H6N6 Avian Influenza Virus Infection Induced Pyroptosis of M1 Macrophages by Activating Caspase-1
Abstract
1. Introduction
2. Materials and Methods
2.1. Virus and Cell Lines
2.2. H6N6 Avian Influenza Virus Replication in Human Lung Tissue
2.3. The THP-1 Cells’ Polarization into M1 Macrophages
2.4. CCK8 Detection of M1 Macrophage Viability
2.5. Lactate Dehydrogenase (LDH) Release Assay
2.6. Scanning Electron Microscopy (SEM) for Cell Morphology
2.7. Detection of Genes and Proteins in the Caspase-1-Dependent Pyroptosis Pathway
2.8. Statistical Analysis
3. Results
3.1. H6N6 Subtype Avian Influenza Virus Effectively Replicates in Human Lung Tissue
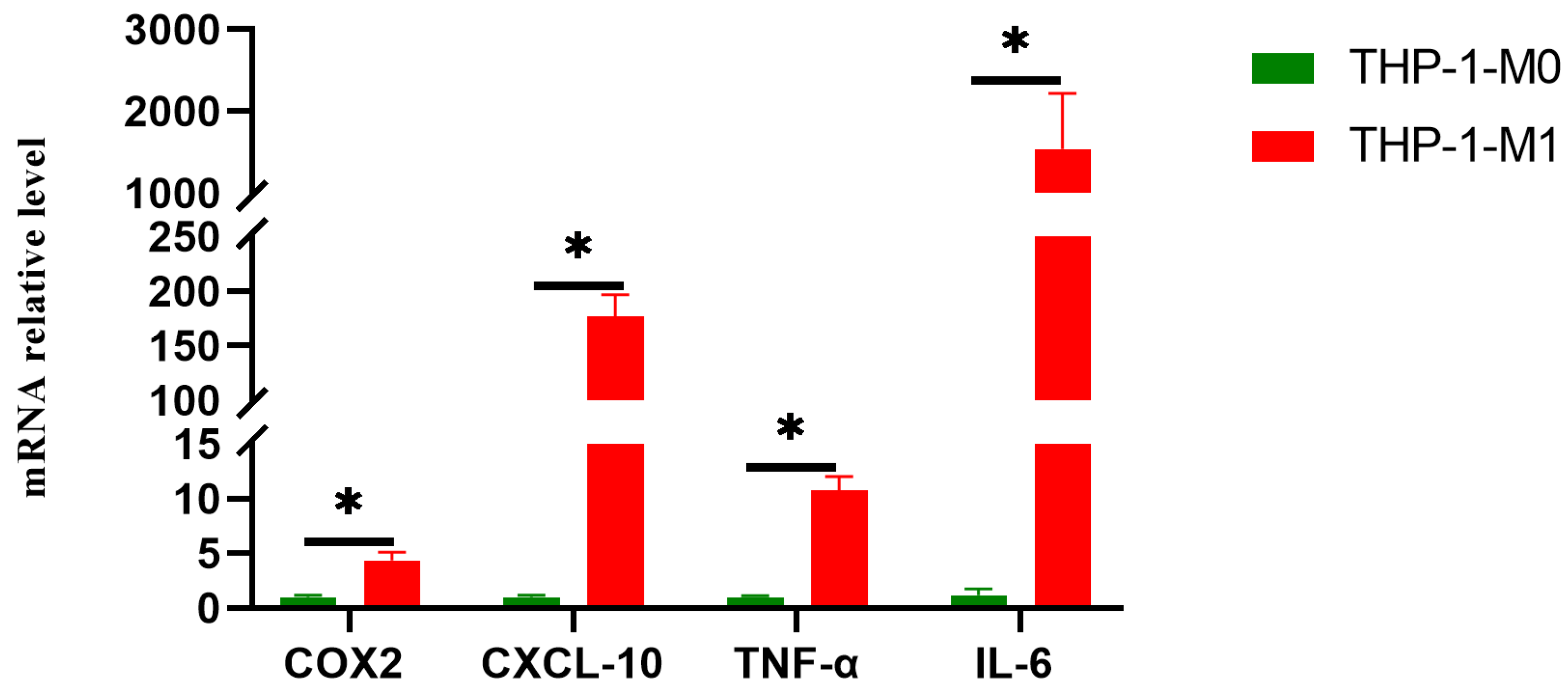
3.2. The THP-1 Cells Successfully Polarized into M1 Macrophages
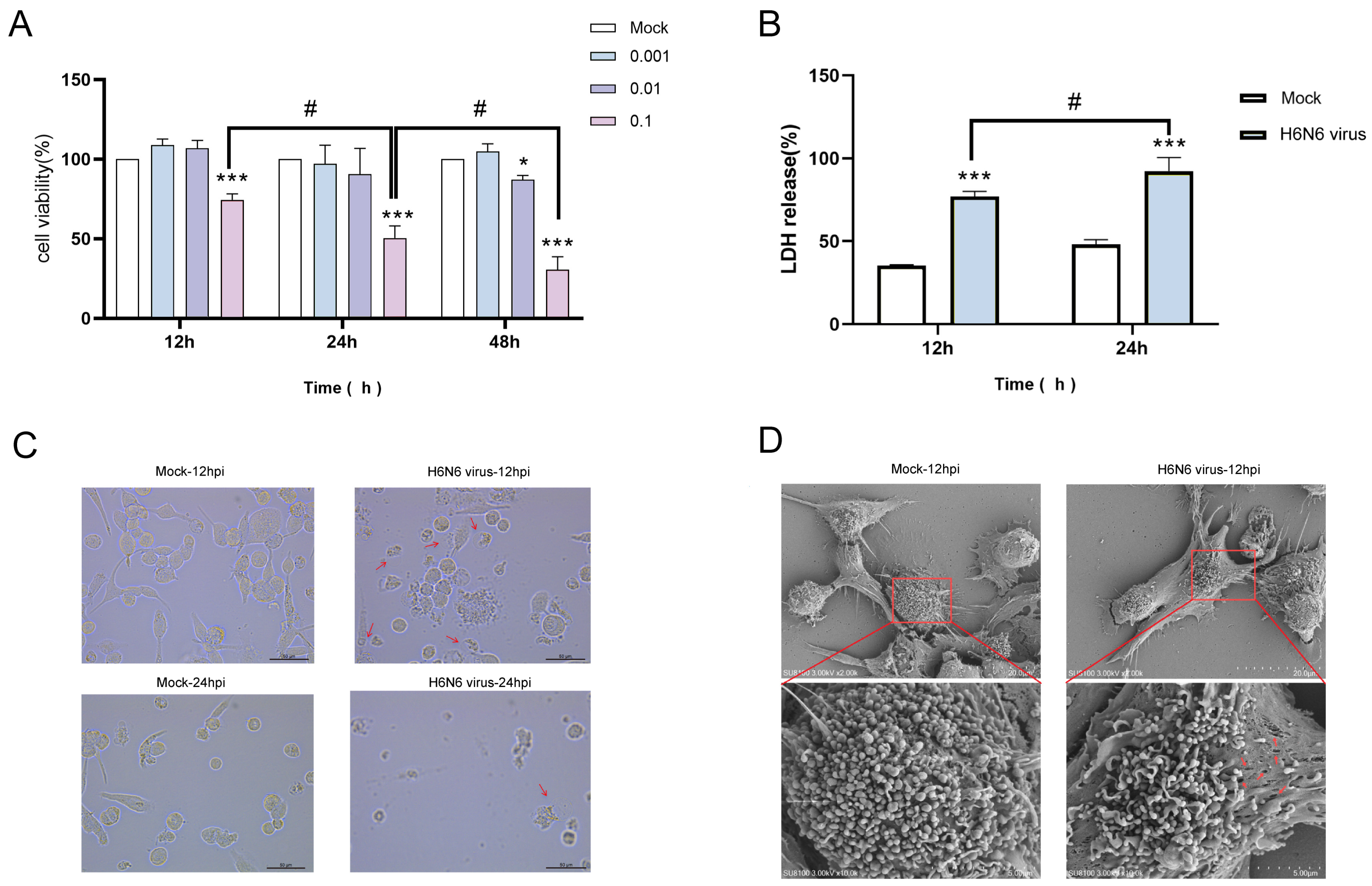
3.3. H6N6 AIV Infection Induces Cytotoxicity and Pyroptotic Morphology in M1 Macrophages
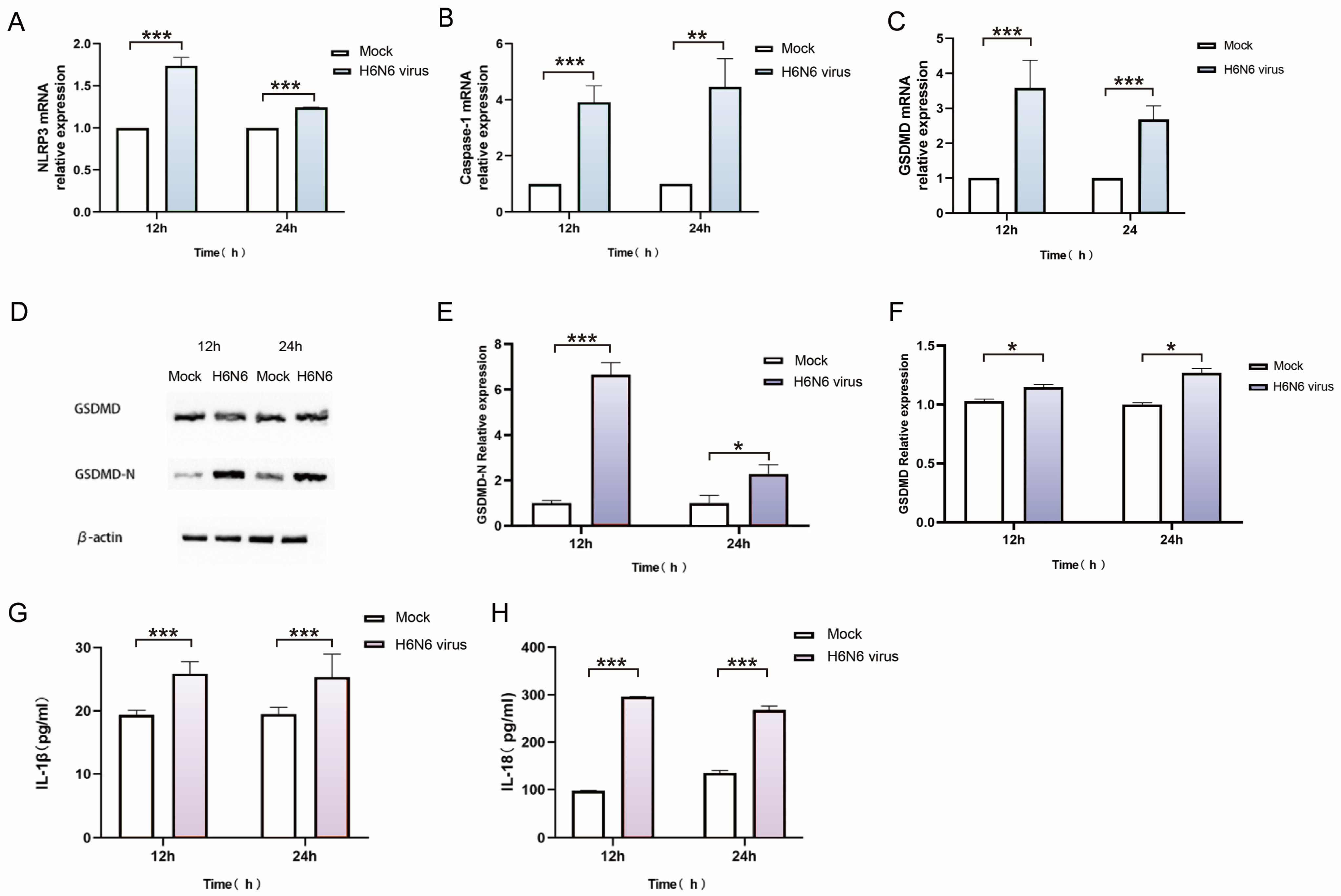
3.4. H6N6 Avian Influenza Virus Triggers Caspase-1-Dependent Pyroptosis in M1 Macrophages
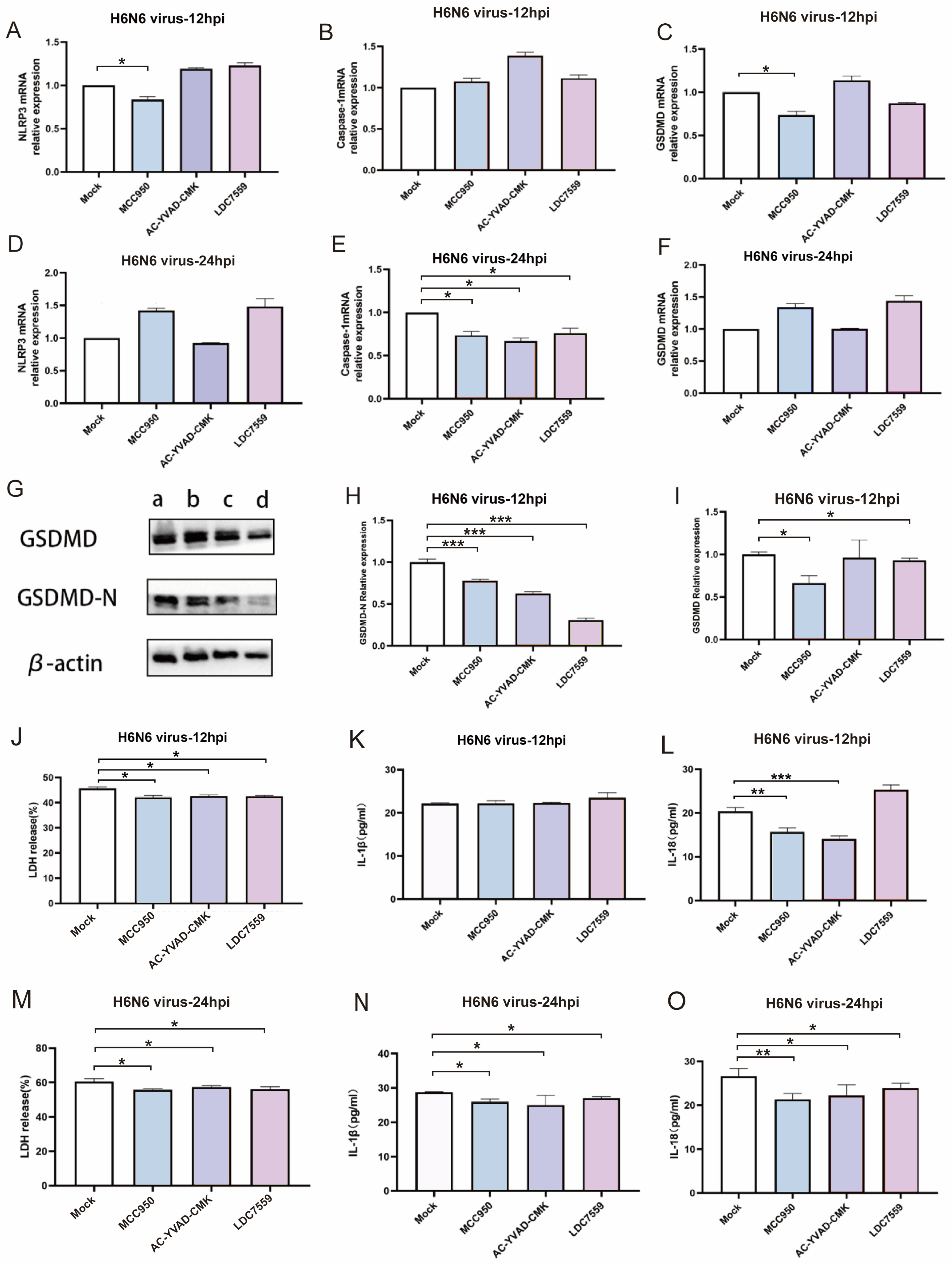
3.5. Pharmacological Inhibition Confirms the Essential Role of NLRP3/Caspase-1/GSDMD Pathway in H6N6-Induced Pyroptosis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AIV | avian influenza virus |
| BSA | bovine serum albumin |
| CARD | caspase activation and recruitment domain |
| GSDMD | Gasdermin D |
| HPAIV | high-pathogenic avian influenza virus |
| hpi | hours post-infection |
| IHC | immunohistochemistry |
| LDH | Lactate Dehydrogenase |
| LPAIV | low-pathogenic avian influenza virus |
| LPS | lipopolysaccharides |
| MCSF | macrophage colony-stimulating facto |
| MDCK | Madin-Darby Canine Kidney |
| MOI | multiplicities of infection |
| NP | nucleoprotein |
| OD | optical density |
| PAMPs | pathogen-associated molecular patterns |
| PCD | programmed cell death |
