Ad6-Based GM-CSF Expressing Vector Displays Oncolytic and Immunostimulatory Effects in an Immunocompetent Syrian Hamster Model of Cholangiocarcinoma
Abstract
1. Introduction
2. Materials and Methods
2.1. Viruses
2.2. Cells Lines
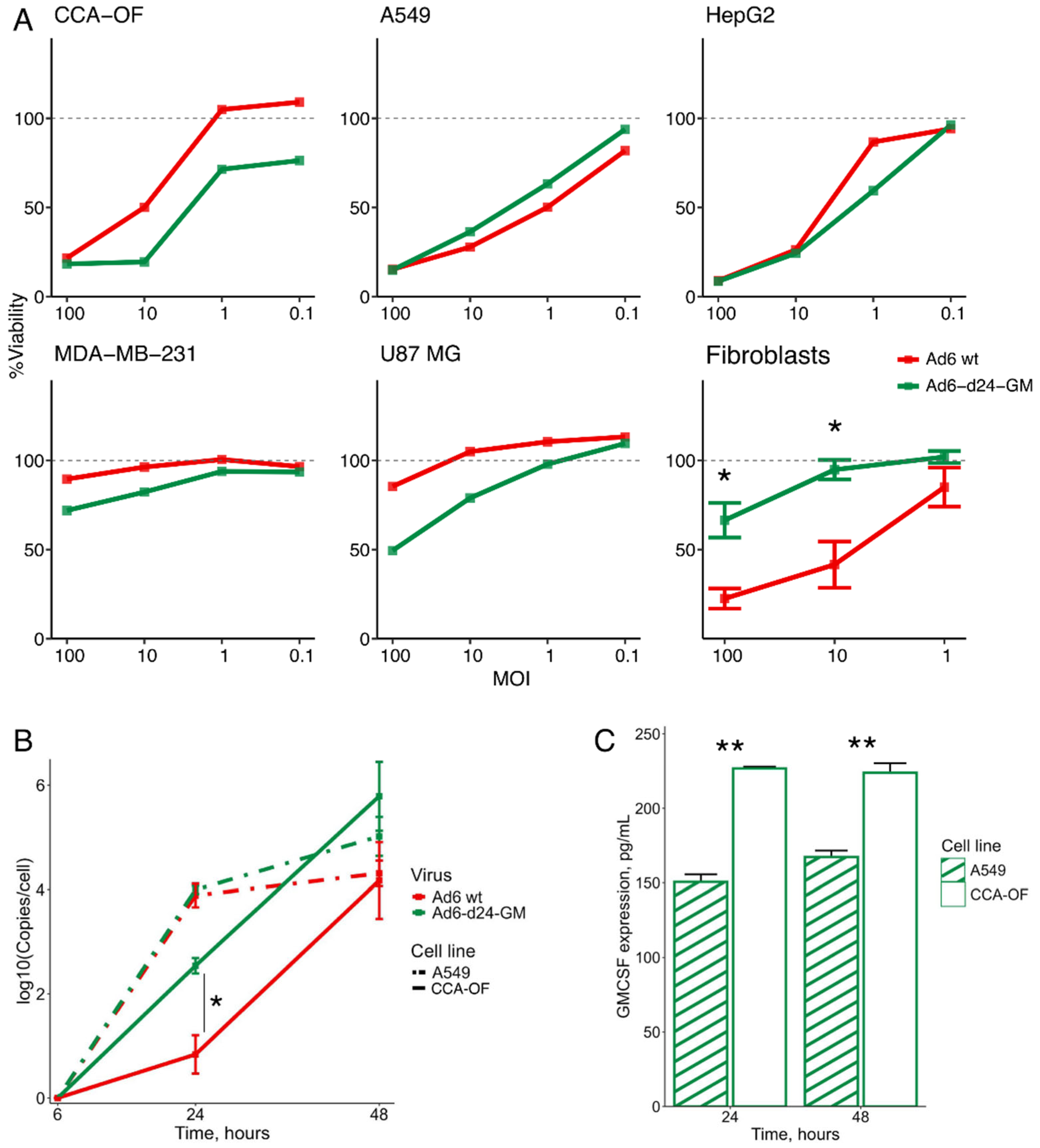
2.3. Cell Viability Assay
2.4. GM-CSF Expression
2.5. Viral Replication Study
2.6. Animals
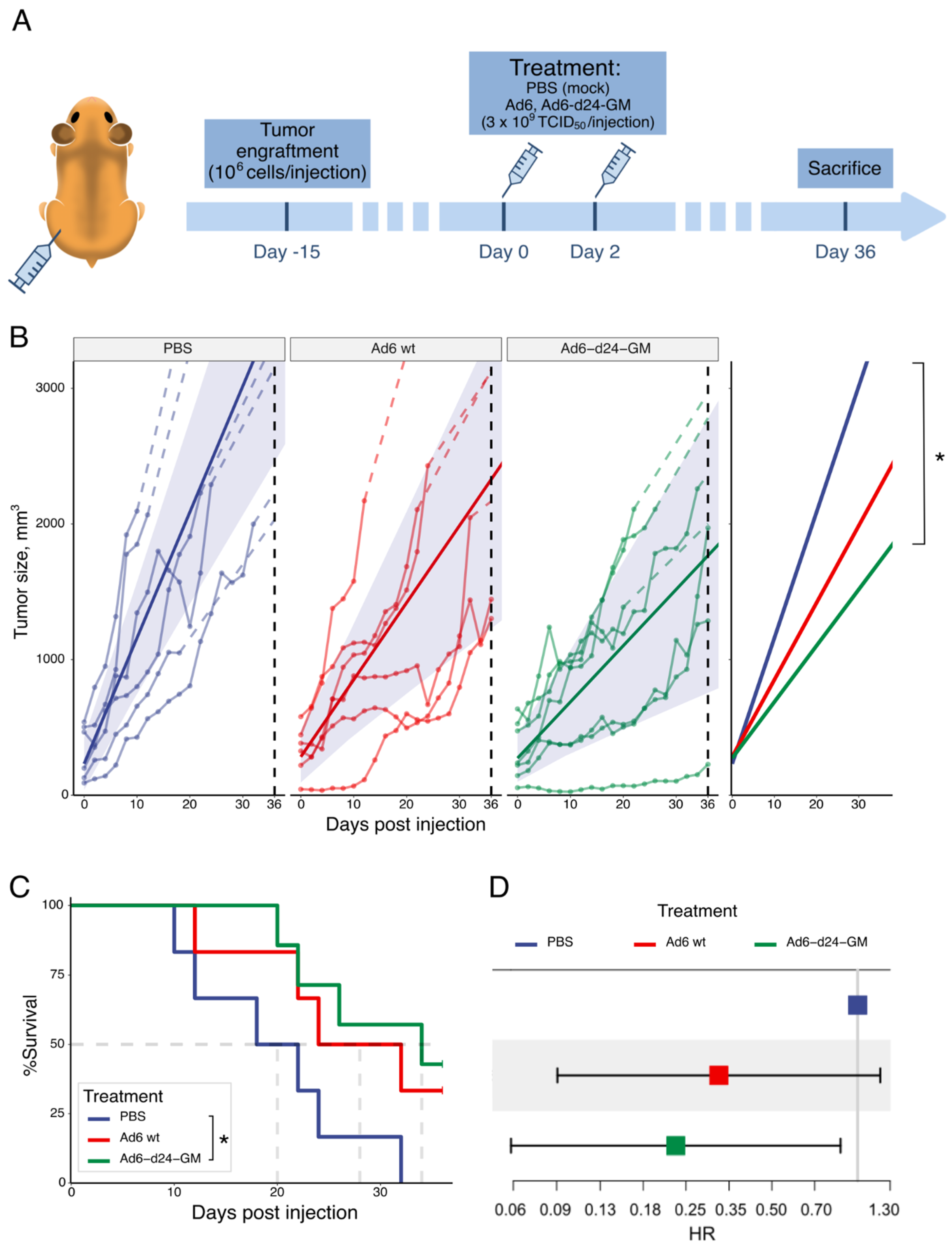
2.7. In Vivo Study
2.8. Viral Biodistribution
2.9. Histopathological Analysis
- Adenovirus (EMD Millipore Corp., #4034661, Temecula, CA, USA);
- Epithelial CK19 (Abcam, #ab220193, Waltham, MA, USA);
- Immune response (CD4 (Elabscience, #E-AB-F1105A, Houston, TX, USA), CD8a (Cloud-Clone Corp., #PAB099Ra01, Wuhan, Hubei, China), CD68 (Affinity, #DF7518, Buckingham, UK)).
