Cytokine and Antibody Isotype Responses in Vaccinated Healthcare Workers with SARS-CoV-2 Breakthrough Infections
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Information and Ethics Statement
2.2. Ethical Statement
2.3. Sample Processing
2.4. Cytokine Assay
2.5. SARS-CoV-2 Antibody Responses
Data Handling and Statistical Analysis
3. Results
3.1. Demographic and Clinical Characteristics of SARS-CoV-2+ HCWs
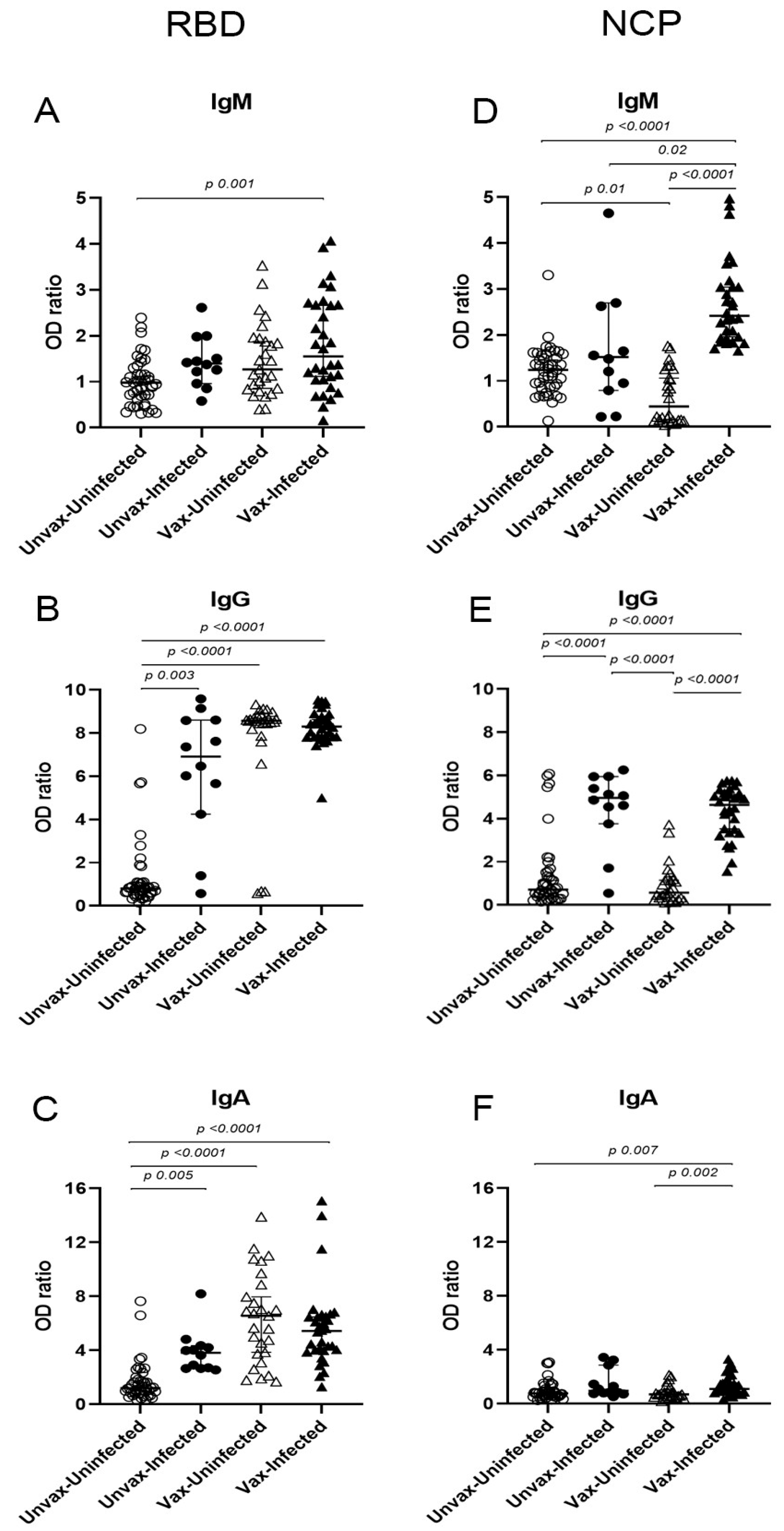
3.2. Anti-SARS-CoV-2 Specific Antibodies
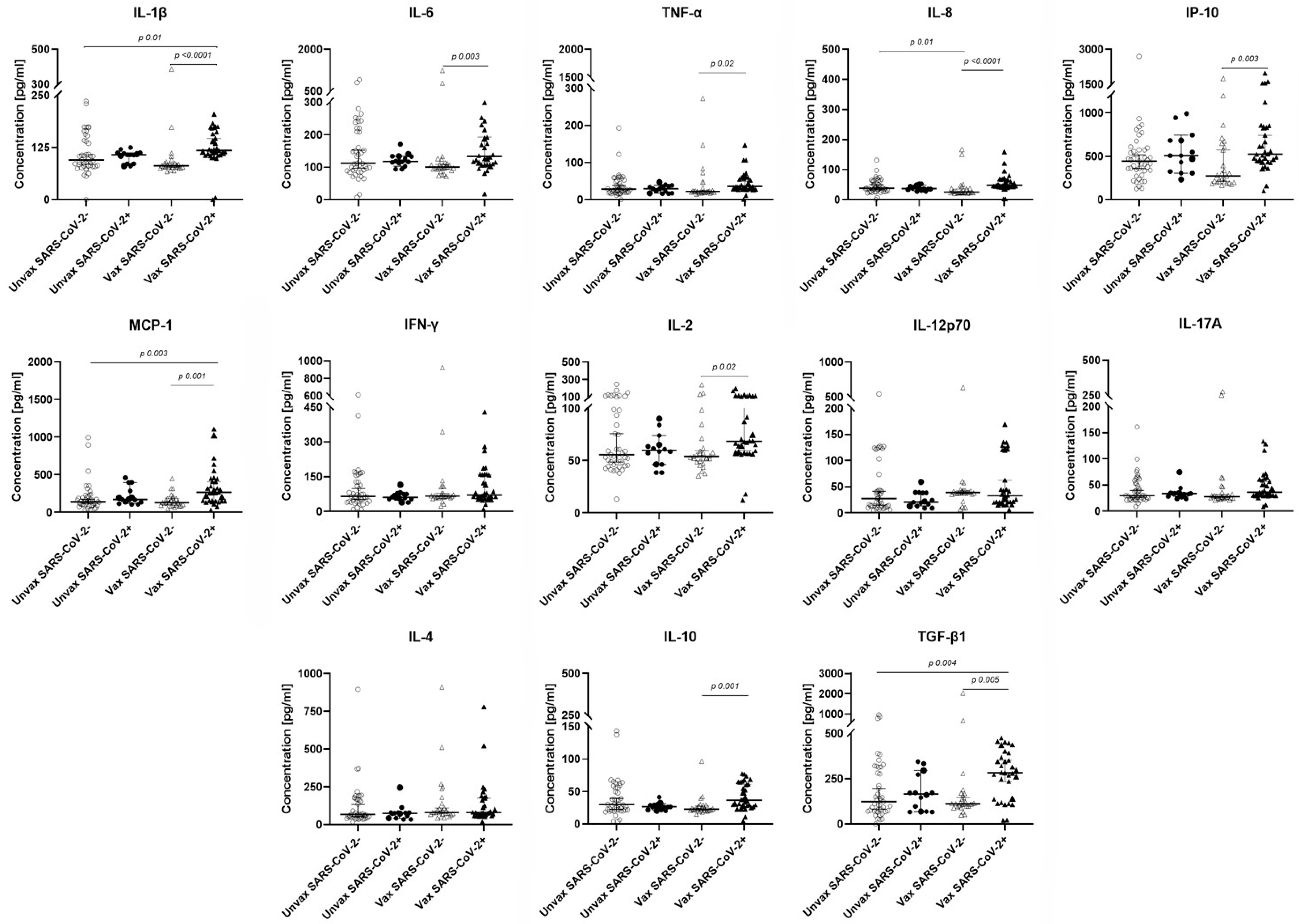
3.3. Cytokine Levels in HCWs
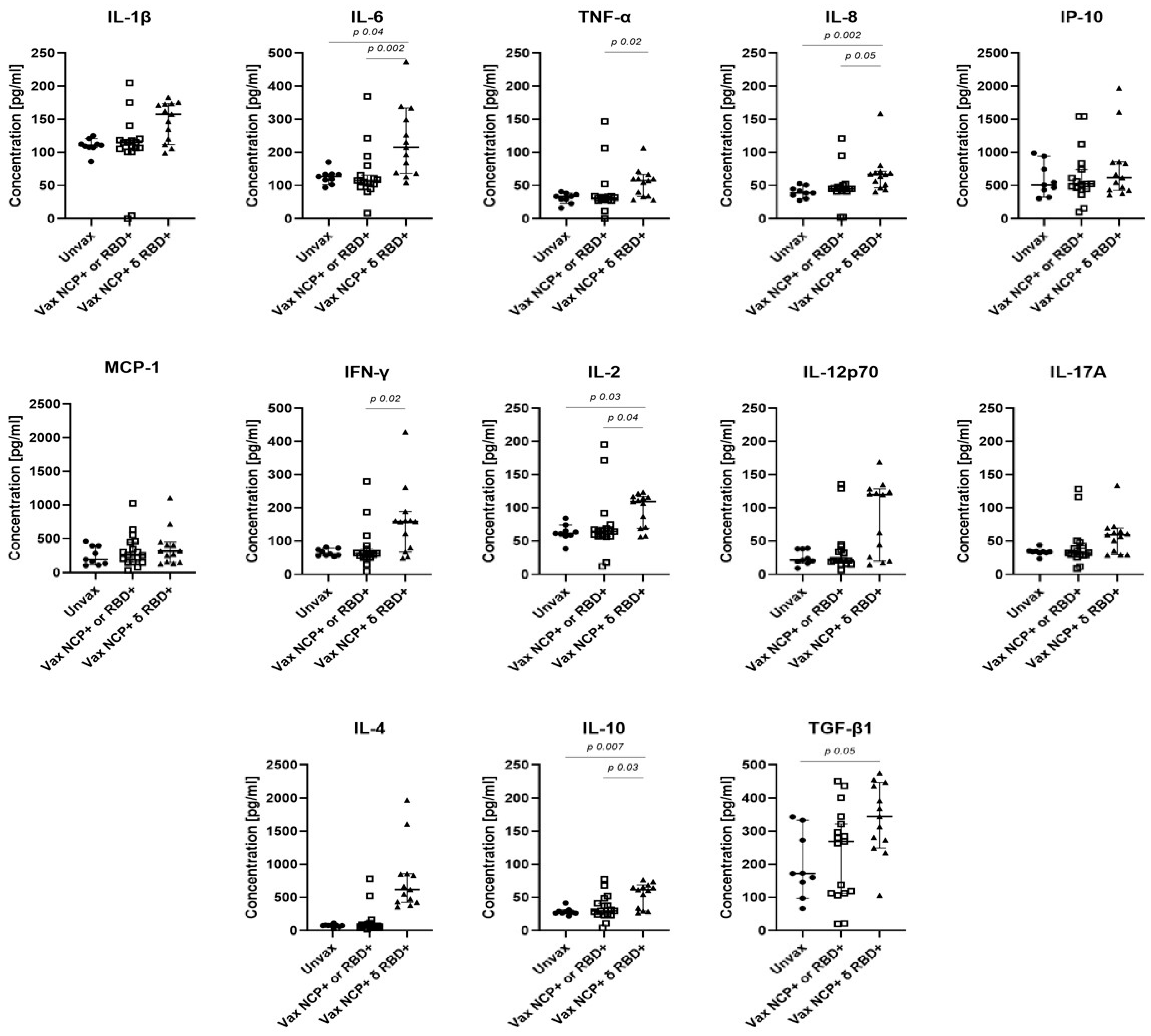
3.4. Association of the IgM and IgA Responses with Cytokine Concentrations
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grupo Técnico Asesor de Vacunación COVID-19. Priorización inicial y consecutiva para la vacunación contra SARS-CoV-2 en la población mexicana. Recomendaciones preliminares. Salud Publica Mex. 2020, 63, 288–309. [Google Scholar] [CrossRef] [PubMed]
- Widyasari, K.; Jang, J.; Lee, S.; Kang, T.; Kim, S. Evaluation of the T Cell and B Cell Response Following the Administration of COVID-19 Vaccines in Korea. J. Microbiol. Immunol. Infect. 2022, 55, 1013–1024. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.-W.; Widyasari, K.; Jang, J.; Lee, S.; Kang, T.; Kim, S. Kinetics of Adaptive Immune Responses after Administering mRNA-Based COVID-19 Vaccination in Individuals with and without Prior SARS-CoV-2 Infections. BMC Infect. Dis. 2023, 23, 732. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-de Prada, L.; Martínez-García, A.M.; González-Fernández, B.; Gutiérrez-Ballesteros, J.; Rojo-Rello, S.; Garcinuño-Pérez, S.; Álvaro-Meca, A.; Ortiz De Lejarazu, R.; Sanz-Muñoz, I.; Eiros, J.M. Impact on the Time Elapsed since SARS-CoV-2 Infection, Vaccination History, and Number of Doses, on Protection against Reinfection. Sci. Rep. 2024, 14, 353. [Google Scholar] [CrossRef]