| PMA | phorbol 12-myristate 13-acetate |
| SEM | Scanning Electron Microscopy |
| TCID50 | 50% tissue culture infectious dose |
| WB | western blot |
| WHO | World Health Organization |
References
- Mallapaty, S. The pathogens that could spark the next pandemic. Nature 2024, 632, 488. [Google Scholar] [CrossRef] [PubMed]
- Claas, E.C.; Osterhaus, A.D.; van Beek, R.; De Jong, J.C.; Rimmelzwaan, G.F.; Senne, D.A.; Krauss, S.; Shortridge, K.F.; Webster, R.G. Human influenza A H5N1 virus related to a highly pathogenic avian influenza virus. Lancet 1998, 351, 472–477. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.; Cao, B.; Hu, Y.; Feng, Z.; Wang, D.; Hu, W.; Chen, J.; Jie, Z.; Qiu, H.; Xu, K.; et al. Human infection with a novel avian-origin influenza A (H7N9) virus. N. Engl. J. Med. 2013, 368, 1888–1897. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.Y.; Ke, C.W.; Li, Q.; Yuan, R.Y.; Xiang, D.; Jia, W.X.; Yu, Y.D.; Liu, L.; Huang, C.; Qi, W.B.; et al. Novel reassortant avian influenza A(H5N6) viruses in Humans, Guangdong, China 2015. Emerg. Infect. Dis. 2016, 22, 1507–1509. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Deng, G.; Shi, J.; Luo, W.; Zhang, G.; Zhang, Q.; Liu, L.; Jiang, Y.; Li, C.; Sriwilaijaroen, N.; et al. H6 influenza viruses pose a potential threat to human health. J. Virol. 2014, 88, 3953–3964. [Google Scholar] [CrossRef] [PubMed]
- Zhu, P.; He, X.; Chen, Y.; Yan, Z.; Li, Q.; Zhou, Q.; Lin, W.; Chen, F. Characterization and Pathogenicity of Novel Reassortment H6N6 Avian Influenza Viruses in Southern China. Transbound. Emerg. Dis. 2024, 2024, 4005909. [Google Scholar] [CrossRef] [PubMed]
- Shinya, K.; Ebina, M.; Yamada, S.; Ono, M.; Kasai, N.; Kawaoka, Y. Avian flu: Influenza virus receptors in the human airway. Nature 2006, 440, 435–436. [Google Scholar] [CrossRef] [PubMed]
