Characterizing the Bat Virome of Vietnam: A Systematic Review of Viral Diversity and Zoonotic Potential
Abstract
1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Sources of Information and Search Strategy
2.3. Article Choice and Data Collection
2.4. Data Items
2.5. Study Risk of Bias Assessment
2.6. Effect Measures and Synthesis of Results
2.7. Reporting Bias and Certainty Assessment
3. Results
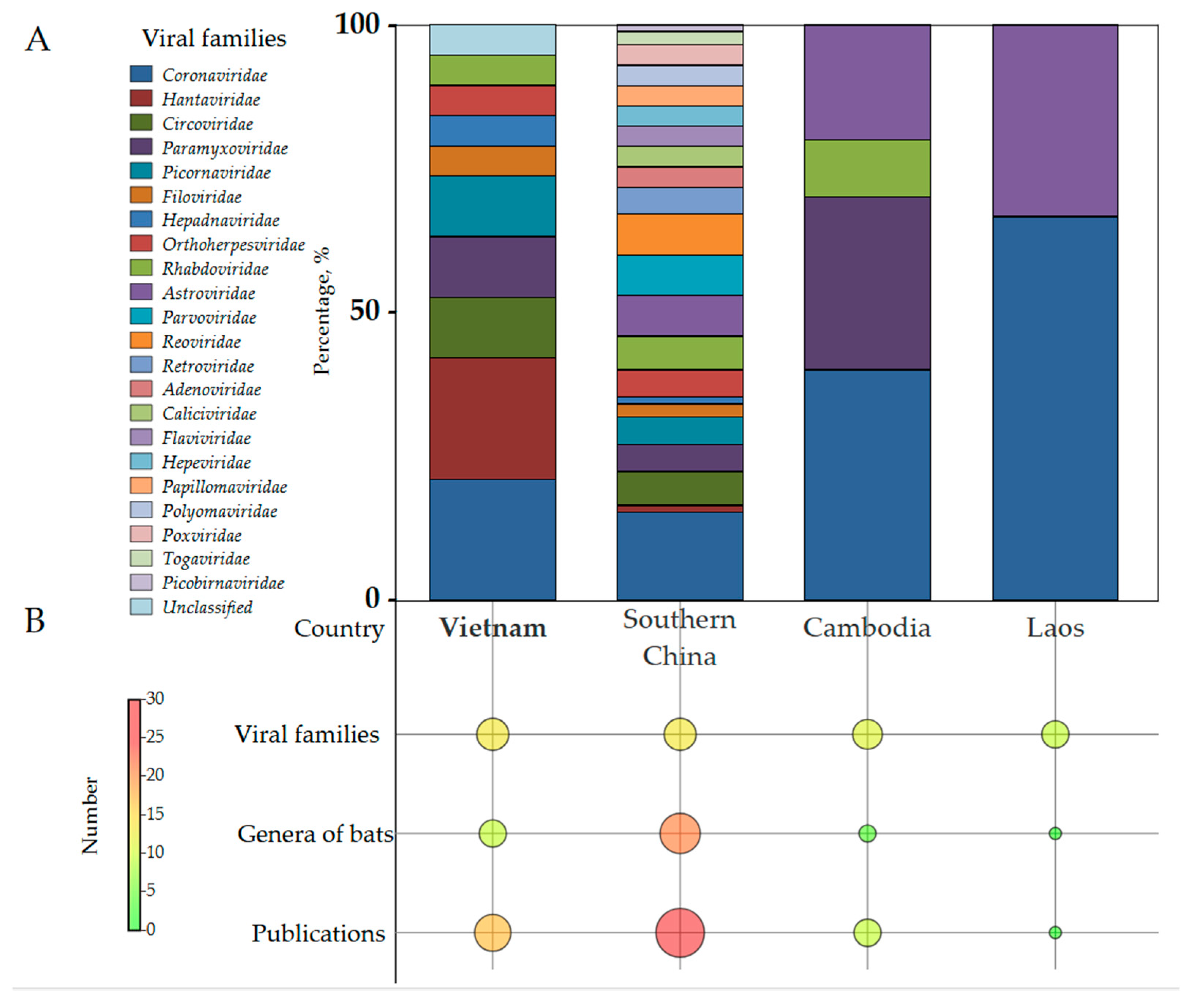
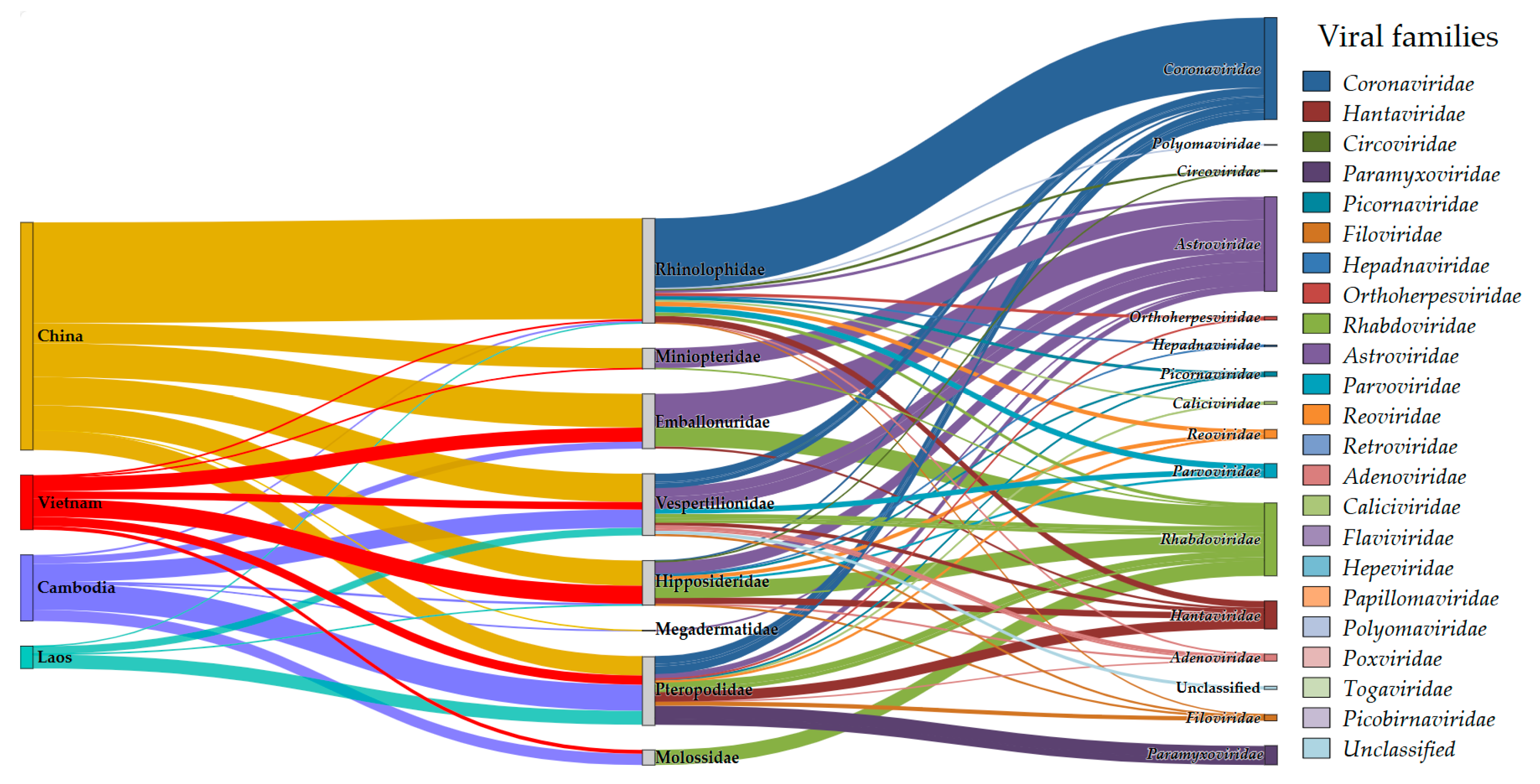
3.1. Bat Virome Composition Overview
3.2. High-Priority Viral Families with Pandemic Potential
3.2.1. Coronaviridae
3.2.2. Paramyxoviridae
3.2.3. Hantaviridae
3.2.4. Filoviridae
3.2.5. Flaviviridae
3.2.6. Poxviridae
3.2.7. Togaviridae
3.3. Viral Families with Moderate Pandemic Potential
3.3.1. Picornaviridae
3.3.2. Retroviridae
3.3.3. Adenoviridae
3.4. Viral Families with Low Pandemic Potential
3.4.1. Astroviridae
3.4.2. Rhabdoviridae
3.4.3. Parvoviridae
3.4.4. Spinareoviridae and Sedoreoviridae
3.4.5. Orthoherpesviridae (Herpesviridae)
3.4.6. Hepeviridae
3.4.7. Papillomaviridae
3.4.8. Polyomaviridae
3.4.9. Hepadnaviridae
3.5. Viral Families with No Demonstrated Pandemic Potential
3.5.1. Circoviridae
3.5.2. Caliciviridae
3.5.3. Other Taxonomic Groups
4. Summary
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Calisher, C.H.; Childs, J.E.; Field, H.E.; Holmes, K.V.; Schountz, T. Bats: Important Reservoir Hosts of Emerging Viruses. Clin. Microbiol. Rev. 2006, 19, 531–545. [Google Scholar] [CrossRef] [PubMed]
- Hayman, D.T.S. Bats as Viral Reservoirs. Annu. Rev. Virol. 2016, 3, 77–99. [Google Scholar] [CrossRef]
- Van Brussel, K.; Holmes, E.C. Zoonotic Disease and Virome Diversity in Bats. Curr. Opin. Virol. 2022, 52, 192–202. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Yang, L.; Ren, X.; He, G.; Zhang, J.; Yang, J.; Qian, Z.; Dong, J.; Sun, L.; Zhu, Y.; et al. Deciphering the Bat Virome Catalog to Better Understand the Ecological Diversity of Bat Viruses and the Bat Origin of Emerging Infectious Diseases. ISME J. 2016, 10, 609–620. [Google Scholar] [CrossRef]
- Hu, D.; Zhu, C.; Wang, Y.; Ai, L.; Yang, L.; Ye, F.; Ding, C.; Chen, J.; He, B.; Zhu, J.; et al. Virome Analysis for Identification of Novel Mammalian Viruses in Bats from Southeast China. Sci. Rep. 2017, 7, 10917. [Google Scholar] [CrossRef]
- Peng, C.; Zhang, D.; Li, C.; Li, Y.; Zhang, H.; Li, N.; Xiao, P. Rhinolophus Sinicus Virome Revealed Multiple Novel Mosquito-Borne Zoonotic Viruses. Front. Cell. Infect. Microbiol. 2022, 12, 960507. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Pan, Y.; Yang, L.; Yang, W.; Lv, K.; Luo, C.; Wang, J.; Kuang, G.; Wu, W.; Gou, Q.; et al. Individual Bat Virome Analysis Reveals Co-Infection and Spillover among Bats and Virus Zoonotic Potential. Nat. Commun. 2023, 14, 4079. [Google Scholar] [CrossRef]
- Li, Z.; Tang, C.; Li, Y.; Zhang, Y.; Wang, G.; Peng, R.; Huang, Y.; Hu, X.; Xin, H.; Cao, X.; et al. Virome Survey of the Bat, Rhinolophus Affinis, in Hainan Province, China. Microbes Infect. 2024, 26, 105331. [Google Scholar] [CrossRef]
- Cui, X.; Fan, K.; Liang, X.; Gong, W.; Chen, W.; He, B.; Chen, X.; Wang, H.; Wang, X.; Zhang, P.; et al. Virus Diversity, Wildlife-Domestic Animal Circulation and Potential Zoonotic Viruses of Small Mammals, Pangolins and Zoo Animals. Nat. Commun. 2023, 14, 2488. [Google Scholar] [CrossRef]
- Solari, S.; Baker, R.J. Mammal Species of the World: A Taxonomic and Geographic Reference. J. Mammal. 2007, 88, 824–830. [Google Scholar] [CrossRef]
- Kruskop Sergei, V. Bats Of Vietnam. In Биoразнooбразие Вьетнама, 2nd ed.; Joint Russian-Vietnamese Science and Technological Tropical Centre Zoological Museum of Moscow M.V. Lomonosov State University: Moscow, Russia, 2013; Volume 1, ISBN 978-5-87317-901-5. [Google Scholar]
- Corbet, G.B.; Hill, J.E. The Mammals of the Indomalayan Region: A Systematic Review; Natural History Museum Publishing: London, UK; Oxford University Press: Oxford, UK, 1992; ISBN 978-0-19-854693-1. [Google Scholar]
- Lim, V.-C.; Ramli, R.; Bhassu, S.; Wilson, J.-J. A Checklist of the Bats of Peninsular Malaysia and Progress towards a DNA Barcode Reference Library. PLoS ONE 2017, 12, e0179555. [Google Scholar] [CrossRef]
- List of Vietnamese Bats. Available online: https://zmmu.msu.ru/bats/science/fauna/vietnam/taxlist.htm (accessed on 15 July 2024).
