Beyond Pathogenesis: The Nematode Immune Network as the Arbiter of a Host–Virus Truce
Abstract
1. Introduction
2. The Arbiter of the Truce: Architecture of the Nematode Antiviral Defense Network
2.1. The First Tier: The Direct-Acting Effector Arsenal
2.1.1. Barricading the Gates: Defenses Against Viral Entry
2.1.2. The Intracellular Battlefield: Suppressing Viral Replication and Gene Expression
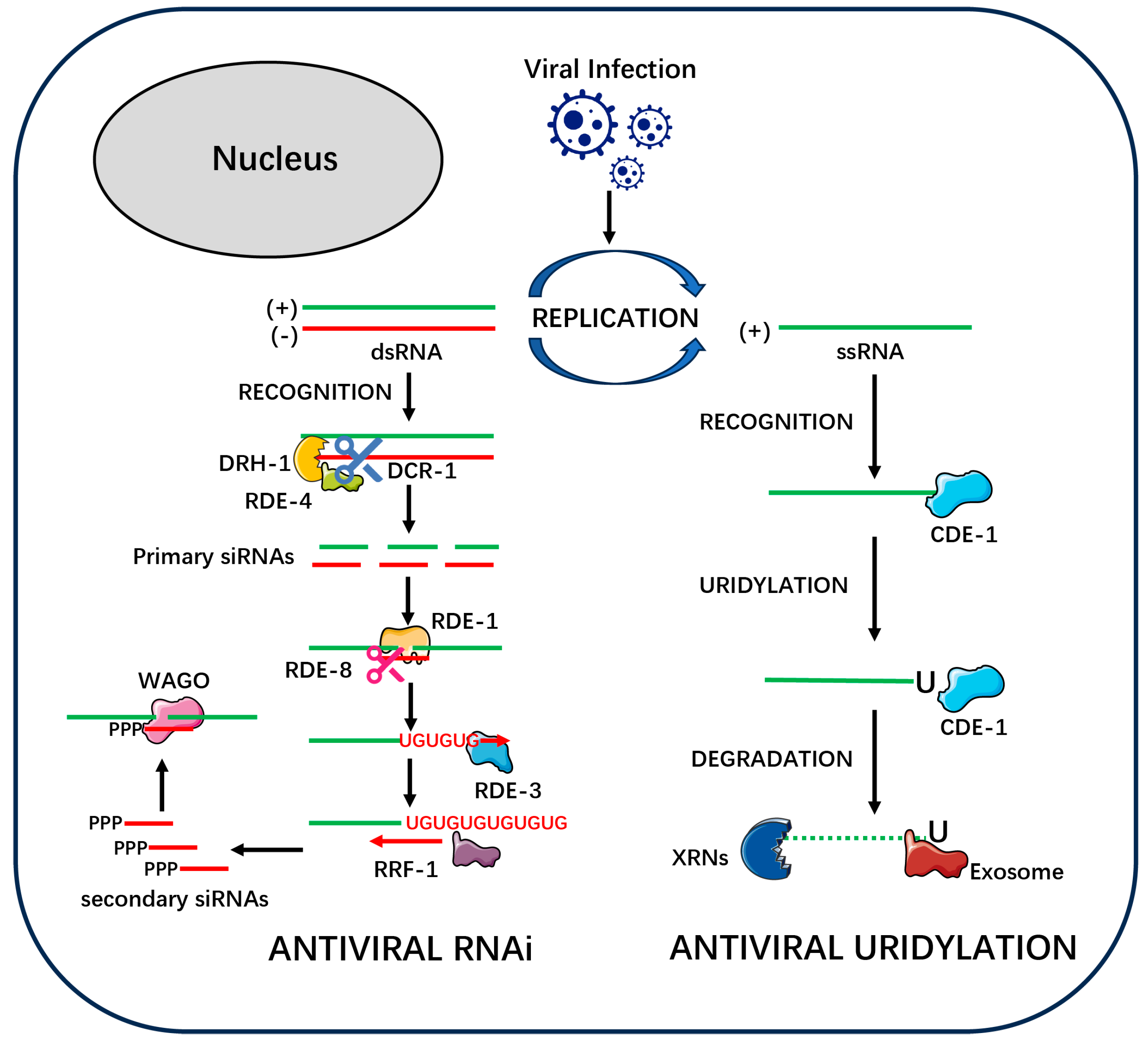
- RNA Interference (RNAi): The Precision-Guided System
- Primary siRNA Biogenesis: A Conserved Core with Architectural Variations
- Signal Amplification: A Specialized Innovation Versus a Basal Response
- CDE-1: The Uridylation “Tagging” Weapon
- Different RNA Substrates: CDE-1 acts directly on the viral single-stranded RNA (ssRNA) genome. Conversely, the RDE-3 in the RNAi pathway acts on viral RNA fragments that have been previously cleaved by the nuclease RDE-8 [17].
- Different Chemical Modifications: CDE-1 adds a simple, short U-tail (1-2 uridines) to its target. In stark contrast, RDE-3 adds a structurally distinct poly(UG) tail, composed of alternating uridine and guanosine nucleotides [23].
