The Role of Innate Cells During Alphavirus Chikungunya Infection
Abstract
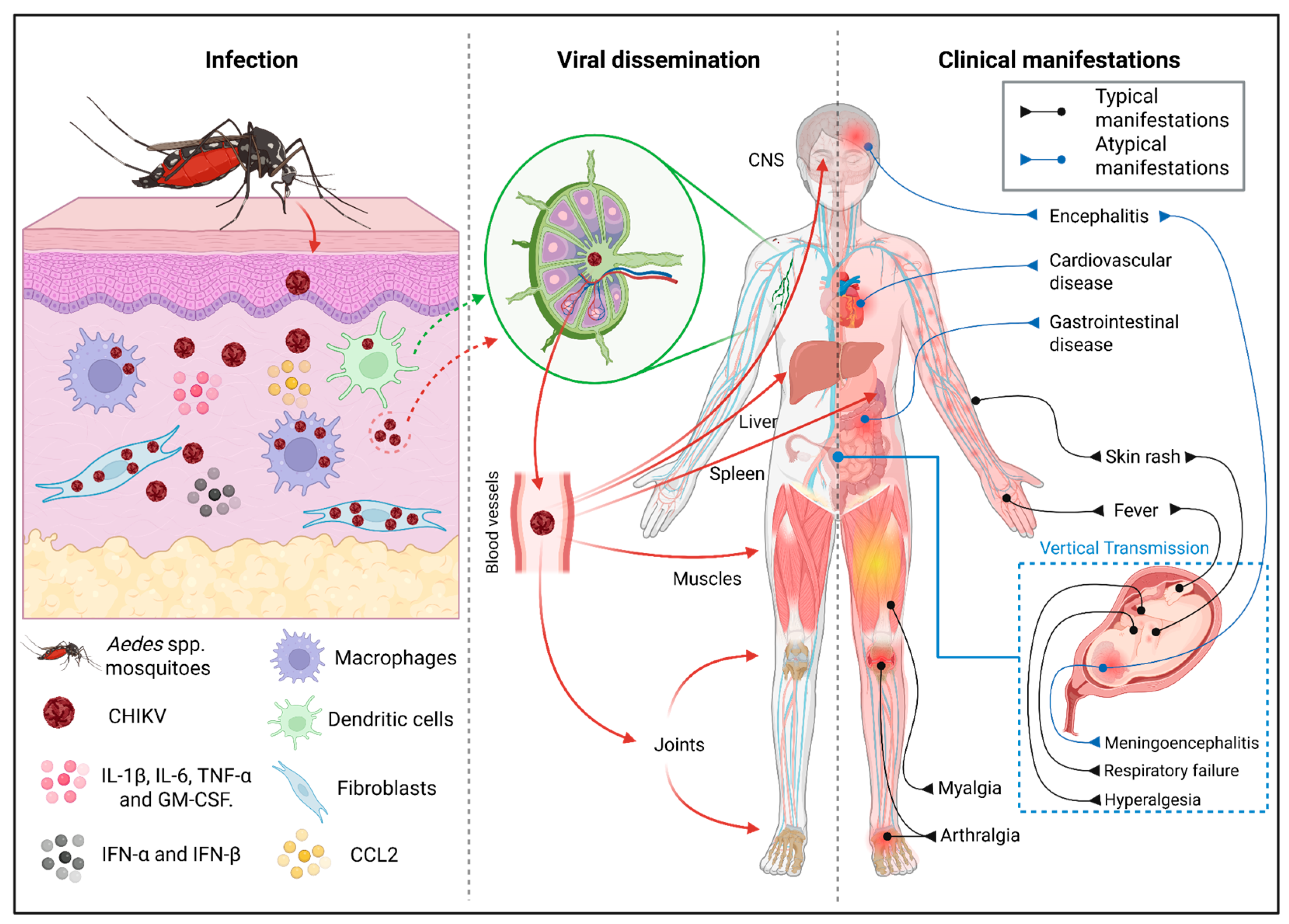
1. Introduction
2. Search Strategy
3. Fibroblasts as Central Hubs in CHIKV Infection
4. Macrophages and Monocytes in CHIKV Infection: Roles in Inflammation, Viral Persistence and Dissemination
5. Dendritic Cell Dynamics During CHIKV Infection
6. The Ambiguous Role of Neutrophils in CHIKV Immunity and Pathogenesis
7. Cytotoxic Innate Cells in CHIKV Control
8. Immunomodulatory Effects of Ae. aegypti Saliva During CHIKV Infection
9. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CHIKD | Chikungunya Disease |
| CHIKV | Chikungunya Virus |
| CXCL8 | C-X-C Motif Chemokine Ligand 8 |
| DCIR | Dendritic Cell Immunoreceptor |
| DCIR−/− | DCIR Knockout Mice |
| DC-SIGN | Dendritic Cell-Specific Intercellular Adhesion Molecule-3-Grabbing Non-Integrin |
| DCs | Dendritic Cells |
| DNase | Deoxyribonuclease |
| E2 | CHIKV Envelope Protein E2 |
| GM-CSF | Granulocyte-Macrophage Colony-Stimulating Factor |
| HLA-DR | Human Leukocyte Antigen–DR Isotype |
| IFN-I | Type I Interferon |
| IFN-β | Interferon Beta |
| IFN-γ | Interferon Gamma |
| IFNAR−/− | Type I Interferon Receptor Knockout Mice |
| IL-6 | Interleukin 6 |
| IL-8 | Interleukin 8 |
| IL-10 | Interleukin 10 |
| IL-12 | Interleukin 12 |
| IL-12p70 | Interleukin 12p70 |
| IL-17A | Interleukin 17A |
| iNOS | Inducible Nitric Oxide Synthase |
| IRF7 | Interferon Regulatory Factor 7 |
| ISG56 | Interferon-Stimulated Gene 56 |
| Ly6C+ | Lymphocyte Antigen 6 Complex, Locus C Positive (mouse monocyte marker) |
| M2 | Alternatively Activated (anti-inflammatory) Macrophage Phenotype |
| MARCO | Macrophage Receptor with Collagenous Structure |
| MRC5 | Human Fetal Lung Fibroblast Cell Line |
| Msr1 | Macrophage Scavenger Receptor 1 |
| mDCs | Myeloid Dendritic Cells |
| NKG2A | NK Cell Inhibitory Receptor |
| NKRp44+ | NK Cell Activating Receptor Marker |
| NK cells | Natural Killer Cells |
| NKT-like | Natural Killer T-like Cells |
| NETs | Neutrophil Extracellular Traps |
| NF-κB | Nuclear Factor Kappa B |
| OPG | Osteoprotegerin |
| pDCs | Plasmacytoid Dendritic Cells |
| RANKL | Receptor Activator of Nuclear Factor Kappa-B Ligand |
| RNA | Ribonucleic Acid |
| ROS | Reactive Oxygen Species |
| SGE | Salivary Gland Extract |
| SIGNR3 | SIGN-related 3 (mouse homolog of DC-SIGN) |
| TGF-β | Transforming Growth Factor Beta |
| TNF-α | Tumor Necrosis Factor Alpha |
| TRAF6 | TNF Receptor-Associated Factor 6 |
| TLR3 | Toll-Like Receptor 3 |
| TLR7 | Toll-Like Receptor 7 |
| Wdfy4 | WD Repeat and FYVE Domain Containing 4 (critical for cDC1 development) |
| Ym1 | Chitinase-Like Protein Ym1 (mouse marker for M2 macrophages) |
References
- International Committee on Taxonomy of Viruses (ICTV). Taxon Details: Alphavirus chikungunya. ICTV—Current ICTV Taxonomy Release 40. 2025. Available online: https://ictv.global/taxonomy/taxondetails?taxnode_id=202405087&taxon_name=Alphavirus%20chikungunya#release_40 (accessed on 29 October 2025).
- Maure, C.; Khazhidinov, K.; Kang, H.; Auzenbergs, M.; Moyersoen, P.; Abbas, K.; Santos, G.M.L.; Medina, L.M.H.; Wartel, T.A.; Kim, J.H.; et al. Chikungunya Vaccine Development, Challenge and Pathway toward Public Health Impact. Vaccine 2024, 42, 126483. [Google Scholar] [CrossRef]
- World Health Organization. Report on the Global Arbovirus Surveillance and Response Capacity Survey 2021–2022; World Health Organization: Geneva, Switzerland, 2025; ISBN 978-92-4010-7380. [Google Scholar]
- Mehta, R.; Gerardin, P.; de Brito, C.A.A.; Soares, C.N.; Ferreira, M.L.B.; Solomon, T. The Neurological Complications of Chikungunya Virus: A Systematic Review. Rev. Med. Virol. 2018, 28, e1978. [Google Scholar] [CrossRef]
