Detecting Arboviruses Through Screening Asymptomatic Blood Donors in Rio de Janeiro/Brazil During a Dengue Outbreak
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Description
2.2. RNA Extraction
2.3. Reverse Transcription and PCR Amplification
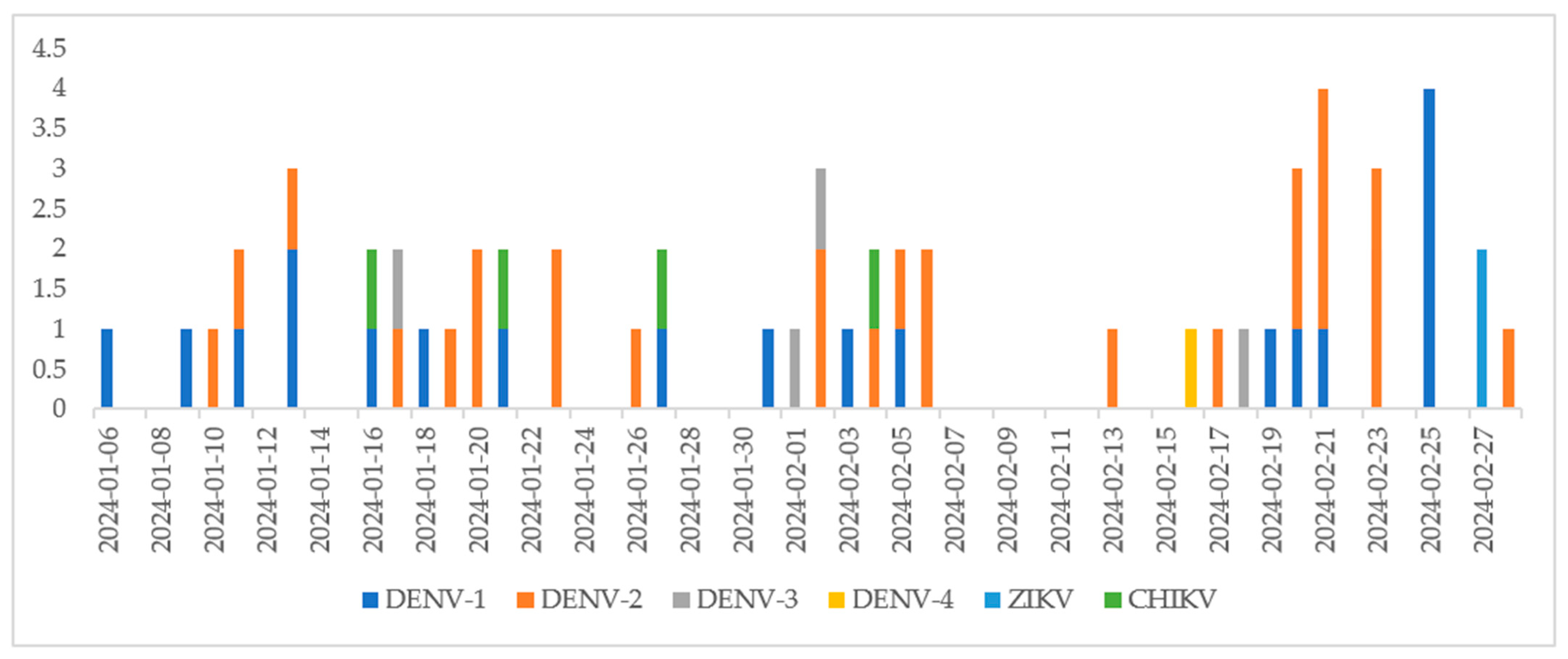
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mayer, S.V.; Tesh, R.B.; Vasilakis, N. The emergence of arthropod-borne viral diseases: A global prospective on dengue, chikungunya and zika fevers. Acta Trop. 2017, 166, 155–163. [Google Scholar] [CrossRef]
- Sharma, R.; Costa Santos, L.; da Silva, R.A.; Gonçalves, C.V.; de Melo Calado, S.; Santos, D.P.; de Andrade de Melo, J.P.; de Cássia Pontello Rampazzo, R.; Requião, L.; Krieger, M.A.; et al. Surveillance of donated blood during the 2016 arbovirus outbreak in Brazil. J. Med. Virol. 2018, 90, 1406–1410. [Google Scholar] [CrossRef] [PubMed]
- WHO. Vector-Borne Diseases. 2020. Available online: https://www.who.int/news-room/fact-sheets/detail/vector-borne-diseases (accessed on 10 September 2024).