- Rubio, R.; Macià, D.; Barrios, D.; Vidal, M.; Jiménez, A.; Molinos-Albert, L.M.; Díaz, N.; Canyelles, M.; Lara-Escandell, M.; Planchais, C.; et al. High-Resolution Kinetics and Cellular Determinants of SARS-CoV-2 Antibody Response over Two Years after COVID-19 Vaccination. Microbes Infect. 2025, 27, 105423. [Google Scholar] [CrossRef]
- Serwanga, J.; Ankunda, V.; Katende, J.S.; Baine, C.; Oluka, G.K.; Odoch, G.; Nantambi, H.; Mugaba, S.; Namuyanja, A.; Ssali, I.; et al. Sustained S-IgG and S-IgA Antibodies to Moderna’s mRNA-1273 Vaccine in a Sub-Saharan African Cohort Suggests Need for Booster Timing Reconsiderations. Front. Immunol. 2024, 15, 1348905. [Google Scholar] [CrossRef]
- Ruggiero, A.; Piubelli, C.; Calciano, L.; Accordini, S.; Valenti, M.T.; Carbonare, L.D.; Siracusano, G.; Temperton, N.; Tiberti, N.; Longoni, S.S.; et al. SARS-CoV-2 Vaccination Elicits Unconventional IgM Specific Responses in Naïve and Previously COVID-19-Infected Individuals. EBioMedicine 2022, 77, 103888. [Google Scholar] [CrossRef]
- Chaudhury, S.; Hutter, J.; Bolton, J.S.; Hakre, S.; Mose, E.; Wooten, A.; O’Connell, W.; Hudak, J.; Krebs, S.J.; Darden, J.M.; et al. Serological Profiles of Pan-Coronavirus-Specific Responses in COVID-19 Patients Using a Multiplexed Electro-Chemiluminescence-Based Testing Platform. PLoS ONE 2021, 16, e0252628. [Google Scholar] [CrossRef]
- Kryukova, N.; Baranova, I.; Abramova, N.; Khromova, E.; Pachomov, D.; Svitich, O.; Chuchalin, A.; Kostinov, M. Mucosal Immunity in Health Care Workers’ Respiratory Tracts in the Post-COVID-19 Period. Sci. Rep. 2023, 13, 7162. [Google Scholar] [CrossRef]