- Zachos, F.E. D.E. Wilson and R.A. Mittermeier (Chief Editors): Handbook of the Mammals of the World. Vol. 9. Bats. Mamm. Biol. 2020, 100, 335. [Google Scholar] [CrossRef]
- Kuznetsov, G. Mammals of Coastal Islands of Vietnam: Zoogeographical and Ecological Aspects; Bonner Zoologische Monograp; Zoologisches Forschungsmuseum Alexander Koenig: Bonn, Germany, 2020. [Google Scholar]
- Smith, B.T.; McCormack, J.E.; Cuervo, A.M.; Hickerson, M.J.; Aleixo, A.; Cadena, C.D.; Pérez-Emán, J.; Burney, C.W.; Xie, X.; Harvey, M.G.; et al. The Drivers of Tropical Speciation. Nature 2014, 515, 406–409. [Google Scholar] [CrossRef] [PubMed]
- Kruskop, S.V.; Eger, J.L. A New Species of Tube-Nosed Bat Murina (Vespertilionidae, Chiroptera) from Vietnam. Acta 2008, 10, 213–220. [Google Scholar] [CrossRef]
- Borisenko, A.V.; Kruskop, S.V.; Ivanova, N.V. A New Mouse-Eared Bat (Mammalia: Chiroptera: Vespertilionidae) from Vietnam. Russ. J. Theriol. 2008, 7, 57–69. [Google Scholar] [CrossRef]
- Thong, V.D.; Puechmaille, S.J.; Denzinger, A.; Dietz, C.; Csorba, G.; Bates, P.J.J.; Teeling, E.C.; Schnitzler, H.-U. A New Species of Hipposideros (Chiroptera: Hipposideridae) from Vietnam. J. Mammal. 2012, 93, 1–11. [Google Scholar] [CrossRef]
- Thong, V.D.; Puechmaille, S.J.; Denzinger, A.; Bates, P.J.J.; Dietz, C.; Csorba, G.; Soisook, P.; Teeling, E.C.; Matsumura, S.; Furey, N.; et al. Systematics of the Hipposideros Turpis Complex and a Description of a New Subspecies from Vietnam. Mammal. Review 2012, 42, 166–192. [Google Scholar] [CrossRef]
- Fleming, T.H. Ecology of Bat Migration. In Bat Ecology; University of Chicago Press: Chicago, IL, USA, 2003; Volume 4. [Google Scholar]
- Chaerephon Plicata. Available online: https://www.bio.bris.ac.uk/research/bats/China%20bats/chaerephonplicata.htm (accessed on 24 August 2024).
- Fleming, T.H. Bat Migration. In Encyclopedia of Animal Behavior; Elsevier: Amsterdam, The Netherlands, 2019; pp. 605–610. ISBN 978-0-12-813252-4. [Google Scholar]
- Phan, M.V.T.; Ngo Tri, T.; Hong Anh, P.; Baker, S.; Kellam, P.; Cotten, M. Identification and Characterization of Coronaviridae Genomes from Vietnamese Bats and Rats Based on Conserved Protein Domains. Virus Evol. 2018, 4, vey035. [Google Scholar] [CrossRef]
- Berto, A.; Anh, P.H.; Carrique-Mas, J.J.; Simmonds, P.; Van Cuong, N.; Tue, N.T.; Van Dung, N.; Woolhouse, M.E.; Smith, I.; Marsh, G.A.; et al. Detection of Potentially Novel Paramyxovirus and Coronavirus Viral RNA in Bats and Rats in the Mekong Delta Region of Southern Viet Nam. Zoonoses Public Health 2018, 65, 30–42. [Google Scholar] [CrossRef]
- Huong, N.Q.; Nga, N.T.T.; Long, N.V.; Luu, B.D.; Latinne, A.; Pruvot, M.; Phuong, N.T.; Quang, L.T.V.; Hung, V.V.; Lan, N.T.; et al. Coronavirus Testing Indicates Transmission Risk Increases along Wildlife Supply Chains for Human Consumption in Viet Nam, 2013–2014. PLoS ONE 2020, 15, e0237129. [Google Scholar] [CrossRef]
- Latinne, A.; Nga, N.T.T.; Long, N.V.; Ngoc, P.T.B.; Thuy, H.B.; PREDICT Consortium; Long, N.V.; Long, P.T.; Phuong, N.T.; Quang, L.T.V.; et al. One Health Surveillance Highlights Circulation of Viruses with Zoonotic Potential in Bats, Pigs, and Humans in Viet Nam. Viruses 2023, 15, 790. [Google Scholar] [CrossRef]
- Arai, S.; Nguyen, S.T.; Boldgiv, B.; Fukui, D.; Araki, K.; Dang, C.N.; Ohdachi, S.D.; Nguyen, N.X.; Pham, T.D.; Boldbaatar, B.; et al. Novel Bat-Borne Hantavirus, Vietnam. Emerg. Infect. Dis. 2013, 19, 1159–1161. [Google Scholar] [CrossRef]
- Gu, S.; Lim, B.; Kadjo, B.; Arai, S.; Kim, J.-A.; Nicolas, V.; Lalis, A.; Denys, C.; Cook, J.; Dominguez, S.; et al. Molecular Phylogeny of Hantaviruses Harbored by Insectivorous Bats in Côte d’Ivoire and Vietnam. Viruses 2014, 6, 1897–1910. [Google Scholar] [CrossRef]
- Arai, S.; Aoki, K.; Sơn, N.T.; Tú, V.T.; Kikuchi, F.; Kinoshita, G.; Fukui, D.; Thành, H.T.; Gu, S.H.; Yoshikawa, Y.; et al. Đakrông Virus, a Novel Mobatvirus (Hantaviridae) Harbored by the Stoliczka’s Asian Trident Bat (Aselliscus Stoliczkanus) in Vietnam. Sci. Rep. 2019, 9, 10239. [Google Scholar] [CrossRef]
- Arai, S.; Kikuchi, F.; Bawm, S.; Sơn, N.; Lin, K.; Tú, V.; Aoki, K.; Tsuchiya, K.; Tanaka-Taya, K.; Morikawa, S.; et al. Molecular Phylogeny of Mobatviruses (Hantaviridae) in Myanmar and Vietnam. Viruses 2019, 11, 228. [Google Scholar] [CrossRef] [PubMed]
- Pathogens Prioritization: A Scientific Framework for Epidemic and Pandemic Research Preparedness. Available online: https://www.who.int/publications/m/item/pathogens-prioritization-a-scientific-framework-for-epidemic-and-pandemic-research-preparedness (accessed on 20 March 2025).
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Waltham, T.; Middleton, J. The Khammouan Karst of Laos. Cave Karst Sci. 2000, 27, 113–120. [Google Scholar]
- Arias, M.E.; Holtgrieve, G.W.; Ngor, P.B.; Dang, T.D.; Piman, T. Maintaining Perspective of Ongoing Environmental Change in the Mekong Floodplains. Curr. Opin. Environ. Sustain. 2019, 37, 1–7. [Google Scholar] [CrossRef]
- Indomalaya|Realm & Subrealms. Available online: https://www.oneearth.org/realms/indomalaya/ (accessed on 21 March 2025).
- Laos: Total Arable Land. Available online: https://www.statista.com/statistics/687985/laos-arable-land/ (accessed on 7 August 2024).
- Covidence. Available online: https://www.covidence.org (accessed on 29 July 2024).