2.10. Data Analysis
3. Results
3.1. Characterization of Ad6-d24-GM In Vitro
3.2. Ad6-d24-GM Suppressed Tumor Growth and Prolonged Survival in a Hamster Syngeneic Subcutaneous Tumor Model
3.3. Biodistribution and Safety
3.4. Treatment with Ad6-d24-GM Promotes T-Lymphocyte and CD68+ Macrophage Infiltration
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Turgeon, M.K.; Maithel, S.K. Cholangiocarcinoma: A Site-Specific Update on the Current State of Surgical Management and Multi-Modality Therapy. Chin. Clin. Oncol. 2020, 9, 4. [Google Scholar] [CrossRef]
- Manthopoulou, E.; Ramai, D.; Dhar, J.; Samanta, J.; Ioannou, A.; Lusina, E.; Sacco, R.; Facciorusso, A. Cholangiocarcinoma in the Era of Immunotherapy. Vaccines 2023, 11, 1062. [Google Scholar] [CrossRef] [PubMed]
- Uchio, E.M.; Lamm, D.L.; Shore, N.D.; Kamat, A.M.; Tyson, M.; Tran, B.; Anderson, P.; Grandi, P.; Burke, J.M. A Phase 3, Single-Arm Study of CG0070 in Subjects with Nonmuscle Invasive Bladder Cancer (NMIBC) Unresponsive to Bacillus Calmette-Guerin (BCG). J. Clin. Oncol. 2022, 40, TPS598. [Google Scholar] [CrossRef]
- Nassiri, F.; Patil, V.; Yefet, L.S.; Singh, O.; Liu, J.; Dang, R.M.A.; Yamaguchi, T.N.; Daras, M.; Cloughesy, T.F.; Colman, H.; et al. Oncolytic DNX-2401 Virotherapy plus Pembrolizumab in Recurrent Glioblastoma: A Phase 1/2 Trial. Nat. Med. 2023, 29, 1370–1378. [Google Scholar] [CrossRef]
- Shah, M.A.; Eads, J.R.; Sarkar, S.; Khan, S.; Sharaiha, R.; Carr-Locke, D.; Chang, L.; Ginsberg, G.; DiCicco, L.; Garcia-Marcano, L.; et al. Phase II Study of Telomelysin (OBP-301) in Combination with Pembrolizumab in Gastroesophageal (GEA) Adenocarcinoma. J. Clin. Oncol. 2023, 41, 4052. [Google Scholar] [CrossRef]
- Musher, B.L.; Rowinsky, E.K.; Smaglo, B.G.; Abidi, W.; Othman, M.; Patel, K.; Jawaid, S.; Jing, J.; Brisco, A.; Leen, A.M.; et al. LOAd703, an Oncolytic Virus-Based Immunostimulatory Gene Therapy, Combined with Chemotherapy for Unresectable or Metastatic Pancreatic Cancer (LOKON001): Results from Arm 1 of a Non-Randomised, Single-Centre, Phase 1/2 Study. Lancet Oncol. 2024, 25, 488–500. [Google Scholar] [CrossRef] [PubMed]
- Naing, A.; Khalil, D.; Rosen, O.; Camidge, D.R.; Lillie, T.; Ji, R.-R.; Stacey, A.; Thomas, M.; Rosen, L. First-in-Human Clinical Outcomes with NG-350A, an Anti-CD40 Expressing Tumor-Selective Vector Designed to Remodel Immunosuppressive Tumor Microenvironments. J. Immunother. Cancer 2024, 12, e010016. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Chen, Y.; Makhija, S.; Lu, B.; Wang, M.; Rivera, A.; Yamamoto, M.; Wang, S.; Siegal, G.; Curiel, D.; et al. Survivin Promoter-Based Conditionally Replicative Adenoviruses Target Cholangiocarcinoma. Int. J. Oncol. 2006, 29, 1319–1329. [Google Scholar] [CrossRef]
- Kojima, Y.; Honda, K.; Hamada, H.; Kobayashi, N. Oncolytic Gene Therapy Combined with Double Suicide Genes for Human Bile Duct Cancer in Nude Mouse Models. J. Surg. Res. 2009, 157, e63–e70. [Google Scholar] [CrossRef]
- Wang, J.; Zhu, Y.; Chen, Y.; Huang, Y.; Guo, Q.; Wang, Y.; Chen, A.; Zhou, Y.; Xu, L.; Wang, L.; et al. Three-in-One Oncolytic Adenovirus System Initiates a Synergetic Photodynamic Immunotherapy in Immune-Suppressive Cholangiocarcinoma. Small 2023, 19, e2207668. [Google Scholar] [CrossRef]