- Fernández-Rojas, M.A.; Ávila, G.; Romero-Valdovinos, M.; Plett-Torres, T.; Salazar, A.M.; Sordo, M.; Chávez-Vargas, M.; Coeto Ángeles, C.J.; Cruz-Rivera, M.; Santiago-Olivares, C.; et al. Elevated Levels of Cytotoxicity, Cytokines, and Anti-SARS-CoV-2 Antibodies in Mild Cases of COVID-19. Viral Immunol. 2023, 36, 550–561. [Google Scholar] [CrossRef]
- Bergamaschi, C.; Terpos, E.; Rosati, M.; Angel, M.; Bear, J.; Stellas, D.; Karaliota, S.; Apostolakou, F.; Bagratuni, T.; Patseas, D.; et al. Systemic IL-15, IFN-γ, and IP-10/CXCL10 Signature Associated with Effective Immune Response to SARS-CoV-2 in BNT162b2 mRNA Vaccine Recipients. Cell Rep. 2021, 36, 109504. [Google Scholar] [CrossRef] [PubMed]
- Castro-Trujillo, S.; Castro-Meneses, J.; Rojas, M.C.; Castro-Amaya, M.; Lastra, G.; Narváez, C.F. Regulatory Cytokines Modulate Early Isotype-Specific Response Associated with COVID-19 Survival. Front. Immunol. 2025, 16, 1543626. [Google Scholar] [CrossRef] [PubMed]
- Samaan, P.; Korosec, C.S.; Budylowski, P.; Chau, S.L.L.; Pasculescu, A.; Qi, F.; Delgado-Brand, M.; Tursun, T.R.; Mailhot, G.; Dayam, R.M.; et al. mRNA Vaccine-Induced SARS-CoV-2 Spike-Specific IFN-γ and IL-2 T-Cell Responses Are Predictive of Serological Neutralization and Are Transiently Enhanced by Pre-Existing Cross-Reactive Immunity. J. Virol. 2025, 99, e0168524. [Google Scholar] [CrossRef] [PubMed]
- Alghamdi, A.; Hussain, S.D.; Wani, K.; Sabico, S.; Alnaami, A.M.; Amer, O.E.; Al-Daghri, N.M. Altered Circulating Cytokine Profile Among mRNA-Vaccinated Young Adults: A Year-Long Follow-Up Study. Immun. Inflamm. Dis. 2025, 13, e70194. [Google Scholar] [CrossRef]
- González-Arenas, N.R.; Chavez-Vargas, M.D.; Prado-Calleros, H.; Ramírez-Hinojosa, J.P.; Martinez-Hernandez, F.; Olivo-Díaz, A.; Maravilla, P.; Romero-Valdovinos, M.; Ávila-Ramírez, G. Titers of IgG, IgM, and IgA Against SARS-CoV-2 in Healthcare Workers from a General Hospital in Mexico City. Diseases 2025, 13, 276. [Google Scholar] [CrossRef]
