Comparative Analysis of Extracellular Vesicle and Virus Co-Purified Fractions Produced by Contemporary Influenza A and B Viruses in Different Human Cell Lines
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines
2.2. Viruses
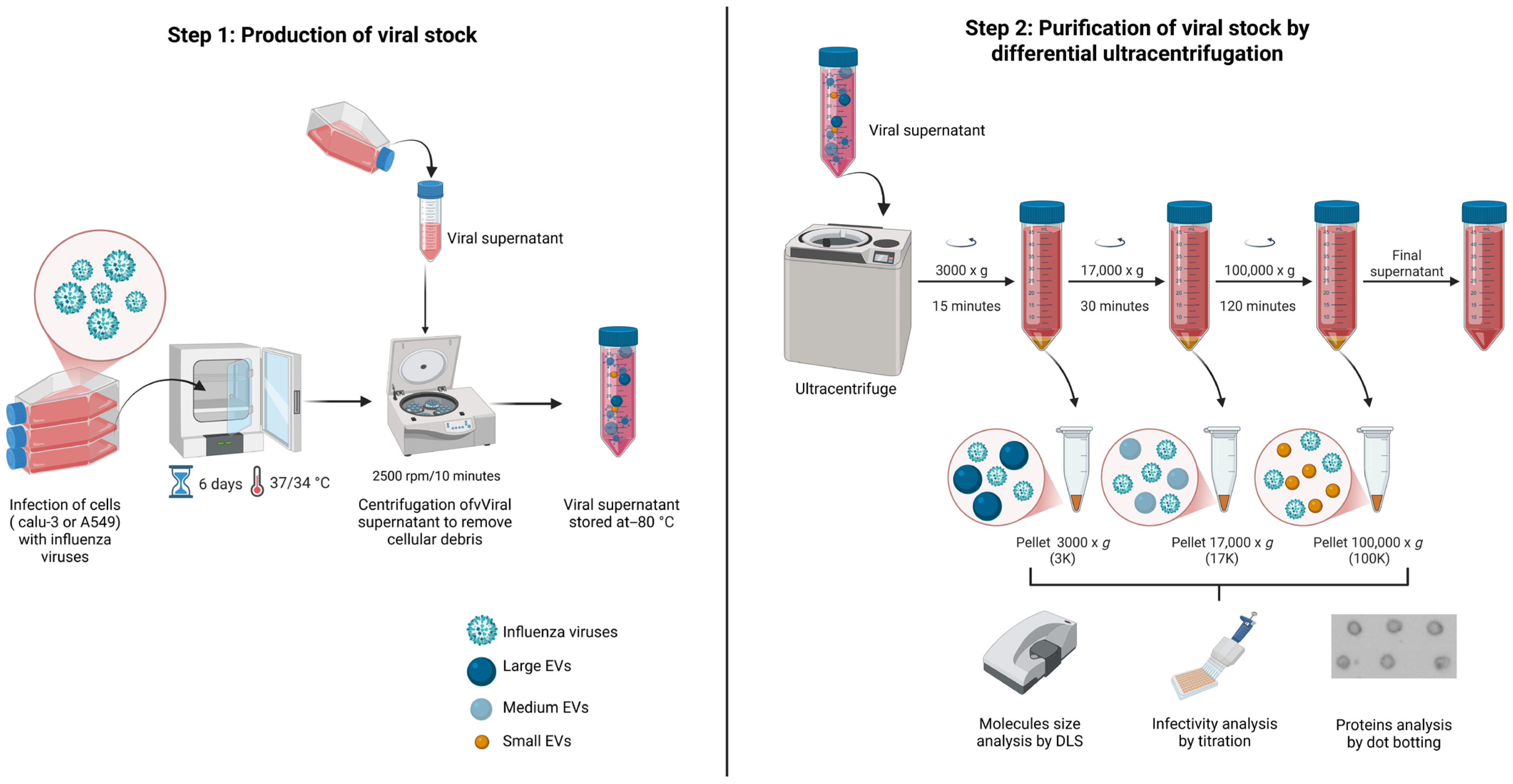
2.3. Stock Viral Production
2.4. Virus Titration
2.5. Purification of EVs by Ultracentrifugation
2.6. Dynamic Light Scattering Analysis
2.7. Antibodies Analyzed