- Beyer, R.M.; Manica, A.; Mora, C. Shifts in Global Bat Diversity Suggest a Possible Role of Climate Change in the Emergence of SARS-CoV-1 and SARS-CoV-2. Sci. Total Environ. 2021, 767, 145413. [Google Scholar] [CrossRef]
- Latinne, A.; Morand, S. Climate Anomalies and Spillover of Bat-Borne Viral Diseases in the Asia–Pacific Region and the Arabian Peninsula. Viruses 2022, 14, 1100. [Google Scholar] [CrossRef] [PubMed]
- Azuero, O.C.; Lefrancq, N.; Nikolay, B.; McKee, C.; Cappelle, J.; Hul, V.; Ou, T.P.; Hoem, T.; Lemey, P.; Rahman, M.Z.; et al. The Genetic Diversity of Nipah Virus across Spatial Scales. medRxiv 2023. [Google Scholar] [CrossRef]
- Hu, Y.; Liu, K.; Han, P.; Xu, Z.; Zheng, A.; Pan, X.; Jia, Y.; Su, C.; Tang, L.; Wu, L.; et al. Host Range and Structural Analysis of Bat-origin RshSTT182/200 Coronavirus Binding to Human ACE2 and Its Animal Orthologs. EMBO J. 2023, 42, e111737. [Google Scholar] [CrossRef]
- Barua, S.; Dénes, A. Global Dynamics of a Compartmental Model for the Spread of Nipah Virus. Heliyon 2023, 9, e19682. [Google Scholar] [CrossRef]
- Quan, P.-L.; Firth, C.; Conte, J.M.; Williams, S.H.; Zambrana-Torrelio, C.M.; Anthony, S.J.; Ellison, J.A.; Gilbert, A.T.; Kuzmin, I.V.; Niezgoda, M.; et al. Bats Are a Major Natural Reservoir for Hepaciviruses and Pegiviruses. Proc. Natl. Acad. Sci. USA 2013, 110, 8194–8199. [Google Scholar] [CrossRef] [PubMed]
- Gouilh, M.A.; Puechmaille, S.J.; Gonzalez, J.-P.; Teeling, E.; Kittayapong, P.; Manuguerra, J.-C. SARS-Coronavirus Ancestor’s Foot-Prints in South-East Asian Bat Colonies and the Refuge Theory. Infect. Genet. Evol. 2011, 11, 1690–1702. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, S.; Zhao, J.; Zhang, F.; Hu, R. Isolation of Irkut Virus from a Murina Leucogaster Bat in China. PLoS Negl. Trop. Dis. 2013, 7, e2097. [Google Scholar] [CrossRef]
- Armero, A.; Li, R.; Bienes, K.M.; Chen, X.; Li, J.; Xu, S.; Chen, Y.; Hughes, A.C.; Berthet, N.; Wong, G. Myotis Fimbriatus Virome, a Window to Virus Diversity and Evolution in the Genus Myotis. Viruses 2022, 14, 1899. [Google Scholar] [CrossRef]
- Li, W.; Shi, Z.; Yu, M.; Ren, W.; Smith, C.; Epstein, J.H.; Wang, H.; Crameri, G.; Hu, Z.; Zhang, H.; et al. Bats Are Natural Reservoirs of SARS-like Coronaviruses. Science 2005, 310, 676–679. [Google Scholar] [CrossRef]
- Lau, S.K.P.; Li, K.S.M.; Huang, Y.; Shek, C.-T.; Tse, H.; Wang, M.; Choi, G.K.Y.; Xu, H.; Lam, C.S.F.; Guo, R.; et al. Ecoepidemiology and Complete Genome Comparison of Different Strains of Severe Acute Respiratory Syndrome-Related Rhinolophus Bat Coronavirus in China Reveal Bats as a Reservoir for Acute, Self-Limiting Infection That Allows Recombination Events. J. Virol. 2010, 84, 2808–2819. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Hon, C.-C.; Li, Y.; Wang, D.; Xu, G.; Zhang, H.; Zhou, P.; Poon, L.L.M.; Lam, T.T.-Y.; Leung, F.C.-C.; et al. Intraspecies Diversity of SARS-like Coronaviruses in Rhinolophus Sinicus and Its Implications for the Origin of SARS Coronaviruses in Humans. J. Gen. Virol. 2010, 91, 1058–1062. [Google Scholar] [CrossRef]
- Luo, Y.; Li, B.; Jiang, R.-D.; Hu, B.-J.; Luo, D.-S.; Zhu, G.-J.; Hu, B.; Liu, H.-Z.; Zhang, Y.-Z.; Yang, X.-L.; et al. Longitudinal Surveillance of Betacoronaviruses in Fruit Bats in Yunnan Province, China During 2009–2016. Virol. Sin. 2018, 33, 87–95. [Google Scholar] [CrossRef]
- He, B.; Zhang, Y.; Xu, L.; Yang, W.; Yang, F.; Feng, Y.; Xia, L.; Zhou, J.; Zhen, W.; Feng, Y.; et al. Identification of Diverse Alphacoronaviruses and Genomic Characterization of a Novel Severe Acute Respiratory Syndrome-Like Coronavirus from Bats in China. J. Virol. 2014, 88, 7070–7082. [Google Scholar] [CrossRef] [PubMed]
- Lacroix, A.; Duong, V.; Hul, V.; San, S.; Davun, H.; Omaliss, K.; Chea, S.; Hassanin, A.; Theppangna, W.; Silithammavong, S.; et al. Genetic Diversity of Coronaviruses in Bats in Lao PDR and Cambodia. Infect. Genet. Evol. 2017, 48, 10–18. [Google Scholar] [CrossRef]
- Afelt, A.; Lacroix, A.; Zawadzka-Pawlewska, U.; Pokojski, W.; Buchy, P.; Frutos, R. Distribution of Bat-Borne Viruses and Environment Patterns. Infect. Genet. Evol. 2018, 58, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Zhu, C.; Ai, L.; He, T.; Wang, Y.; Ye, F.; Yang, L.; Ding, C.; Zhu, X.; Lv, R.; et al. Genomic Characterization and Infectivity of a Novel SARS-like Coronavirus in Chinese Bats. Emerg. Microbes Infect. 2018, 7, 1–10. [Google Scholar] [CrossRef]
- Lau, S.K.P.; Wong, A.C.P.; Zhang, L.; Luk, H.K.H.; Kwok, J.S.L.; Ahmed, S.S.; Cai, J.-P.; Zhao, P.S.H.; Teng, J.L.L.; Tsui, S.K.W.; et al. Novel Bat Alphacoronaviruses in Southern China Support Chinese Horseshoe Bats as an Important Reservoir for Potential Novel Coronaviruses. Viruses 2019, 11, 423. [Google Scholar] [CrossRef]
- Wang, N.; Luo, C.; Liu, H.; Yang, X.; Hu, B.; Zhang, W.; Li, B.; Zhu, Y.; Zhu, G.; Shen, X.; et al. Characterization of a New Member of Alphacoronavirus with Unique Genomic Features in Rhinolophus Bats. Viruses 2019, 11, 379. [Google Scholar] [CrossRef]
- Delaune, D.; Hul, V.; Karlsson, E.A.; Hassanin, A.; Ou, T.P.; Baidaliuk, A.; Gámbaro, F.; Prot, M.; Tu, V.T.; Chea, S.; et al. A Novel SARS-CoV-2 Related Coronavirus in Bats from Cambodia. Nat. Commun. 2021, 12, 6563. [Google Scholar] [CrossRef]
- Zhou, H.; Ji, J.; Chen, X.; Bi, Y.; Li, J.; Wang, Q.; Hu, T.; Song, H.; Zhao, R.; Chen, Y.; et al. Identification of Novel Bat Coronaviruses Sheds Light on the Evolutionary Origins of SARS-CoV-2 and Related Viruses. Cell 2021, 184, 4380–4391.e14. [Google Scholar] [CrossRef]
- Zhu, F.; Duong, V.; Lim, X.F.; Hul, V.; Chawla, T.; Keatts, L.; Goldstein, T.; Hassanin, A.; Tu, V.T.; Buchy, P.; et al. Presence of Recombinant Bat Coronavirus GCCDC1 in Cambodian Bats. Viruses 2022, 14, 176. [Google Scholar] [CrossRef] [PubMed]
- Latinne, A.; Hu, B.; Olival, K.J.; Zhu, G.; Zhang, L.-B.; Li, H.; Chmura, A.A.; Field, H.E.; Zambrana-Torrelio, C.; Epstein, J.H.; et al. Origin and Cross-Species Transmission of Bat Coronaviruses in China. Nat. Commun. 2024, 15, 10705. [Google Scholar] [CrossRef]
- Olson, J.G.; Rupprecht, C.; Rollin, P.E.; An, U.S.; Niezgoda, M.; Clemins, T.; Walston, J.; Ksiazek, T.G. Antibodies to Nipah-like Virus in Bats (Pteropus Lylei), Cambodia. Emerg. Infect. Dis. 2002, 8, 987–988. [Google Scholar] [CrossRef]
- Reynes, J.-M.; Counor, D.; Ong, S.; Faure, C.; Seng, V.; Molia, S.; Walston, J.; Georges-Courbot, M.C.; Deubel, V.; Sarthou, J.-L. Nipah Virus in Lyle’s Flying Foxes, Cambodia. Emerg. Infect. Dis. 2005, 11, 1042–1047. [Google Scholar] [CrossRef]
- Hasebe, F.; Thuy, N.T.T.; Inoue, S.; Yu, F.; Kaku, Y.; Watanabe, S.; Akashi, H.; Dat, D.T.; Mai, L.T.Q.; Morita, K. Serologic Evidence of Nipah Virus Infection in Bats, Vietnam. Emerg. Infect. Dis. 2012, 18, 536–537. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Li, M.; Li, L.; Monagin, C.; Chmura, A.; Schneider, B.; Epstein, J.; Mei, X.; Shi, Z.; Daszak, P.; et al. Evidence for Retrovirus and Paramyxovirus Infection of Multiple Bat Species in China. Viruses 2014, 6, 2138–2154. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, Z.; Cai, J.; Yan, X.; Zhang, F.; Wu, J.; Xu, L.; Zhao, Z.; Hu, T.; Tu, C.; et al. Seroreactive Profiling of Filoviruses in Chinese Bats Reveals Extensive Infection of Diverse Viruses. J. Virol. 2020, 94, e02042-19. [Google Scholar] [CrossRef]
- Cappelle, J.; Hoem, T.; Hul, V.; Furey, N.; Nguon, K.; Prigent, S.; Dupon, L.; Ken, S.; Neung, C.; Hok, V.; et al. Nipah Virus Circulation at Human–Bat Interfaces, Cambodia. Bull. World Health Organ. 2020, 98, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Wu, J.; Li, Q.; Wei, Y.; Tan, Z.; Cai, J.; Guo, H.; Yang, L.; Huang, X.; Chen, J.; et al. Seroprevalence, Cross Antigenicity and Circulation Sphere of Bat-Borne Hantaviruses Revealed by Serological and Antigenic Analyses. PLoS Pathog. 2019, 15, e1007545. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Zhang, Y.; Li, J.; Zhang, Y.; Wang, L.-F.; Shi, Z. Serological Evidence of Ebolavirus Infection in Bats, China. Virol. J. 2012, 9, 236. [Google Scholar] [CrossRef]
- Makenov, M.T.; Le, L.A.T.; Stukolova, O.A.; Radyuk, E.V.; Morozkin, E.S.; Bui, N.T.T.; Zhurenkova, O.B.; Dao, M.N.; Nguyen, C.V.; Luong, M.T.; et al. Detection of Filoviruses in Bats in Vietnam. Viruses 2023, 15, 1785. [Google Scholar] [CrossRef]
- Lu, L.; Van Dung, N.; Ivens, A.; Bogaardt, C.; O’Toole, A.; Bryant, J.E.; Carrique-Mas, J.; Van Cuong, N.; Anh, P.H.; Rabaa, M.A.; et al. Genetic Diversity and Cross-Species Transmission of Kobuviruses in Vietnam. Virus Evol. 2018, 4, vey002. [Google Scholar] [CrossRef]
- Lu, L.; Ashworth, J.; Nguyen, D.; Li, K.; Smith, D.B.; Woolhouse, M.; on behalf of the VIZIONS Consortium. No Exchange of Picornaviruses in Vietnam between Humans and Animals in a High-Risk Cohort with Close Contact despite High Prevalence and Diversity. Viruses 2021, 13, 1709. [Google Scholar] [CrossRef]