- Silva, L.A.; Dermody, T.S. Chikungunya Virus: Epidemiology, Replication, Disease Mechanisms and Prospective Intervention Strategies. J. Clin. Investig. 2017, 127, 737–749. [Google Scholar] [CrossRef]
- De Almeida Di Maio Ferreira, F.C.P.; Da Silva, A.S.V.; Recht, J.; Guaraldo, L.; Moreira, M.E.L.; De Siqueira, A.M.; Gerardin, P.; Brasil, P. Vertical Transmission of Chikungunya Virus: A Systematic Review. PLoS ONE 2021, 16, e0249166. [Google Scholar] [CrossRef]
- Torres, J.R.; Falleiros-Arlant, L.H.; Dueñas, L.; Pleitez-Navarrete, J.; Salgado, D.M.; Castillo, J.B. Del Congenital and Perinatal Complications of Chikungunya Fever: A Latin American Experience. Int. J. Infect. Dis. 2016, 51, 85–88. [Google Scholar] [CrossRef]
- Wahid, B.; Ali, A.; Rafique, S.; Idrees, M. Global Expansion of Chikungunya Virus: Mapping the 64-Year History. Int. J. Infect. Dis. 2017, 58, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro dos Santos, G.; Jawed, F.; Mukandavire, C.; Deol, A.; Scarponi, D.; Mboera, L.E.G.; Seruyange, E.; Poirier, M.J.P.; Bosomprah, S.; Udeze, A.O.; et al. Global Burden of Chikungunya Virus Infections and the Potential Benefit of Vaccination Campaigns. Nat. Med. 2025, 31, 2342–2349. [Google Scholar] [CrossRef]
- Krambrich, J.; Mihalič, F.; Gaunt, M.W.; Bohlin, J.; Hesson, J.C.; Lundkvist, Å.; de Lamballerie, X.; Li, C.; Shi, W.; Pettersson, J.H.O. The Evolutionary and Molecular History of a Chikungunya Virus Outbreak Lineage. PLoS Negl. Trop. Dis. 2024, 18, e0012349. [Google Scholar] [CrossRef]
- Pedí, V.D.; Porto, D.L.; de Jesus Martins, W.; de França, G.V.A. Epidemiology of Chikungunya Hospitalizations, Brazil, 2014–2024. Emerg. Infect. Dis. 2025, 31, 1718–1728. [Google Scholar] [CrossRef] [PubMed]
- Lim, A.; Shearer, F.M.; Sewalk, K.; Pigott, D.M.; Clarke, J.; Ghouse, A.; Judge, C.; Kang, H.; Messina, J.P.; Kraemer, M.U.G.; et al. The Overlapping Global Distribution of Dengue, Chikungunya, Zika and Yellow Fever. Nat. Commun. 2025, 16, 3418. [Google Scholar] [CrossRef] [PubMed]
- Rama, K.; de Roo, A.M.; Louwsma, T.; Hofstra, H.S.; Gurgel Do Amaral, G.S.; Vondeling, G.T.; Postma, M.J.; Freriks, R.D. Clinical Outcomes of Chikungunya: A Systematic Literature Review and Meta-Analysis. PLoS Negl. Trop. Dis. 2024, 18, e0012254. [Google Scholar] [CrossRef]
- Tiozzo, G.; de Roo, A.M.; Gurgel Do Amaral, G.S.; Hofstra, H.; Vondeling, G.T.; Postma, M.J. Assessing Chikungunya’s Economic Burden and Impact on Health-Related Quality of Life: Two Systematic Literature Reviews. PLoS Negl. Trop. Dis. 2025, 19, e0012990. [Google Scholar] [CrossRef]
- Yodtaweepornanan, P.; Pongsittisak, W.; Satpanich, P. Incidence and Factors Associated with Chronic Chikungunya Arthritis Following Chikungunya Virus Infection. Trop. Med. Int. Health 2023, 28, 653–659. [Google Scholar] [CrossRef] [PubMed]
- Micheleto, J.P.C.; Melo, K.A.; Veloso, F.C.S.; Kassar, S.B.; Oliveira, M.J.C. Risk Factors for Mortality in Patients with Chikungunya: A Systematic Review and Meta-Analysis. Trop. Med. Int. Health 2025, 30, 235–245. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Guidelines for Clinical Management of Arboviral Diseases: Dengue, Chikungunya, Zika and Yellow Fever; World Health Organization: Geneva, Switzerland, 2025; ISBN 978-92-4-011111-0. [Google Scholar]
- U.S. Food and Drug Administration (FDA). FDA Update on the Safety of IXCHIQ (Chikungunya Vaccine, Live). Silver Spring (MD): FDA. 2025. Available online: https://www.fda.gov/vaccines-blood-biologics/safety-availability-biologics/fda-update-safety-ixchiq-chikungunya-vaccine-live (accessed on 12 October 2025).