- Gubler, D.J. The global emergence/resurgence of arboviral diseases as public health problems. Arch. Med. Res. 2002, 33, 330–342. [Google Scholar] [CrossRef] [PubMed]
- Weaver, S.C. Urbanization and geographic expansion of zoonotic arboviral diseases: Mechanisms and potential strategies for prevention. Trends Microbiol. 2013, 21, 360–363. [Google Scholar] [CrossRef] [PubMed]
- Murugesan, A.; Manoharan, M. Dengue virus. In Emerging and Reemerging Viral Pathogens; Academic Press: Cambridge, MA, USA, 2020; pp. 281–359. [Google Scholar]
- Brazil. Ministry of Health. Atualização de Casos de Arboviroses. 2024. Available online: https://www.gov.br/saude/pt-br/assuntos/saude-de-a-a-z/a/aedes-aegypti/monitoramento-das-arboviroses (accessed on 11 September 2024).
- Madariaga, M.; Ticona, E.; Resurrecion, C. Chikungunya: Bending over the Americas and the rest of the world. Braz. J. Infect. Dis. 2016, 20, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Dick, G.W.; Kitchen, S.F.; Haddow, A.J. Zika virus I. Isolations and serological specificity. Trans. R. Soc. Trop. Med. Hyg. 1952, 46, 509–520. [Google Scholar] [CrossRef] [PubMed]
- Slavov, S.N.; Otaguiri, K.K.; Kashima, S.; Covas, D.T. Overview of Zika virus (ZIKV) infection in regard to the Brazilian epidemic. Braz. J. Med. Biol. Res. 2016, 49, e5420. [Google Scholar] [CrossRef]
- Pealer, L.N.; Marfin, A.A.; Petersen, L.R.; Lanciotti, R.S.; Page, P.L.; Stramer, S.L.; Stobierski, M.G.; Signs, K.; Newman, B.; Kapoor, H.; et al. Transmission of West Nile virus through blood transfusion in the United States in 2002. N. Engl. J. Med. 2003, 25, 1236–1245. [Google Scholar] [CrossRef]
- Williamson, P.C.; Linnen, J.M.; Kessler, D.A.; Shaz, B.H.; Kamel, H.; Vassallo, R.R.; Winkelman, V.; Gao, K.; Ziermann, R.; Menezes, J.; et al. First cases of Zika virus-infected US blood donors outside states with areas of active transmission. Transfusion 2017, 57 Pt 2, 770–778. [Google Scholar] [CrossRef]
- Costa, E.; Rocha, D.; Lopes, J.I.F.; Andrade, E.; Cardoso, P.; Ribeiro, M.; Fontana-Maurell, M.; Vicentino, A.R.R.; Calazans, A.R.; Arruda, M.B.; et al. Detection of Plasmodium spp. in asymptomatic blood donors by the new Brazilian NAT PLUS HIV/HBV/HCV/Malaria Bio-Manguinhos kit. Transfusion 2024, 64, 501–509. [Google Scholar] [CrossRef]
- Weaver, S.C.; Reisen, W.K. Present and future arboviral threats. Antiviral Res. 2010, 85, 328–345. [Google Scholar] [CrossRef] [PubMed]
- Giménez-Richarte, Á.; de Salazar, M.O.; Arbona, C.; Giménez-Richarte, M.P.; Collado, M.; Fernández, P.L.; Quiles, F.; Clavijo, C.; Marco, P.; Ramos-Rincon, J.M. Prevalence of Chikungunya, Dengue and Zika viruses in blood donors: A systematic literature review and meta-analysis. Blood Transfus. 2022, 20, 267–280. [Google Scholar]