- Martinez-Cajas, J.L.; Perez-Patrigeon, S.; Evans, G.A.; Stoner, B.; Jolly, A.; Alvarado, B.; Guan, H.; Gong, Y. Anti-SARS-CoV-2 Antibody Levels in a Cohort of Health Care Workers Before and After the Omicron Wave in Canada. J. Assoc. Med. Microbiol. Infect. Dis. Can. 2025, 10, 127–145. [Google Scholar] [CrossRef]
- Tuck, M.K.; Chan, D.W.; Chia, D.; Godwin, A.K.; Grizzle, W.E.; Krueger, K.E.; Rom, W.; Sanda, M.; Sorbara, L.; Stass, S.; et al. Standard Operating Procedures for Serum and Plasma Collection: Early Detection Research Network Consensus Statement Standard Operating Procedure Integration Working Group. J. Proteome Res. 2009, 8, 113–117. [Google Scholar] [CrossRef]
- Ramirez-Hinojosa, J.P.; Rodriguez-Sanchez, Y.; Romero-Gonzalez, A.K.; Chavez-Gutierrez, M.; Gonzalez-Arenas, N.R.; Ibarra-Arce, A.; Arroyo-Escalante, S.; Zavaleta-Villa, B.; Leon-Juarez, M.; Cruz-Holguin, V.J.; et al. Association between Cycle Threshold (Ct) Values and Clinical and Laboratory Data in Inpatients with COVID-19 and Asymptomatic Health Workers. J. Med. Virol. 2021, 93, 5969–5976. [Google Scholar] [CrossRef]
- Sun, B.; Feng, Y.; Mo, X.; Zheng, P.; Wang, Q.; Li, P.; Peng, P.; Liu, X.; Chen, Z.; Huang, H.; et al. Kinetics of SARS-CoV-2 Specific IgM and IgG Responses in COVID-19 Patients. Emerg. Microbes Infect. 2020, 9, 940–948. [Google Scholar] [CrossRef]
- Li, Y.; Lai, D.-Y.; Lei, Q.; Xu, Z.-W.; Wang, F.; Hou, H.; Chen, L.; Wu, J.; Ren, Y.; Ma, M.-L.; et al. Systematic Evaluation of IgG Responses to SARS-CoV-2 Spike Protein-Derived Peptides for Monitoring COVID-19 Patients. Cell Mol. Immunol. 2021, 18, 621–631. [Google Scholar] [CrossRef]