2.8. Dot Blotting Assay
2.9. Statistical Analysis
3. Results
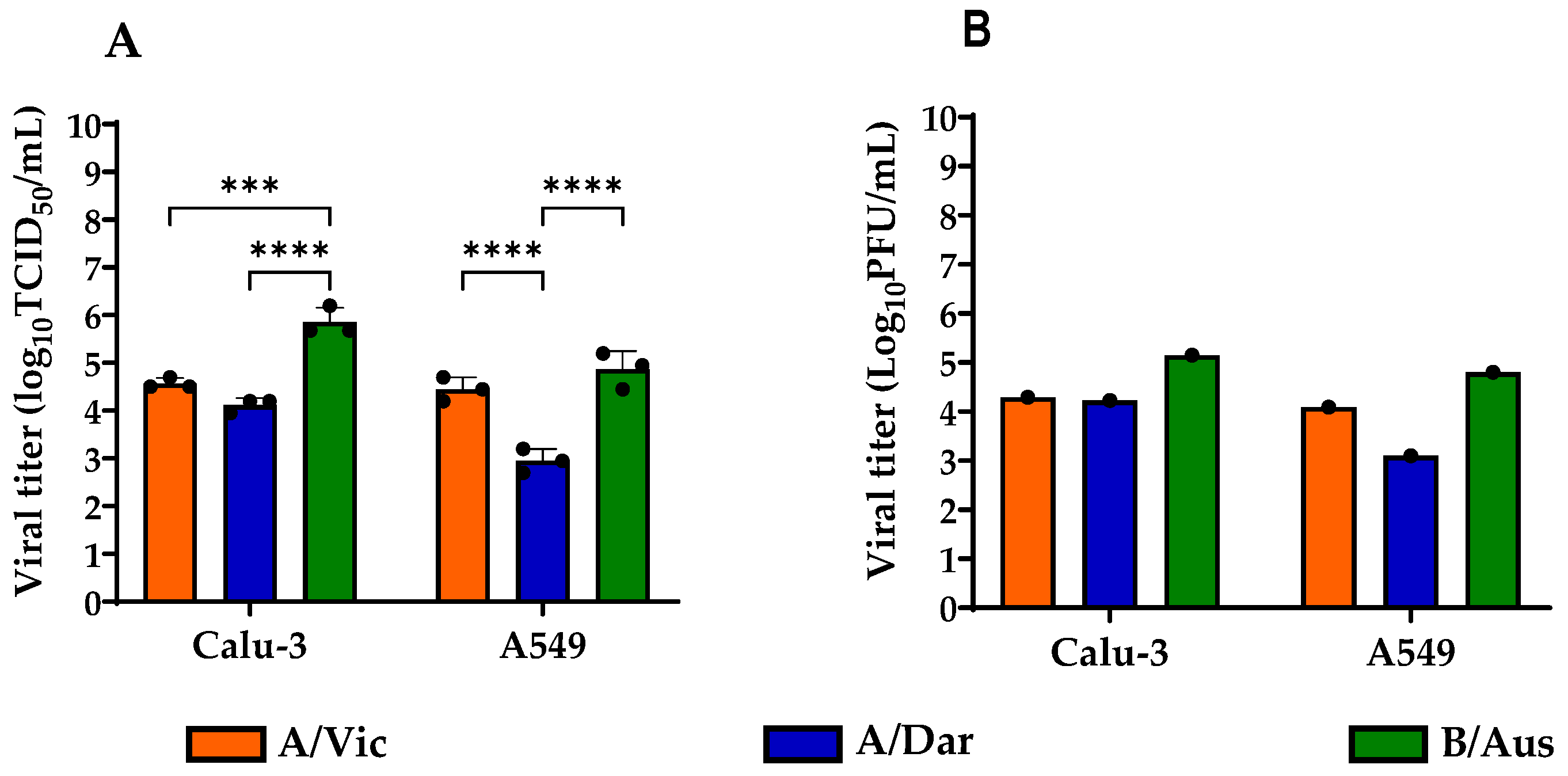
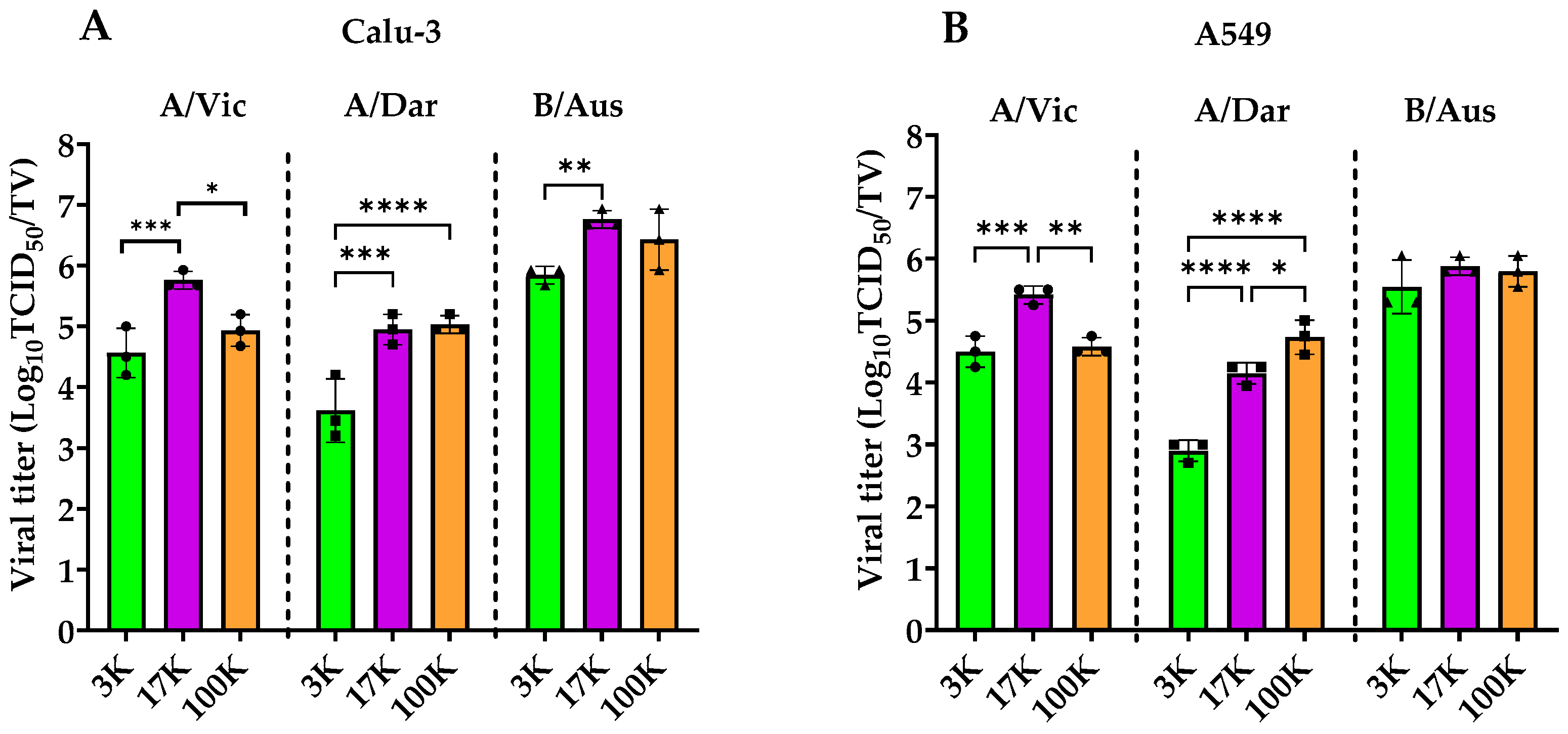
3.1. Viral Load Comparison of Influenza Strains
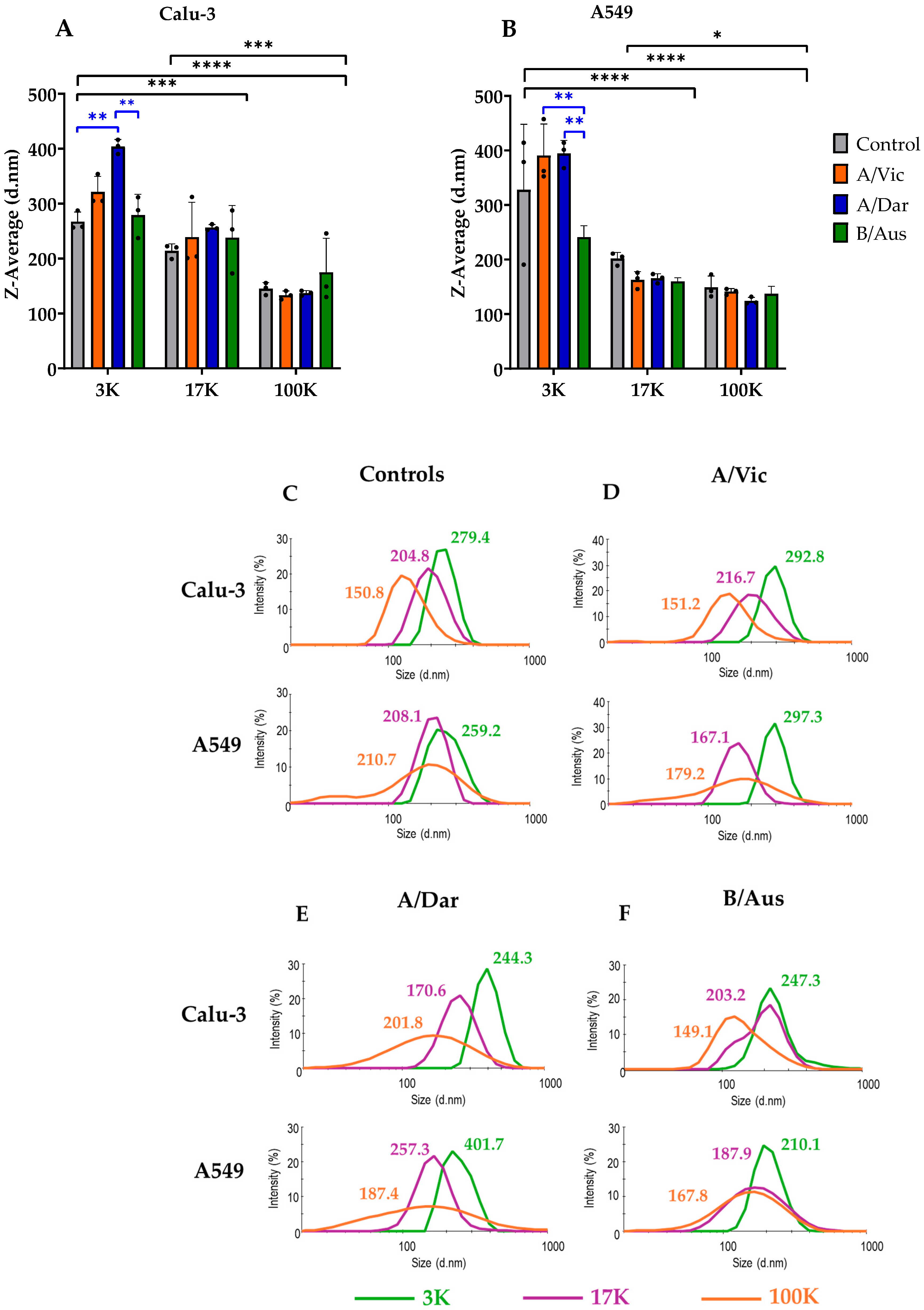
3.2. Analysis of Size Distribution and Heterogeneity of Co-Purified EVs and Viruses
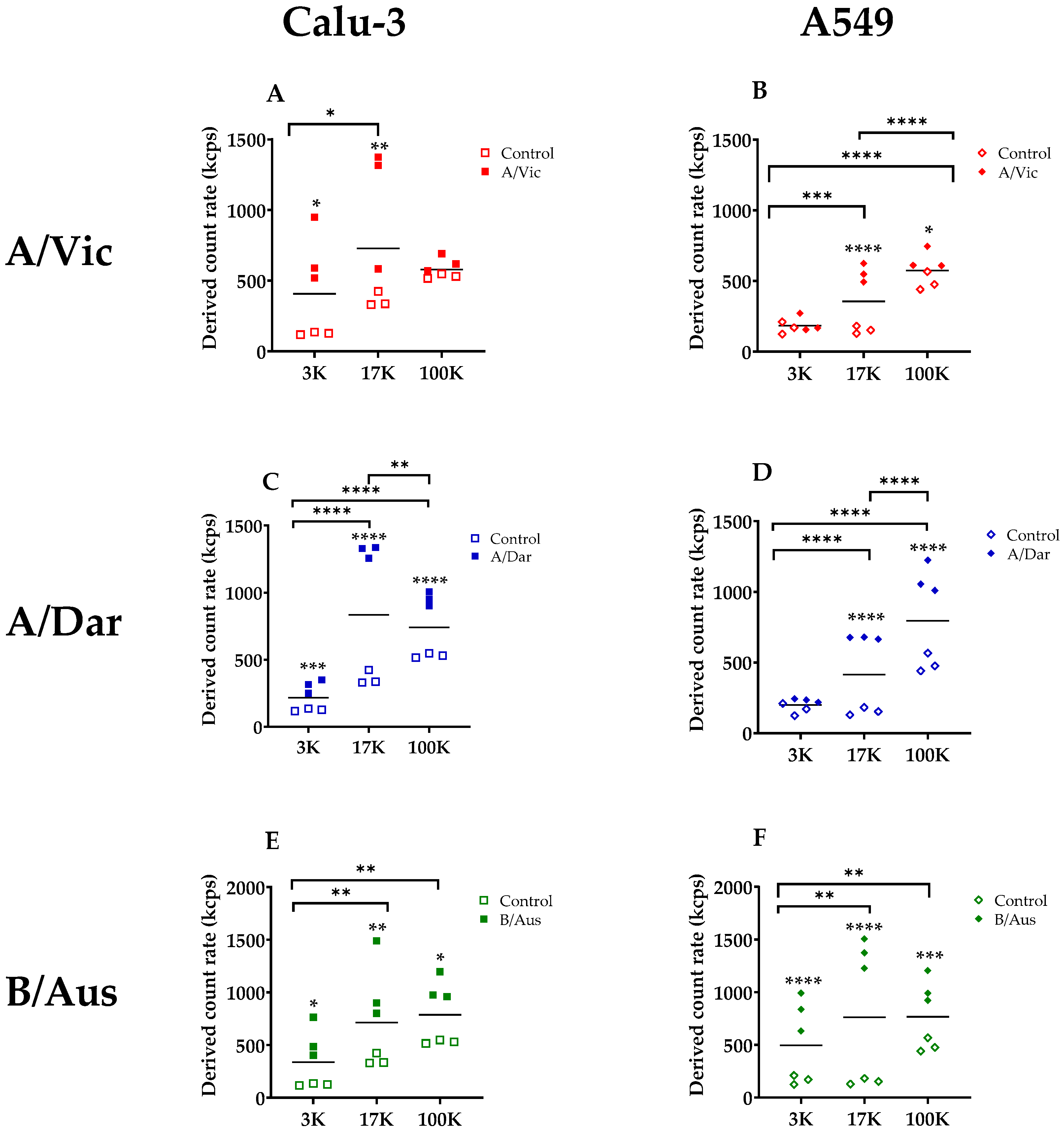
3.3. Relative Quantification of Nanoparticles in EV and Virus Pellets
3.4. Assessment of Infectivity in EV-Associated Fractions
3.5. Characterization of Co-Purified EVs and Viral Particles by Immunoblotting
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 3K | 3000× g |
| 17K | 17,000× g |
| 100K | 100,000× g |
| A/Vic | A/Victoria/4897/2022 (H1N1) |
| A/Dar | A/Darwin/9/2021 (H3N2) |
| B/Aus | B/Austria/1359417/2021 (B/Victoria) |
| DLS | Dynamic Light Scattering |
| EVs | Extracellular vesicles |
| HA | Hemagglutinin |
| ISEV | International Society for Extracellular Vesicles |
| MOI | Multiplicity of infection |
| NA | Neuraminidase |
| NP | Nucleoprotein |
| PDI | Polydispersity index |
| PFU | Plaque-forming units |
| TCID50 | 50% Tissue Culture Infectious Dose |
| TV | Total volume |
References
- WHO. Grippe Saisonnière. 2023. Available online: https://www.who.int/fr/news-room/fact-sheets/detail/influenza-(seasonal) (accessed on 28 February 2025).