- Li, Y.; Ge, X.; Zhang, H.; Zhou, P.; Zhu, Y.; Zhang, Y.; Yuan, J.; Wang, L.-F.; Shi, Z. Host Range, Prevalence, and Genetic Diversity of Adenoviruses in Bats. J. Virol. 2010, 84, 3889–3897. [Google Scholar] [CrossRef] [PubMed]
- Chu, D.K.W.; Poon, L.L.M.; Guan, Y.; Peiris, J.S.M. Novel Astroviruses in Insectivorous Bats. J. Virol. 2008, 82, 9107–9114. [Google Scholar] [CrossRef]
- Zhu, H.C.; Chu, D.K.W.; Liu, W.; Dong, B.Q.; Zhang, S.Y.; Zhang, J.X.; Li, L.F.; Vijaykrishna, D.; Smith, G.J.D.; Chen, H.L.; et al. Detection of Diverse Astroviruses from Bats in China. J. Gen. Virol. 2009, 90, 883–887. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Chmura, A.A.; Li, J.; Zhu, G.; Desmond, J.S.; Zhang, Y.; Zhang, W.; Epstein, J.H.; Daszak, P.; Shi, Z. Detection of Diverse Novel Astroviruses from Small Mammals in China. J. Gen. Virol. 2014, 95, 2442–2449. [Google Scholar] [CrossRef] [PubMed]
- Lacroix, A.; Duong, V.; Hul, V.; San, S.; Davun, H.; Omaliss, K.; Chea, S.; Hassanin, A.; Theppangna, W.; Silithammavong, S.; et al. Diversity of Bat Astroviruses in Lao PDR and Cambodia. Infect. Genet. Evol. 2017, 47, 41–50. [Google Scholar] [CrossRef]
- Reynes, J.-M.; Molia, S.; Audry, L.; Hout, S.; Ngin, S.; Walston, J.; Bourhy, H. Serologic Evidence of Lyssavirus Infection in Bats, Cambodia. Emerg. Infect. Dis. 2004, 10, 2231–2234. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, L.; Lu, Z.; Xuan, H.; Han, X.; Xia, X.; Zhao, F.; Tu, C. Seroprevalence of Rabies Virus Antibodies in Bats from Southern China. Vector Borne Zoonotic Dis. 2010, 10, 177–181. [Google Scholar] [CrossRef]
- Nguyen, A.T.K.; Nguyen, T.T.; Noguchi, A.; Nguyen, D.V.; Ngo, G.C.; Thong, V.D.; Olowokure, B.; Inoue, S. Bat Lyssaviruses, Northern Vietnam. Emerg. Infect. Dis. 2014, 20, 161–163. [Google Scholar] [CrossRef]
- Xu, L.; Wu, J.; Jiang, T.; Qin, S.; Xia, L.; Li, X.; He, B.; Tu, C. Molecular Detection and Sequence Characterization of Diverse Rhabdoviruses in Bats, China. Virus Res. 2018, 244, 208–212. [Google Scholar] [CrossRef]
- Li, L.-L.; Xu, Y.-L.; Lu, X.-X.; Deng, H.-Y.; Li, J.-S.; Song, J.-D.; Ma, X.-H.; Zhu, W.-Y.; Wang, J.-L.; Duan, Z.-J. Isolation of a Novel Bat Rhabdovirus with Evidence of Human Exposure in China. mBio 2022, 13, e02875-21. [Google Scholar] [CrossRef]
- Li, Y.; Ge, X.; Hon, C.-C.; Zhang, H.; Zhou, P.; Zhang, Y.; Wu, Y.; Wang, L.-F.; Shi, Z. Prevalence and Genetic Diversity of Adeno-Associated Viruses in Bats from China. J. Gen. Virol. 2010, 91, 2601–2609. [Google Scholar] [CrossRef] [PubMed]
- Lau, S.K.P.; Ahmed, S.S.; Yeung, H.C.; Li, K.S.M.; Fan, R.Y.Y.; Cheng, T.Y.C.; Cai, J.-P.; Wang, M.; Zheng, B.-J.; Wong, S.S.Y.; et al. Identification and Interspecies Transmission of a Novel Bocaparvovirus among Different Bat Species in China. J. Gen. Virol. 2016, 97, 3345–3358. [Google Scholar] [CrossRef]
- Wang, L.; Fu, S.; Cao, L.; Lei, W.; Cao, Y.; Song, J.; Tang, Q.; Zhang, H.; Feng, Y.; Yang, W.; et al. Isolation and Identification of a Natural Reassortant Mammalian Orthoreovirus from Least Horseshoe Bat in China. PLoS ONE 2015, 10, e0118598. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.-L.; Tan, B.; Wang, B.; Li, W.; Wang, N.; Luo, C.-M.; Wang, M.-N.; Zhang, W.; Li, B.; Peng, C.; et al. Isolation and Identification of Bat Viruses Closely Related to Human, Porcine and Mink Orthoreoviruses. J. Gen. Virol. 2015, 96, 3525–3531. [Google Scholar] [CrossRef]
- Inagaki, T.; Yamada, S.; Fujii, H.; Yoshikawa, T.; Shibamura, M.; Harada, S.; Fukushi, S.; Le, M.Q.; Nguyen, C.T.; Nguyen, T.T.T.; et al. Characterization of a Novel Alphaherpesvirus Isolated from the Fruit Bat Pteropus Lylei in Vietnam. J. Virol. 2020, 94, e00673-20. [Google Scholar] [CrossRef]
- Duan, S.; Li, Z.; Zhang, X.; Yu, X.-J. Novel Betaherpesviruses and Gammaherpesviruses in Bats from Central China. Sci. Rep. 2024, 14, 10651. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Yang, X.-L.; Li, W.; Zhu, Y.; Ge, X.-Y.; Zhang, L.-B.; Zhang, Y.-Z.; Bock, C.-T.; Shi, Z.-L. Detection and Genome Characterization of Four Novel Bat Hepadnaviruses and a Hepevirus in China. Virol. J. 2017, 14, 40. [Google Scholar] [CrossRef]
- Van Nguyen, D.; Van Nguyen, C.; Bonsall, D.; Ngo, T.; Carrique-Mas, J.; Pham, A.; Bryant, J.; Thwaites, G.; Baker, S.; Woolhouse, M.; et al. Detection and Characterization of Homologues of Human Hepatitis Viruses and Pegiviruses in Rodents and Bats in Vietnam. Viruses 2018, 10, 102. [Google Scholar] [CrossRef]
- Kemenesi, G.; Kurucz, K.; Zana, B.; Tu, V.T.; Görföl, T.; Estók, P.; Földes, F.; Sztancsik, K.; Urbán, P.; Fehér, E.; et al. Highly Divergent Cyclo-like Virus in a Great Roundleaf Bat (Hipposideros Armiger) in Vietnam. Arch. Virol. 2017, 162, 2403–2407. [Google Scholar] [CrossRef]
- Zhu, A.; Jiang, T.; Hu, T.; Mi, S.; Zhao, Z.; Zhang, F.; Feng, J.; Fan, Q.; He, B.; Tu, C. Molecular Characterization of a Novel Bat-Associated Circovirus with a Poly-T Tract in the 3′ Intergenic Region. Virus Res. 2018, 250, 95–103. [Google Scholar] [CrossRef]
- Vidovszky, M.Z.; Kapitány, S.; Gellért, Á.; Harrach, B.; Görföl, T.; Boldogh, S.A.; Kohl, C.; Wibbelt, G.; Mühldorfer, K.; Kemenesi, G.; et al. Detection and Genetic Characterization of Circoviruses in More than 80 Bat Species from Eight Countries on Four Continents. Vet. Res. Commun. 2023, 47, 1561–1573. [Google Scholar] [CrossRef]
- Osborne, J.C.; Rupprecht, C.E.; Olson, J.G.; Ksiazek, T.G.; Rollin, P.E.; Niezgoda, M.; Goldsmith, C.S.; An, U.S.; Nichol, S.T. Isolation of Kaeng Khoi Virus from Dead Chaerephon Plicata Bats in Cambodia. J. General. Virol. 2003, 84, 2685–2689. [Google Scholar] [CrossRef] [PubMed]
- Oude Munnink, B.B.; Phan, M.V.T.; The VIZIONS Consortium; Simmonds, P.; Koopmans, M.P.G.; Kellam, P.; Van Der Hoek, L.; Cotten, M. Characterization of Posa and Posa-like Virus Genomes in Fecal Samples from Humans, Pigs, Rats, and Bats Collected from a Single Location in Vietnam. Virus Evol. 2017, 3, vex022. [Google Scholar] [CrossRef]
- Zhou, S.; Liu, B.; Han, Y.; Wang, Y.; Chen, L.; Wu, Z.; Yang, J. ZOVER: The Database of Zoonotic and Vector-Borne Viruses. Nucleic Acids Res. 2022, 50, D943–D949. [Google Scholar] [CrossRef]
- Letko, M.; Seifert, S.N.; Olival, K.J.; Plowright, R.K.; Munster, V.J. Bat-Borne Virus Diversity, Spillover and Emergence. Nat. Rev. Microbiol. 2020, 18, 461–471. [Google Scholar] [CrossRef]
- Bukhari, K.; Mulley, G.; Gulyaeva, A.A.; Zhao, L.; Shu, G.; Jiang, J.; Neuman, B.W. Description and Initial Characterization of Metatranscriptomic Nidovirus-like Genomes from the Proposed New Family Abyssoviridae, and from a Sister Group to the Coronavirinae, the Proposed Genus Alphaletovirus. Virology 2018, 524, 160–171. [Google Scholar] [CrossRef]
- Mordecai, G.J.; Miller, K.M.; Di Cicco, E.; Schulze, A.D.; Kaukinen, K.H.; Ming, T.J.; Li, S.; Tabata, A.; Teffer, A.; Patterson, D.A.; et al. Endangered Wild Salmon Infected by Newly Discovered Viruses. eLife 2019, 8, e47615. [Google Scholar] [CrossRef] [PubMed]
- Woo, P.C.Y.; de Groot, R.J.; Haagmans, B.; Lau, S.K.P.; Neuman, B.W.; Perlman, S.; Sola, I.; van der Hoek, L.; Wong, A.C.P.; Yeh, S.-H. ICTV Virus Taxonomy Profile: Coronaviridae 2023: This Article Is Part of the ICTV Virus Taxonomy Profiles Collection. J. Gen. Virol. 2023, 104, 001843. [Google Scholar] [CrossRef] [PubMed]
- Wacharapluesadee, S.; Duengkae, P.; Rodpan, A.; Kaewpom, T.; Maneeorn, P.; Kanchanasaka, B.; Yingsakmongkon, S.; Sittidetboripat, N.; Chareesaen, C.; Khlangsap, N.; et al. Diversity of Coronavirus in Bats from Eastern Thailand. Virol. J. 2015, 12, 57. [Google Scholar] [CrossRef]
- Rima, B.; Balkema-Buschmann, A.; Dundon, W.G.; Duprex, P.; Easton, A.; Fouchier, R.; Kurath, G.; Lamb, R.; Lee, B.; Rota, P.; et al. ICTV Virus Taxonomy Profile: Paramyxoviridae. J. General. Virol. 2019, 100, 1593–1594. [Google Scholar] [CrossRef]
- Enders, G. Paramyxoviruses. In Medical Microbiology; Baron, S., Ed.; University of Texas Medical Branch at Galveston: Galveston TX, USA, 1996; ISBN 978-0-9631172-1-2. [Google Scholar]
- Chua, K.B.; Bellini, W.J.; Rota, P.A.; Harcourt, B.H.; Tamin, A.; Lam, S.K.; Ksiazek, T.G.; Rollin, P.E.; Zaki, S.R.; Shieh, W.-J.; et al. Nipah Virus: A Recently Emergent Deadly Paramyxovirus. Science 2000, 288, 1432–1435. [Google Scholar] [CrossRef]
- Halpin, K.; Young, P.L.; Field, H.E.; Mackenzie, J.S. Isolation of Hendra Virus from Pteropid Bats: A Natural Reservoir of Hendra Virus. J. General. Virol. 2000, 81, 1927–1932. [Google Scholar] [CrossRef] [PubMed]
- Faus-Cotino, J.; Reina, G.; Pueyo, J. Nipah Virus: A Multidimensional Update. Viruses 2024, 16, 179. [Google Scholar] [CrossRef]
- Nipah Virus. Available online: https://www.who.int/news-room/fact-sheets/detail/nipah-virus (accessed on 26 July 2024).