- Busch, M.P.; Sabino, E.C.; Brambilla, D.; Lopes, M.E.; Capuani, L.; Chowdhury, D.; McClure, C.; Linnen, J.M.; Prince, H.; Simmons, G.; et al. Duration of Dengue Viremia in Blood Donors and Relationships Between Donor Viremia, Infection Incidence and Clinical Case Reports During a Large Epidemic. J. Infect. Dis. 2016, 214, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Lamarão, L.M.; Corrêa, A.S.M.; de Castro, R.B.H.; de Melo Amaral, C.E.; Monteiro, P.D.J.; Palmeira, M.K.; Lopes, L.N.; Oliveira, A.N.; de Lima, M.S.M.; Moreira-Nunes, C.A.; et al. Prevalence of Dengue, Chikungunya and Zika Viruses in Blood Donors in the State of Pará, Northern Brazil: 2018–2020. Medicina 2022, 59, 79. [Google Scholar] [CrossRef]
- Custer, B.; Grebe, E.; Buccheri, R.; Bakkour, S.; Stone, M.; Capuani, L.; Alencar, C.; Amorim, L.; Loureiro, P.; Carneiro-Proietti, A.B.; et al. Surveillance for Zika, Chikungunya, and Dengue Virus Incidence and RNAemia in Blood Donors at 4 Brazilian Blood Centers During 2016–2019. J. Infect Dis. 2023, 227, 696–707. [Google Scholar] [CrossRef] [PubMed]
- Almeida, F.J.; Pacheco, J.T.; Farias, C.G.A.; de Matos, S.F.; de Morais, C.O.; Guerra, G.G.; Dos Santos, A.C.C.; Jarovsky, D.; Bezerra, R.F.; Furlanetto, B.H.S.; et al. Dengue: A hidden threat in blood transfusions amidst Brazil’s largest outbreak? Lancet Infect. Dis. 2025, 25, e10. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Health of Brazil. Boletim Epidemiológico; Volume 55, No. 10. 2024. Available online: https://www.gov.br/saude/pt-br/centrais-de-conteudo/publicacoes/boletins/epidemiologicos/edicoes/2024/boletim-epidemiologico-volume-55-no-10.pdf/view (accessed on 1 October 2024).
- Appassakij, H.; Promwong, C.; Rujirojindakul, P.; Khuntikij, P.; Silpapojakul, K. Risk of transfusion-transmitted chikungunya infection and efficacy of blood safety implementation measures: Experience from the 2009 epidemic in Songkhla Province, Thailand. Transfusion 2016, 56, 2100–2107. [Google Scholar] [CrossRef] [PubMed]
- Yoon, I.K.; Alera, M.T.; Lago, C.B.; Tac-An, I.A.; Villa, D.; Fernandez, S.; Thaisomboonsuk, B.; Klungthong, C.; Levy, J.W.; Velasco, J.M.; et al. High rate of subclinical chikungunya virus infection and association of neutralizing antibody with protection in a prospective cohort in the Philippines. PLoS Negl. Trop. Dis. 2015, 9, e0003764. [Google Scholar] [CrossRef]
- Asish, P.R.; Dasgupta, S.; Rachel, G.; Bagepally, B.S.; Girish Kumar, C.P. Global prevalence of asymptomatic dengue infections—A systematic review and meta-analysis. Int. J. Infect. Dis. 2023, 134, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Subissi, L.; Daudens-Vaysse, E.; Cassadou, S.; Ledrans, M.; Bompard, P.; Gustave, J.; Aubry, M.; Cao-Lormeau, V.-M.; Mallet, H.-P. Revising rates of asymptomatic Zika virus infection based on sentinel surveillance data from French Overseas Territories. Int. J. Infect. Dis. 2017, 65, 116–118. [Google Scholar] [CrossRef] [PubMed]