- Wang, T.T.; Palese, P. Unraveling the mystery of swine influenza virus. Cell 2009, 137, 983–985. [Google Scholar] [CrossRef]
- Taubenberger, J.K.; Morens, D.M. 1918 Influenza: The Mother of All Pandemics. Emerg. Infect. Dis. 2006, 12, 15–22. [Google Scholar] [CrossRef]
- Vabret, A.; Dina, J.; Cuvillon-Nimal, D.; Nguyen, E.; Gouarin, S.; Petitjean, J.; Brouard, J.; Freymuth, F. La grippe saisonnière [Seasonal flu]. Pathol Biol 2010, 58, e51–e57. [Google Scholar] [CrossRef]
- Bouvier, N.M.; Palese, P. The biology of influenza viruses. Vaccine 2008, 26, D49–D53. [Google Scholar] [CrossRef]
- Maciej, B. Vaccination and antigenic drift in influenza. Vaccine 2008, 26, C8–C14. [Google Scholar] [CrossRef] [PubMed]
- Barr, I.G.; McCauley, J.; Cox, N.; Daniels, R.; Engelhardt, O.G.; Fukuda, K.; Grohmann, G.; Hay, A.; Kelso, A.; Klimov, A.; et al. Epidemiological, antigenic and genetic characteristics of seasonal influenza A(H1N1), A(H3N2) and B influenza viruses: Basis for the WHO recommendation on the composition of influenza vaccines for use in the 2009-2010 northern hemisphere season. Vaccine 2010, 28, 1156–1167. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.J.; Vijaykrishna, D.; Bahl, J.; Lycett, S.J.; Worobey, M.; Pybus, O.G.; Ma, S.K.; Cheung, C.L.; Raghwani, J.; Bhatt, S.; et al. Origins and evolutionary genomics of the 2009 swine-origin H1N1 influenza A epidemic. Nature 2009, 459, 1122–1125. [Google Scholar] [CrossRef] [PubMed]
- Bedford, J.G.; Infusini, G.; Dagley, L.F.; Villalon-Letelier, F.; Zheng, M.Z.M.; Bennett-Wood, V.; Reading, P.C.; Wakim, L.M. Airway Exosomes Released During Influenza Virus Infection Serve as a Key Component of the Antiviral Innate Immune Response. Front. Immunol. 2020, 11, 887. [Google Scholar] [CrossRef]
- Zabrodskaya, Y.; Plotnikova, M.; Gavrilova, N.; Lozhkov, A.; Klotchenko, S.; Kiselev, A.; Burdakov, V.; Ramsay, E.; Purvinsh, L.; Egorova, M.; et al. Exosomes Released by Influenza-Virus-Infected Cells Carry Factors Capable of Suppressing Immune Defense Genes in Naive Cells. Viruses 2022, 14, 2690. [Google Scholar] [CrossRef]
- Zheng, B.; Zhou, J.; Wang, H. Host microRNAs and exosomes that modulate influenza virus infection. Virus Res. 2020, 279, 197885. [Google Scholar] [CrossRef]
- Raposo, G.; Stoorvogel, W. Extracellular vesicles: Exosomes, microvesicles, and friends. J. Cell Biol. 2013, 200, 373–383. [Google Scholar] [CrossRef]
- Welsh, J.A.; Goberdhan, D.C.I.; O’Driscoll, L.; Buzas, E.I.; Blenkiron, C.; Bussolati, B.; Cai, H.; Di Vizio, D.; Driedonks, T.A.P.; Erdbrugger, U.; et al. Minimal information for studies of extracellular vesicles (MISEV2023): From basic to advanced approaches. J. Extracell. Vesicles 2024, 13, e12404. [Google Scholar] [CrossRef]
- Thery, C.; Amigorena, S.; Raposo, G.; Clayton, A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr. Protoc. Cell Biol. 2006, 30, 3–22. [Google Scholar] [CrossRef]
- Yanez-Mo, M.; Siljander, P.R.; Andreu, Z.; Zavec, A.B.; Borras, F.E.; Buzas, E.I.; Buzas, K.; Casal, E.; Cappello, F.; Carvalho, J.; et al. Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles 2015, 4, 27066. [Google Scholar] [CrossRef]
- Thery, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef]
- Gurunathan, S.; Kang, M.H.; Qasim, M.; Khan, K.; Kim, J.H. Biogenesis, Membrane Trafficking, Functions, and Next Generation Nanotherapeutics Medicine of Extracellular Vesicles. Int. J. Nanomed. 2021, 16, 3357–3383. [Google Scholar] [CrossRef] [PubMed]
- Doyle, L.M.; Wang, M.Z. Overview of Extracellular Vesicles, Their Origin, Composition, Purpose, and Methods for Exosome Isolation and Analysis. Cells 2019, 8, 727. [Google Scholar] [CrossRef] [PubMed]
- Butreddy, A.; Kommineni, N.; Dudhipala, N. Exosomes as Naturally Occurring Vehicles for Delivery of Biopharmaceuticals: Insights from Drug Delivery to Clinical Perspectives. Nanomaterials 2021, 11, 1481. [Google Scholar] [CrossRef]
- Serretiello, E.; Ballini, A.; Smimmo, A.; Acunzo, M.; Raimo, M.; Cantore, S.; Di Domenico, M. Extracellular Vesicles as a Translational Approach for the Treatment of COVID-19 Disease: An Updated Overview. Viruses 2023, 15, 1976. [Google Scholar] [CrossRef] [PubMed]
- van der Pol, E.; Böing, A.N.; Harrison, P.; Sturk, A.; Nieuwland, R. Classification, Functions, and Clinical Relevance of Extracellular Vesicles. Pharmacol. Rev. 2012, 64, 676–705. [Google Scholar] [CrossRef]
- Zhao, Z.; Wijerathne, H.; Godwin, A.K.; Soper, S.A. Isolation and analysis methods of extracellular vesicles (EVs). Extracell. Vesicles Circ. Nucl. Acids 2021, 2, 80–103. [Google Scholar] [CrossRef]