- Gardner, C.L.; Burke, C.W.; Tesfay, M.Z.; Glass, P.J.; Klimstra, W.B.; Ryman, K.D. Eastern and Venezuelan Equine Encephalitis Viruses Differ in Their Ability to Infect Dendritic Cells and Macrophages: Impact of Altered Cell Tropism on Pathogenesis. J. Virol. 2008, 82, 10634–10646. [Google Scholar] [CrossRef] [PubMed]
- Kafai, N.M.; Diamond, M.S.; Fox, J.M. Distinct Cellular Tropism and Immune Responses to Alphavirus Infection. Annu. Rev. Immunol. 2022, 40, 615–649. [Google Scholar] [CrossRef]
- Trobaugh, D.W.; Klimstra, W.B. Alphaviruses Suppress Host Immunity by Preventing Myeloid Cell Replication and Antagonizing Innate Immune Responses. Curr. Opin. Virol. 2017, 23, 30–34. [Google Scholar] [CrossRef]
- Felipe, V.L.J.; Paula, A.V.; Silvio, U.I. Chikungunya Virus Infection Induces Differential Inflammatory and Antiviral Responses in Human Monocytes and Monocyte-Derived Macrophages. Acta Trop. 2020, 211, 105619. [Google Scholar] [CrossRef]
- Young, A.R.; Locke, M.C.; Cook, L.E.; Hiller, B.E.; Zhang, R.; Hedberg, M.L.; Monte, K.J.; Veis, D.J.; Diamond, M.S.; Lenschow, D.J. Dermal and Muscle Fibroblasts and Skeletal Myofibers Survive Chikungunya Virus Infection and Harbor Persistent RNA. PLoS Pathog. 2019, 15, e1007993. [Google Scholar] [CrossRef]
- Lentscher, A.J.; McCarthy, M.K.; May, N.A.; Davenport, B.J.; Montgomery, S.A.; Raghunathan, K.; McAllister, N.; Silva, L.A.; Morrison, T.E.; Dermody, T.S. Chikungunya Virus Replication in Skeletal Muscle Cells Is Required for Disease Development. J. Clin. Investig. 2020, 130, 1466–1478. [Google Scholar] [CrossRef]
- Sourisseau, M.; Schilte, C.; Casartelli, N.; Trouillet, C.; Guivel-Benhassine, F. Characterization of Reemerging Chikungunya Virus. PLoS Pathog. 2007, 3, 89. [Google Scholar] [CrossRef]
- de Souza, W.M.; Lecuit, M.; Weaver, S.C. Chikungunya Virus and Other Emerging Arthritogenic Alphaviruses. Nat. Rev. Microbiol. 2025, 23, 585–601. [Google Scholar] [CrossRef]
- Chopra, A.; Anuradha, V.; Lagoo-Joshi, V.; Kunjir, V.; Salvi, S.; Saluja, M. Chikungunya Virus Aches and Pains: An Emerging Challenge. Arthritis Rheum. 2008, 58, 2921–2922. [Google Scholar] [CrossRef]
- Hoarau, J.-J.; Jaffar Bandjee, M.-C.; Krejbich Trotot, P.; Das, T.; Li-Pat-Yuen, G.; Dassa, B.; Denizot, M.; Guichard, E.; Ribera, A.; Henni, T.; et al. Persistent Chronic Inflammation and Infection by Chikungunya Arthritogenic Alphavirus in Spite of a Robust Host Immune Response. J. Immunol. 2010, 184, 5914–5927. [Google Scholar] [CrossRef] [PubMed]
- Amaral, J.K.; Bingham, C.O.; Taylor, P.C.; Vilá, L.M.; Weinblatt, M.E.; Schoen, R.T. Pathogenesis of Chronic Chikungunya Arthritis: Resemblances and Links with Rheumatoid Arthritis. Travel. Med. Infect. Dis. 2023, 52, 102534. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Morales, A.J.; Cardona-Ospina, J.A.; Fernanda Urbano-Garzón, S.; Sebastian Hurtado-Zapata, J. Prevalence of Post-Chikungunya Infection Chronic Inflammatory Arthritis: A Systematic Review and Meta-Analysis. Arthritis Care Res. 2016, 68, 1849–1858. [Google Scholar] [CrossRef]
- Schilte, C.; Couderc, T.; Chretien, F.; Sourisseau, M.; Gangneux, N.; Guivel-Benhassine, F.; Kraxner, A.; Tschopp, J.; Higgs, S.; Michault, A.; et al. Type I IFN Controls Chikungunya Virus via Its Action on Nonhematopoietic Cells. J. Exp. Med. 2010, 207, 429–442. [Google Scholar] [CrossRef]
- Rulli, N.E.; Rolph, M.S.; Srikiatkhachorn, A.; Anantapreecha, S.; Guglielmotti, A.; Mahalingam, S. Protection from Arthritis and Myositis in a Mouse Model of Acute Chikungunya Virus Disease by Bindarit, an Inhibitor of Monocyte Chemotactic Protein-1 Synthesis. J. Infect. Dis. 2011, 204, 1026–1030. [Google Scholar] [CrossRef] [PubMed]