- Mennechet, F.J.D.; Paris, O.; Ouoba, A.R.; Salazar Arenas, S.; Sirima, S.B.; Takoudjou Dzomo, G.R.; Diarra, A.; Traore, I.T.; Kania, D.; Eichholz, K.; et al. A Review of 65 Years of Human Adenovirus Seroprevalence. Expert Rev. Vaccines 2019, 18, 597–613. [Google Scholar] [CrossRef] [PubMed]
- Weaver, E.A.; Hillestad, M.L.; Khare, R.; Palmer, D.; Ng, P.; Barry, M.A. Characterization of Species C Human Adenovirus Serotype 6 (Ad6). Virology 2011, 412, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Romanenko, M.; Osipov, I.; Netesov, S.V.; Davydova, J. Adenovirus Type 6: Subtle Structural Distinctions from Adenovirus Type 5 Result in Essential Differences in Properties and Perspectives for Gene Therapy. Pharmaceutics 2021, 13, 1641. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Tian, J.; Smith, J.S.; Byrnes, A.P. Clearance of Adenovirus by Kupffer Cells Is Mediated by Scavenger Receptors, Natural Antibodies, and Complement. J. Virol. 2008, 82, 11705–11713. [Google Scholar] [CrossRef]
- Khare, R.; May, S.M.; Vetrini, F.; Weaver, E.A.; Palmer, D.; Rosewell, A.; Grove, N.; Ng, P.; Barry, M.A. Generation of a Kupffer Cell-Evading Adenovirus for Systemic and Liver-Directed Gene Transfer. Mol. Ther. 2011, 19, 1254–1262. [Google Scholar] [CrossRef]
- Romanenko, M.V.; Dolgova, E.V.; Osipov, I.D.; Ritter, G.S.; Sizova, M.S.; Proskurina, A.S.; Efremov, Y.R.; Bayborodoin, S.I.; Potter, E.A.; Tatanov, O.S.; et al. Oncolytic Effect of Adenoviruses Serotypes 5 and 6 Against U87 Glioblastoma Cancer Stem Cells. Anticancer Res. 2019, 39, 6073–6086. [Google Scholar] [CrossRef]
- Osipov, I.D.; Vasikhovskaia, V.A.; Zabelina, D.S.; Kutseikin, S.S.; Grazhdantseva, A.A.; Kochneva, G.V.; Davydova, J.; Netesov, S.V.; Romanenko, M.V. Development of Oncolytic Vectors Based on Human Adenovirus Type 6 for Cancer Treatment. Viruses 2023, 15, 182. [Google Scholar] [CrossRef]
- Nguyen, T.V.; Heller, G.J.; Barry, M.E.; Crosby, C.M.; Turner, M.A.; Barry, M.A. Evaluation of Polymer Shielding for Adenovirus Serotype 6 (Ad6) for Systemic Virotherapy against Human Prostate Cancers. Mol. Ther.-Oncolytics 2016, 3, 15021. [Google Scholar] [CrossRef]
- Nguyen, T.V.; Crosby, C.M.; Heller, G.J.; Mendel, Z.I.; Barry, M.E.; Barry, M.A. Oncolytic Adenovirus Ad657 for Systemic Virotherapy against Prostate Cancer. Oncolytic Virother. 2018, 7, 43–51. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, X.; Newman, K.; Liu, X. MicroRNA Regulation of Oncolytic Adenovirus 6 for Selective Treatment of Castration-Resistant Prostate Cancer. Mol. Cancer Ther. 2012, 11, 2410–2418. [Google Scholar] [CrossRef]
- Cress, D.; Engel, B.; Santiago-Cardona, P. The Retinoblastoma Protein: A Master Tumor Suppressor Acts as a Link between Cell Cycle and Cell Adhesion. Cell Health Cytoskelet. 2014, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Urdinguio, R.G.; Fernandez, A.F.; Moncada-Pazos, A.; Huidobro, C.; Rodriguez, R.M.; Ferrero, C.; Martinez-Camblor, P.; Obaya, A.J.; Bernal, T.; Parra-Blanco, A.; et al. Immune-Dependent and Independent Antitumor Activity of GM-CSF Aberrantly Expressed by Mouse and Human Colorectal Tumors. Cancer Res. 2013, 73, 395–405. [Google Scholar] [CrossRef]