- Different Functional Purposes: The purpose of the CDE-1-mediated U-tail is to directly mark the viral RNA for degradation, serving as a terminal signal that operates independently of the RNAi pathway [28]. The purpose of the RDE-3-generated poly(UG) tail, however, is to create a unique molecular beacon that serves as a template for the RNA-dependent RNA polymerase (RdRP) RRF-1 to amplify the production of secondary siRNAs within the RNAi pathway [24].
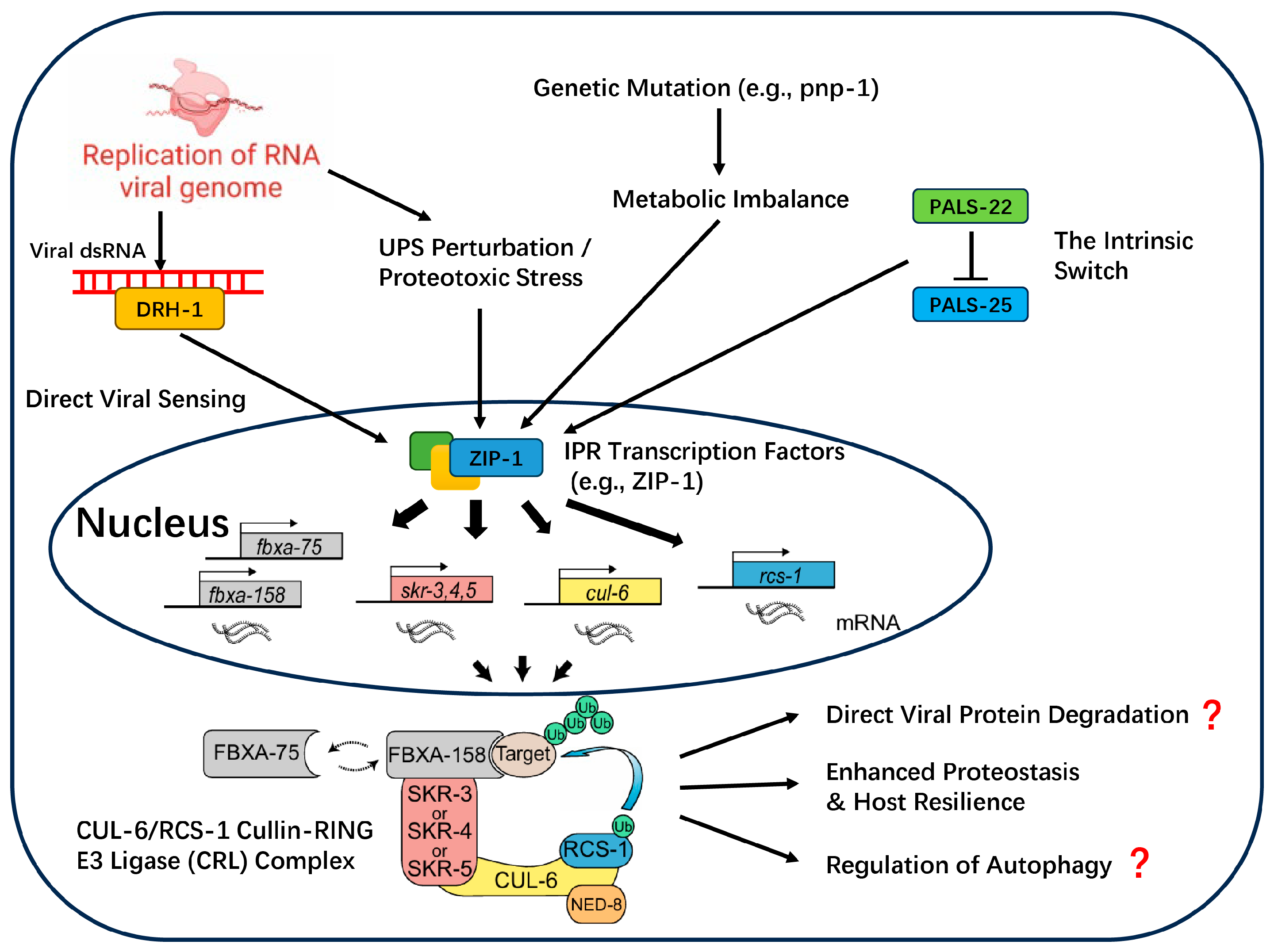
- The Intracellular Pathogen Response (IPR): A Fortress Defense
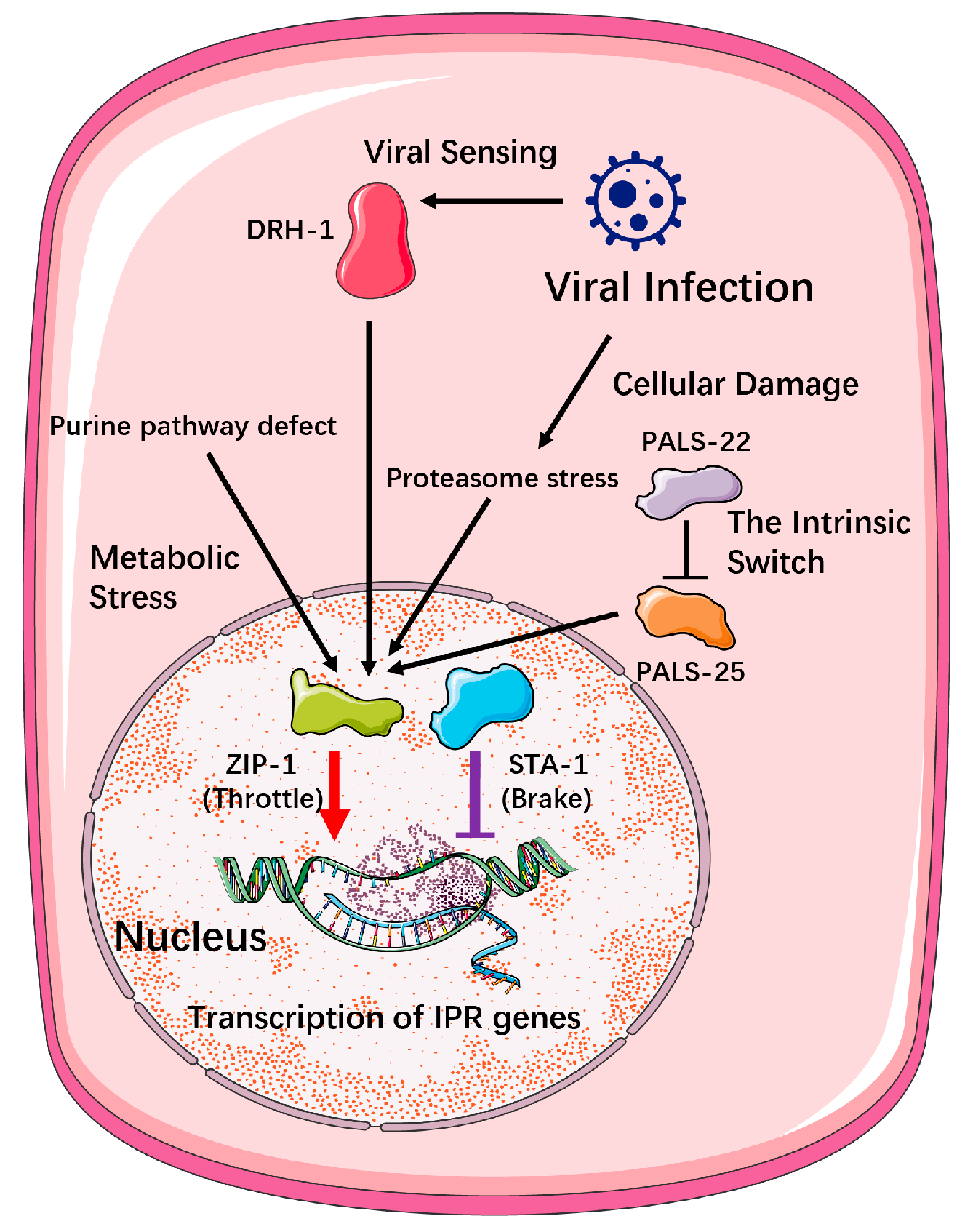
- Activation of the IPR: A Multi-Trigger, Parallel Input System
- The Effector Machinery and Speculative Antiviral Mechanisms
- Cross-functional Immunity: Repurposing Core Cellular Machinery
2.2. The Second Tier: The Command and Control Network of Trade-Offs
2.2.1. The Rationale for Regulation: The Inevitable Cost of an Antiviral Response
- Metabolic and Physiological Costs: The clearest evidence for this cost comes from the constitutive activation of the immune system, which severely impairs host fitness even in the absence of a pathogen. For example, in mutants of the key IPR repressor gene pals-22, the worm gains powerful resistance to viruses but pays a steep price in the form of slowed development and a significantly shortened lifespan [29]. Likewise, in mutants of another repressive transcription factor, sta-1, the constitutively active antiviral genes also lead to a shortened lifespan [37]. However, this fitness cost cannot be attributed solely to immune hyperactivation due to the other physiological processes regulated by these key repressor genes. This cost–benefit