- Hiroki, C.H.; Toller-Kawahisa, J.E.; Fumagalli, M.J.; Colon, D.F.; Figueiredo, L.T.M.; Fonseca, B.A.L.D.; Franca, R.F.O.; Cunha, F.Q. Neutrophil Extracellular Traps Effectively Control Acute Chikungunya Virus Infection. Front. Immunol. 2020, 10, 3108. [Google Scholar] [CrossRef]
- Petitdemange, C.; Becquart, P.; Wauquier, N.; Béziat, V.; Debré, P.; Leroy, E.M.; Vieillard, V. Unconventional Repertoire Profile Is Imprinted during Acute Chikungunya Infection for Natural Killer Cells Polarization toward Cytotoxicity. PLoS Pathog. 2011, 7, e1002268. [Google Scholar] [CrossRef]
- Briant, L.; Desprès, P.; Choumet, V.; Missé, D. Role of Skin Immune Cells on the Host Susceptibility to Mosquito-Borne Viruses. Virology 2014, 464–465, 26–32. [Google Scholar] [CrossRef]
- Couderc, T.; Chrétien, F.; Schilte, C.; Disson, O.; Brigitte, M.; Guivel-Benhassine, F.; Touret, Y.; Barau, G.; Cayet, N.; Schuffenecker, I.; et al. A Mouse Model for Chikungunya: Young Age and Inefficient Type-I Interferon Signaling Are Risk Factors for Severe Disease. PLoS Pathog. 2008, 4, e29. [Google Scholar] [CrossRef]
- Selvamani, S.P.; Mishra, R.; Singh, S.K. Chikungunya Virus Exploits MiR-146a to Regulate NF-ΚB Pathway in Human Synovial Fibroblasts. PLoS ONE 2014, 9, e103624. [Google Scholar] [CrossRef]
- Bedoui, Y.; Septembre-Malaterre, A.; Giry, C.; Jaffar-Bandjee, M.C.; Selambarom, J.; Guiraud, P.; Gasque, P. Robust Cox-2-Mediated Prostaglandin Response May Drive Arthralgia and Bone Destruction in Patients with Chronic Inflammation Post-Chikungunya. PLoS Negl. Trop. Dis. 2021, 15, e0009115. [Google Scholar] [CrossRef]
- Phuklia, W.; Kasisith, J.; Modhiran, N.; Rodpai, E.; Thannagith, M.; Thongsakulprasert, T.; Smith, D.R.; Ubol, S. Osteoclastogenesis Induced by CHIKV-Infected Fibroblast-like Synoviocytes: A Possible Interplay between Synoviocytes and Monocytes/Macrophages in CHIKV-Induced Arthralgia/Arthritis. Virus Res. 2013, 177, 179–188. [Google Scholar] [CrossRef]
- Schett, G.; Teitelbaum, S.L. Osteoclasts and Arthritis. J. Bone Miner. Res. 2009, 24, 1142–1146. [Google Scholar] [CrossRef] [PubMed]
- Noret, M.; Herrero, L.; Rulli, N.; Rolph, M.; Smith, P.N.; Li, R.W.; Roques, P.; Gras, G.; Mahalingam, S. Interleukin 6, RANKL and Osteoprotegerin Expression by Chikungunya Virus-Infected Human Osteoblasts. J. Infect. Dis. 2012, 206, 455–457. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Jaffar-Bandjee, M.C.; Giry, C.; Connen De Kerillis, L.; Merits, A.; Gasque, P.; Hoarau, J.J. Mouse Macrophage Innate Immune Response to Chikungunya Virus Infection. Virol. J. 2012, 9, 313. [Google Scholar] [CrossRef] [PubMed]
- Salomão, N.G.; Araújo, L.; de Souza, L.J.; Luiza Young, A.; Basílio-de-Oliveira, C.; Basílio-de-Oliveira, R.P.; de Carvalho, J.J.; Nunes, P.C.G.; da Silva Amorim, J.F.; dos Santos Barbosa, D.V.; et al. Chikungunya Virus Infection in the Skin: Histopathology and Cutaneous Immunological Response. Front. Microbiol. 2025, 16, 1497354. [Google Scholar] [CrossRef]
- Gardner, J.; Anraku, I.; Le, T.T.; Larcher, T.; Major, L.; Roques, P.; Schroder, W.A.; Higgs, S.; Suhrbier, A. Chikungunya Virus Arthritis in Adult Wild-Type Mice. J. Virol. 2010, 84, 8021–8032. [Google Scholar] [CrossRef]
- Poo, Y.S.; Nakaya, H.; Gardner, J.; Larcher, T.; Schroder, W.A.; Le, T.T.; Major, L.D.; Suhrbier, A. CCR2 Deficiency Promotes Exacerbated Chronic Erosive Neutrophil-Dominated Chikungunya Virus Arthritis. J. Virol. 2014, 88, 6862–6872. [Google Scholar] [CrossRef]
- Stoermer, K.A.; Burrack, A.; Oko, L.; Montgomery, S.A.; Borst, L.B.; Gill, R.G.; Morrison, T.E. Genetic Ablation of Arginase 1 in Macrophages and Neutrophils Enhances Clearance of an Arthritogenic Alphavirus. J. Immunol. 2012, 189, 4047–4059. [Google Scholar] [CrossRef]