- Kalsi, S.; Galenkamp, A.L.; Singh, R.; Khosla, A.A.; McGranaghan, P.; Cintolo-Gonzalez, J. Talimogene Laherparepvec (T-VEC) and Emerging Intralesional Immunotherapies for Metastatic Melanoma: A Review. Curr. Oncol. Rep. 2024, 26, 1651–1663. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Gupta, R.; Petrik, S.; Laiko, M.; Leatherman, J.M.; Asquith, J.M.; Daphtary, M.M.; Garrett-Mayer, E.; Davidson, N.E.; Hirt, K.; et al. A Feasibility Study of Cyclophosphamide, Trastuzumab, and an Allogeneic GM-CSF–Secreting Breast Tumor Vaccine for HER2+ Metastatic Breast Cancer. Cancer Immunol. Res. 2014, 2, 949–961. [Google Scholar] [CrossRef] [PubMed]
- Koodie, L.; Robertson, M.G.; Chandrashekar, M.; Ruth, G.; Dunning, M.; Bianco, R.W.; Davydova, J. Rodents Versus Pig Model for Assessing the Performance of Serotype Chimeric Ad5/3 Oncolytic Adenoviruses. Cancers 2019, 11, 198. [Google Scholar] [CrossRef]
- Wold, W.S.M.; Toth, K. Syrian Hamster as an Animal Model to Study Oncolytic Adenoviruses and to Evaluate the Efficacy of Antiviral Compounds. Adv. Cancer Res. 2012, 115, 69–92. [Google Scholar]
- Prince, G.A.; Porter, D.D.; Jenson, A.B.; Horswood, R.L.; Chanock, R.M.; Ginsberg, H.S. Pathogenesis of Adenovirus Type 5 Pneumonia in Cotton Rats (Sigmodon Hispidus). J. Virol. 1993, 67, 101–111. [Google Scholar] [CrossRef]
- Thomas, M.A.; Spencer, J.F.; La Regina, M.C.; Dhar, D.; Tollefson, A.E.; Toth, K.; Wold, W.S.M. Syrian Hamster as a Permissive Immunocompetent Animal Model for the Study of Oncolytic Adenovirus Vectors. Cancer Res. 2006, 66, 1270–1276. [Google Scholar] [CrossRef]
- Jia, Y.; Wang, Y.; Dunmall, L.S.C.; Lemoine, N.R.; Wang, P.; Wang, Y. Syrian Hamster as an Ideal Animal Model for Evaluation of Cancer Immunotherapy. Front. Immunol. 2023, 14, 1126969. [Google Scholar] [CrossRef]
- Phillips, L.M.; Li, S.; Gumin, J.; Daou, M.; Ledbetter, D.; Yang, J.; Singh, S.; Parker Kerrigan, B.C.; Hossain, A.; Yuan, Y.; et al. An Immune-Competent, Replication-Permissive Syrian Hamster Glioma Model for Evaluating Delta-24-RGD Oncolytic Adenovirus. Neuro. Oncol. 2021, 23, 1911–1921. [Google Scholar] [CrossRef]
- Cervera-Carrascon, V.; Quixabeira, D.C.A.; Havunen, R.; Santos, J.M.; Kutvonen, E.; Clubb, J.H.A.; Siurala, M.; Heiniö, C.; Zafar, S.; Koivula, T.; et al. Comparison of Clinically Relevant Oncolytic Virus Platforms for Enhancing T Cell Therapy of Solid Tumors. Mol. Ther.-Oncolytics 2020, 17, 47–60. [Google Scholar] [CrossRef]
- Nakano, K.; Todo, T.; Zhao, G.; Yamaguchi, K.; Kuroki, S.; Cohen, J.B.; Glorioso, J.C.; Tanaka, M. Enhanced Efficacy of Conditionally Replicating Herpes Simplex Virus (G207) Combined with 5-fluorouracil and Surgical Resection in Peritoneal Cancer Dissemination Models. J. Gene Med. 2005, 7, 638–648. [Google Scholar] [CrossRef] [PubMed]
- Mordvinov, V.A.; Minkova, G.A.; Kovner, A.V.; Ponomarev, D.V.; Lvova, M.N.; Zaparina, O.; Romanenko, S.A.; Shilov, A.G.; Pakharukova, M.Y. A Tumorigenic Cell Line Derived from a Hamster Cholangiocarcinoma Associated with Opisthorchis Felineus Liver Fluke Infection. Life Sci. 2021, 277, 119494. [Google Scholar] [CrossRef] [PubMed]
- Kovner, A.; Zaparina, O.; Kapushchak, Y.; Minkova, G.; Mordvinov, V.; Pakharukova, M. Jagged-1/Notch Pathway and Key Transient Markers Involved in Biliary Fibrosis during Opisthorchis Felineus Infection. Trop. Med. Infect. Dis. 2022, 7, 364. [Google Scholar] [CrossRef] [PubMed]
