Primordial Biochemicals Within Coacervate-Like Droplets and the Origins of Life
Abstract
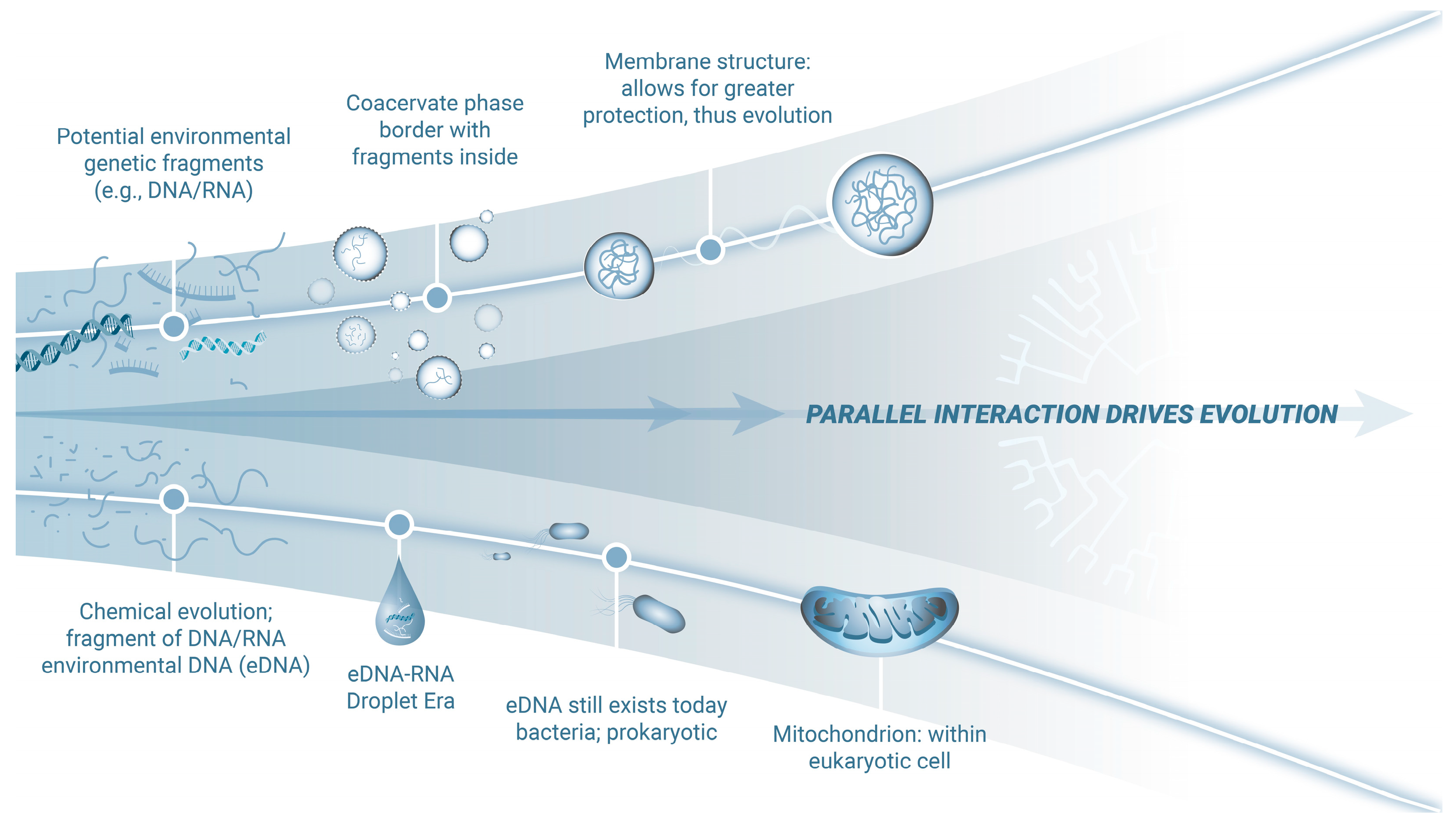
1. Environmental DNA and the Origins of Life
2. Environmental DNA and the Emergence of Viruses
3. Environmental DNA and the Evolution of Bacteria
4. Facilitating Genetic Evolution: The Droplet Era
5. Evolution of Chemical Communication
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Stefano, G.B.; Kream, R.M. Prebiotic Formation of Protoalkaloids within Alkaline Oceanic Hydrothermal Vents in the Hadean Seafloor as a Prerequisite for Evolutionary Biodiversity. Med. Sci. Monit. 2020, 26, e928415. [Google Scholar] [CrossRef] [PubMed]
- Whitmore, L.; McCauley, M.; Farrell, J.A.; Stammnitz, M.R.; Koda, S.A.; Mashkour, N.; Summers, V.; Osborne, T.; Whilde, J.; Duffy, D.J. Inadvertent human genomic bycatch and intentional capture raise beneficial applications and ethical concerns with environmental DNA. Nat. Ecol. Evol. 2023, 7, 873–888. [Google Scholar] [CrossRef]
- Duffy, D.J.; Martindale, M.Q. Perspectives on the expansion of human precision oncology and genomic approaches to sea turtle fibropapillomatosis. Commun. Biol. 2019, 2, 54. [Google Scholar] [CrossRef] [PubMed]
- Duffy, D.J.; Burkhalter, B. When is a lab animal not a lab animal? Lab. Anim. 2020, 49, 95–98. [Google Scholar] [CrossRef] [PubMed]
- Wessner, D.R. The Origin of Viruses. Nat. Educ. 2010, 3, 37–39. [Google Scholar]
- Koonin, E.V.; Martin, W. On the origin of genomes and cells within inorganic compartments. Trends Genet. 2005, 21, 647–654. [Google Scholar] [CrossRef]
- Iyer, L.M.; Koonin, E.V.; Leipe, D.D.; Aravind, L. Origin and evolution of the archaeo-eukaryotic primase superfamily and related palm-domain proteins: Structural insights and new members. Nucleic Acids Res. 2005, 33, 3875–3896. [Google Scholar] [CrossRef] [PubMed]
- George, J.; Halami, P.M. Presence of extracellular DNA & protein in biofilm formation by gentamicin-resistant Lactobacillus plantarum. Indian J. Med. Res. 2019, 149, 257–262. [Google Scholar] [CrossRef]
- Yang, K.; Wang, L.; Cao, X.; Gu, Z.; Zhao, G.; Ran, M.; Yan, Y.; Yan, J.; Xu, L.; Gao, C.; et al. The Origin, Function, Distribution, Quantification, and Research Advances of Extracellular DNA. Int. J. Mol. Sci. 2022, 23, 3690. [Google Scholar] [CrossRef]
- Atlante, A.; Valenti, D. Mitochondria Have Made a Long Evolutionary Path from Ancient Bacteria Immigrants within Eukaryotic Cells to Essential Cellular Hosts and Key Players in Human Health and Disease. Curr. Issues Mol. Biol. 2023, 45, 4451–4479. [Google Scholar] [CrossRef]