- Xavier, L.L.; Honório, N.A.; Pessanha, J.F.M.; Peiter, P.C. Analysis of climate factors and dengue incidence in the metropolitan region of Rio de Janeiro, Brazil. PLoS ONE 2021, 16, e0251403. [Google Scholar] [CrossRef] [PubMed]
| Targets (Positive Pool by Target) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Month/Year | Number of Pools | Number of Samples | Positive Pools | ZIKV | CHIKV | D1 | D2 | D3 | D4 |
| December 2023 | 179 | 1074 | 1 | - | - | - | 1 | - | - |
| January 2024 | 3291 | 19,746 | 26 | - | 3 | 12 | 10 | 1 | - |
| February 2024 | 2788 | 16,728 | 36 * | 2 | 1 | 12 | 18 | 3 | 1 |
| March 2024 | 745 | 4470 | 15 | 1 | 1 | 9 | 3 | 1 | - |
| April 2024 | 1633 | 9798 | 15 | - | 1 | 8 | 6 | - | - |
| May 2024 | 827 | 4962 | 2 | - | - | - | 2 | - | - |
| Total | 9463 | 56,778 | 95 * | 3 | 6 | 41 | 40 | 5 | 1 |
| Month/Year | Epidemiological Week | Number of Probable Cases |
|---|---|---|
| December 2023 | 49–52 | 76,387 |
| January 2024 | 1–5 | 497,340 |
| February 2024 | 6–9 | 1,116,098 |
| March 2024 | 10–13 | 1,581,219 |
| April 2024 | 14–18 | 1,885,120 |
| May 2024 | 19–22 | 940,567 |
| June 2024 | 23–26 | 276,907 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ribeiro, M.d.O.; Arruda, M.B.; Calazans, A.R.; Frederico, A.V.; Brito, A.F.; Barreto, B.V.d.S.; Brandão, É.M.d.V.; Athayde, H.; Nascimento, K.C.S.; Souza, L.P.d.B.O.; et al. Detecting Arboviruses Through Screening Asymptomatic Blood Donors in Rio de Janeiro/Brazil During a Dengue Outbreak. Viruses 2025, 17, 224. https://doi.org/10.3390/v17020224
Ribeiro MdO, Arruda MB, Calazans AR, Frederico AV, Brito AF, Barreto BVdS, Brandão ÉMdV, Athayde H, Nascimento KCS, Souza LPdBO, et al. Detecting Arboviruses Through Screening Asymptomatic Blood Donors in Rio de Janeiro/Brazil During a Dengue Outbreak. Viruses. 2025; 17(2):224. https://doi.org/10.3390/v17020224
Chicago/Turabian StyleRibeiro, Marisa de Oliveira, Mônica Barcellos Arruda, Alexandre Rodrigues Calazans, Alexandre Vicente Frederico, Anielly Ferreira Brito, Beatriz Vasconcello de Souza Barreto, Élida Millena de Vasconcelos Brandão, Hamilton Athayde, Kátia Cristina Silva Nascimento, Luiz Paulo de Brito Oliveira Souza, and et al. 2025. "Detecting Arboviruses Through Screening Asymptomatic Blood Donors in Rio de Janeiro/Brazil During a Dengue Outbreak" Viruses 17, no. 2: 224. https://doi.org/10.3390/v17020224
APA StyleRibeiro, M. d. O., Arruda, M. B., Calazans, A. R., Frederico, A. V., Brito, A. F., Barreto, B. V. d. S., Brandão, É. M. d. V., Athayde, H., Nascimento, K. C. S., Souza, L. P. d. B. O., Cardoso, P. H., Guimarães, P. L. d. S., Costa, V. D. d., Silva, C. A. d. C., Soares, A. M., Iole, J., Louzada, G., Filho, L. A., & Alvarez, P. (2025). Detecting Arboviruses Through Screening Asymptomatic Blood Donors in Rio de Janeiro/Brazil During a Dengue Outbreak. Viruses, 17(2), 224. https://doi.org/10.3390/v17020224