- Kovner, A.V.; Pakharukova, M.Y.; Maksimova, G.A.; Mordvinov, V.A. Characteristics of Liver Fibrosis Associated with Chronic Opisthorchis Felineus Infection in Syrian Hamsters and Humans. Exp. Mol. Pathol. 2019, 110, 104274. [Google Scholar] [CrossRef]
- Kapushchak, Y.K.; Zaparina, O.G.; Mordvinov, V.A.; Pakharukova, M.Y. Time-Dependent Renal Pathologies Associated with the Liver Fluke Infection, Opisthorchiasis Felinea. Acta Trop. 2022, 228, 106282. [Google Scholar] [CrossRef]
- R Core Team. R Core Team R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023. [Google Scholar]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using Lme4. J. Stat. Softw. 2015, 67, 48. [Google Scholar] [CrossRef]
- Kuznetsova, A.; Brockhoff, P.B.; Christensen, R.H.B. LmerTest Package: Tests in Linear Mixed Effects Models. J. Stat. Softw. 2017, 82, 13. [Google Scholar] [CrossRef]
- Washington, I.M.; Van Hoosier, G. Clinical Biochemistry and Hematology. In The Laboratory Rabbit, Guinea Pig, Hamster, and Other Rodents; Elsevier: Amsterdam, The Netherlands, 2012; pp. 57–116. [Google Scholar]
- Marin, J.J.G.; Lozano, E.; Herraez, E.; Asensio, M.; Di Giacomo, S.; Romero, M.R.; Briz, O.; Serrano, M.A.; Efferth, T.; Macias, R.I.R. Chemoresistance and Chemosensitization in Cholangiocarcinoma. Biochim. Biophys. Acta-Mol. Basis Dis. 2018, 1864, 1444–1453. [Google Scholar] [CrossRef]
- Diggs, L.P.; Ruf, B.; Ma, C.; Heinrich, B.; Cui, L.; Zhang, Q.; McVey, J.C.; Wabitsch, S.; Heinrich, S.; Rosato, U.; et al. CD40-Mediated Immune Cell Activation Enhances Response to Anti-PD-1 in Murine Intrahepatic Cholangiocarcinoma. J. Hepatol. 2021, 74, 1145–1154. [Google Scholar] [CrossRef]
- Zhou, G.; Sprengers, D.; Mancham, S.; Erkens, R.; Boor, P.P.C.; van Beek, A.A.; Doukas, M.; Noordam, L.; Campos Carrascosa, L.; de Ruiter, V.; et al. Reduction of Immunosuppressive Tumor Microenvironment in Cholangiocarcinoma by Ex Vivo Targeting Immune Checkpoint Molecules. J. Hepatol. 2019, 71, 753–762. [Google Scholar] [CrossRef] [PubMed]
- Supimon, K.; Sangsuwannukul, T.; Sujjitjoon, J.; Phanthaphol, N.; Chieochansin, T.; Poungvarin, N.; Wongkham, S.; Junking, M.; Yenchitsomanus, P. Anti-Mucin 1 Chimeric Antigen Receptor T Cells for Adoptive T Cell Therapy of Cholangiocarcinoma. Sci. Rep. 2021, 11, 6276. [Google Scholar] [CrossRef] [PubMed]
- Solinas, C.; Pusole, G.; Demurtas, L.; Puzzoni, M.; Mascia, R.; Morgan, G.; Giampieri, R.; Scartozzi, M. Tumor Infiltrating Lymphocytes in Gastrointestinal Tumors: Controversies and Future Clinical Implications. Crit. Rev. Oncol. Hematol. 2017, 110, 106–116. [Google Scholar] [CrossRef]
- Nagi, P.; Vickers, S.M.; Davydova, J.; Adachi, Y.; Takayama, K.; Barker, S.; Krasnykh, V.; Curiel, D.T.; Yamamoto, M. Development of a Therapeutic Adenoviral Vector for Cholangiocarcinoma Combining Tumor-Restricted Gene Expression and Infectivity Enhancement. J. Gastrointest. Surg. 2003, 7, 364–371. [Google Scholar] [CrossRef]
- Yamamoto, K.; Katayose, Y.; Suzuki, M.; Unno, M.; Sasaki, T.; Mizuma, M.; Shiraso, S.; Ohtuka, H.; Cowan, K.H.; Seth, P.; et al. Adenovirus Expressing P27KIP1 Induces Apoptosis against Cholangiocarcinoma Cells by Triggering Fas Ligand on the Cell Surface. Hepatogastroenterology 2003, 50, 1847–1853. [Google Scholar] [PubMed]
- Chen, C.Y.; Weaver, E.A.; Khare, R.; May, S.M.; Barry, M.A. Mining the Adenovirus Virome for Oncolytics against Multiple Solid Tumor Types. Cancer Gene Ther. 2011, 18, 744–750. [Google Scholar] [CrossRef]