- Stefano, G.B.; Kream, R.M. Viruses Broaden the Definition of Life by Genomic Incorporation of Artificial Intelligence and Machine Learning Processes. Curr. Neuropharmacol. 2022, 20, 1888–1893. [Google Scholar] [CrossRef]
- Margulis, L.; Bermudes, D. Symbiosis as a mechanism of evolution: Status of cell symbiosis theory. Symbiosis 1985, 1, 101–124. [Google Scholar]
- Cutler, R.G. Evolutionary Biology of Senescence; Behnke, J.A., Finch, C.E., Moment, G.B., Eds.; Plenum Press: New York, NY, USA, 1978; 388p. [Google Scholar]
- Stefano, G.; Büttiker, P.; Weissenberger, S.; Esch, T.; Anders, M.; Raboch, J.; Kream, R.; Ptacek, R. Independent and sensory human mitochondrial functions reflecting symbiotic evolution. Front. Cell. Infect. Microbiol. 2023, 13, 1130197. [Google Scholar] [CrossRef] [PubMed]
- Stefano, G.B.; Kream, R.M. Mitochondrial DNA Heteroplasmy as an Informational Reservoir Dynamically Linked to Metabolic and Immunological Processes Associated with COVID-19 Neurological Disorders. Cell. Mol. Neurobiol. 2022, 42, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, A.; Bandyopadhaya, A.; Singh, V.K.; Kovacic, F.; Cha, S.; Oldham, W.M.; Tzika, A.A.; Rahme, L.G. The bacterial quorum sensing signal 2’-aminoacetophenone rewires immune cell bioenergetics through the Ppargc1a/Esrra axis to mediate tolerance to infection. eLife 2024, 13, RP97568. [Google Scholar] [CrossRef] [PubMed]
- Maurice, N.M.; Sadikot, R.T. Mitochondrial Dysfunction in Bacterial Infections. Pathogens 2023, 12, 1005. [Google Scholar] [CrossRef] [PubMed]
- Stefano, G.B. Antibiotics and Antiviral Agents Can Trigger Mitochondrial Dysfunction that Leads to Psychiatric Disorders. Mind Bull. Mind-Body Med. Res. 2023, 2, 8–10. [Google Scholar] [CrossRef]
- Stefano, G.B.; Samuel, J.; Kream, R.M. Antibiotics May Trigger Mitochondrial Dysfunction Inducing Psychiatric Disorders. Med. Sci. Monit. 2017, 23, 101–106. [Google Scholar] [CrossRef]
- Warstler, A.; Bean, J. Antimicrobial-Induced Cognitive Side Effects. Ment. Health Clin. 2016, 6, 207–214. [Google Scholar] [CrossRef]
- Jenkins, H.L.; Graham, R.; Porter, J.S.; Vieira, L.M.; De Almeida, A.C.S.; Hall, A.; O’Dea, A.; Coppard, S.E.; Waeschenbach, A. Unprecedented Frequency of Mitochondrial Introns in Colonial Bilaterians. Sci. Rep. 2022, 12, 10889. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Schon, K.R.; Elgar, G.; Orioli, A.; Tanguy, M.; Giess, A.; Tischkowitz, M.; Caulfield, M.J.; Chinnery, P.F. Nuclear-Embedded Mitochondrial DNA Sequences in 66,083 Human Genomes. Nature 2022, 611, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Karan, K.R.; Gu, W.; Klein, H.-U.; Sturm, G.; De Jager, P.L.; Bennett, D.A.; Hirano, M.; Picard, M.; Mills, R.E. Somatic Nuclear Mitochondrial DNA Insertions Are Prevalent in the Human Brain and Accumulate over Time in Fibroblasts. PLoS Biol. 2024, 22, e3002723. [Google Scholar] [CrossRef]