- Fraser, D.D.; Singh, D.; Cela, E.; Patel, M.A.; Assaf, M.; Quintero, M.; Knauer, M.; Miller, M.R.; Bellini, M.; Li, A.; et al. Neutralizing Antibodies to SARS-CoV-2 Variants of Concern: A Pediatric Surveillance Study. Sci. Rep. 2025, 15, 11588. [Google Scholar] [CrossRef]
- Fernández-Rojas, M.A.; Luna-Ruiz Esparza, M.A.; Campos-Romero, A.; Calva-Espinosa, D.Y.; Moreno-Camacho, J.L.; Mendlovic, F.; Plett-Torres, T.; Alcántar-Fernández, J. Seroconversion Dynamic and SARS-CoV-2 Seropositivity in Unvaccinated Population during the First and Second Outbreaks in Mexico. Sci. Rep. 2022, 12, 5241. [Google Scholar] [CrossRef]
- Goel, R.R.; Apostolidis, S.A.; Painter, M.M.; Mathew, D.; Pattekar, A.; Kuthuru, O.; Gouma, S.; Hicks, P.; Meng, W.; Rosenfeld, A.M.; et al. Distinct Antibody and Memory B Cell Responses in SARS-CoV-2 Naïve and Recovered Individuals Following mRNA Vaccination. Sci. Immunol. 2021, 6, eabi6950. [Google Scholar] [CrossRef]
- Harrington, W.E.; Trakhimets, O.; Andrade, D.V.; Dambrauskas, N.; Raappana, A.; Jiang, Y.; Houck, J.; Selman, W.; Yang, A.; Vigdorovich, V.; et al. Rapid Decline of Neutralizing Antibodies Is Associated with Decay of IgM in Adults Recovered from Mild COVID-19. Cell Rep. Med. 2021, 2, 100253. [Google Scholar] [CrossRef] [PubMed]
- Kared, H.; Wolf, A.-S.; Alirezaylavasani, A.; Ravussin, A.; Solum, G.; Tran, T.T.; Lund-Johansen, F.; Vaage, J.T.; Nissen-Meyer, L.S.; Nygaard, U.C.; et al. Immune Responses in Omicron SARS-CoV-2 Breakthrough Infection in Vaccinated Adults. Nat. Commun. 2022, 13, 4165. [Google Scholar] [CrossRef] [PubMed]
- WHO Interim Statement on Hybrid Immunity and Increasing Population Seroprevalence Rates. Available online: https://www.who.int/news/item/01-06-2022-interim-statement-on-hybrid-immunity-and-increasing-population-seroprevalence-rates (accessed on 2 July 2025).