picture becomes more complex during an active infection, where it is challenging to deconvolve the cost of immunity from direct viral pathology. For instance, dynamic transcriptomic studies of an ongoing viral infection paint a clear picture of massive physiological disruption, triggering a large-scale reprogramming of host metabolism, such as the strategic downregulation of genes related to lipid metabolism and fatty acid elongation during peak viral replication [11]. Besides, it was observed that lipid abundance was significantly decreased in infected worms, which supported the cost of the immune activation. However, this specific metabolic shift could represent a strategic host resource reallocation (a cost), but it is also plausible that it is a pathology induced by the virus to promote its own replication.
- Evolutionary Cost: This fundamental trade-off is so profound that it has left an indelible mark on the species’ genome. Population genetic analyses of wild C. elegans isolates revealed that the genomic locus containing the core IPR regulators, pals-22 and pals-25, is under strong balancing selection [38]. Direct evidence for this includes the atypically high Tajima’s D values for multiple pals genes, which indicates that evolution has actively maintained multiple different versions (haplotypes) of this immune switch in the population, rather than selecting a single “best” version [38]. This finding provides powerful evidence that the cost–benefit analysis of immunity is a dynamic challenge optimized at the evolutionary scale, with different strategies (e.g., “always ready” versus “induce on-demand”) being favored in different pathogenic environments [38].