- Maes, P.; Adkins, S.; Alkhovsky, S.V.; Avšič-Županc, T.; Ballinger, M.J.; Bente, D.A.; Beer, M.; Bergeron, É.; Blair, C.D.; Briese, T.; et al. Taxonomy of the Order Bunyavirales: Second Update 2018. Arch. Virol. 2019, 164, 927–941. [Google Scholar] [CrossRef] [PubMed]
- Nichol, S.T.; Spiropoulou, C.F.; Morzunov, S.; Rollin, P.E.; Ksiazek, T.G.; Feldmann, H.; Sanchez, A.; Childs, J.; Zaki, S.; Peters, C.J. Genetic Identification of a Hantavirus Associated with an Outbreak of Acute Respiratory Illness. Science 1993, 262, 914–917. [Google Scholar] [CrossRef] [PubMed]
- Duchin, J.S.; Koster, F.T.; Peters, C.J.; Simpson, G.L.; Tempest, B.; Zaki, S.R.; Ksiazek, T.G.; Rollin, P.E.; Nichol, S.; Umland, E.T.; et al. Hantavirus Pulmonary Syndrome: A Clinical Description of 17 Patients with a Newly Recognized Disease. N. Engl. J. Med. 1994, 330, 949–955. [Google Scholar] [CrossRef]
- Vial, P.A.; Ferrés, M.; Vial, C.; Klingström, J.; Ahlm, C.; López, R.; Le Corre, N.; Mertz, G.J. Hantavirus in Humans: A Review of Clinical Aspects and Management. Lancet Infect. Dis. 2023, 23, e371–e382. [Google Scholar] [CrossRef]
- Kuhn, J.H.; Amarasinghe, G.K.; Basler, C.F.; Bavari, S.; Bukreyev, A.; Chandran, K.; Crozier, I.; Dolnik, O.; Dye, J.M.; Formenty, P.B.H.; et al. ICTV Virus Taxonomy Profile: Filoviridae. J. Gen. Virol. 2019, 100, 911–912. [Google Scholar] [CrossRef]
- Ozharovskaia, T.A.; Zubkova, O.V.; Popova, O.; Kovyrshina, A.V.; Goldovskaya, P.P.; Vavilova, I.V.; Dolzhikova, I.V.; Ermolova, E.I.; Kunda, M.S.; Ryzhova, N.N.; et al. Immunogenicity of various variants of Ebola and Marburg virus glycoprotein genes in recombinant adenoviral vectors. Biol. Products. Prev. Diagn. Treat. 2024, 24, 294–311. [Google Scholar] [CrossRef]
- Marburg Virus Disease. Available online: https://www.who.int/news-room/fact-sheets/detail/marburg-virus-disease (accessed on 3 April 2025).
- Burgueño-Sosa, E.E.; Esquivel-Gómez, L.R.; Rivadeneyra-Gutiérrez, E.; León-López, A.A. Generalidades de La Familia Filoviridae y El Virus Del Ébola: Una Actualización de Sus Implicaciones En La Población Humana. Rev. Biomed. 2020, 31, 58–68. [Google Scholar] [CrossRef]
- Dovih, P.; Laing, E.D.; Chen, Y.; Low, D.H.W.; Ansil, B.R.; Yang, X.; Shi, Z.; Broder, C.C.; Smith, G.J.D.; Linster, M.; et al. Filovirus-Reactive Antibodies in Humans and Bats in Northeast India Imply Zoonotic Spillover. PLoS Negl. Trop. Dis. 2019, 13, e0007733. [Google Scholar] [CrossRef]
- Simmonds, P.; Becher, P.; Bukh, J.; Gould, E.A.; Meyers, G.; Monath, T.; Muerhoff, S.; Pletnev, A.; Rico-Hesse, R.; Smith, D.B.; et al. ICTV Virus Taxonomy Profile: Flaviviridae. J. Gen. Virol. 2017, 98, 2–3. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, Q.; Wang, H.; Yao, X. Severe Zoonotic Viruses Carried by Different Species of Bats and Their Regional Distribution. Clin. Microbiol. Infect. 2024, 30, 206–210. [Google Scholar] [CrossRef]
- McInnes, C.J.; Damon, I.K.; Smith, G.L.; McFadden, G.; Isaacs, S.N.; Roper, R.L.; Evans, D.H.; Damaso, C.R.; Carulei, O.; Wise, L.M.; et al. ICTV Virus Taxonomy Profile: Poxviridae 2023: This Article Is Part of the ICTV Virus Taxonomy Profiles Collection. J. Gen. Virol. 2023, 104, 001849. [Google Scholar] [CrossRef]
- Mpox Outbreak. Available online: https://www.who.int/emergencies/situations/mpox-outbreak (accessed on 19 February 2025).
- Hou, W.; Wu, N.; Liu, Y.; Tang, Y.; Quan, Q.; Luo, Y.; Jin, C. Mpox: Global Epidemic Situation and Countermeasures. Virulence 2025, 16, 2457958. [Google Scholar] [CrossRef]
- WHO. Multi-Country Outbreak of Mpox, External Situation Report #47–13 February 2025; WHO: Geneva, Switzerland, 2025. [Google Scholar]
- Board on Health Promotion and Disease Prevention; Institute of Medicine (United States). Scientific Background on Smallpox and Smallpox Vaccination. In Scientific and Policy Considerations in Developing Smallpox Vaccination Options: A Workshop Report; National Academies Press: Washington, DC, USA, 2002. [Google Scholar]
- Smallpox. Available online: https://www.who.int/health-topics/smallpox (accessed on 19 February 2025).
- Baker, K.; Murcia, P. Poxviruses in Bats … so What? Viruses 2014, 6, 1564–1577. [Google Scholar] [CrossRef] [PubMed]
- David, D.; Davidson, I.; Karniely, S.; Edery, N.; Rosenzweig, A.; Sol, A. Israeli Rousettus Aegyptiacus Pox Virus (IsrRAPXV) Infection in Juvenile Egyptian Fruit Bat (Rousettus Aegyptiacus): Clinical Findings and Molecular Detection. Viruses 2021, 13, 407. [Google Scholar] [CrossRef] [PubMed]
- O’Dea, M.A.; Tu, S.-L.; Pang, S.; De Ridder, T.; Jackson, B.; Upton, C. Genomic Characterization of a Novel Poxvirus from a Flying Fox: Evidence for a New Genus? J. Gen. Virol. 2016, 97, 2363–2375. [Google Scholar] [CrossRef] [PubMed]
- David, D.; Davidson, I.; Berkowitz, A.; Karniely, S.; Edery, N.; Bumbarov, V.; Laskar, O.; Elazari-Volcani, R. A Novel Poxvirus Isolated from an Egyptian Fruit Bat in Israel. Vet. Med. Sci. 2020, 6, 587–590. [Google Scholar] [CrossRef] [PubMed]
- Paran, Y.; David, D.; Rudoler, N.; Ingbir, M.; Khoury, N.; Halutz, O.; Ben-Ami, R.; Berkowitz, A.; Sol, A. Human Infection with IsrRAPXV: A Novel Zoonotic Bat-Derived Poxvirus. J. Infect. Dis. 2025, 231, 495–500. [Google Scholar] [CrossRef] [PubMed]
- Nurmukanova, V.; Matsvay, A.; Gordukova, M.; Shipulin, G. Square the Circle: Diversity of Viral Pathogens Causing Neuro-Infectious Diseases. Viruses 2024, 16, 787. [Google Scholar] [CrossRef]
- Renault, P.; Josseran, L.; Pierre, V. Chikungunya-Related Fatality Rates, Mauritius, India, and Reunion Island. Emerg. Infect. Dis. 2008, 14, 1327. [Google Scholar] [CrossRef]
- Silva, L.A.; Dermody, T.S. Chikungunya Virus: Epidemiology, Replication, Disease Mechanisms, and Prospective Intervention Strategies. J. Clin. Investig. 2017, 127, 737–749. [Google Scholar] [CrossRef]
- Chang, A.Y.; Encinales, L.; Porras, A.; Pacheco, N.; Reid, S.P.; Martins, K.A.O.; Pacheco, S.; Bravo, E.; Navarno, M.; Rico Mendoza, A.; et al. Frequency of Chronic Joint Pain Following Chikungunya Virus Infection: A Colombian Cohort Study. Arthritis Rheumatol. 2018, 70, 578–584. [Google Scholar] [CrossRef]
- Tritsch, S.R.; Encinales, L.; Pacheco, N.; Cadena, A.; Cure, C.; McMahon, E.; Watson, H.; Porras Ramirez, A.; Mendoza, A.R.; Li, G.; et al. Chronic Joint Pain 3 Years after Chikungunya Virus Infection Largely Characterized by Relapsing-Remitting Symptoms. J. Rheumatol. 2020, 47, 1267–1274. [Google Scholar] [CrossRef]
- Maes, P.; Alkhovsky, S.V.; Bào, Y.; Beer, M.; Birkhead, M.; Briese, T.; Buchmeier, M.J.; Calisher, C.H.; Charrel, R.N.; Choi, I.R.; et al. Taxonomy of the Family Arenaviridae and the Order Bunyavirales: Update 2018. Arch. Virol. 2018, 163, 2295–2310. [Google Scholar] [CrossRef]
- Fagre, A.C.; Kading, R.C. Can Bats Serve as Reservoirs for Arboviruses? Viruses 2019, 11, 215. [Google Scholar] [CrossRef]
- Yeh-Gorocica, A.; Torres-Castro, M.; Carrillo-Chan, C.; Suarez-Galaz, A.; Suarez-Galaz, M.; Moguel-Chin, W.; Panti-May, A.; Lugo-Caballero, C.; Puerta-Guardo, H.; Chable-Santos, J.; et al. Prevalence of Flavivirus and Alphavirus in Bats Captured in the State of Yucatan, Southeastern Mexico. One Health 2024, 19, 100876. [Google Scholar] [CrossRef] [PubMed]
- Zell, R.; Delwart, E.; Gorbalenya, A.E.; Hovi, T.; King, A.M.Q.; Knowles, N.J.; Lindberg, A.M.; Pallansch, M.A.; Palmenberg, A.C.; Reuter, G.; et al. ICTV Virus Taxonomy Profile: Picornaviridae. J. Gen. Virol. 2017, 98, 2421–2422. [Google Scholar] [CrossRef]
- Lukashev, A.N.; Vakulenko, Y.A. Molecular Evolution of Types in Non-Polio Enteroviruses. J. Gen. Virol. 2017, 98, 2968–2981. [Google Scholar] [CrossRef]
- Rabaa, M.A.; Tue, N.T.; Phuc, T.M.; Carrique-Mas, J.; Saylors, K.; Cotten, M.; Bryant, J.E.; Nghia, H.D.T.; Cuong, N.V.; Pham, H.A.; et al. The Vietnam Initiative on Zoonotic Infections (VIZIONS): A Strategic Approach to Studying Emerging Zoonotic Infectious Diseases. EcoHealth 2015, 12, 726–735. [Google Scholar] [CrossRef] [PubMed]