- Shashkova, E.V.; May, S.M.; Barry, M.A. Characterization of Human Adenovirus Serotypes 5, 6, 11, and 35 as Anticancer Agents. Virology 2009, 394, 311–320. [Google Scholar] [CrossRef]
- Capone, S.; Meola, A.; Ercole, B.B.; Vitelli, A.; Pezzanera, M.; Ruggeri, L.; Davies, M.E.; Tafi, R.; Santini, C.; Luzzago, A.; et al. A Novel Adenovirus Type 6 (Ad6)-Based Hepatitis C Virus Vector That Overcomes Preexisting Anti-Ad5 Immunity and Induces Potent and Broad Cellular Immune Responses in Rhesus Macaques. J. Virol. 2006, 80, 1688–1699. [Google Scholar] [CrossRef]
- Fueyo, J.; Gomez-Manzano, C.; Alemany, R.; Lee, P.S.; McDonnell, T.J.; Mitlianga, P.; Shi, Y.-X.; Levin, V.A.; Yung, W.K.A.; Kyritsis, A.P. A Mutant Oncolytic Adenovirus Targeting the Rb Pathway Produces Anti-Glioma Effect In Vivo. Oncogene 2000, 19, 2–12. [Google Scholar] [CrossRef]
- Kang, Y.K.; Kim, W.H.; Jang, J.J. Expression of G1-S Modulators (P53, P16, P27, Cyclin D1, Rb) and Smad4/Dpc4 in Intrahepatic Cholangiocarcinoma. Hum. Pathol. 2002, 33, 877–883. [Google Scholar] [CrossRef]
- Boonmars, T.; Wu, Z.; Boonjaruspinyo, S.; Pinlaor, S.; Nagano, I.; Takahashi, Y.; Kaewsamut, B.; Yongvanit, P. Alterations of Gene Expression of RB Pathway in Opisthorchis Viverrini Infection-Induced Cholangiocarcinoma. Parasitol. Res. 2009, 105, 1273–1281. [Google Scholar] [CrossRef]
- van Putten, E.H.P.; Kleijn, A.; van Beusechem, V.W.; Noske, D.; Lamers, C.H.J.; de Goede, A.L.; Idema, S.; Hoefnagel, D.; Kloezeman, J.J.; Fueyo, J.; et al. Convection Enhanced Delivery of the Oncolytic Adenovirus Delta24-RGD in Patients with Recurrent GBM: A Phase I Clinical Trial Including Correlative Studies. Clin. Cancer Res. 2022, 28, 1572–1585. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Dmitriev, I.P.; Saddekni, S.; Kashentseva, E.A.; Harris, R.D.; Aurigemma, R.; Bae, S.; Singh, K.P.; Siegal, G.P.; Curiel, D.T.; et al. A Phase I Clinical Trial of Ad5/3-Δ24, a Novel Serotype-Chimeric, Infectivity-Enhanced, Conditionally-Replicative Adenovirus (CRAd), in Patients with Recurrent Ovarian Cancer. Gynecol. Oncol. 2013, 130, 518–524. [Google Scholar] [CrossRef]
- Cho, S.-A.; Park, J.-H.; Seok, S.-H.; Juhn, J.-H.; Kim, S.-J.; Ji, H.-J.; Choo, Y.-S.; Park, J.-H. Effect of Granulocyte Macrophage-Colony Stimulating Factor (GM-CSF) on 5-FU-Induced Ulcerative Mucositis in Hamster Buccal Pouches. Exp. Toxicol. Pathol. 2006, 57, 321–328. [Google Scholar] [CrossRef]
- Kumar, A.; Taghi Khani, A.; Sanchez Ortiz, A.; Swaminathan, S. GM-CSF: A Double-Edged Sword in Cancer Immunotherapy. Front. Immunol. 2022, 13, 901277. [Google Scholar] [CrossRef]
- Ranki, T.; Pesonen, S.; Hemminki, A.; Partanen, K.; Kairemo, K.; Alanko, T.; Lundin, J.; Linder, N.; Turkki, R.; Ristimäki, A.; et al. Phase I Study with ONCOS-102 for the Treatment of Solid Tumors—An Evaluation of Clinical Response and Exploratory Analyses of Immune Markers. J. Immunother. Cancer 2016, 4, 17. [Google Scholar] [CrossRef]
- van den Bossche, W.B.L.; Kleijn, A.; Teunissen, C.E.; Voerman, J.S.A.; Teodosio, C.; Noske, D.P.; van Dongen, J.J.M.; Dirven, C.M.F.; Lamfers, M.L.M. Oncolytic Virotherapy in Glioblastoma Patients Induces a Tumor Macrophage Phenotypic Shift Leading to an Altered Glioblastoma Microenvironment. Neuro. Oncol. 2018, 20, 1494–1504. [Google Scholar] [CrossRef]