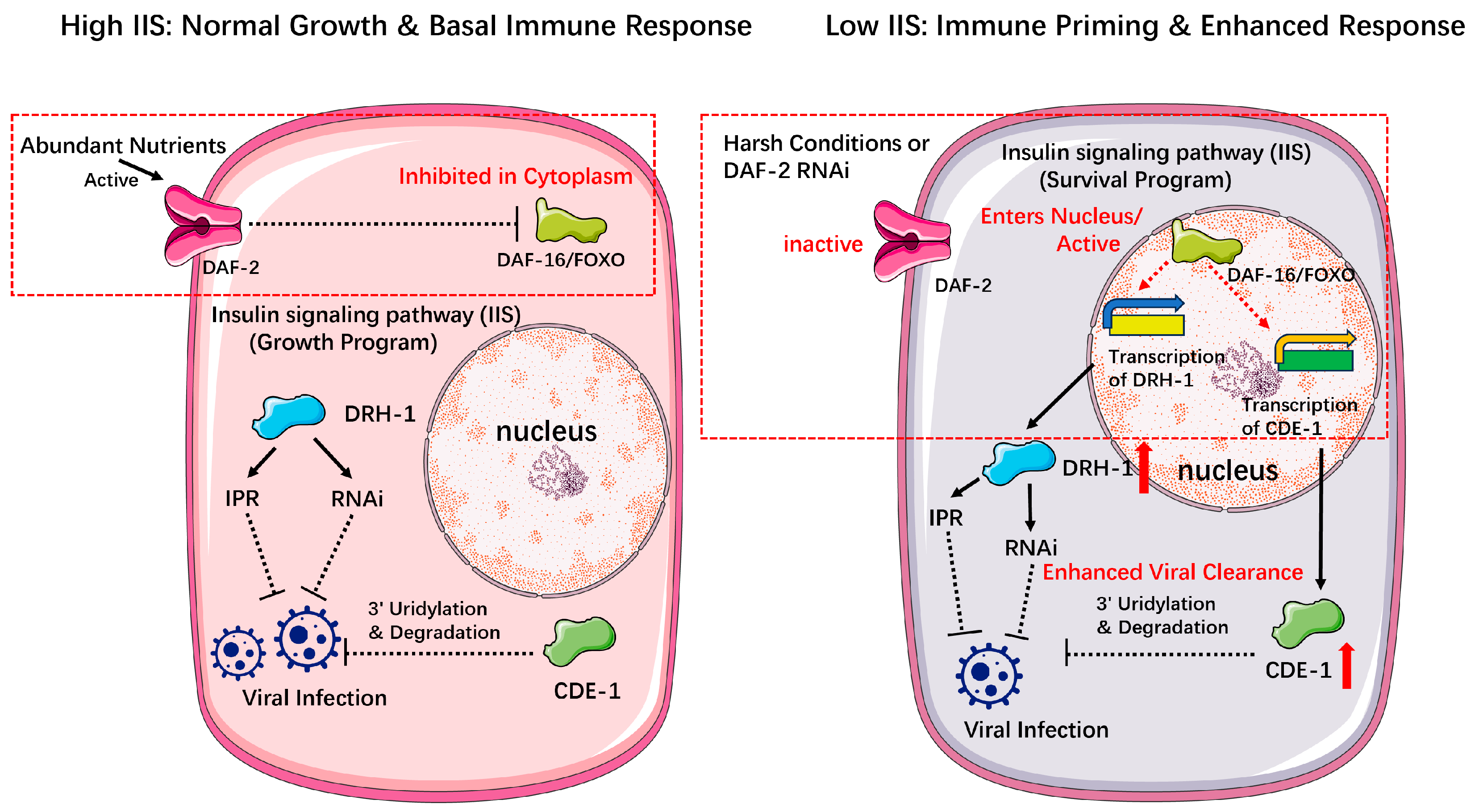
2.2.2. The Command Hierarchy: A Multi-Input Regulatory Decision System
- Direct Threat-Responsive Mechanisms
- Global State-Dependent Modulation
3. Summary and Outlook
3.1. Summary: The Architecture of a Negotiated Truce
3.2. Outlook: Unraveling the Secrets of the Truce
3.2.1. The Arms Race Full Picture: Viral Counter-Weapons and Host Back-Up Defenses
3.2.2. Beyond C. elegans: Deciphering the Immune Logic and Application Potential in Parasitic Nematodes
3.2.3. The “Trojan Horse” on a Bigger Stage: The Impact of the Nematode Virome on Final Hosts
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AME | Antimicrobial Effector |
| AMEs | Antimicrobial Effectors |
| CRL | Cullin-RING E3 Ligase |
| CRLs | Cullin-RING E3 Ligases |
| DAMP | Danger-Associated Molecular Pattern |
| dsRNA | double-stranded RNA |
| IAV | Influenza A Virus |
| IFN-I | type I interferon |
| IIS | Insulin/IGF-1 Signaling |
| IPR | Intracellular Pathogen Response |
| OrV | Orsay Virus |
| PAMPs | Pathogen-Associated Molecular Patterns |
| PCD | Programmed Cell Death |
| RdRP | RNA-dependent RNA polymerase |
| RIAD | RNAi-independent antiviral defense |
| RNAi | RNA interference |
| TUT | Terminal Uridylyltransferase |
| UPS | Ubiquitin–Proteasome System |
| VV | Vaccinia Virus |
| vsiRNA | virus-derived siRNA |
| WAGO | worm-specific Argonaute protein |
References
- Félix, M.-A.; Ashe, A.; Piffaretti, J.; Wu, G.; Nuez, I.; Bélicard, T.; Jiang, Y.; Zhao, G.; Franz, C.J.; Goldstein, L.D.; et al. Natural and Experimental Infection of Caenorhabditis Nematodes by Novel Viruses Related to Nodaviruses. PLoS Biol. 2011, 9, e1000586. [Google Scholar] [CrossRef]
- Quek, S.; Hadermann, A.; Wu, Y.; De Coninck, L.; Hegde, S.; Boucher, J.R.; Cresswell, J.; Foreman, E.; Steven, A.; LaCourse, E.J.; et al. Diverse RNA viruses of parasitic nematodes can elicit antibody responses in vertebrate hosts. Nat. Microbiol. 2024, 9, 2488–2505. [Google Scholar] [CrossRef]
- Vieira, P.; Nemchinov, L.G. A novel species of RNA virus associated with root lesion nematode Pratylenchus penetrans. J. Gen. Virol. 2019, 100, 704–708. [Google Scholar] [CrossRef]
- Bekal, S.; Domier, L.L.; Niblack, T.L.; Lambert, K.N. Discovery and initial analysis of novel viral genomes in the soybean cyst nematode. J. Gen. Virol. 2011, 92, 1870–1879. [Google Scholar] [CrossRef] [PubMed]
- Kud, J.; Dahan, J.; Orellana, G.E.; Dandurand, L.-M.; Karasev, A.V. A Novel Rhabdovirus Associated with the Idaho Population of Potato Cyst Nematode Globodera pallida. Viruses 2022, 14, 2718. [Google Scholar] [CrossRef] [PubMed]
- Franz, C.J.; Zhao, G.; Félix, M.-A.; Wang, D. Complete Genome Sequence of Le Blanc Virus, a Third Caenorhabditis Nematode-Infecting Virus. J. Virol. 2012, 86, 11940. [Google Scholar] [CrossRef]
- Frézal, L.; Jung, H.; Tahan, S.; Wang, D.; Félix, M.-A. Noda-Like RNA Viruses Infecting Caenorhabditis Nematodes: Sympatry, Diversity, and Reassortment. J. Virol. 2019, 93, 21. [Google Scholar] [CrossRef]
- Guo, Y.R.; Hryc, C.F.; Jakana, J.; Jiang, H.; Wang, D.; Chiu, W.; Zhong, W.; Tao, Y.J. Crystal structure of a nematode-infecting virus. Proc. Natl. Acad. Sci. USA 2014, 111, 12781–12786. [Google Scholar] [CrossRef]