- van Riel, D.; Munster, V.J.; de Wit, E.; Rimmelzwaan, G.F.; Fouchier, R.A.; Osterhaus, A.D.; Kuiken, T. H5N1 virus attachment to lower respiratory tract. Science 2006, 312, 399. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Jian, Z.; Xu, T.; Li, F.; Deng, H.; Zhou, Y.; Lai, S.; Xu, Z.; Zhu, L. Macrophage polarization: An important candidate regulator for lung diseases. Molecules 2023, 28, 2379. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, S.; Trivedi, A.K. Origin, production and molecular determinants of macrophages for their therapeutic targeting. Cell Biol. Int. 2022, 47, 15–29. [Google Scholar] [CrossRef] [PubMed]
- Ni, R.; Jiang, L.; Zhang, C.; Liu, M.; Luo, Y.; Hu, Z.; Mou, X.; Zhu, Y. Biologic mechanisms of macrophage phenotypes responding to infection and the novel therapies to moderate inflammation. Int. J. Mol. Sci. 2023, 24, 8358. [Google Scholar] [CrossRef] [PubMed]
- Cron, R.Q.; Goyal, G.; Chatham, W.W. Cytokine storm syndrome. Annu. Rev. Med. 2022, 74, 321–337. [Google Scholar] [CrossRef] [PubMed]
- Malireddi, R.K.S.; Kesavardhana, S.; Kanneganti, T.D. ZBP1 and TAK1: Master regulators of NLRP3 Inflammasome/Pyroptosis, Apoptosis, and Necroptosis (PANoptosis). Front. Cell. Infect. Microbiol. 2019, 9, 406. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Gao, W.; Shao, F. Pyroptosis: Gasdermin-mediated programmed necrotic cell death. Trends Biochem. Sci. 2017, 42, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Zhao, Y.; Wang, K.; Shi, X.; Wang, Y.; Huang, H.; Zhuang, Y.; Cai, T.; Wang, F.; Shao, F. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 2015, 526, 660–665. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Zhao, Y.; Wang, Y.; Gao, W.; Ding, J.; Li, P.; Hu, L.; Shao, F. Inflammatory caspases are innate immune receptors for intracellular LPS. Nature 2014, 514, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Chu, L.H.; Indramohan, M.; Ratsimandresy, R.A.; Gangopadhyay, A.; Morris, E.P.; Monack, D.M.; Dorfleutner, A.; Stehlik, C. The oxidized phospholipid oxPAPC protects from septic shock by targeting the non-canonical inflammasome in macrophages. Nat. Commun. 2018, 9, 996. [Google Scholar] [CrossRef] [PubMed]
- Kayagaki, N.; Stowe, I.B.; Lee, B.L.; O’Rourke, K.; Anderson, K.; Warming, S.; Cuellar, T.; Haley, B.; Roose-Girma, M.; Phung, Q.T.; et al. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature 2015, 526, 666–671. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Gao, W.; Shi, X.; Ding, J.; Liu, W.; He, H.; Wang, K.; Shao, F. Chemotherapy drugs induce pyroptosis through caspase-3 cleavage of a gasdermin. Nature 2017, 547, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Zhao, R.; Xia, W.; Chang, C.W.; You, Y.; Hsu, J.M.; Nie, L.; Chen, Y.; Wang, Y.C.; Liu, C.; et al. PD-L1-mediated gasdermin C expression switches apoptosis to pyroptosis in cancer cells and facilitates tumour necrosis. Nat. Cell Biol. 2020, 22, 1264–1275. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Fang, Y.; Chen, X.; Wang, Z.; Liang, X.; Zhang, T.; Liu, M.; Zhou, N.; Lv, J.; Tang, K.; et al. Gasdermin E-mediated target cell pyroptosis by CAR T cells triggers cytokine release syndrome. Sci. Immunol. 2020, 5, eaax7969. [Google Scholar] [CrossRef] [PubMed]
- Cerato, J.A.; da Silva, E.F.; Porto, B.N. Breaking bad: Inflammasome activation by respiratory viruses. Biology 2023, 12, 943. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; Li, J.; Wang, Y.; Yu, X.; He, X.; Shi, J.; Deng, G.; Zeng, X.; Tian, G.; Li, Y.; et al. H7N9 virus infection triggers lethal cytokine storm by activating gasdermin E-mediated pyroptosis of lung alveolar epithelial cells. Natl. Sci. Rev. 2021, 9, nwab137. [Google Scholar] [CrossRef] [PubMed]