- Packiam, V.T.; Lamm, D.L.; Barocas, D.A.; Trainer, A.; Fand, B.; Davis, R.L.; Clark, W.; Kroeger, M.; Dumbadze, I.; Chamie, K.; et al. An Open Label, Single-Arm, Phase II Multicenter Study of the Safety and Efficacy of CG0070 Oncolytic Vector Regimen in Patients with BCG-Unresponsive Non–Muscle-Invasive Bladder Cancer: Interim Results. Urol. Oncol. Semin. Orig. Investig. 2018, 36, 440–447. [Google Scholar] [CrossRef]
- Machiels, J.-P.; Salazar, R.; Rottey, S.; Duran, I.; Dirix, L.; Geboes, K.; Wilkinson-Blanc, C.; Pover, G.; Alvis, S.; Champion, B.; et al. A Phase 1 Dose Escalation Study of the Oncolytic Adenovirus Enadenotucirev, Administered Intravenously to Patients with Epithelial Solid Tumors (EVOLVE). J. Immunother. Cancer 2019, 7, 20. [Google Scholar] [CrossRef]
- Garcia-Carbonero, R.; Bazan-Peregrino, M.; Gil-Martín, M.; Álvarez, R.; Macarulla, T.; Riesco-Martinez, M.C.; Verdaguer, H.; Guillén-Ponce, C.; Farrera-Sal, M.; Moreno, R.; et al. Phase I, Multicenter, Open-Label Study of Intravenous VCN-01 Oncolytic Adenovirus with or without Nab-Paclitaxel plus Gemcitabine in Patients with Advanced Solid Tumors. J. Immunother. Cancer 2022, 10, e003255. [Google Scholar] [CrossRef]
- LaRocca, C.J.; Han, J.; Gavrikova, T.; Armstrong, L.; Oliveira, A.R.; Shanley, R.; Vickers, S.M.; Yamamoto, M.; Davydova, J. Oncolytic Adenovirus Expressing Interferon Alpha in a Syngeneic Syrian Hamster Model for the Treatment of Pancreatic Cancer. Surgery 2015, 157, 888–898. [Google Scholar] [CrossRef] [PubMed]
- Bründler, M.-A.; Rodriguez-Baez, N.; Jaffe, R.; Weinberg, A.G.; Rogers, B.B. Adenovirus Ascending Cholangiohepatitis. Pediatr. Dev. Pathol. 2003, 6, 156–159. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zabelina, D.S.; Osipov, I.D.; Maslov, D.E.; Kovner, A.V.; Vasikhovskaia, V.A.; Demina, D.S.; Romanov, S.E.; Shishkina, E.V.; Davydova, J.; Netesov, S.V.; et al. Ad6-Based GM-CSF Expressing Vector Displays Oncolytic and Immunostimulatory Effects in an Immunocompetent Syrian Hamster Model of Cholangiocarcinoma. Viruses 2025, 17, 162. https://doi.org/10.3390/v17020162
Zabelina DS, Osipov ID, Maslov DE, Kovner AV, Vasikhovskaia VA, Demina DS, Romanov SE, Shishkina EV, Davydova J, Netesov SV, et al. Ad6-Based GM-CSF Expressing Vector Displays Oncolytic and Immunostimulatory Effects in an Immunocompetent Syrian Hamster Model of Cholangiocarcinoma. Viruses. 2025; 17(2):162. https://doi.org/10.3390/v17020162
Chicago/Turabian StyleZabelina, Daria S., Ivan D. Osipov, Denis E. Maslov, Anna V. Kovner, Valeriia A. Vasikhovskaia, Diana S. Demina, Stanislav E. Romanov, Ekaterina V. Shishkina, Julia Davydova, Sergey V. Netesov, and et al. 2025. "Ad6-Based GM-CSF Expressing Vector Displays Oncolytic and Immunostimulatory Effects in an Immunocompetent Syrian Hamster Model of Cholangiocarcinoma" Viruses 17, no. 2: 162. https://doi.org/10.3390/v17020162
APA StyleZabelina, D. S., Osipov, I. D., Maslov, D. E., Kovner, A. V., Vasikhovskaia, V. A., Demina, D. S., Romanov, S. E., Shishkina, E. V., Davydova, J., Netesov, S. V., & Romanenko, M. V. (2025). Ad6-Based GM-CSF Expressing Vector Displays Oncolytic and Immunostimulatory Effects in an Immunocompetent Syrian Hamster Model of Cholangiocarcinoma. Viruses, 17(2), 162. https://doi.org/10.3390/v17020162