- Bekal, S.; Domier, L.L.; Gonfa, B.; McCoppin, N.K.; Lambert, K.N.; Bhalerao, K. A novel flavivirus in the soybean cyst nematode. J. Gen. Virol. 2014, 95, 1272–1280. [Google Scholar] [CrossRef]
- Ruark, C.L.; Koenning, S.R.; Davis, E.L.; Opperman, C.H.; Lommel, S.A.; Mitchum, M.G.; Sit, T.L. Soybean cyst nematode culture collections and field populations from North Carolina and Missouri reveal high incidences of infection by viruses. PLoS ONE 2017, 12, e0171514. [Google Scholar] [CrossRef] [PubMed]
- Castiglioni, V.G.; Olmo-Uceda, M.J.; Villena-Giménez, A.; Muñoz-Sánchez, J.C.; Legarda, E.G.; Elena, S.F. Story of an infection: Viral dynamics and host responses in the Caenorhabditis elegans-Orsay virus pathosystem. Sci. Adv. 2024, 10, eadn5945. [Google Scholar] [CrossRef]
- Williams, S.H.; Che, X.; Oleynik, A.; Garcia, J.A.; Muller, D.; Zabka, T.S.; Firth, C.; Corrigan, R.M.; Briese, T.; Jain, K.; et al. Discovery of two highly divergent negative-sense RNA viruses associated with the parasitic nematode, Capillaria hepatica, in wild Mus musculus from New York City. J. Gen. Virol. 2019, 100, 1350–1362. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Chen, H.; Zhong, W.; Tao, Y.J. Collagen and actin network mediate antiviral immunity against Orsay virus in C. elegans intestinal cells. PLoS Pathog. 2024, 20, e1011366. [Google Scholar] [CrossRef]
- Le Pen, J.; Jiang, H.; Di Domenico, T.; Kneuss, E.; Kosałka, J.; Leung, C.; Morgan, M.; Much, C.; Rudolph, K.L.M.; Enright, A.J.; et al. Terminal uridylyltransferases target RNA viruses as part of the innate immune system. Nat. Struct. Mol. Biol. 2018, 25, 778–786. [Google Scholar] [CrossRef]
- Reddy, K.C.; Dror, T.; Sowa, J.N.; Panek, J.; Chen, K.; Lim, E.S.; Wang, D.; Troemel, E.R. An Intracellular Pathogen Response Pathway Promotes Proteostasis in C. elegans. Curr. Biol. 2017, 27, 3544–3553.e3545. [Google Scholar] [CrossRef]
- Liu, W.-H.; Lin, Y.-L.; Wang, J.-P.; Liou, W.; Hou, R.F.; Wu, Y.-C.; Liao, C.-L. Restriction of vaccinia virus replication by a ced-3 and ced-4-dependent pathway in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 2006, 103, 4174–4179. [Google Scholar] [CrossRef]
- Yan, T.; Lu, R. Shared and unique mechanisms of RNAi-mediated antiviral immunity in C. elegans. Virology 2025, 605, 110459. [Google Scholar] [CrossRef]
- Guo, X.; Zhang, R.; Wang, J.; Ding, S.-W.; Lu, R. Homologous RIG-I-like helicase proteins direct RNAi-mediated antiviral immunity in C. elegans by distinct mechanisms. Proc. Natl. Acad. Sci. USA 2013, 110, 16085–16090. [Google Scholar] [CrossRef]
- Ashe, A.; Bélicard, T.; Le Pen, J.; Sarkies, P.; Frézal, L.; Lehrbach, N.J.; Félix, M.-A.; Miska, E.A. A deletion polymorphism in the Caenorhabditis elegans RIG-I homolog disables viral RNA dicing and antiviral immunity. eLife 2013, 2, e00994. [Google Scholar] [CrossRef] [PubMed]
- Dalzell, J.J.; McVeigh, P.; Warnock, N.D.; Mitreva, M.; Bird, D.M.; Abad, P.; Fleming, C.C.; Day, T.A.; Mousley, A.; Marks, N.J.; et al. RNAi Effector Diversity in Nematodes. PLoS Neglected Trop. Dis. 2011, 5, e1176. [Google Scholar] [CrossRef] [PubMed]
- Steiner, F.A.; Okihara, K.L.; Hoogstrate, S.W.; Sijen, T.; Ketting, R.F. RDE-1 slicer activity is required only for passenger-strand cleavage during RNAi in Caenorhabditis elegans. Nat. Struct. Mol. Biol. 2009, 16, 207–211. [Google Scholar] [CrossRef]