- Krejbich-Trotot, P.; Denizot, M.; Hoarau, J.; Jaffar-Bandjee, M.; Das, T.; Gasque, P. Chikungunya Virus Mobilizes the Apoptotic Machinery to Invade Host Cell Defenses. FASEB J. 2011, 25, 314–325. [Google Scholar] [CrossRef]
- Labadie, K.; Larcher, T.; Joubert, C.; Mannioui, A.; Delache, B.; Brochard, P.; Guigand, L.; Dubreil, L.; Lebon, P.; Verrier, B.; et al. Chikungunya Disease in Nonhuman Primates Involves Long-Term Viral Persistence in Macrophages. J. Clin. Investig. 2010, 120, 894–906. [Google Scholar] [CrossRef]
- Holmes, A.C.; Lucas, C.J.; Brisse, M.E.; Ware, B.C.; Hickman, H.D.; Morrison, T.E.; Diamond, M.S. Ly6C+ Monocytes in the Skin Promote Systemic Alphavirus Dissemination. Cell Rep. 2024, 43, 113876. [Google Scholar] [CrossRef]
- de Souza, W.M.; Fumagalli, M.J.; de Lima, S.T.S.; Parise, P.L.; Carvalho, D.C.M.; Hernandez, C.; de Jesus, R.; Delafiori, J.; Candido, D.S.; Carregari, V.C.; et al. Pathophysiology of Chikungunya Virus Infection Associated with Fatal Outcomes. Cell Host Microbe 2024, 32, 606–622.e8. [Google Scholar] [CrossRef] [PubMed]
- Michlmayr, D.; Pak, T.R.; Rahman, A.H.; Amir, E.D.; Kim, E.; Kim-Schulze, S.; Suprun, M.; Stewart, M.G.; Thomas, G.P.; Balmaseda, A.; et al. Comprehensive Innate Immune Profiling of Chikungunya Virus Infection in Pediatric Cases. Mol. Syst. Biol. 2018, 14, e7862. [Google Scholar] [CrossRef]
- Johnston, L.J.; Halliday, G.M.; King, N.J.C. Phenotypic Changes in Langerhans’ Cells after Infection with Arboviruses: A Role in the Immune Response to Epidermally Acquired Viral Infection. J. Virol. 1996, 70, 4761–4766. [Google Scholar] [CrossRef] [PubMed]
- Johnston, L.J.; Halliday, G.M.; King, N.J.C. Langerhans Cells Migrate to Local Lymph Nodes Following Cutaneous Infection with an Arbovirus. J. Investig. Dermatol. 2000, 114, 560–568. [Google Scholar] [CrossRef]
- Sadanand, B.J.P.S. Chikungunya Infection: A Re-Emerging Epidemic. Rheumatol. Ther. 2018, 5, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Lucas, C.J.; Sheridan, R.M.; Reynoso, G.V.; Davenport, B.J.; McCarthy, M.K.; Martin, A.; Hesselberth, J.R.; Hickman, H.D.; Tamburini, B.A.J.; Morrison, T.E. Chikungunya Virus Infection Disrupts Lymph Node Lymphatic Endothelial Cell Composition and Function via MARCO. JCI Insight 2024, 9, e176537. [Google Scholar] [CrossRef]
- Messaoudi, I.; Vomaske, J.; Totonchy, T.; Kreklywich, C.N.; Haberthur, K.; Springgay, L.; Brien, J.D.; Diamond, M.S.; DeFilippis, V.R.; Streblow, D.N. Chikungunya Virus Infection Results in Higher and Persistent Viral Replication in Aged Rhesus Macaques Due to Defects in Anti-Viral Immunity. PLoS Negl. Trop. Dis. 2013, 7, e2343. [Google Scholar] [CrossRef]
- Beddingfield, B.J.; Sugimoto, C.; Kuroda, M.J.; Wang, E.; Weaver, S.C.; Russell-Lodrigue, K.E.; Killeen, S.Z.; Roy, C.J. Phenotypic and Kinetic Changes of Myeloid Lineage Cells in Innate Response to Chikungunya Infection in Cynomolgus Macaques. Viral Immunol. 2022, 35, 192–199. [Google Scholar] [CrossRef]
- Swiecki, M.; Colonna, M. The Multifaceted Biology of Plasmacytoid Dendritic Cells. Nat. Rev. Immunol. 2015, 15, 471–485. [Google Scholar] [CrossRef]
- Webster, B.; Werneke, S.W.; Zafirova, B.; This, S.; Coléon, S.; Decembre, E.; Paidassi, H.; Bouvier, I.; Joubert, P.E.; Duffy, D.; et al. Plasmacytoid Dendritic Cells Control Dengue and Chikungunya Virus Infections via IRF7-Regulated Interferon Responses. eLife 2018, 7, e34273. [Google Scholar] [CrossRef] [PubMed]