- D’Orso, S.; Pirronello, M.; Verdiani, A.; Rossini, A.; Guerrera, G.; Picozza, M.; Sambucci, M.; Misiti, A.; De Marco, L.; Salvia, A.; et al. Primary and Recall Immune Responses to SARS-CoV-2 in Breakthrough Infection. Vaccines 2023, 11, 1705. [Google Scholar] [CrossRef]
- Denis, J.; Garnier, A.; Cheutin, L.; Ferrier, A.; Timera, H.; Jarjaval, F.; Hejl, C.; Billon-Denis, E.; Percy ImmunoCovid group; Ricard, D.; et al. Long-Term Systemic and Mucosal SARS-CoV-2 IgA Response and Its Association with Persistent Smell and Taste Disorders. Front. Immunol. 2023, 14, 1140714. [Google Scholar] [CrossRef] [PubMed]
- Hsu, R.-J.; Yu, W.-C.; Peng, G.-R.; Ye, C.-H.; Hu, S.; Chong, P.C.T.; Yap, K.Y.; Lee, J.Y.C.; Lin, W.-C.; Yu, S.-H. The Role of Cytokines and Chemokines in Severe Acute Respiratory Syndrome Coronavirus 2 Infections. Front. Immunol. 2022, 13, 832394. [Google Scholar] [CrossRef]
- Branchett, W.J.; Saraiva, M.; O’Garra, A. Regulation of Inflammation by Interleukin-10 in the Intestinal and Respiratory Mucosa. Curr. Opin. Immunol. 2024, 91, 102495. [Google Scholar] [CrossRef]
- Rosati, M.; Terpos, E.; Homan, P.; Bergamaschi, C.; Karaliota, S.; Ntanasis-Stathopoulos, I.; Devasundaram, S.; Bear, J.; Burns, R.; Bagratuni, T.; et al. Rapid Transient and Longer-Lasting Innate Cytokine Changes Associated with Adaptive Immunity after Repeated SARS-CoV-2 BNT162b2 mRNA Vaccinations. Front. Immunol. 2023, 14, 1292568. [Google Scholar] [CrossRef]
- Benhamouda, N.; Besbes, A.; Bauer, R.; Mabrouk, N.; Gadouas, G.; Desaint, C.; Chevrier, L.; Lefebvre, M.; Radenne, A.; Roelens, M.; et al. Cytokine Profile of Anti-Spike CD4+T Cells Predicts Humoral and CD8+T Cell Responses after Anti-SARS-CoV-2 mRNA Vaccination. iScience 2024, 27, 110441. [Google Scholar] [CrossRef]
- Tarke, A.; Ramezani-Rad, P.; Alves Pereira Neto, T.; Lee, Y.; Silva-Moraes, V.; Goodwin, B.; Bloom, N.; Siddiqui, L.; Avalos, L.; Frazier, A.; et al. SARS-CoV-2 Breakthrough Infections Enhance T Cell Response Magnitude, Breadth, and Epitope Repertoire. Cell Rep. Med. 2024, 5, 101583. [Google Scholar] [CrossRef] [PubMed]
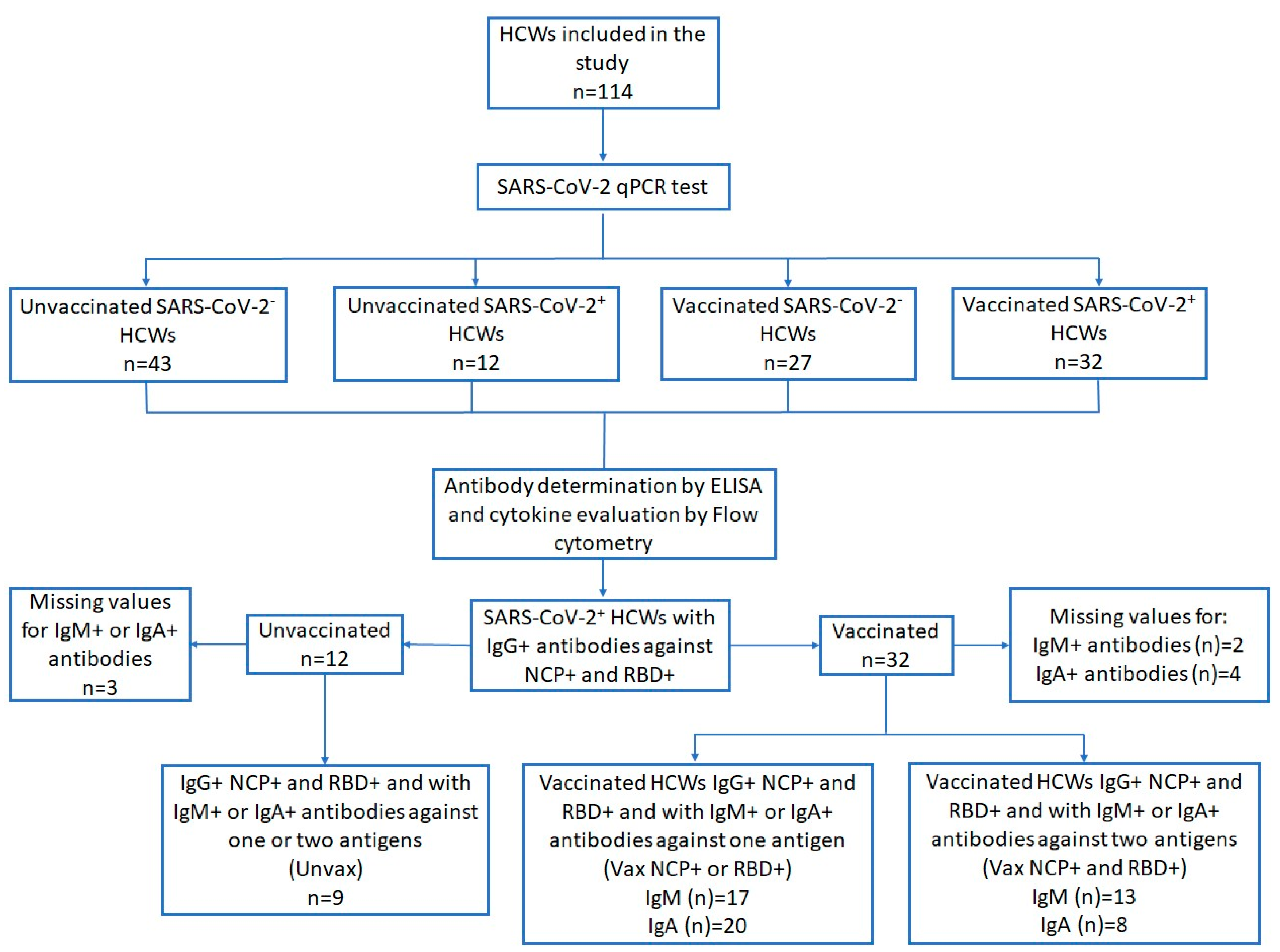
| Characteristic n = 114 (n; %) | Unvaccinated SARS-CoV-2− n = 43 n (%; 95% CI) | Unvaccinated SARS-CoV-2+ n = 12 n (%; 95% CI) | Vaccinated SARS-CoV-2− n = 27 n (%; 95% CI) | Vaccinated SARS-CoV-2+ n = 32 n (%; 95% CI) | p Value |
|---|---|---|---|---|---|