- Tsai, H.-Y.; Chen, C.-C.G.; Conte, D., Jr.; Moresco, J.J.; Chaves, D.A.; Mitani, S.; Yates, J.R., III; Tsai, M.-D.; Mello, C.C. A Ribonuclease Coordinates siRNA Amplification and mRNA Cleavage during RNAi. Cell 2015, 160, 407–419. [Google Scholar] [CrossRef]
- Preston, M.A.; Porter, D.F.; Chen, F.; Buter, N.; Lapointe, C.P.; Keles, S.; Kimble, J.; Wickens, M. Unbiased screen of RNA tailing activities reveals a poly(UG) polymerase. Nat. Methods 2019, 16, 437–445. [Google Scholar] [CrossRef]
- Shukla, A.; Yan, J.; Pagano, D.J.; Dodson, A.E.; Fei, Y.; Gorham, J.; Seidman, J.G.; Wickens, M.; Kennedy, S. poly(UG)-tailed RNAs in genome protection and epigenetic inheritance. Nature 2020, 582, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Gu, W.; Shirayama, M.; Conte, D., Jr.; Vasale, J.; Batista, P.J.; Claycomb, J.M.; Moresco, J.J.; Youngman, E.M.; Keys, J.; Stoltz, M.J.; et al. Distinct Argonaute-Mediated 22G-RNA Pathways Direct Genome Surveillance in the C. elegans Germline. Mol. Cell 2009, 36, 231–244. [Google Scholar] [CrossRef] [PubMed]
- Pak, J.; Fire, A. Distinct Populations of Primary and Secondary Effectors During RNAi in C. elegans. Science 2007, 315, 241–244. [Google Scholar] [CrossRef]
- Yigit, E.; Batista, P.J.; Bei, Y.; Pang, K.M.; Chen, C.-C.G.; Tolia, N.H.; Joshua-Tor, L.; Mitani, S.; Simard, M.J.; Mello, C.C. Analysis of the C. elegans Argonaute Family Reveals that Distinct Argonautes Act Sequentially during RNAi. Cell 2006, 127, 747–757. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Liu, H.; Guo, Y.R. Insights into Virus-Host Interactions: Lessons from Caenorhabditis elegans-Orsay Virus Model. Curr. Med. Sci. 2025, 45, 169–184. [Google Scholar] [CrossRef]
- Reddy, K.C.; Dror, T.; Underwood, R.S.; Osman, G.A.; Elder, C.R.; Desjardins, C.A.; Cuomo, C.A.; Barkoulas, M.; Troemel, E.R. Antagonistic paralogs control a switch between growth and pathogen resistance in C. elegans. PLoS Pathog. 2019, 15, e1007528. [Google Scholar] [CrossRef]
- Sowa, J.N.; Jiang, H.; Somasundaram, L.; Tecle, E.; Xu, G.; Wang, D.; Troemel, E.R. The Caenorhabditis elegans RIG-I Homolog DRH-1 Mediates the Intracellular Pathogen Response upon Viral Infection. J. Virol. 2020, 94, 2. [Google Scholar] [CrossRef]
- Tecle, E.; Chhan, C.B.; Franklin, L.; Underwood, R.S.; Hanna-Rose, W.; Troemel, E.R. The purine nucleoside phosphorylase pnp-1 regulates epithelial cell resistance to infection in C. elegans. PLoS Pathog. 2021, 17, e1009350. [Google Scholar] [CrossRef] [PubMed]
- Wernet, N.D.; Tecle, E.; Sarmiento, M.B.; Kuo, C.-J.; Chhan, C.B.; Baick, I.; Batachari, L.E.; Franklin, L.; Herneisen, A.; Bhabha, G.; et al. Adenosine deaminase and deoxyadenosine regulate intracellular immune response in C. elegans. iScience 2025, 28, 3. [Google Scholar] [CrossRef] [PubMed]
- Bakowski, M.A.; Desjardins, C.A.; Smelkinson, M.G.; Dunbar, T.A.; Lopez-Moyado, I.F.; Rifkin, S.A.; Cuomo, C.A.; Troemel, E.R. Ubiquitin-Mediated Response to Microsporidia and Virus Infection in C. elegans. PLoS Pathog. 2014, 10, e1004200. [Google Scholar] [CrossRef]
- Panek, J.; Gang, S.S.; Reddy, K.C.; Luallen, R.J.; Fulzele, A.; Bennett, E.J.; Troemel, E.R. A cullin-RING ubiquitin ligase promotes thermotolerance as part of the intracellular pathogen response in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 2020, 117, 7950–7960. [Google Scholar] [CrossRef]