- Untergasser, A.; Zedler, U.; Langenkamp, A.; Hösel, M.; Quasdorff, M.; Esser, K.; Dienes, H.P.; Tappertzhofen, B.; Kolanus, W.; Protzer, U. Dendritic cells take up viral antigens but do not support the early steps of hepatitis B virus infection. Hepatology 2006, 43, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Thi Xuan, N.; Xuan Nghia, V.; Van Giang, N.; Xuan Canh, N.; Hai Ha, N.; Thuy Duong, N.; Huy Hoang, N. Stimulation of Dendritic Cell Functional Maturation by Capsid Protein from Chikungunya Virus. Iran. J. Basic Med. Sci. 2020, 23, 1268. [Google Scholar] [CrossRef]
- Davenport, B.J.; Bullock, C.; Mccarthy, M.K.; Hawman, D.W.; Murphy, K.M.; Kedl, R.M.; Diamond, M.S.; Morrison, T.E. Chikungunya Virus Evades Antiviral CD8 T Cell Responses to Establish Persistent Infection in Joint-Associated Tissues. J. Virol. 2020, 94, 10–1128. [Google Scholar] [CrossRef]
- Long, K.M.; Whitmore, A.C.; Ferris, M.T.; Sempowski, G.D.; McGee, C.; Trollinger, B.; Gunn, B.; Heise, M.T. Dendritic Cell Immunoreceptor Regulates Chikungunya Virus Pathogenesis in Mice. J. Virol. 2013, 87, 5697–5706. [Google Scholar] [CrossRef]
- Muralidharan, A.; Patrick Reid, S.; Groseth, A. Complex Roles of Neutrophils during Arboviral Infections. Cells 2021, 10, 1324. [Google Scholar] [CrossRef]
- Liu, X.; Poo, Y.S.; Alves, J.C.; Almeida, R.P.; Mostafavi, H.; Tang, P.C.H.; Bucala, R.; Teixeira, M.M.; Taylor, A.; Zaid, A.; et al. Interleukin-17 Contributes to Chikungunya Virus-Induced Disease. mBio 2022, 13, e0028922. [Google Scholar] [CrossRef]
- Her, Z.; Teng, T.; Tan, J.J.; Teo, T.; Kam, Y.; Lum, F.; Lee, W.W.; Gabriel, C.; Melchiotti, R.; Andiappan, A.K.; et al. Loss of TLR3 Aggravates CHIKV Replication and Pathology Due to an Altered Virus-specific Neutralizing Antibody Response. EMBO Mol. Med. 2015, 7, 24–41. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, M.K.; Reynoso, G.V.; Winkler, E.S.; Mack, M.; Diamond, M.S.; Hickman, H.D.; Morrison, T.E. MyD88-Dependent Influx of Monocytes and Neutrophils Impairs Lymph Node B Cell Responses to Chikungunya Virus Infection via Irf5, Nos2 and Nox2. PLoS Pathog. 2020, 16, e1008292. [Google Scholar] [CrossRef] [PubMed]
- Palha, N.; Guivel-Benhassine, F.; Briolat, V.; Lutfalla, G.; Sourisseau, M.; Ellett, F.; Wang, C.H.; Lieschke, G.J.; Herbomel, P.; Schwartz, O.; et al. Real-Time Whole-Body Visualization of Chikungunya Virus Infection and Host Interferon Response in Zebrafish. PLoS Pathog. 2013, 9, e1003619. [Google Scholar] [CrossRef]
- Maucourant, C.; Petitdemange, C.; Yssel, H.; Vieillard, V. Control of Acute Arboviral Infection by Natural Killer Cells. Viruses 2019, 11, 131. [Google Scholar] [CrossRef]
- Petitdemange, C.; Wauquier, N.; Devilliers, H.; Yssel, H.; Mombo, I.; Caron, M.; Nkoghé, D.; Debré, P.; Leroy, E.; Vieillard, V. Longitudinal Analysis of Natural Killer Cells in Dengue Virus-Infected Patients in Comparison to Chikungunya and Chikungunya/Dengue Virus-Infected Patients. PLoS Negl. Trop. Dis. 2016, 10, e0004499. [Google Scholar] [CrossRef] [PubMed]
- Thanapati, S.; Ganu, M.A.; Tripathy, A.S. Differential Inhibitory and Activating NK Cell Receptor Levels and NK/NKT-like Cell Functionality in Chronic and Recovered Stages of Chikungunya. PLoS ONE 2017, 12, e0188342. [Google Scholar] [CrossRef]
- Teo, T.-H.; Her, Z.; Tan, J.J.L.; Lum, F.-M.; Lee, W.W.L.; Chan, Y.-H.; Ong, R.-Y.; Kam, Y.-W.; Leparc-Goffart, I.; Gallian, P.; et al. Caribbean and La Réunion Chikungunya Virus Isolates Differ in Their Capacity To Induce Proinflammatory Th1 and NK Cell Responses and Acute Joint Pathology. J. Virol. 2015, 89, 7955–7969. [Google Scholar] [CrossRef]
- Schanoski, A.S.; Le, T.T.; Kaiserman, D.; Rowe, C.; Prow, N.A.; Barboza, D.D.; Santos, C.A.; Zanotto, P.M.A.; Magalhães, K.G.; Aurelio, L.; et al. Granzyme A in Chikungunya and Other Arboviral Infections. Front. Immunol. 2020, 10, 3083. [Google Scholar] [CrossRef]