- Olival, K.J.; Hosseini, P.R.; Zambrana-Torrelio, C.; Ross, N.; Bogich, T.L.; Daszak, P. Host and Viral Traits Predict Zoonotic Spillover from Mammals. Nature 2017, 546, 646–650. [Google Scholar] [CrossRef]
- Lau, S.K.P.; Woo, P.C.Y.; Lai, K.K.Y.; Huang, Y.; Yip, C.C.Y.; Shek, C.-T.; Lee, P.; Lam, C.S.F.; Chan, K.-H.; Yuen, K.-Y. Complete Genome Analysis of Three Novel Picornaviruses from Diverse Bat Species. J. Virol. 2011, 85, 8819–8828. [Google Scholar] [CrossRef] [PubMed]
- Drexler, J.F.; Corman, V.M.; Lukashev, A.N.; van den Brand, J.M.A.; Gmyl, A.P.; Brünink, S.; Rasche, A.; Seggewiβ, N.; Feng, H.; Leijten, L.M.; et al. Evolutionary Origins of Hepatitis A Virus in Small Mammals. Proc. Natl. Acad. Sci. USA 2015, 112, 15190–15195. [Google Scholar] [CrossRef]
- Zeghbib, S.; Herczeg, R.; Kemenesi, G.; Zana, B.; Kurucz, K.; Urbán, P.; Madai, M.; Földes, F.; Papp, H.; Somogyi, B.; et al. Genetic Characterization of a Novel Picornavirus in Algerian Bats: Co-Evolution Analysis of Bat-Related Picornaviruses. Sci. Rep. 2019, 9, 15706. [Google Scholar] [CrossRef]
- Rosenberg, N.; Jolicoeur, P. Retroviral Pathogenesis. In Retroviruses; Coffin, J.M., Hughes, S.H., Varmus, H.E., Eds.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 1997; ISBN 978-0-87969-571-2. [Google Scholar]
- Kaplan, M. Human Retroviruses: A Common Virology. Transfus. Med. Rev. 1989, 3, 4–8. [Google Scholar] [CrossRef]
- Coffin, J.; Blomberg, J.; Fan, H.; Gifford, R.; Hatziioannou, T.; Lindemann, D.; Mayer, J.; Stoye, J.; Tristem, M.; Johnson, W.; et al. ICTV Virus Taxonomy Profile: Retroviridae 2021: This Article Is Part of the ICTV Virus Taxonomy Profiles Collection. J. Gen. Virol. 2021, 102, 001712. [Google Scholar] [CrossRef]
- Harrach, B.; Tarján, Z.L.; Benkő, M. Adenoviruses across the Animal Kingdom: A Walk in the Zoo. FEBS Lett. 2019, 593, 3660–3673. [Google Scholar] [CrossRef] [PubMed]
- Benkő, M.; Aoki, K.; Arnberg, N.; Davison, A.J.; Echavarría, M.; Hess, M.; Jones, M.S.; Kaján, G.L.; Kajon, A.E.; Mittal, S.K.; et al. ICTV Virus Taxonomy Profile: Adenoviridae 2022: This Article Is Part of the ICTV Virus Taxonomy Profiles Collection. J. Gen. Virol. 2022, 103, 001721. [Google Scholar] [CrossRef]
- Medkour, H.; Amona, I.; Akiana, J.; Davoust, B.; Bitam, I.; Levasseur, A.; Tall, M.L.; Diatta, G.; Sokhna, C.; Hernandez-Aguilar, R.A.; et al. Adenovirus Infections in African Humans and Wild Non-Human Primates: Great Diversity and Cross-Species Transmission. Viruses 2020, 12, 657. [Google Scholar] [CrossRef]
- Kozak, R.A.; Ackford, J.G.; Slaine, P.; Li, A.; Carman, S.; Campbell, D.; Welch, M.K.; Kropinski, A.M.; Nagy, É. Characterization of a Novel Adenovirus Isolated from a Skunk. Virology 2015, 485, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Nwachuku, N.; Gerba, C.P.; Oswald, A.; Mashadi, F.D. Comparative Inactivation of Adenovirus Serotypes by UV Light Disinfection. Appl. Environ. Microbiol. 2005, 71, 5633–5636. [Google Scholar] [CrossRef]
- Gray, G.C. Adenovirus Transmission–Worthy of Our Attention. J. Infect. Dis. 2006, 194, 871–873. [Google Scholar] [CrossRef]
- De Benedictis, P.; Schultz-Cherry, S.; Burnham, A.; Cattoli, G. Astrovirus Infections in Humans and Animals–Molecular Biology, Genetic Diversity, and Interspecies Transmissions. Infect. Genet. Evol. 2011, 11, 1529–1544. [Google Scholar] [CrossRef]
- Allocati, N.; Petrucci, A.G.; Di Giovanni, P.; Masulli, M.; Di Ilio, C.; De Laurenzi, V. Bat–Man Disease Transmission: Zoonotic Pathogens from Wildlife Reservoirs to Human Populations. Cell Death Discov. 2016, 2, 16048. [Google Scholar] [CrossRef]
- Walker, P.J.; Freitas-Astúa, J.; Bejerman, N.; Blasdell, K.R.; Breyta, R.; Dietzgen, R.G.; Fooks, A.R.; Kondo, H.; Kurath, G.; Kuzmin, I.V.; et al. ICTV Virus Taxonomy Profile: Rhabdoviridae 2022: This Article Is Part of the ICTV Virus Taxonomy Profiles Collection. J. Gen. Virol. 2022, 103, 001689. [Google Scholar] [CrossRef] [PubMed]
- Kuzmin, I.V.; Novella, I.S.; Dietzgen, R.G.; Padhi, A.; Rupprecht, C.E. The Rhabdoviruses: Biodiversity, Phylogenetics, and Evolution. Infect. Genet. Evol. 2009, 9, 541–553. [Google Scholar] [CrossRef] [PubMed]
- Jackson, A.C.; Warrell, M.J.; Rupprecht, C.E.; Ertl, H.C.J.; Dietzschold, B.; O’Reilly, M.; Leach, R.P.; Fu, Z.F.; Wunner, W.H.; Bleck, T.P.; et al. Management of Rabies in Humans. Clin. Infect. Dis. 2003, 36, 60–63. [Google Scholar] [CrossRef]
- Dacheux, L.; Delmas, O.; Bourhy, H. Human Rabies Encephalitis Prevention and Treatment: Progress Since Pasteurs Discovery. Infect. Disord. Drug Targets 2011, 11, 251–299. [Google Scholar] [CrossRef]
- Grard, G.; Fair, J.N.; Lee, D.; Slikas, E.; Steffen, I.; Muyembe, J.-J.; Sittler, T.; Veeraraghavan, N.; Ruby, J.G.; Wang, C.; et al. A Novel Rhabdovirus Associated with Acute Hemorrhagic Fever in Central Africa. PLoS Pathog. 2012, 8, e1002924. [Google Scholar] [CrossRef]
- Sapkal, G.N.; Sawant, P.M.; Mourya, D.T. Chandipura Viral Encephalitis: A Brief Review. Open Virol. J. 2018, 12, 44–51. [Google Scholar] [CrossRef]
- Badrane, H.; Tordo, N. Host Switching in Lyssavirus History from the Chiroptera to the Carnivora Orders. J. Virol. 2001, 75, 8096–8104. [Google Scholar] [CrossRef]
- Luo, D.-S.; Li, B.; Shen, X.-R.; Jiang, R.-D.; Zhu, Y.; Wu, J.; Fan, Y.; Bourhy, H.; Hu, B.; Ge, X.-Y.; et al. Characterization of Novel Rhabdoviruses in Chinese Bats. Viruses 2021, 13, 64. [Google Scholar] [CrossRef]
- Shipley, R.; Wright, E.; Selden, D.; Wu, G.; Aegerter, J.; Fooks, A.R.; Banyard, A.C. Bats and Viruses: Emergence of Novel Lyssaviruses and Association of Bats with Viral Zoonoses in the EU. Trop. Med. 2019, 4, 31. [Google Scholar] [CrossRef] [PubMed]
- Nel, L.H.; Markotter, W. Lyssaviruses. Crit. Rev. Microbiol. 2007, 33, 301–324. [Google Scholar] [CrossRef]
- Fischer, M.; Freuling, C.M.; Müller, T.; Schatz, J.; Rasmussen, T.B.; Chriel, M.; Balkema-Buschmann, A.; Beer, M.; Hoffmann, B. Identification of Rhabdoviral Sequences in Oropharyngeal Swabs from German and Danish Bats. Virol. J. 2014, 11, 196. [Google Scholar] [CrossRef] [PubMed]
- Aznar-Lopez, C.; Vazquez-Moron, S.; Marston, D.A.; Juste, J.; Ibáñez, C.; Berciano, J.M.; Salsamendi, E.; Aihartza, J.; Banyard, A.C.; McElhinney, L.; et al. Detection of Rhabdovirus Viral RNA in Oropharyngeal Swabs and Ectoparasites of Spanish Bats. J. Gen. Virol. 2013, 94, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Woodruff, A.W.; Ansdell, V.E.; Bowen, E.T. Le Dantec Virus Infection in a Patient Who Had Not Been to West Africa. BMJ 1977, 2, 1632–1633. [Google Scholar] [CrossRef]
- Cropp, C.B.; Prange, W.C.; Monath, T.P. LeDantec Virus: Identification as a Rhabdovirus Associated with Human Infection and Formation of a New Serogroup. J. Gen. Virol. 1985, 66, 2749–2754. [Google Scholar] [CrossRef]
- Shepherd, J.G.; Ashraf, S.; Salazar-Gonzalez, J.F.; Salazar, M.G.; Downing, R.G.; Bukenya, H.; Jerome, H.; Mpanga, J.T.; Davis, C.; Tong, L.; et al. Widespread Human Exposure to Ledanteviruses in Uganda: A Population Study. PLoS Negl. Trop. Dis. 2024, 18, e0012297. [Google Scholar] [CrossRef] [PubMed]
- Cotmore, S.F.; Agbandje-McKenna, M.; Canuti, M.; Chiorini, J.A.; Eis-Hubinger, A.-M.; Hughes, J.; Mietzsch, M.; Modha, S.; Ogliastro, M.; Pénzes, J.J.; et al. ICTV Virus Taxonomy Profile: Parvoviridae. J. Gen. Virol. 2019, 100, 367–368. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Söderlund-Venermo, M.; Young, N.S. Human Parvoviruses. Clin. Microbiol. Rev. 2017, 30, 43–113. [Google Scholar] [CrossRef]
- Shackelton, L.A.; Parrish, C.R.; Truyen, U.; Holmes, E.C. High Rate of Viral Evolution Associated with the Emergence of Carnivore Parvovirus. Proc. Natl. Acad. Sci. USA 2005, 102, 379–384. [Google Scholar] [CrossRef] [PubMed]
- Kailasan, S.; Agbandje-McKenna, M.; Parrish, C.R. Parvovirus Family Conundrum: What Makes a Killer? Annu. Rev. Virol. 2015, 2, 425–450. [Google Scholar] [CrossRef]