- van Swaay, D.; Tang, T.Y.; Mann, S.; de Mello, A. Microfluidic Formation of Membrane-Free Aqueous Coacervate Droplets in Water. Angew. Chem. Int. Ed. Engl. 2015, 54, 8398–8401. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, A.; Douglas, J.F.; Tirrell, M.; Karim, A. Manipulation of coacervate droplets with an electric field. Proc. Natl. Acad. Sci. USA 2022, 119, e2203483119. [Google Scholar] [CrossRef]
- Moulik, S.P.; Rakshit, A.K.; Pan, A.; Naskar, B. An Overview of Coacervates: The Special Disperse State of Amphiphilic and Polymeric Materials in Solution. Colloids Interfaces 2022, 6, 45. [Google Scholar] [CrossRef]
- Stefano, G.B. (Ed.) Conformational matching: A possible evolutionary force in the evolvement of signal systems. In CRC Handbook of Comparative Opioid and Related Neuropeptide Mechanisms; CRC Press Inc.: Boca Raton, FL, USA, 1986; Volume 2, pp. 271–277. [Google Scholar]
- Stefano, G.B. The evolvement of signal systems: Conformational matching a determining force stabilizing families of signal molecules. Comp. Biochem. Physiol. C 1988, 90, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Büttiker, P.; Boukherissa, A.; Weissenberger, S.; Ptacek, R.; Anders, M.; Raboch, J.; Stefano, G.B. Cognitive Impact of Neurotropic Pathogens: Investigating Molecular Mimicry through Computational Methods. Cell. Mol. Neurobiol. 2024, 44, 72. [Google Scholar] [CrossRef] [PubMed]
- Stefano, G.B.; Büttiker, P.; Weissenberger, S.; Anders, M.; Raboch, J.; Kream, R.M. Viruses May Be Redefined as Self-Replicating Entities: Expanding the Definition of Life. Mind-Bull. Mind-Body Med. Res. 2024, 3, 2–8. [Google Scholar] [CrossRef]
- Darwin, C. Origin of the Species; The Easton Press: Norwalk, CT, USA, 1976. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stefano, G.B.; Kream, R.M. Primordial Biochemicals Within Coacervate-Like Droplets and the Origins of Life. Viruses 2025, 17, 146. https://doi.org/10.3390/v17020146
Stefano GB, Kream RM. Primordial Biochemicals Within Coacervate-Like Droplets and the Origins of Life. Viruses. 2025; 17(2):146. https://doi.org/10.3390/v17020146
Chicago/Turabian StyleStefano, George B., and Richard M. Kream. 2025. "Primordial Biochemicals Within Coacervate-Like Droplets and the Origins of Life" Viruses 17, no. 2: 146. https://doi.org/10.3390/v17020146
APA StyleStefano, G. B., & Kream, R. M. (2025). Primordial Biochemicals Within Coacervate-Like Droplets and the Origins of Life. Viruses, 17(2), 146. https://doi.org/10.3390/v17020146