- Thanapati, S.; Das, R.; Tripathy, A.S. Phenotypic and Functional Analyses of NK and NKT-like Populations during the Early Stages of Chikungunya Infection. Front. Microbiol. 2015, 6, 895. [Google Scholar] [CrossRef]
- Guo, J.; He, X.; Tao, J.; Sun, H.; Yang, J. Unraveling the Molecular Mechanisms of Mosquito Salivary Proteins: New Frontiers in Disease Transmission and Control. Biomolecules 2025, 15, 82. [Google Scholar] [CrossRef] [PubMed]
- Fong, S.-W.; Tan, J.J.L.; Sridhar, V.; Amrun, S.N.; Neo, V.K.X.; Wong, N.; Lee, B.; Chan, Y.-H.; Torres-Ruesta, A.; Loo, L.H.; et al. Mosquito Salivary Sialokinin Reduces Monocyte Activation and Chikungunya Virus-Induced Inflammation via Neurokinin Receptors. Nat. Commun. 2025, 16, 8644. [Google Scholar] [CrossRef]
- Martin-Martin, I.; Alves E Silva, T.L.; Williams, A.E.; Vega-Rodriguez, J.; Calvo, E. Performing Immunohistochemistry in Mosquito Salivary Glands. Cold Spring Harb. Protoc. 2022, 2022, pdb.top107699. [Google Scholar] [CrossRef]
- Agarwal, A.; Joshi, G.; Nagar, D.P.; Sharma, A.K.; Sukumaran, D.; Pant, S.C.; Parida, M.M.; Dash, P.K. Mosquito Saliva Induced Cutaneous Events Augment Chikungunya Virus Replication and Disease Progression. Infect. Genet. Evol. 2016, 40, 126–135. [Google Scholar] [CrossRef]
- Gavor, E.; Choong, Y.K.; Liu, Y.; Pompon, J.; Ooi, E.E.; Mok, Y.K.; Liu, H.; Kini, R.M.; Sivaraman, J. Identification of Aedes aegypti Salivary Gland Proteins Interacting with Human Immune Receptor Proteins. PLoS Negl. Trop. Dis. 2022, 16, e0010743. [Google Scholar] [CrossRef]
- Champagne, D.E.; Ribeiro, J.M. Sialokinin I and II: Vasodilatory Tachykinins from the Yellow Fever Mosquito Aedes aegypti. Proc. Natl. Acad. Sci. USA 1994, 91, 138–142. [Google Scholar] [CrossRef]
- Pingen, M.; Bryden, S.R.; Pondeville, E.; Schnettler, E.; Kohl, A.; Merits, A.; Fazakerley, J.K.; Graham, G.J.; McKimmie, C.S. Host Inflammatory Response to Mosquito Bites Enhances the Severity of Arbovirus Infection. Immunity 2016, 44, 1455–1469. [Google Scholar] [CrossRef]
- Ribeiro, J.M. Characterization of a Vasodilator from the Salivary Glands of the Yellow Fever Mosquito Aedes aegypti. J. Exp. Biol. 1992, 165, 61–71. [Google Scholar] [CrossRef]
- Martin-Martin, I.; Valenzuela Leon, P.C.; Amo, L.; Shrivastava, G.; Iniguez, E.; Aryan, A.; Brooks, S.; Kojin, B.B.; Williams, A.E.; Bolland, S.; et al. Aedes aegypti Sialokinin Facilitates Mosquito Blood Feeding and Modulates Host Immunity and Vascular Biology. Cell Rep. 2022, 39, 110648. [Google Scholar] [CrossRef] [PubMed]
- Lefteri, D.A.; Bryden, S.R.; Pingen, M.; Terry, S.; McCafferty, A.; Beswick, E.F.; Georgiev, G.; Van der Laan, M.; Mastrullo, V.; Campagnolo, P.; et al. Mosquito Saliva Enhances Virus Infection through Sialokinin-Dependent Vascular Leakage. Proc. Natl. Acad. Sci. USA 2022, 119, e2114309119. [Google Scholar] [CrossRef] [PubMed]
- Schneider, B.S.; Soong, L.; Coffey, L.L.; Stevenson, H.L.; McGee, C.E.; Higgs, S. Aedes aegypti Saliva Alters Leukocyte Recruitment and Cytokine Signaling by Antigen-Presenting Cells during West Nile Virus Infection. PLoS ONE 2010, 5, e11704. [Google Scholar] [CrossRef]
- Shrivastava, G.; Valenzuela-Leon, P.C.; Botello, K.; Calvo, E. Aedes aegypti Saliva Modulates Inflammasome Activation and Facilitates Flavivirus Infection In Vitro. iScience 2023, 27, 108620. [Google Scholar] [CrossRef]
- Wichit, S.; Diop, F.; Hamel, R.; Talignani, L.; Ferraris, P.; Cornelie, S.; Liegeois, F.; Thomas, F.; Yssel, H.; Missé, D. Aedes aegypti Saliva Enhances Chikungunya Virus Replication in Human Skin Fibroblasts via Inhibition of the Type I Interferon Signaling Pathway. Infect. Genet. Evol. 2017, 55, 68–70. [Google Scholar] [CrossRef] [PubMed]