- Lau, S.K.P.; Ahmed, S.S.; Tsoi, H.-W.; Yeung, H.C.; Li, K.S.M.; Fan, R.Y.Y.; Zhao, P.S.H.; Lau, C.C.C.; Lam, C.S.F.; Choi, K.K.F.; et al. Bats Host Diverse Parvoviruses as Possible Origin of Mammalian Dependoparvoviruses and Source for Bat–Swine Interspecies Transmission. J. Gen. Virol. 2017, 98, 3046–3059. [Google Scholar] [CrossRef]
- Ramos, E.d.S.F.; Abreu, W.U.; Rodrigues, L.R.R.; Marinho, L.F.; Morais, V.d.S.; Villanova, F.; Pandey, R.P.; Araújo, E.L.L.; Deng, X.; Delwart, E.; et al. Novel Chaphamaparvovirus in Insectivorous Molossus Molossus Bats, from the Brazilian Amazon Region. Viruses 2023, 15, 606. [Google Scholar] [CrossRef]
- Matthijnssens, J.; Attoui, H.; Bányai, K.; Brussaard, C.P.D.; Danthi, P.; del Vas, M.; Dermody, T.S.; Duncan, R.; Fang, Q.; Johne, R.; et al. ICTV Virus Taxonomy Profile: Spinareoviridae 2022: This Article Is Part of the ICTV Virus Taxonomy Profiles Collection. J. Gen. Virol. 2022, 103, 001781. [Google Scholar] [CrossRef]
- Matthijnssens, J.; Attoui, H.; Bányai, K.; Brussaard, C.P.D.; Danthi, P.; del Vas, M.; Dermody, T.S.; Duncan, R.; Fang, Q.; Johne, R.; et al. ICTV Virus Taxonomy Profile: Sedoreoviridae 2022: This Article Is Part of the ICTV Virus Taxonomy Profiles Collection. J. Gen. Virol. 2022, 103, 001782. [Google Scholar] [CrossRef]
- Johnson, A.J.; Karabatsos, N.; Lanciotti, R.S. Detection of Colorado Tick Fever Virus by Using Reverse Transcriptase PCR and Application of the Technique in Laboratory Diagnosis. J. Clin. Microbiol. 1997, 35, 1203–1208. [Google Scholar] [CrossRef]
- Rosen, L.; Evans, H.E.; Spickard, A. Reovirus Infections In Human Volunteers. Am. J. Epidemiol. 1963, 77, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Tai, J.H.; Williams, J.V.; Edwards, K.M.; Wright, P.F.; Crowe, J.E., Jr.; Dermody, T.S. Prevalence of Reovirus-Specific Antibodies in Young Children in Nashville, Tennessee. J. Infect. Dis. 2005, 191, 1221–1224. [Google Scholar] [CrossRef] [PubMed]
- MacLachlan, N.J.; Guthrie, A.J. Re-Emergence of Bluetongue, African Horse Sickness, and Other Orbivirus Diseases. Vet. Res. 2010, 41, 35. [Google Scholar] [CrossRef]
- Eremyan, A.A.; Lvov, D.K.; Shchetinin, A.M.; Deryabin, P.G.; Aristova, V.A.; Gitelman, A.K.; Botikov, A.G.; Alkhovsky, S.V. Genetic Diversity of Viruses of Chenuda Virus Species (Orbivirus, Reoviridae) Circulating in Central Asia. Vopr. Virusol. 2017, 62, 81–86. [Google Scholar]
- LeClair, C.E.; McConnell, K.A. Rotavirus. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Belaganahalli, M.N.; Maan, S.; Maan, N.S.; Brownlie, J.; Tesh, R.; Attoui, H.; Mertens, P.P.C. Genetic Characterization of the Tick-Borne Orbiviruses. Viruses 2015, 7, 2185–2209. [Google Scholar] [CrossRef]
- Steyer, A.; Gutiérrez-Aguire, I.; Kolenc, M.; Koren, S.; Kutnjak, D.; Pokorn, M.; Poljšak-Prijatelj, M.; Rački, N.; Ravnikar, M.; Sagadin, M.; et al. High Similarity of Novel Orthoreovirus Detected in a Child Hospitalized with Acute Gastroenteritis to Mammalian Orthoreoviruses Found in Bats in Europe. J. Clin. Microbiol. 2013, 51, 3818–3825. [Google Scholar] [CrossRef]
- Chua, K.B.; Voon, K.; Crameri, G.; Tan, H.S.; Rosli, J.; McEachern, J.A.; Suluraju, S.; Yu, M.; Wang, L.-F. Identification and Characterization of a New Orthoreovirus from Patients with Acute Respiratory Infections. PLoS ONE 2008, 3, e3803. [Google Scholar] [CrossRef]
- King, A.M. Virus Taxonomy: Ninth Report of the International Committee on Taxonomy of Virst; Elsevier: St. Louis, MO, USA, 2011; ISBN 978-0-12-384685-3. [Google Scholar]
- Gatherer, D.; Depledge, D.P.; Hartley, C.A.; Szpara, M.L.; Vaz, P.K.; Benkő, M.; Brandt, C.R.; Bryant, N.A.; Dastjerdi, A.; Doszpoly, A.; et al. ICTV Virus Taxonomy Profile: Herpesviridae 2021. J. Gen. Virol. 2021, 102, 001673. [Google Scholar] [CrossRef] [PubMed]
- Drexler, J.F.; Seelen, A.; Corman, V.M.; Fumie Tateno, A.; Cottontail, V.; Melim Zerbinati, R.; Gloza-Rausch, F.; Klose, S.M.; Adu-Sarkodie, Y.; Oppong, S.K.; et al. Bats Worldwide Carry Hepatitis E Virus-Related Viruses That Form a Putative Novel Genus within the Family Hepeviridae. J. Virol. 2012, 86, 9134–9147. [Google Scholar] [CrossRef]
- Purdy, M.A.; Drexler, J.F.; Meng, X.-J.; Norder, H.; Okamoto, H.; Van der Poel, W.H.M.; Reuter, G.; de Souza, W.M.; Ulrich, R.G.; Smith, D.B. ICTV Virus Taxonomy Profile: Hepeviridae 2022. J. Gen. Virol. 2022, 103, 001778. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.-J.; Halbur, P.G.; Shapiro, M.S.; Govindarajan, S.; Bruna, J.D.; Mushahwar, I.K.; Purcell, R.H.; Emerson, S.U. Genetic and Experimental Evidence for Cross-Species Infection by Swine Hepatitis E Virus. J. Virol. 1998, 72, 9714–9721. [Google Scholar] [CrossRef]
- Primadharsini, P.P.; Nagashima, S.; Okamoto, H. Mechanism of Cross-Species Transmission, Adaptive Evolution and Pathogenesis of Hepatitis E Virus. Viruses 2021, 13, 909. [Google Scholar] [CrossRef]
- Van Doorslaer, K.; Chen, Z.; Bernard, H.-U.; Chan, P.K.S.; DeSalle, R.; Dillner, J.; Forslund, O.; Haga, T.; McBride, A.A.; Villa, L.L.; et al. ICTV Virus Taxonomy Profile: Papillomaviridae. J. Gen. Virol. 2018, 99, 989–990. [Google Scholar] [CrossRef]
- Peh, W.L.; Middleton, K.; Christensen, N.; Nicholls, P.; Egawa, K.; Sotlar, K.; Brandsma, J.; Percival, A.; Lewis, J.; Liu, W.J.; et al. Life Cycle Heterogeneity in Animal Models of Human Papillomavirus-Associated Disease. J. Virol. 2002, 76, 10401–10416. [Google Scholar] [CrossRef]
- Munday, J.S.; Hanlon, E.M.; Howe, L.; Squires, R.A.; French, A.F. Feline Cutaneous Viral Papilloma Associated with Human Papillomavirus Type 9. Vet. Pathol. 2007, 44, 924–927. [Google Scholar] [CrossRef]
- Van Dyk, E.; Bosman, A.-M.; Van Wilpe, E.; Williams, J.H.; Bengis, R.G.; Van Heerden, J.; Venter, E.H. Detection and Characterisation of Papillomavirus in Skin Lesions of Giraffe and Sable Antelope in South Africa. J. S. Afr. Vet. Assoc. 2011, 82, 80–85. [Google Scholar] [CrossRef]
- Alves, C.D.B.T.; Weber, M.N.; Guimarães, L.L.B.; Cibulski, S.P.; da Silva, F.R.C.; Daudt, C.; Budaszewski, R.F.; Silva, M.S.; Mayer, F.Q.; Bianchi, R.M.; et al. Canine Papillomavirus Type 16 Associated to Squamous Cell Carcinoma in a Dog: Virological and Pathological Findings. Braz. J. Microbiol. 2020, 51, 2087–2094. [Google Scholar] [CrossRef] [PubMed]
- de Camargo, L.J.; Alves, R.S.; dos Santos, R.N.; Baumbach, L.F.; Olegário, J.d.C.; Rabaioli, V.; Silva, M.d.O.; Witt, A.A.; Godinho, F.M.; Salvato, R.S.; et al. Characterization of Three Novel Papillomavirus Genomes in Vampire Bats (Desmodus Rotundus). Animals 2024, 14, 3604. [Google Scholar] [CrossRef]
- García-Pérez, R.; Ibáñez, C.; Godínez, J.M.; Aréchiga, N.; Garin, I.; Pérez-Suárez, G.; de Paz, O.; Juste, J.; Echevarría, J.E.; Bravo, I.G. Novel Papillomaviruses in Free-Ranging Iberian Bats: No Virus–Host Co-Evolution, No Strict Host Specificity, and Hints for Recombination. Genome Biol. Evol. 2014, 6, 94–104. [Google Scholar] [CrossRef] [PubMed]
- Buigues, J.; Viñals, A.; Martínez-Recio, R.; Monrós, J.S.; Sanjuán, R.; Cuevas, J.M. Full-Genome Sequencing of Dozens of New DNA Viruses Found in Spanish Bat Feces. Microbiol. Spectr. 2024, 12, e00675-24. [Google Scholar] [CrossRef]
- McKnight, C.A.; Wise, A.G.; Maes, R.K.; Howe, C.; Rector, A.; Van Ranst, M.; Kiupel, M. Papillomavirus-Associated Basosquamous Carcinoma in an Egyptian Fruit Bat (Rousettus Aegyptiacus). J. Zoo. Wildl. Med. 2006, 37, 193–196. [Google Scholar] [CrossRef]
- Moens, U.; Calvignac-Spencer, S.; Lauber, C.; Ramqvist, T.; Feltkamp, M.C.W.; Daugherty, M.D.; Verschoor, E.J.; Ehlers, B. ICTV Report Consortium ICTV Virus Taxonomy Profile: Polyomaviridae. J. Gen. Virol. 2017, 98, 1159–1160. [Google Scholar] [CrossRef] [PubMed]
- Krumbholz, A.; Bininda-Emonds, O.R.P.; Wutzler, P.; Zell, R. Phylogenetics, Evolution, and Medical Importance of Polyomaviruses. Infect. Genet. Evol. 2009, 9, 784–799. [Google Scholar] [CrossRef] [PubMed]