- Thomas, P.G.; Shubina, M.; Balachandran, S. ZBP1/DAI-dependent cell death pathways in influenza A virus immunity and pathogenesis. Curr. Top. Microbiol. Immunol. 2023, 442, 41–63. [Google Scholar] [CrossRef] [PubMed]
- Ren, R.; Wu, S.; Cai, J.; Yang, Y.; Ren, X.; Feng, Y.; Chen, L.; Qin, B.; Xu, C.; Yang, H.; et al. The H7N9 influenza A virus infection results in lethal inflammation in the mammalian host via the NLRP3-caspase-1 inflammasome. Sci. Rep. 2017, 7, 7625. [Google Scholar] [CrossRef] [PubMed]
- Quan, C.; Wang, Q.; Zhang, J.; Zhao, M.; Dai, Q.; Huang, T.; Zhang, Z.; Mao, S.; Nie, Y.; Liu, J.; et al. Avian influenza A viruses among occupationally exposed populations, China, 2014–2016. Emerg. Infect. Dis. 2019, 25, 2215–2225. [Google Scholar] [CrossRef] [PubMed]
- Zhong, W.; Gao, L.; Wang, X.; Su, S.; Lin, Y.; Huang, K.; Zhou, S.; Fan, X.; Zhang, Z. Influenza A (H6N6) viruses isolated from chickens replicate in mice and human lungs without prior adaptation. J. Virus Erad. 2022, 8, 100086. [Google Scholar] [CrossRef]
- Rayamajhi, M.; Zhang, Y.; Miao, E.A. Detection of pyroptosis by measuring released lactate dehydrogenase activity. Methods Mol. Biol. 2013, 1040, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Li, Y.; Huang, S.; Wen, F. Global distribution, receptor binding, and cross-species transmission of H6 influenza viruses: Risks and implications for humans. J. Virol. 2023, 97, e0137023. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Xia, J.; Wang, Z.; Xu, J.; Ji, Y.; Jin, Y.; Pu, L.; Xu, S. Evolution of H6N6 viruses in China between 2014 and 2019 involves multiple reassortment events. Emerg. Microbes Infect. 2024, 13, 2341142. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Ci, Y.; Ma, Y.; Liu, L.; Wang, D.; Ma, J.; Li, Y.; Chen, H. Phylogenetic analysis of a novel H6N6 avian influenza virus isolated from a green peafowl in China and its pathogenic potential in mice. Infect. Genet. Evol. 2014, 28, 107–112. [Google Scholar] [CrossRef] [PubMed]