- Thangamani, S.; Higgs, S.; Ziegler, S.; Vanlandingham, D.; Tesh, R.; Wikel, S. Host Immune Response to Mosquito-Transmitted Chikungunya Virus Differs from That Elicited by Needle Inoculated Virus. PLoS ONE 2010, 5, e12137. [Google Scholar] [CrossRef] [PubMed]
- Bizzarro, B.; Barros, M.S.; Maciel, C.; Gueroni, D.I.; Lino, C.N.; Campopiano, J.; Kotsyfakis, M.; Amarante-Mendes, G.P.; Calvo, E.; Capurro, M.L.; et al. Effects of Aedes aegypti Salivary Components on Dendritic Cell and Lymphocyte Biology. Parasites Vectors 2013, 6, 329. [Google Scholar] [CrossRef] [PubMed]
- Wasserman, H.A.; Singh, S.; Champagne, D.E. Saliva of the Yellow Fever Mosquito, Aedes aegypti, Modulates Murine Lymphocyte Function. Parasite Immunol. 2004, 26, 295–306. [Google Scholar] [CrossRef]
- Vogt, M.B.; Lahon, A.; Arya, R.P.; Kneubehl, A.R.; Spencer Clinton, J.L.; Paust, S.; Rico-Hesse, R. Mosquito Saliva Alone Has Profound Effects on the Human Immune System. PLoS Negl. Trop. Dis. 2018, 12, e0006439. [Google Scholar] [CrossRef]
| Cell Type | Productively Infected | Activated | Main Immune Function(s) |
|---|---|---|---|
| Fibroblasts | Yes | Yes | Major viral replication site; produce cytokines and chemokines; act as long-term reservoirs contributing to chronic inflammation. |
| Macrophages | Yes | Yes | Participate in viral clearance; secrete inflammatory mediators; may harbor persistent viral RNA during chronic phase. |
| Monocytes | Unclear | Yes | Produce cytokines and chemokines; contribute to viral dissemination and CNS entry via the “Trojan horse” mechanism. |
| Langerhans cells | Unclear | Yes (antigen carriers) | Likely involved in viral transport to draining lymph nodes. |
| Plasmacytoid DCs (pDCs) | No | Yes | Detect infected cells and produce large amounts of type I interferons. |
| Conventional DCs (cDCs) | Unclear | Yes | Present antigens to T cells and bridge innate and adaptive responses. |
| Neutrophils | No | Yes | Release extracellular traps (NETs) with antiviral effects; excessive recruitment contributes to tissue damage. |
| NK and NKT-like cells | No | Yes | Contribute to infection control; persistent and prolonged activation; high cytotoxic potential; cause tissue damage; pathogenic role during the chronic phase. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, J.S.d.F.d.; Santos, L.M.S.d.; Ferreira Junior, C.V.; Pereira, N.d.S.; Yaochite, J.N.U.; Andrade Neto, V.F.d.; Guedes, P.M.d.M.; França, R.F.D.O.; Brito, R.M.d.M.; Nascimento, M.S.L. The Role of Innate Cells During Alphavirus Chikungunya Infection. Viruses 2025, 17, 1469. https://doi.org/10.3390/v17111469
Silva JSdFd, Santos LMSd, Ferreira Junior CV, Pereira NdS, Yaochite JNU, Andrade Neto VFd, Guedes PMdM, França RFDO, Brito RMdM, Nascimento MSL. The Role of Innate Cells During Alphavirus Chikungunya Infection. Viruses. 2025; 17(11):1469. https://doi.org/10.3390/v17111469
Chicago/Turabian StyleSilva, Juliane Santos de França da, Livian Maria Silva dos Santos, Célio Valdevino Ferreira Junior, Nathalie de Sena Pereira, Juliana Navarro Ueda Yaochite, Valter Ferreira de Andrade Neto, Paulo Marcos da Matta Guedes, Rafael Freitas De Oliveira França, Ramayana Morais de Medeiros Brito, and Manuela Sales Lima Nascimento. 2025. "The Role of Innate Cells During Alphavirus Chikungunya Infection" Viruses 17, no. 11: 1469. https://doi.org/10.3390/v17111469
APA StyleSilva, J. S. d. F. d., Santos, L. M. S. d., Ferreira Junior, C. V., Pereira, N. d. S., Yaochite, J. N. U., Andrade Neto, V. F. d., Guedes, P. M. d. M., França, R. F. D. O., Brito, R. M. d. M., & Nascimento, M. S. L. (2025). The Role of Innate Cells During Alphavirus Chikungunya Infection. Viruses, 17(11), 1469. https://doi.org/10.3390/v17111469