- Buck, C.B.; Van Doorslaer, K.; Peretti, A.; Geoghegan, E.M.; Tisza, M.J.; An, P.; Katz, J.P.; Pipas, J.M.; McBride, A.A.; Camus, A.C.; et al. The Ancient Evolutionary History of Polyomaviruses. PLoS Pathog. 2016, 12, e1005574. [Google Scholar] [CrossRef]
- Shah, P.T.; Ejaz, M.; Tamanna, K.; Riaz, M.N.; Wu, Z.; Wu, C. Insights into the Genetic Characteristics, Clustering Patterns, and Phylogeographic Dynamics of the JC Polyomavirus, 1993 to 2023. Virus Res. 2024, 346, 199414. [Google Scholar] [CrossRef]
- Carr, M.J.; Gonzalez, G.; Teeling, E.C.; Sawa, H. Bat Polyomaviruses: A Challenge to the Strict Host-Restriction Paradigm within the Mammalian Polyomaviridae. In Bats and Viruses: Current Research and Future Trends; Caister Academic Press: Norfolk, UK, 2020; ISBN 978-1-912530-14-4. [Google Scholar]
- Surján, A.; Gonzalez, G.; Gellért, Á.; Boldogh, S.; Carr, M.J.; Harrach, B.; Vidovszky, M.Z. First Detection and Genome Analysis of Simple Nosed Bat Polyomaviruses in Central Europe. Infect. Genet. Evol. 2023, 112, 31–42. [Google Scholar] [CrossRef]
- Magnius, L.; Mason, W.S.; Taylor, J.; Kann, M.; Glebe, D.; Dény, P.; Sureau, C.; Norder, H. ICTV Report Consortium ICTV Virus Taxonomy Profile: Hepadnaviridae. J. Gen. Virol. 2020, 101, 571–572. [Google Scholar] [CrossRef] [PubMed]
- Drexler, J.F.; Geipel, A.; König, A.; Corman, V.M.; van Riel, D.; Leijten, L.M.; Bremer, C.M.; Rasche, A.; Cottontail, V.M.; Maganga, G.D.; et al. Bats Carry Pathogenic Hepadnaviruses Antigenically Related to Hepatitis B Virus and Capable of Infecting Human Hepatocytes. Proc. Natl. Acad. Sci. USA 2013, 110, 16151–16156. [Google Scholar] [CrossRef]
- He, B.; Zhang, F.; Xia, L.; Hu, T.; Chen, G.; Qiu, W.; Fan, Q.; Feng, Y.; Guo, H.; Tu, C. Identification of a Novel Orthohepadnavirus in Pomona Roundleaf Bats in China. Arch. Virol. 2015, 160, 335–337. [Google Scholar] [CrossRef]
- Breitbart, M.; Delwart, E.; Rosario, K.; Segalés, J.; Varsani, A. ICTV Report Consortium ICTV Virus Taxonomy Profile: Circoviridae. J. Gen. Virol. 2017, 98, 1997–1998. [Google Scholar] [CrossRef]
- Fogell, D.J.; Martin, R.O.; Groombridge, J.J. Beak and Feather Disease Virus in Wild and Captive Parrots: An Analysis of Geographic and Taxonomic Distribution and Methodological Trends. Arch. Virol. 2016, 161, 2059–2074. [Google Scholar] [CrossRef]
- Segalés, J.; Sibila, M. Revisiting Porcine Circovirus Disease Diagnostic Criteria in the Current Porcine Circovirus 2 Epidemiological Context. Vet. Sci. 2022, 9, 110. [Google Scholar] [CrossRef]
- Li, L.; Victoria, J.G.; Wang, C.; Jones, M.; Fellers, G.M.; Kunz, T.H.; Delwart, E. Bat Guano Virome: Predominance of Dietary Viruses from Insects and Plants plus Novel Mammalian Viruses. J. Virol. 2010, 84, 6955–6965. [Google Scholar] [CrossRef]
- Ge, X.; Li, J.; Peng, C.; Wu, L.; Yang, X.; Wu, Y.; Zhang, Y.; Shi, Z. Genetic Diversity of Novel Circular SsDNA Viruses in Bats in China. J. Gen. Virol. 2011, 92, 2646–2653. [Google Scholar] [CrossRef]
- Li, L.; Shan, T.; Soji, O.B.; Alam, M.M.; Kunz, T.H.; Zaidi, S.Z.; Delwart, E. Possible Cross-Species Transmission of Circoviruses and Cycloviruses among Farm Animals. J. Gen. Virol. 2011, 92, 768–772. [Google Scholar] [CrossRef] [PubMed]
- Silva, D.F.D.; Ciola, M.; Lopes, V.D.O.; Matias, D.R.M.; Oliveira, T.S.; Castro, A.M.M.G.D. Canine Circovirus: Emergence, Adaptation, and Challenges for Animal and Public Health. Front. Vet. Sci. 2025, 12, 1535650. [Google Scholar] [CrossRef]
- Vinjé, J.; Estes, M.K.; Esteves, P.; Green, K.Y.; Katayama, K.; Knowles, N.J.; L’Homme, Y.; Martella, V.; Vennema, H.; White, P.A.; et al. ICTV Virus Taxonomy Profile: Caliciviridae. J. Gen. Virol. 2019, 100, 1469–1470. [Google Scholar] [CrossRef]
- Mukhina, A.A.; Shipulin, G.A.; Podkolzin, A.T.; Malaev, V.V. Calicivirus infection. Infect. Dis. 2004, 2, 64–73. [Google Scholar]
- Kocher, J.F.; Lindesmith, L.C.; Debbink, K.; Beall, A.; Mallory, M.L.; Yount, B.L.; Graham, R.L.; Huynh, J.; Gates, J.E.; Donaldson, E.F.; et al. Bat Caliciviruses and Human Noroviruses Are Antigenically Similar and Have Overlapping Histo-Blood Group Antigen Binding Profiles. mBio 2018, 9, e00869-18. [Google Scholar] [CrossRef] [PubMed]
| Viral Family | Genome | Host | Publication Number | References |
|---|---|---|---|---|
| High risk | ||||
| Coronaviridae | (+)RNA | Mammals, birds, amphibians, fish | 21 | [5,7,8,25,26,27,28,49,50,51,52,53,54,55,56,57,58,59,60,61,62] |
| Paramyxoviridae | (−)RNA | Mammals, birds, reptiles, fish | 9 | [7,8,26,28,63,64,65,66,67,68] |
| Hantaviridae | (−)RNA | Mammals, reptiles, fish | 5 | [29,30,31,32,69] |
| Filoviridae | (−)RNA | Mammals, reptiles, fish | 3 | [67,70,71] |
| Flaviviridae | (+)RNA | Mammals; most Orthoflavivirus are arthropod-borne | 3 | [5,6,8] |
| Poxviridae | dsDNA | Vertebrates, arthropods | 3 | [5,6,8] |
| Togaviridae | (+)RNA | Vertebrates, arthropods; most alphaviruses are mosquito-borne | 2 | [5,6] |
| Medium risk | ||||
| Picornaviridae | (+)RNA | Vertebrates | 6 | [5,6,7,8,72,73] |
| Retroviridae | ssRNA-RT | Mammals, birds, signs of ERVs in genomes of other vertebrates and invertebrates | 4 | [5,6,8,66] |
| * Adenoviridae | dsDNA | Vertebrates | 3 | [5,7,74] |
| Low risk | ||||
| Astroviridae | (+)RNA | Birds, Mammals | 8 | [5,7,8,55,75,76,77,78] |
| Rhabdoviridae | (−)RNA | Vertebrates, insects and plants; many vertebrate and plant rhabdoviruses are arthropod-borne | 7 | [7,67,79,80,81,82,83] |
| Parvoviridae | dsDNA | Mammals, birds, reptiles, insects, crustacea, echinoderms | 6 | [5,6,7,8,84,85] |
| Spinareoviridae and Sedoreoviridae | dsDNA | Mammals, fish, birds, reptiles, arthropods, plants, fungi | 6 | [5,6,7,8,86,87] |
| Orthoherpesviridae | dsDNA | Mammals, birds, reptiles | 5 | [5,6,8,88,89] |
| Hepeviridae | (+)RNA | Mammals, birds, fish | 3 | [5,6,8] |
| Papillomaviridae | dsDNA | Mammals, birds, reptiles, fish | 3 | [5,6,8] |
| Polyomaviridae | dsDNA | Mammals, birds, fish | 3 | [7,8,66] |
| Hepadnaviridae | dsDNA | Vertebrates | 2 | [90,91] |
| Anelloviridae | ssDNA | Mammals, birds | 2 | [7,8] |
| Picobirnaviridae | dsDNA | Mammals, birds, reptiles, invertebrates | 1 | [8] |
| Other | ||||
| Circoviridae | ssDNA | Mammals, birds, fish, arthropods | 6 | [5,6,7,8,92,93,94] |
| Caliciviridae | (+)RNA | Mammals, birds, fish | 3 | [5,7,8] |
| Unclassified Order: Picornavirales, Class: Bunyaviricetes | RNA | Vertebrates, invertebrates, protists, plants | 2 | [95,96] |
| Asfarviridae | dsDNA | Suidae | 1 | [5] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lapshina, V.K.; Guskova, N.I.; Stetsenko, I.F.; Luong, M.T.; Tran, T.V.; Matsvay, A.D.; Shipulin, G.A.; Yudin, S.M.; Skvortsova, V.I. Characterizing the Bat Virome of Vietnam: A Systematic Review of Viral Diversity and Zoonotic Potential. Viruses 2025, 17, 1532. https://doi.org/10.3390/v17121532
Lapshina VK, Guskova NI, Stetsenko IF, Luong MT, Tran TV, Matsvay AD, Shipulin GA, Yudin SM, Skvortsova VI. Characterizing the Bat Virome of Vietnam: A Systematic Review of Viral Diversity and Zoonotic Potential. Viruses. 2025; 17(12):1532. https://doi.org/10.3390/v17121532
Chicago/Turabian StyleLapshina, Vasilina K., Natalia I. Guskova, Ivan F. Stetsenko, Mo T. Luong, Truong V. Tran, Alina D. Matsvay, German A. Shipulin, Sergey M. Yudin, and Veronika I. Skvortsova. 2025. "Characterizing the Bat Virome of Vietnam: A Systematic Review of Viral Diversity and Zoonotic Potential" Viruses 17, no. 12: 1532. https://doi.org/10.3390/v17121532
APA StyleLapshina, V. K., Guskova, N. I., Stetsenko, I. F., Luong, M. T., Tran, T. V., Matsvay, A. D., Shipulin, G. A., Yudin, S. M., & Skvortsova, V. I. (2025). Characterizing the Bat Virome of Vietnam: A Systematic Review of Viral Diversity and Zoonotic Potential. Viruses, 17(12), 1532. https://doi.org/10.3390/v17121532