- de Jong, M.D.; Simmons, C.P.; Thanh, T.T.; Hien, V.M.; Smith, G.J.; Chau, T.N.; Hoang, D.M.; Chau, N.V.; Khanh, T.H.; Dong, V.C.; et al. Fatal outcome of human influenza A (H5N1) is associated with high viral load and hypercytokinemia. Nat. Med. 2006, 12, 1203–1207. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Huang, F.; Liu, J.; Chen, Y.; Wang, W.; Cao, B.; Zou, Z.; Liu, S.; Pan, J.; Bao, C.; et al. The serum profile of hypercytokinemia factors identified in H7N9-infected patients can predict fatal outcomes. Sci. Rep. 2015, 5, 10942. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Zuo, X.; Zhang, S.; Ouyang, Z.; Jiang, S.; Wang, F.; Wang, G. The mechanism behind influenza virus cytokine storm. Viruses 2021, 13, 1362. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, K.; van Eeden, S.F. Lung macrophage phenotypes and functional responses: Role in the pathogenesis of COPD. Int. J. Mol. Sci. 2018, 19, 582. [Google Scholar] [CrossRef] [PubMed]
- Rivera, A.; Siracusa, M.C.; Yap, G.S.; Gause, W.C. Innate cell communication kick-starts pathogen-specific immunity. Nat. Immunol. 2016, 17, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Liegeois, M.; Legrand, C.; Desmet, C.J.; Marichal, T.; Bureau, F. The interstitial macrophage: A long-neglected piece in the puzzle of lung immunity. Cell. Immunol. 2018, 330, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Byrne, A.J.; Mathie, S.A.; Gregory, L.G.; Lloyd, C.M. Pulmonary macrophages: Key players in the innate defence of the airways. Thorax 2015, 70, 1189–1196. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Qiu, H.; Hao, S.; Zhu, F.; Huang, Y.; Xu, K.; Yu, H.; Wang, D.; Zhou, L.; Dai, Q.; et al. Human infection with an avian-origin influenza A (H10N3) virus. N. Engl. J. Med. 2022, 386, 1087–1088. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhang, Z.; Wang, M.; Pan, X.; Jiang, X. Second identified human infection with the avian influenza virus H10N3: A case report. Ann. Intern. Med. 2023, 176, 429–431. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Luo, S.; Gao, Y.; Dai, M.; Yan, J.; Yang, Y.; Li, H.; Zhang, Y.; Mao, Z. A case report of human infection with avian influenza H10N3 with a complex respiratory disease history. BMC Infect. Dis. 2024, 24, 918. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Yuan, H.; Gao, R.; Zhang, J.; Wang, D.; Xiong, Y.; Fan, G.; Yang, F.; Li, X.; Zhou, J.; et al. Clinical and epidemiological characteristics of a fatal case of avian influenza A H10N8 virus infection: A descriptive study. Lancet 2014, 383, 714–721. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Deobald, K.; Re, F. Gasdermin D protects from melioidosis through pyroptosis and direct killing of bacteria. J. Immunol. 2019, 202, 3468–3473. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Zheng, M.; Balakrishnan, A.; Karki, R.; Kanneganti, T.D. Gasdermin D promotes AIM2 inflammasome activation and is required for host protection against francisella novicida. J. Immunol. 2018, 201, 3662–3668. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Chen, Q.; Tan, M.; Liu, J.; Li, X.; Yang, L.; Shu, Y.; Wang, D.; Zhu, W. Epidemiology, evolution, and biological characteristics of H6 avian influenza viruses in China. Emerg. Microbes Infect. 2023, 12, 2151380. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Gao, R.; Sun, Z.; Yang, W.; He, Q.; Wang, C.; Zhang, J.; Zhang, X.; Guo, L.; Wang, S. Baicalin inhibits influenza A (H1N1)-induced pyroptosis of lung alveolar epithelial cells via caspase-3/GSDME pathway. J. Med. Virol. 2023, 95, e28790. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Xu, G.; Zhou, X.; Luo, M.; Ma, N.; Wang, X.; Wang, Z.; Tang, H.; Wang, X.; Li, Y.; et al. Mesenchymal stem cells ameliorate H9N2-induced acute lung injury by inhibiting caspase-3-GSDME-mediated pyroptosis of lung alveolar epithelial cells. Eur. J. Pharmacol. 2023, 960, 176148. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, X.; Hao, X.; Gao, R.; Lu, X.; Yang, W.; Chen, Y.; Hu, J.; Gu, M.; Liu, X.; et al. The Novel H10N3 Avian Influenza Virus Triggers Lethal Cytokine Storm by Activating Multiple Forms of Programmed Cell Death in Mammalian Lungs. Int. J. Mol. Sci. 2025, 26, 1977. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, H.; He, D.; Liu, S.; Fan, X.; Gao, L.; Guo, L.; Zhang, Z. H6N6 Avian Influenza Virus Infection Induced Pyroptosis of M1 Macrophages by Activating Caspase-1. Viruses 2025, 17, 1492. https://doi.org/10.3390/v17111492
Zhu H, He D, Liu S, Fan X, Gao L, Guo L, Zhang Z. H6N6 Avian Influenza Virus Infection Induced Pyroptosis of M1 Macrophages by Activating Caspase-1. Viruses. 2025; 17(11):1492. https://doi.org/10.3390/v17111492
Chicago/Turabian StyleZhu, Hui, Dongfang He, Sicong Liu, Xiaohui Fan, Lingxi Gao, Liping Guo, and Zengfeng Zhang. 2025. "H6N6 Avian Influenza Virus Infection Induced Pyroptosis of M1 Macrophages by Activating Caspase-1" Viruses 17, no. 11: 1492. https://doi.org/10.3390/v17111492
APA StyleZhu, H., He, D., Liu, S., Fan, X., Gao, L., Guo, L., & Zhang, Z. (2025). H6N6 Avian Influenza Virus Infection Induced Pyroptosis of M1 Macrophages by Activating Caspase-1. Viruses, 17(11), 1492. https://doi.org/10.3390/v17111492