| Sex Female (60; 52.6) Male (54; 47.4) | 26 (60.5; 45.2–75.7) 17 (39.5; 24.3–54.8) | 4 (33.3; 2.05–64.6) 8 (66.3; 35.4–97.9) | 17 (63.0; 43.5–82.4) 10 (37.0; 17.6–56.5) | 13 (40.6; 22.6–58.6) 19 (59.4; 41.4–77.4) | 0.121 |
| Age Groups a 20–29 (29; 29.6) 30–39 (28; 28.6) 40–49 (11; 11.2) 50–59 (20; 20.4) ≥60 (10; 10.2) | 7 (20.6; 6.3–34.9) 14 (41.2; 23.7–58.6) 2 (5.9; −2.4–14.2) 7 (20.6; 6.3–34.9) 4 (11.8; 0.3–23.2) | 3 (27.3; −4.1–58.6) 4 (36.4; 2.5–70.3) 2 (18.8; −8.9–45.4) 2 (18.8; −8.9–45.4) 0 | 9 (33.3; 14.3–52.3) 4 (14.8; 5.0–29.1) 2 (7.4; −3.1–17.9) 7 (25.9; 8.3–43.6) 2 (7.4; −3.1–17.9) | 10 (31.2; 14.3–48.2) 6 (18.8; 4.5–33.1) 5 (15.6; 2.3–28.9) 4 (12.5; 4.0–24.6) 4 (12.5; 4.0–24.6) | 0.497 0.141 0.434 0.587 0.610 |
| Comorbidities b No (56; 56.6) Yes (43; 43.4) Types c Overweight/Obesity (42; 97.7) Hypertension (3; 7.0) Dyslipidemia (1; 2.3) | 21 (63.6; 46.3–80.9) 12 (36.4; 19.0–53.7) 12 (36.4; 19.0–53.7) 0 0 | 4 (57.1; 7.7–106.6) 3 (42.9; 6.6–92.3) 3 (42.9; −6.6–92.3) 0 0 | 17 (63.0; 43.5–82.4) 10 (37.0; 17.6–56.5) 10 (37.0; 17.6–56.5) 1 (3.7; −3.9–11.3) 1 (3.7; −3.9–11.3) | 14 (43.7; 25.6–61.9) 18 (56.3; 38.1–74.4) 17 (53.1; 34.8–71.4) 2 (6.3; −2.6–15.1) 0 | 0.358 |
| Smoking No (103; 90.4) Yes (11; 9.6) | 40 (93.0; 85.1–100.9) 3 (7.0; −0.9–14.9) | 11 (91.7; 73.3–110) 1 (8.3; −10.0–26.7) | 24 (88.9; 76.2–101.6) 3 (11.1; −1.6–23.8) | 28 (87.5; 75.4–99.6) 4 (12.5; 3.9–24.6) | 0.865 |
| Symptoms Asymptomatic (57; 50.0) Symptomatic (57; 50.0) Fever (25; 21.9) Myalgia (40; 35.1) Anosmia (22; 19.3) Rhinorrhea (28; 24.6) Diarrhea (14; 12.3) Fatigue (22; 19.3) Erythema (2; 1.8) Odynophagia (31; 27.2) Headache (48; 42.1) Dysgeusia (22; 19.3) | 36 (83.7; 72.2–95.2) 7 (16.3; 4.8–27.8) 2 (4.6; −1.9–11.2) 1 (2.3; −2.4–7.0) 1 (2.3; −2.4–7.0) 6 (13.9; 3.2–24.7) 2 (4.6; −1.9–11.2) 6 (13.9; 3.2–24.7) 0 3 (6.9; −0.9–14.1) 6 (13.9; 3.2–24.7) 0 | 2 (16.7; −8.1–41.4) 10 (83.3; 58.6–108.1) 6 (50.0; 16.8–83.2) 9 (75.0; 46.3–103.7) 7 (58.3; 25.6–91.1) 2 (16.7; −8.1–41.4) 2 (16.7; −8.1–41.4) 4 (33.3; 2.1–64.6) 1 (8.3; −10.0–26.7) 4 (33.3; 2.1–64.6) 7 (58.3; 25.6–91.1) 7 (58.3; 25.6–91.1) | 16 (59.3; 39.5–79.1) 11 (40.7; 20.9–60.5) 1 (3.6; −3.8–10.9) 5 (17.9; 2.7–32.9) 0 5 (17.9; 2.7–32.9) 1 (3.6; −3.8–10.9) 7 (25.0; 7.9–42.1) 0 3 (10.7; −1.5–22.9) 7 (25.0; 7.9–42.1) 1 (3.6; −3.8–10.9) | 3 (9.4; −1.3–20.0) 29 (90.6; 79.9–101.3) 16 (50.0; 31–7−68.3) 25 (78.1; 62.9–93.3) 14 (43.8; 25.6–61.9) 15 (46.9; 28.6–65.1) 9 (28.1; 11.6–44.6) 5 (12.8; 2.3–28.9) 1 (2.6; −3.2–9.5) 21 (65.6; 48.2–83.0) 28 (87.5; 75.4–99.6) 14 (43.8; 25.6–61.9) | 0.0001 0.0001 0.0001 0.0001 0.007 0.008 0.345 0.206 0.0001 0.0001 0.0001 |