- Sparrer, K.M.J.; Gableske, S.; Zurenski, M.A.; Parker, Z.M.; Full, F.; Baumgart, G.J.; Kato, J.; Pacheco-Rodriguez, G.; Liang, C.; Pornillos, O.; et al. TRIM23 mediates virus-induced autophagy via activation of TBK1. Nat. Microbiol. 2017, 2, 1543–1557. [Google Scholar] [CrossRef] [PubMed]
- Lažetić, V.; Batachari, L.E.; Russell, A.B.; Troemel, E.R. Similarities in the induction of the intracellular pathogen response in Caenorhabditis elegans and the type I interferon response in mammals. BioEssays 2023, 45, 2300097. [Google Scholar] [CrossRef]
- Tanguy, M.; Véron, L.; Stempor, P.; Ahringer, J.; Sarkies, P.; Miska, E.A. An Alternative STAT Signaling Pathway Acts in Viral Immunity in Caenorhabditis elegans. mBio 2017, 8, 5. [Google Scholar] [CrossRef]
- van Sluijs, L.; Bosman, K.J.; Pankok, F.; Blokhina, T.; Wilten, J.I.H.A.; te Molder, D.M.; Riksen, J.A.G.; Snoek, B.L.; Pijlman, G.P.; Kammenga, J.E.; et al. Balancing Selection of the Intracellular Pathogen Response in Natural Caenorhabditis elegans Populations. Front. Cell. Infect. Microbiol. 2022, 11, 758331. [Google Scholar] [CrossRef]
- Lažetić, V.; Wu, F.; Cohen, L.B.; Reddy, K.C.; Chang, Y.-T.; Gang, S.S.; Bhabha, G.; Troemel, E.R. The transcription factor ZIP-1 promotes resistance to intracellular infection in Caenorhabditis elegans. Nat. Commun. 2022, 13, 17. [Google Scholar] [CrossRef]
- Biglou, S.G.; Bendena, W.G.; Chin-Sang, I. An overview of the insulin signaling pathway in model organisms Drosophila melanogaster and Caenorhabditis elegans. Peptides 2021, 145, 170640. [Google Scholar] [CrossRef]
- Duxbury, E.M.L.; Carlsson, H.; Kimberley, A.; Ridge, Y.; Johnson, K.; Maklakov, A.A. Reduced insulin/IGF-1 signalling upregulates two anti-viral immune pathways, decreases viral load and increases survival under viral infection in C. elegans. GeroScience 2024, 46, 5767–5780. [Google Scholar] [CrossRef]
- Castelletto, M.L.; Massey, H.C., Jr.; Lok, J.B. Morphogenesis of Strongyloides stercoralis Infective Larvae Requires the DAF-16 Ortholog FKTF-1. PLoS Pathog. 2009, 5, e1000370. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Lok, J.B.; Ranjit, N.; Massey, H.C.; Sternberg, P.W.; Gasser, R.B. Structural and functional characterisation of the fork head transcription factor-encoding gene, Hc-daf-16, from the parasitic nematode Haemonchus contortus (Strongylida). Int. J. Parasitol. 2010, 40, 405–415. [Google Scholar] [CrossRef]
- Li, F.; Lok, J.B.; Gasser, R.B.; Korhonen, P.K.; Sandeman, M.R.; Shi, D.; Zhou, R.; Li, X.; Zhou, Y.; Zhao, J.; et al. Hc-daf-2 encodes an insulin-like receptor kinase in the barber’s pole worm, Haemonchus contortus, and restores partial dauer regulation. Int. J. Parasitol. 2014, 44, 485–496. [Google Scholar] [CrossRef]
- Marks, N.D.; Winter, A.D.; Gu, H.Y.; Maitland, K.; Gillan, V.; Ambroz, M.; Martinelli, A.; Laing, R.; MacLellan, R.; Towne, J.; et al. Profiling microRNAs through development of the parasitic nematode Haemonchus identifies nematode-specific miRNAs that suppress larval development. Sci. Rep. 2019, 9, 17594. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Sarkis, P.T.N.; Luo, K.; Yu, Y.; Yu, X.-F. Regulation of Apobec3F and Human Immunodeficiency Virus Type 1 Vif by Vif-Cul5-ElonB/C E3 Ubiquitin Ligase. J. Virol. 2005, 79, 9579–9587. [Google Scholar] [CrossRef] [PubMed]