- Crescitelli, R.; Lasser, C.; Szabo, T.G.; Kittel, A.; Eldh, M.; Dianzani, I.; Buzas, E.I.; Lotvall, J. Distinct RNA profiles in subpopulations of extracellular vesicles: Apoptotic bodies, microvesicles and exosomes. J. Extracell. Vesicles 2013, 2, 20677. [Google Scholar] [CrossRef]
- Mitra, P.; Gupta, S.; Samal, P. Methods in Extracellular Vesicle Isolation, Characterization, and Production. In Extracellular Vesicles in Human Health and Diseases; Springer: Singapore, 2024; pp. 23–43. [Google Scholar]
- Boucher, J.; Pépin, G.; Goyer, B.; Hubert, A.; Bazié, W.W.; Vitry, J.; Barabé, F.; Gilbert, C. Exploring the relationship between extracellular vesicles, the dendritic cell immunoreceptor, and microRNA-155 in an in vivo model of HIV-1 infection to understand the disease and develop new treatments. FASEB J. 2025, 39, e70475. [Google Scholar] [CrossRef]
- Boucher, J.; Rousseau, A.; Boucher, C.; Subra, C.; Bazié, W.W.; Hubert, A.; Bourgeault, E.; Benmoussa, A.; Goyer, B.; Tessier, P.A.; et al. Immune Cells Release MicroRNA-155 Enriched Extracellular Vesicles That Promote HIV-1 Infection. Cells 2023, 12, 466. [Google Scholar] [CrossRef] [PubMed]
- Chahar, H.S.; Bao, X.; Casola, A. Exosomes and Their Role in the Life Cycle and Pathogenesis of RNA Viruses. Viruses 2015, 7, 3204–3225. [Google Scholar] [CrossRef]
- Wu, Q.; Glitscher, M.; Tonnemacher, S.; Schollmeier, A.; Raupach, J.; Zahn, T.; Eberle, R.; Krijnse-Locker, J.; Basic, M.; Hildt, E. Presence of Intact Hepatitis B Virions in Exosomes. Cell. Mol. Gastroenterol. Hepatol. 2023, 15, 237–259. [Google Scholar] [CrossRef]
- Jackson, H.K.; Long, H.M.; Yam-Puc, J.C.; Palmulli, R.; Haigh, T.A.; Gerber, P.P.; Lee, J.S.; Matheson, N.J.; Young, L.; Trowsdale, J.; et al. Bioengineered small extracellular vesicles deliver multiple SARS-CoV-2 antigenic fragments and drive a broad immunological response. J. Extracell. Vesicles 2024, 13, e12412. [Google Scholar] [CrossRef]
- Kwasnik, M.; Socha, W.; Czech, B.; Wasiak, M.; Rola, J.; Rozek, W. Protein-Coding Region Derived Small RNA in Exosomes from Influenza A Virus-Infected Cells. Int. J. Mol. Sci. 2023, 24, 867. [Google Scholar] [CrossRef] [PubMed]
- Keshavarz, M.; Dianat-Moghadam, H.; Sofiani, V.H.; Karimzadeh, M.; Zargar, M.; Moghoofei, M.; Biglari, H.; Ghorbani, S.; Nahand, J.S.; Mirzaei, H. miRNA-based strategy for modulation of influenza A virus infection. Epigenomics 2018, 10, 829–844. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Liu, K.; Chi, Y.; Zhu, X.; Wu, T.; Zhao, K.; Qiao, Q.; Wu, B.; Zhu, F.; Cui, L. Exosomal microRNA expression profiles derived from A549 human lung cells in response to influenza A/H1N1pdm09 infection. Virology 2022, 574, 9–17. [Google Scholar] [CrossRef]
- Ujie, M.; Takada, K.; Kiso, M.; Sakai-Tagawa, Y.; Ito, M.; Nakamura, K.; Watanabe, S.; Imai, M.; Kawaoka, Y. Long-term culture of human lung adenocarcinoma A549 cells enhances the replication of human influenza A viruses. J. Gen. Virol. 2019, 100, 1345–1349. [Google Scholar] [CrossRef]
- Zhu, Y.; Chidekel, A.; Shaffer, T.H. Cultured human airway epithelial cells (calu-3): A model of human respiratory function, structure, and inflammatory responses. Crit. Care Res. Pract. 2010, 2010, 394578. [Google Scholar] [CrossRef]
- Hatakeyama, S.; Sakai-Tagawa, Y.; Kiso, M.; Goto, H.; Kawakami, C.; Mitamura, K.; Sugaya, N.; Suzuki, Y.; Kawaoka, Y. Enhanced Expression of an α2,6-Linked Sialic Acid on MDCK Cells Improves Isolation of Human Influenza Viruses and Evaluation of Their Sensitivity to a Neuraminidase Inhibitor. J. Clin. Microbiol. 2005, 43, 4139–4146. [Google Scholar] [CrossRef] [PubMed]
- Baz, M. Zika Virus Isolation, Purification, and Titration. In Methods in Molecular Biology; Kobinger, G., Racine, T., Eds.; Humana: New York, NY, USA, 2020; Volume 2142. [Google Scholar]
- Reed, L.J.; Muench, H. A Simple Method Of Estimating Fifty Per Cent Endpoints. Am. J. Epidemiol. 1938, 27, 493–497. [Google Scholar] [CrossRef]
- Heinemann, M.L.; Ilmer, M.; Silva, L.P.; Hawke, D.H.; Recio, A.; Vorontsova, M.A.; Alt, E.; Vykoukal, J. Benchtop isolation and characterization of functional exosomes by sequential filtration. J. Chromatogr. A 2014, 1371, 125–135. [Google Scholar] [CrossRef]
- CDC. Pandémie de Grippe H1N1 2009 (Virus H1N1pdm09). 2009. Available online: https://archive.cdc.gov/www_cdc_gov/flu/pandemic-resources/2009-h1n1-pandemic.html (accessed on 5 November 2024).