| Antibody | Antigen | A Unvaccinated SARS-CoV-2+ n = 12 (%) | B Unvaccinated SARS-CoV-2− n = 43 (%) | C Vaccinated SARS-CoV-2+ n = 32 (%) | D Vaccinated SARS-CoV-2− n = 27 (%) | p Value | |||
|---|---|---|---|---|---|---|---|---|---|
| A vs. B | A vs. C | C vs. D | B vs. D | ||||||
| IgM | NCP | 4 (33.3) | 2 (4.7) | 30(93.8) | 1 (3.7) | 0.017 | 0.0001 | 0.0001 | 1.000 |
| RBD | 3 (25.0) | 3 (7.0) | 15 (46.9) | 9 (33.3) | 0.110 | 0.303 | 0.425 | 0.008 | |
| IgG | NCP | 10 (83.3) | 5 (11.6) | 30 (93.8) | 2 (7.4) | 0.0001 | 0.297 | 0.0001 | 0.699 |
| RBD | 10 (83.3) | 5 (11.6) | 32 (100) | 24 (88.9) | 0.0001 | 0.070 | 0.090 | 0.0001 | |
| IgA | NCP | 3 (25.0) | 4 (9.3) | 9 (28.1) | 3 (11.1) | 0.168 | 1.000 | 0.193 | 1.000 |
| RBD | 8 (66.7) | 4 (9.3) | 29 (90.6) | 22 (81.5) | 0.0001 | 0.075 | 0.450 | 0.0001 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-Rojas, M.Á.; Plett-Torres, T.; Ávila, G.; Romero-Valdovinos, M.; Salazar, A.M.; Sordo, M.; Chávez-Vargas, M.; Coeto Ángeles, C.J.; Cruz-Rivera, M.; Santiago-Olivares, C.; et al. Cytokine and Antibody Isotype Responses in Vaccinated Healthcare Workers with SARS-CoV-2 Breakthrough Infections. Viruses 2025, 17, 1517. https://doi.org/10.3390/v17111517
Fernández-Rojas MÁ, Plett-Torres T, Ávila G, Romero-Valdovinos M, Salazar AM, Sordo M, Chávez-Vargas M, Coeto Ángeles CJ, Cruz-Rivera M, Santiago-Olivares C, et al. Cytokine and Antibody Isotype Responses in Vaccinated Healthcare Workers with SARS-CoV-2 Breakthrough Infections. Viruses. 2025; 17(11):1517. https://doi.org/10.3390/v17111517
Chicago/Turabian StyleFernández-Rojas, Miguel Ángel, Tanya Plett-Torres, Guillermina Ávila, Mirza Romero-Valdovinos, Ana María Salazar, Monserrat Sordo, Mariana Chávez-Vargas, Cesar Josué Coeto Ángeles, Mayra Cruz-Rivera, Carlos Santiago-Olivares, and et al. 2025. "Cytokine and Antibody Isotype Responses in Vaccinated Healthcare Workers with SARS-CoV-2 Breakthrough Infections" Viruses 17, no. 11: 1517. https://doi.org/10.3390/v17111517
APA StyleFernández-Rojas, M. Á., Plett-Torres, T., Ávila, G., Romero-Valdovinos, M., Salazar, A. M., Sordo, M., Chávez-Vargas, M., Coeto Ángeles, C. J., Cruz-Rivera, M., Santiago-Olivares, C., Hinojosa, J. P. R., Maravilla, P., Ostrosky-Wegman, P., Mendlovic, F., & Flisser, A. (2025). Cytokine and Antibody Isotype Responses in Vaccinated Healthcare Workers with SARS-CoV-2 Breakthrough Infections. Viruses, 17(11), 1517. https://doi.org/10.3390/v17111517