- Hüttenhain, R.; Xu, J.; Burton, L.A.; Gordon, D.E.; Hultquist, J.F.; Johnson, J.R.; Satkamp, L.; Hiatt, J.; Rhee, D.Y.; Baek, K.; et al. ARIH2 Is a Vif-Dependent Regulator of CUL5-Mediated APOBEC3G Degradation in HIV Infection. Cell Host Microbe 2019, 26, 86–99.e87. [Google Scholar] [CrossRef]
- Xia, M.; Yan, T.; Lu, R. A targeted genetic screen identifies Caenorhabditis elegans genes involved in RNAi-independent antiviral defense. Virology 2025, 610, 110597. [Google Scholar] [CrossRef]
- Hagen, J.; Sarkies, P.; Selkirk, M.E. Lentiviral transduction facilitates RNA interference in the nematode parasite Nippostrongylus brasiliensis. PLoS Pathog. 2021, 17, e1009286. [Google Scholar] [CrossRef]
- Desai, P.; Diamond, M.S.; Thackray, L.B. Helminth–virus interactions: Determinants of coinfection outcomes. Gut Microbes 2021, 13, 1961202. [Google Scholar] [CrossRef]
| Tier | Component/Pathway | Key Proteins (Examples) | Primary Function & Role |
|---|---|---|---|
| Tier 1: The Effector Layer | Structural Barriers | COL-51, COL-61, ACT-5 | Physical “barricades” (e.g., collagens, actin) that block or impede viral entry and ingress into intestinal cells. |
| RNA interference (RNAi) | DCR-1, DRH-1, RDE-1, RRF-1, WAGOs | A “precision-guided system” that cleaves viral dsRNA (primary siRNAs) and uses RdRPs for a powerful amplification loop (secondary siRNAs). | |
| Uridylation Pathway | CDE-1 | An RNAi-independent “tag-and-degrade” mechanism; CDE-1 adds a U-tail to viral ssRNA to mark it for destruction by host exonucleases. | |
| Intracellular Pathogen Response (IPR) | ZIP-1, CUL-6/RCS-1 (CRL Complex) | A broad “fortress defense”; a transcriptional program that upregulates an effector module (E3 ligase) to enhance host proteostasis and resilience. | |
| Repurposed Core Machinery | CED-3, CED-4 (PCD); lys-2 (AMEs) | “Moonlighting” proteins from other core systems (e.g., Programmed Cell Death, antibacterial effectors) are recruited for antiviral functions. | |
| Tier 2: The Regulatory Layer | Intrinsic “State-Gating” Switch | PALS-22 (Repressor), PALS-25 (Activator) | An internal “pro-growth” vs. “pro-defense” toggle that manages the high fitness costs of constitutive immunity by keeping the IPR off in the absence of threat. |
| Global State-Dependent Modulation | Insulin/IGF-1 Signaling (IIS) Pathway (DAF-2, DAF-16) | A “life-strategy rheostat” that links the host’s metabolic/environmental state (“growth” vs. “survival”) to immune readiness, “priming” the host for a potent, cost-effective response upon infection. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xi, E.; Meng, T.; Chen, H. Beyond Pathogenesis: The Nematode Immune Network as the Arbiter of a Host–Virus Truce. Viruses 2025, 17, 1485. https://doi.org/10.3390/v17111485
Xi E, Meng T, Chen H. Beyond Pathogenesis: The Nematode Immune Network as the Arbiter of a Host–Virus Truce. Viruses. 2025; 17(11):1485. https://doi.org/10.3390/v17111485
Chicago/Turabian StyleXi, Emma, Tan Meng, and Hanqiao Chen. 2025. "Beyond Pathogenesis: The Nematode Immune Network as the Arbiter of a Host–Virus Truce" Viruses 17, no. 11: 1485. https://doi.org/10.3390/v17111485
APA StyleXi, E., Meng, T., & Chen, H. (2025). Beyond Pathogenesis: The Nematode Immune Network as the Arbiter of a Host–Virus Truce. Viruses, 17(11), 1485. https://doi.org/10.3390/v17111485