- Adeola, O.A.; Olugasa, B.O.; Emikpe, B.O. Detection of pandemic strain of influenza virus (A/H1N1/pdm09) in pigs, West Africa: Implications and considerations for prevention of future influenza pandemics at the source. Infect. Ecol. Epidemiol. 2015, 5, 30227. [Google Scholar] [CrossRef] [PubMed]
- Lorusso, A.; Faaberg, K.S.; Killian, M.L.; Koster, L.; Vincent, A.L. One-step real-time RT-PCR for pandemic influenza A virus (H1N1) 2009 matrix gene detection in swine samples. J. Virol. Methods 2010, 164, 83–87. [Google Scholar] [CrossRef]
- Tambyah, P.A.; Sepramaniam, S.; Mohamed Ali, J.; Chai, S.C.; Swaminathan, P.; Armugam, A.; Jeyaseelan, K. microRNAs in circulation are altered in response to influenza A virus infection in humans. PLoS ONE 2013, 8, e76811. [Google Scholar] [CrossRef]
- Zaborowski, M.P.; Balaj, L.; Breakefield, X.O.; Lai, C.P. Extracellular Vesicles: Composition, Biological Relevance, and Methods of Study. Bioscience 2015, 65, 783–797. [Google Scholar] [CrossRef] [PubMed]
- Gardiner, C.; Di Vizio, D.; Sahoo, S.; Théry, C.; Witwer, K.W.; Wauben, M.; Hill, A.F. Techniques used for the isolation and characterization of extracellular vesicles: Results of a worldwide survey. J. Extracell. Vesicles 2016, 5, 32945. [Google Scholar] [CrossRef]
- Jiang, Y.; Cai, X.; Yao, J.; Guo, H.; Yin, L.; Leung, W.; Xu, C. Role of Extracellular Vesicles in Influenza Virus Infection. Front. Cell. Infect. Microbiol. 2020, 10, 366. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.M.; Tseng, C.H.; Chen, Y.C.; Yu, W.Y.; Ho, M.Y.; Ho, C.Y.; Lai, M.M.C.; Su, W.C. Exosome-delivered and Y RNA-derived small RNA suppresses influenza virus replication. J. Biomed. Sci. 2019, 26, 58. [Google Scholar] [CrossRef]
- Fening, S.W.; Jollick, J.A.; Huang, Y.T. Calu-3/A-549 mixed cells as a replacement for primary rhesus monkey kidney cells for virus detection. J. Clin. Virol. 2008, 42, 254–259. [Google Scholar] [CrossRef]
- Knepper, J.; Schierhorn, K.L.; Becher, A.; Budt, M.; Tonnies, M.; Bauer, T.T.; Schneider, P.; Neudecker, J.; Ruckert, J.C.; Gruber, A.D.; et al. The novel human influenza A(H7N9) virus is naturally adapted to efficient growth in human lung tissue. mBio 2013, 4, e00601–e00613. [Google Scholar] [CrossRef] [PubMed]
- van Niel, G.; D’Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef]
- McNamara, R.P.; Dittmer, D.P. Modern Techniques for the Isolation of Extracellular Vesicles and Viruses. J. Neuroimmune Pharmacol. 2020, 15, 459–472. [Google Scholar] [CrossRef] [PubMed]
- Vajda, J.; Weber, D.; Brekel, D.; Hundt, B.; Muller, E. Size distribution analysis of influenza virus particles using size exclusion chromatography. J. Chromatogr. A 2016, 1465, 117–125. [Google Scholar] [CrossRef]
- Bazie, W.W.; Boucher, J.; Vitry, J.; Goyer, B.; Routy, J.P.; Tremblay, C.; Trottier, S.; Jenabian, M.A.; Provost, P.; Alary, M.; et al. Plasma Extracellular Vesicle Subtypes May be Useful as Potential Biomarkers of Immune Activation in People With HIV. Pathog. Immun. 2021, 6, 1–28. [Google Scholar] [CrossRef]
- Akers, J.C.; Gonda, D.; Kim, R.; Carter, B.S.; Chen, C.C. Biogenesis of extracellular vesicles (EV): Exosomes, microvesicles, retrovirus-like vesicles, and apoptotic bodies. J. Neurooncol 2013, 113, 1–11. [Google Scholar] [CrossRef]
- Caobi, A.; Nair, M.; Raymond, A.D. Extracellular Vesicles in the Pathogenesis of Viral Infections in Humans. Viruses 2020, 12, 1200. [Google Scholar] [CrossRef]
