiological Update: Avian Influenza A(H5N1) in the Americas Region, 15 November 2024; PAHO/WHO: Washington, DC, USA, 2024. [Google Scholar]
- Huang, S.Y.; Yang, J.R.; Lin, Y.J.; Yang, C.H.; Cheng, M.C.; Liu, M.T.; Wu, H.S.; Chang, F.Y. Serological comparison of antibodies to avian influenza viruses, subtypes H5N2, H6N1, H7N3 and H7N9 between poultry workers and non-poultry workers in Taiwan in 2012. Epidemiol. Infect. 2015, 143, 2965–2974. [Google Scholar] [CrossRef]
- Lin, C.-Y.; Chia, M.-Y.; Chen, P.-L.; Yeh, C.-T.; Cheng, M.-C.; Su, I.-J.; Lee, M.-S. Assessment of pathogenicity and antigenicity of American lineage influenza H5N2 viruses in Taiwan. Virology 2017, 508, 159–163. [Google Scholar] [CrossRef]
- Shao, W.; Li, X.; Goraya, M.U.; Wang, S.; Chen, J.-L. Evolution of Influenza A Virus by Mutation and Re-Assortment. Int. J. Mol. Sci. 2017, 18, 1650. [Google Scholar] [CrossRef]

| Data Base | Abbreviation | Use | Link |
|---|---|---|---|
| Influenza network | FluNet | AIV and BIV cases in the medical system, per year and country | https://www.who.int/tools/flunet |
| Food and Agriculture Organization | FAO | Poultry and swine stocks, per year and country Poultry and pork meat exports, per year and country | https://www.fao.org/faostat/en/#data/QCL https://www.fao.org/faostat/en/#data/TI |
| Global Burden of Disease [32] | GBD | Disability-adjusted life years (DALYs), per year and country | https://www.healthdata.org/research-analysis/library/global-burden-369-diseases-and-injuries-1990–2019-systematic-analysis |
| Global Initiative on Sharing All Influenza Data | GISAID | Sources of data for influenza genomic surveillance | https://nextstrain.org/flu/ |
| Influenza Research Database [31] | IRD | Surveillance for AIV in avian hosts and no-human mammals | https://www.bv-brc.org/searches/SurveillanceSearch |
| World Animal Health Information System | WAHIS | Events of avian, equine and porcine influenza tracked in productive systems | https://wahis.woah.org/#/event-management |
| World Bank Databank | World Bank | Population, per year and country | https://data.worldbank.org/indicator/SP.POP.TOTL?end=2022&locations=ZJ&start=2009 |
| Genotype | Host(s) | Positives Confirmed | Deaths | Sacrificed | Sources |
|---|---|---|---|---|---|
| H5(N untyped) | Coati | 16 | 16 | 0 | WAHIS 1 |
| H5(N untyped) | Poultry | 405 | 719 | 870 | WAHIS 1 |
| H5(N untyped) | Sea lion, fur seal, otter, Chilean dolphin, southern river otter | 449 | 449 | 0 | WAHIS 1 |
| H5(N untyped) | Wild birds, multiple species | 604 | 55 | 28 | WAHIS 1 |
| H5N1 | Human | 2 | 0 | --- | [43,44,45,46] |
| H5N1 | Poultry | 4,215,219 | 2,444,659 | 28,880,317 | WAHIS |
| H5N1 | Wild birds: includes Anseriformes, Cathartidae, Falconiadae, Galliformes, Hirundinidae and Pelecanidae | 3027 | 2147 | 700 | WAHIS |
| H5N2 | Human | 2 | 0 | --- | FLUNET |
| H5N2 | Poultry | 130,017 | 41,952 | 1,575,341 | WAHIS |
| H5N2 | Wild birds | 2 | NA | NA | IRD |
| H7N3 | Poultry | 4,562,445 | 1,889,150 | 24,482,538 | WAHIS |
| H7N3 | Wild birds, multiple species: Hirundinidae, Icteridae, Anseriformes | 19 | 19 | 0 | WAHIS |
| H7N6 | Poultry | 57,575 | 5637 | 442,300 | [47], WAHIS |
| NA | Brown pelican | 79 | 73 | 1 | WAHIS 1 |
| NA | Poultry | 94,940 | 52,623 | 199,740 | WAHIS 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoyos-Cerón, T.; Albarrán-Tamayo, F.; Bañuelos-Hernández, B.; Londoño-Avendaño, M.A. Disparities in Influenza Control and Surveillance in Latin America and the Caribbean. Viruses 2025, 17, 225. https://doi.org/10.3390/v17020225
Hoyos-Cerón T, Albarrán-Tamayo F, Bañuelos-Hernández B, Londoño-Avendaño MA. Disparities in Influenza Control and Surveillance in Latin America and the Caribbean. Viruses. 2025; 17(2):225. https://doi.org/10.3390/v17020225
Chicago/Turabian StyleHoyos-Cerón, Tatiana, Froylán Albarrán-Tamayo, Bernardo Bañuelos-Hernández, and María Aurora Londoño-Avendaño. 2025. "Disparities in Influenza Control and Surveillance in Latin America and the Caribbean" Viruses 17, no. 2: 225. https://doi.org/10.3390/v17020225
APA StyleHoyos-Cerón, T., Albarrán-Tamayo, F., Bañuelos-Hernández, B., & Londoño-Avendaño, M. A. (2025). Disparities in Influenza Control and Surveillance in Latin America and the Caribbean. Viruses, 17(2), 225. https://doi.org/10.3390/v17020225