- Vaillancourt, M.; Hubert, A.; Subra, C.; Boucher, J.; Bazie, W.W.; Vitry, J.; Berrazouane, S.; Routy, J.P.; Trottier, S.; Tremblay, C.; et al. Velocity Gradient Separation Reveals a New Extracellular Vesicle Population Enriched in miR-155 and Mitochondrial DNA. Pathogens 2021, 10, 526. [Google Scholar] [CrossRef]
- Böing, A.N.; van der Pol, E.; Grootemaat, A.E.; Coumans, F.A.; Sturk, A.; Nieuwland, R. Single-step isolation of extracellular vesicles by size-exclusion chromatography. J. Extracell. Vesicles 2014, 3, 23430. [Google Scholar] [CrossRef]
- Hong, Y.; Truong, A.D.; Vu, T.H.; Lee, S.; Heo, J.; Kang, S.; Lillehoj, H.S.; Hong, Y.H. Exosomes from H5N1 avian influenza virus-infected chickens regulate antiviral immune responses of chicken immune cells. Dev. Comp. Immunol. 2022, 130, 104368. [Google Scholar] [CrossRef]
- Ao, J.; Ma, A.X.; Li, J.; Wang, C.Y.; Fu, D.D.; Du, L.; Yu, C.; Liu, S.L.; Wang, Z.G.; Pang, D.W. Real-Time Dissection of the Exosome Pathway for Influenza Virus Infection. ACS Nano 2024, 18, 4507–4519. [Google Scholar] [CrossRef] [PubMed]
- Xia, B.; Pan, X.; Luo, R.H.; Shen, X.; Li, S.; Wang, Y.; Zuo, X.; Wu, Y.; Guo, Y.; Xiao, G.; et al. Extracellular vesicles mediate antibody-resistant transmission of SARS-CoV-2. Cell Discov. 2023, 9, 2. [Google Scholar] [CrossRef] [PubMed]
- Kugeratski, F.G.; Hodge, K.; Lilla, S.; McAndrews, K.M.; Zhou, X.; Hwang, R.F.; Zanivan, S.; Kalluri, R. Quantitative proteomics identifies the core proteome of exosomes with syntenin-1 as the highest abundant protein and a putative universal biomarker. Nat. Cell Biol. 2021, 23, 631–641. [Google Scholar] [CrossRef] [PubMed]

| Virus and Cell Lines | A/Vic | A/Dar | B/Aus |
|---|---|---|---|
| Calu-3 | 0.08 | 0.01 | 0.01 |
| A549 | 0.8 | 0.1 | 0.1 |
| Fractions | Calu-3 | A549 | |
|---|---|---|---|
| Control | 3K | 0.31 | 0.38 |
| 17K | 0.27 | 0.28 | |
| 100K | 0.24 | 0.35 | |
| A/Vic | 3K | 0.31 | 0.37 |
| 17K | 0.28 | 0.21 | |
| 100K | 0.18 | 0.36 | |
| A/Dar | 3K | 0.25 | 0.53 |
| 17K | 0.24 | 0.26 | |
| 100K | 0.26 | 0.41 | |
| B/Aus | 3K | 0.36 | 0.32 |
| 17K | 0.28 | 0.19 | |
| 100K | 0.27 | 0.25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wantchecon, A.; Boucher, J.; Rabezanahary, H.; Gilbert, C.; Baz, M. Comparative Analysis of Extracellular Vesicle and Virus Co-Purified Fractions Produced by Contemporary Influenza A and B Viruses in Different Human Cell Lines. Viruses 2025, 17, 1470. https://doi.org/10.3390/v17111470
Wantchecon A, Boucher J, Rabezanahary H, Gilbert C, Baz M. Comparative Analysis of Extracellular Vesicle and Virus Co-Purified Fractions Produced by Contemporary Influenza A and B Viruses in Different Human Cell Lines. Viruses. 2025; 17(11):1470. https://doi.org/10.3390/v17111470
Chicago/Turabian StyleWantchecon, Aude, Julien Boucher, Henintsoa Rabezanahary, Caroline Gilbert, and Mariana Baz. 2025. "Comparative Analysis of Extracellular Vesicle and Virus Co-Purified Fractions Produced by Contemporary Influenza A and B Viruses in Different Human Cell Lines" Viruses 17, no. 11: 1470. https://doi.org/10.3390/v17111470
APA StyleWantchecon, A., Boucher, J., Rabezanahary, H., Gilbert, C., & Baz, M. (2025). Comparative Analysis of Extracellular Vesicle and Virus Co-Purified Fractions Produced by Contemporary Influenza A and B Viruses in Different Human Cell Lines. Viruses, 17(11), 1470. https://doi.org/10.3390/v17111470







